Abstract
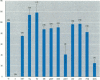
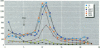
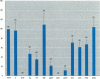
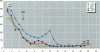
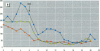
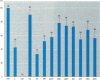
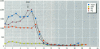
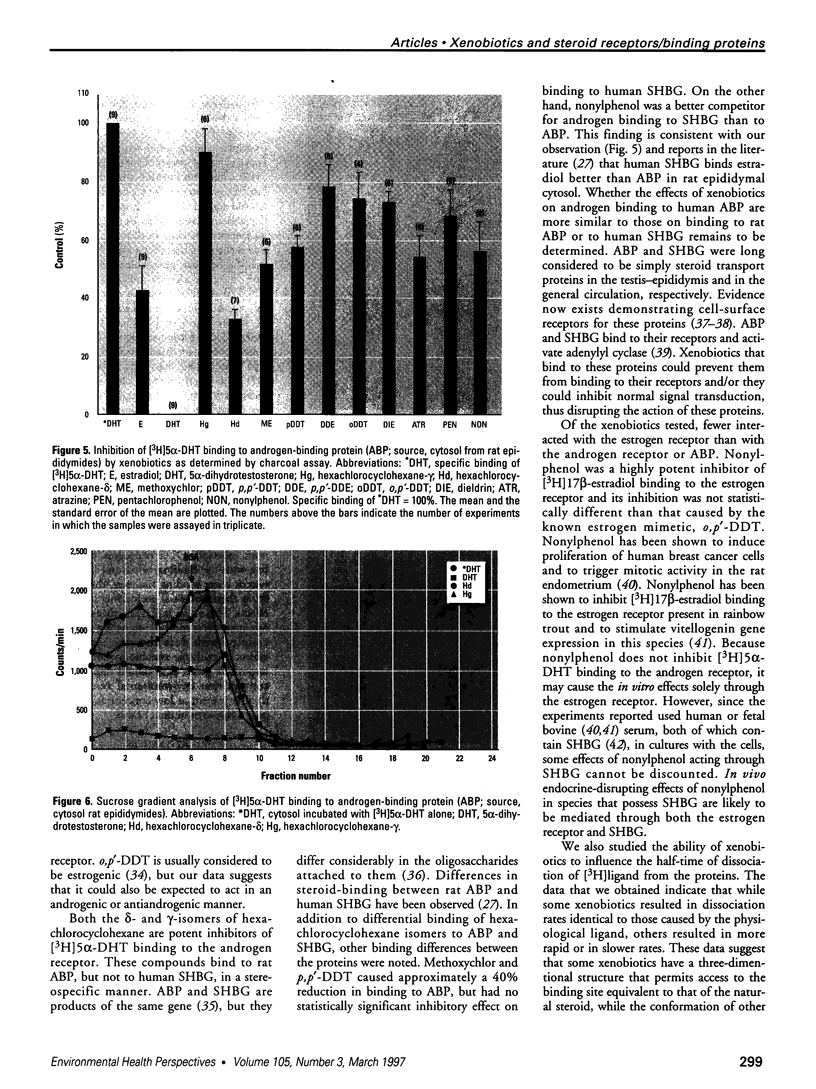
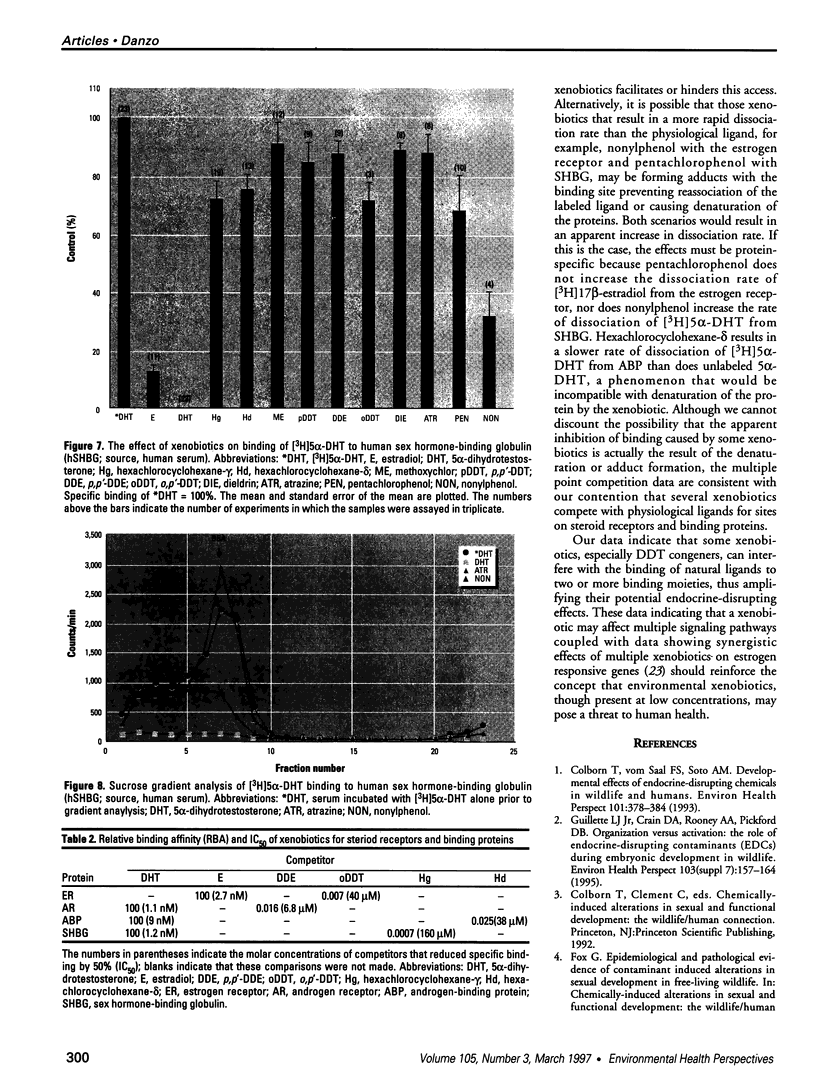
The disruption of the reproductive system of male and female animals in the wild has been attributed to environmental chemicals (xenobiotics). The effects seen mirror alterations one might anticipate if the steroid hormone-dependent processes that regulate these systems were impaired. To determine whether xenobiotics (present at a concentration of 100 microM) exert their action through steroid-mediated pathways, we examined their ability to inhibit the binding of [3H]physiological ligands (present at a concentration of 7 nM) to the androgen and estrogen receptors, rat androgen-binding protein (ABP), and human sex hormone-binding globulin (hSHBG). The gamma- and delta-isomers of hexachlorocyclohexane, congeners of dichlorodiphenyl-trichloroethane (DDT; p,p'-DDT; p,p'-DDE; o,p'-DDT), dieldrin, atrazine, and pentachlorophenol, caused a statistically significant inhibition of specific binding of [3H]5 alpha-DHT to the androgen receptor that ranged from 100% (p,p'-DDE) to 25% (dieldrin). Methoxychlor, o,p'-DDT1, pentachlorophenol, and nonylphenol significantly reduced [3H]17 beta-estradiol binding to the estrogen receptor by 10, 60, 20, and 75%, respectively. The binding of [3H]5 alpha-DHT to ABP was inhibited 70% by the delta-isomer of hexachlorocyclohexane, but the gamma-isomer did not reduce binding significantly. Methoxychlor, p,p'-DDT, atrazine, and nonylphenol reduced [3H]5 alpha-DHT binding to ABP by approximately 40%. Nonylphenol reduced the binding of [3H]5 alpha-DHT to hSHBG by 70%. Hexachlorocyclohexane reduced [3H]5 alpha-DHT binding to hSHBG by 20%, but the stereospecific effects observed with ABP did not occur. o,p'-DDT and pentachlorophenol resulted in a statistically significant 20% inhibition of [3H]5 alpha-DHT binding to hSHBG. Some xenobiotics resulted in dissociation of [3H]ligands from their binding proteins that was statistically identical to that caused by the unlabeled natural ligand, whereas others resulted in slower or more rapid dissociation rates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. R., Hargraves W. A., Hsia M. T., Lin F. S. Comparative toxicology of chlorinated compounds on mammalian species. Pharmacol Ther. 1979;7(3):513–547. doi: 10.1016/0163-7258(79)90041-x. [DOI] [PubMed] [Google Scholar]
- Arnold S. F., Klotz D. M., Collins B. M., Vonier P. M., Guillette L. J., Jr, McLachlan J. A. Synergistic activation of estrogen receptor with combinations of environmental chemicals. Science. 1996 Jun 7;272(5267):1489–1492. doi: 10.1126/science.272.5267.1489. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bibbo M., Gill W. B., Azizi F., Blough R., Fang V. S., Rosenfield R. L., Schumacher G. F., Sleeper K., Sonek M. G., Wied G. L. Follow-up study of male and female offspring of DES-exposed mothers. Obstet Gynecol. 1977 Jan;49(1):1–8. [PubMed] [Google Scholar]
- Bonne C., Raynaud J. P. Assay of androgen binding sites by exchange with methyltrienolone (R 1881). Steroids. 1976 Apr;27(4):497–507. doi: 10.1016/0039-128x(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Bulger W. H., Muccitelli R. M., Kupfer D. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 1978;27(20):2417–2423. doi: 10.1016/0006-2952(78)90354-4. [DOI] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilvers C., Pike M. C., Forman D., Fogelman K., Wadsworth M. E. Apparent doubling of frequency of undescended testis in England and Wales in 1962-81. Lancet. 1984 Aug 11;2(8398):330–332. doi: 10.1016/s0140-6736(84)92697-7. [DOI] [PubMed] [Google Scholar]
- Colborn T., vom Saal F. S., Soto A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993 Oct;101(5):378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol P., Bardin C. W. Species distribution of testosterone-binding globulin. Biol Reprod. 1973 Apr;8(3):277–282. doi: 10.1093/biolreprod/8.3.277. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Black J. H., Bell B. W. Analysis of the oligosaccharides on androgen-binding proteins: implications concerning their role in structure/function relationships. J Steroid Biochem Mol Biol. 1991;40(4-6):821–831. doi: 10.1016/0960-0760(91)90308-r. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Joseph D. R. Structure-function relationships of rat androgen--binding protein/human sex-hormone binding globulin: the effect of mutagenesis on steroid-binding parameters. Endocrinology. 1994 Jul;135(1):157–167. doi: 10.1210/endo.135.1.8013348. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., St Raymond P. A., Davies J. Hormonally responsive areas of the reproductive system of the male guinea pig. III. Presence of cytoplasmic estrogen receptors. Biol Reprod. 1981 Dec;25(5):1159–1168. doi: 10.1095/biolreprod25.5.1159. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Taylor C. A., Jr, Eller B. C. Some physicochemical characteristics of photoaffinity-labeled rabbit testosterone-binding globulin. Endocrinology. 1982 Oct;111(4):1278–1285. doi: 10.1210/endo-111-4-1278. [DOI] [PubMed] [Google Scholar]
- Davies J., Russell M., Davenport G. R. Effects of maternal administration of diethylstilbestrol and estradiol on the newborn guinea pig. Acta Anat (Basel) 1985;122(1):39–61. doi: 10.1159/000145983. [DOI] [PubMed] [Google Scholar]
- Gill W. B., Schumacher G. F., Bibbo M. Structural and functional abnormalities in the sex organs of male offspring of mothers treated with diethylstilbestrol (DES). J Reprod Med. 1976 Apr;16(4):147–153. [PubMed] [Google Scholar]
- Giwercman A., Skakkebaek N. E. The human testis--an organ at risk? Int J Androl. 1992 Oct;15(5):373–375. doi: 10.1111/j.1365-2605.1992.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Crain D. A., Rooney A. A., Pickford D. B. Organization versus activation: the role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ Health Perspect. 1995 Oct;103 (Suppl 7):157–164. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Pickford D. B., Crain D. A., Rooney A. A., Percival H. F. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol. 1996 Jan;101(1):32–42. doi: 10.1006/gcen.1996.0005. [DOI] [PubMed] [Google Scholar]
- Heyns W., De Moor P. Prostatic binding protein. A steriod-binding protein secreted by rat prostate. Eur J Biochem. 1977 Aug 15;78(1):221–230. doi: 10.1111/j.1432-1033.1977.tb11733.x. [DOI] [PubMed] [Google Scholar]
- Hryb D. J., Khan M. S., Romas N. A., Rosner W. Solubilization and partial characterization of the sex hormone-binding globulin receptor from human prostate. J Biol Chem. 1989 Apr 5;264(10):5378–5383. [PubMed] [Google Scholar]
- Joseph D. R. Structure, function, and regulation of androgen-binding protein/sex hormone-binding globulin. Vitam Horm. 1994;49:197–280. doi: 10.1016/s0083-6729(08)61148-6. [DOI] [PubMed] [Google Scholar]
- Kelce W. R., Stone C. R., Laws S. C., Gray L. E., Kemppainen J. A., Wilson E. M. Persistent DDT metabolite p,p'-DDE is a potent androgen receptor antagonist. Nature. 1995 Jun 15;375(6532):581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Korach K. S., Sarver P., Chae K., McLachlan J. A., McKinney J. D. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Mol Pharmacol. 1988 Jan;33(1):120–126. [PubMed] [Google Scholar]
- Krupenko S. A., Krupenko N. I., Danzo B. J. Interaction of sex hormone-binding globulin with plasma membranes from the rat epididymis and other tissues. J Steroid Biochem Mol Biol. 1994 Oct;51(1-2):115–124. doi: 10.1016/0960-0760(94)90122-8. [DOI] [PubMed] [Google Scholar]
- McLachlan J. A., Newbold R. R., Bullock B. Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science. 1975 Dec 5;190(4218):991–992. doi: 10.1126/science.242076. [DOI] [PubMed] [Google Scholar]
- Nakhla A. M., Khan M. S., Romas N. P., Rosner W. Estradiol causes the rapid accumulation of cAMP in human prostate. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5402–5405. doi: 10.1073/pnas.91.12.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A. Effects of dichlorodiphenyltrichloroethane (DDT) analogs and polychlorinated biphenyl (PCB) mixtures on 17beta-(3H)estradiol binding to rat uterine receptor. Biochem Pharmacol. 1974 Jan 15;23(2):447–451. doi: 10.1016/0006-2952(74)90436-5. [DOI] [PubMed] [Google Scholar]
- Robison A. K., Schmidt W. A., Stancel G. M. Estrogenic activity of DDT: estrogen-receptor profiles and the responses of individual uterine cell types following o,p'-DDT administration. J Toxicol Environ Health. 1985;16(3-4):493–508. doi: 10.1080/15287398509530758. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993 May 29;341(8857):1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. A., Jr, Smith H. E., Danzo B. J. Characterization of androgen binding protein in rat epididymal cytosol using a photoaffinity ligand. J Biol Chem. 1980 Aug 25;255(16):7769–7773. [PubMed] [Google Scholar]
- Toney T. W., Danzo B. J. Developmental changes in and hormonal regulation of estrogen and androgen receptors present in the rabbit epididymis. Biol Reprod. 1988 Nov;39(4):818–828. doi: 10.1095/biolreprod39.4.818. [DOI] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]