Abstract
This article consists of two parts: a brief overview of the ways in which free radicals can be involved in chemical carcinogenesis, and a review of cigarette smoke chemistry. Carcinogenesis is generally agreed to involve at least three stages: initiation, promotion, and progression. It is suggested that radicals sometimes are involved in the initiation step, either in the oxidative activation of a procarcinogen (such as benzo[a]pyrene) to its carcinogenic form or in the binding of the carcinogenic species to DNA, or both. The fraction of initiation events that involve radicals, as opposed to two-electron steps, is not known, but radicals probably are involved in a substantial number, although probably not a majority, of cancer initiation reactions. Promotion always involves radicals, at least to some extent. Progression probably does not normally involve radicals. The second part of this article reviews the molecular mechanisms involved in cigarette-induced tumors, particularly by aqueous cigarette tar (ACT) extracts and by a model of these solutions, aged solutions of catechol. ACT solutions as well as aged solutions of catechol contain a quinone-hydroquinone-semiquinone system that can reduce oxygen to produce superoxide and hence hydrogen peroxide and the hydroxyl radical. Both the cigarette tar radical and the catechol-derived radical can penetrate viable cells, bind to DNA, and cause nicks.
Full text
PDF

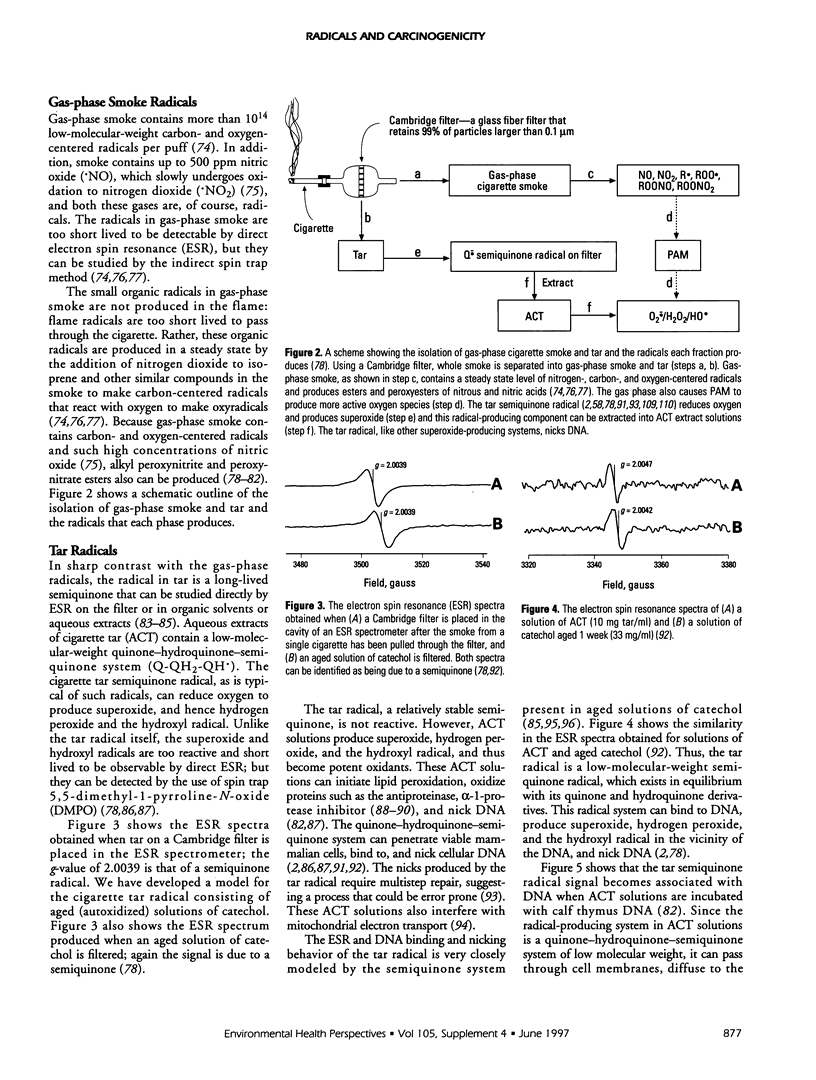
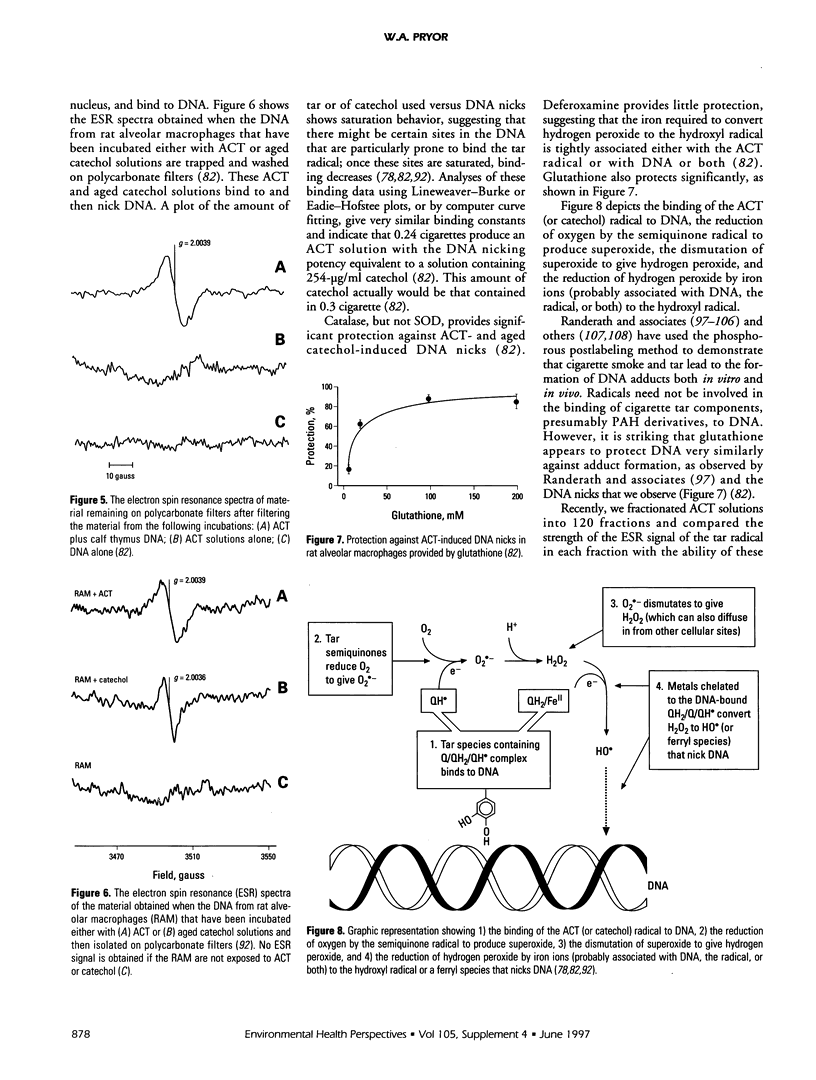
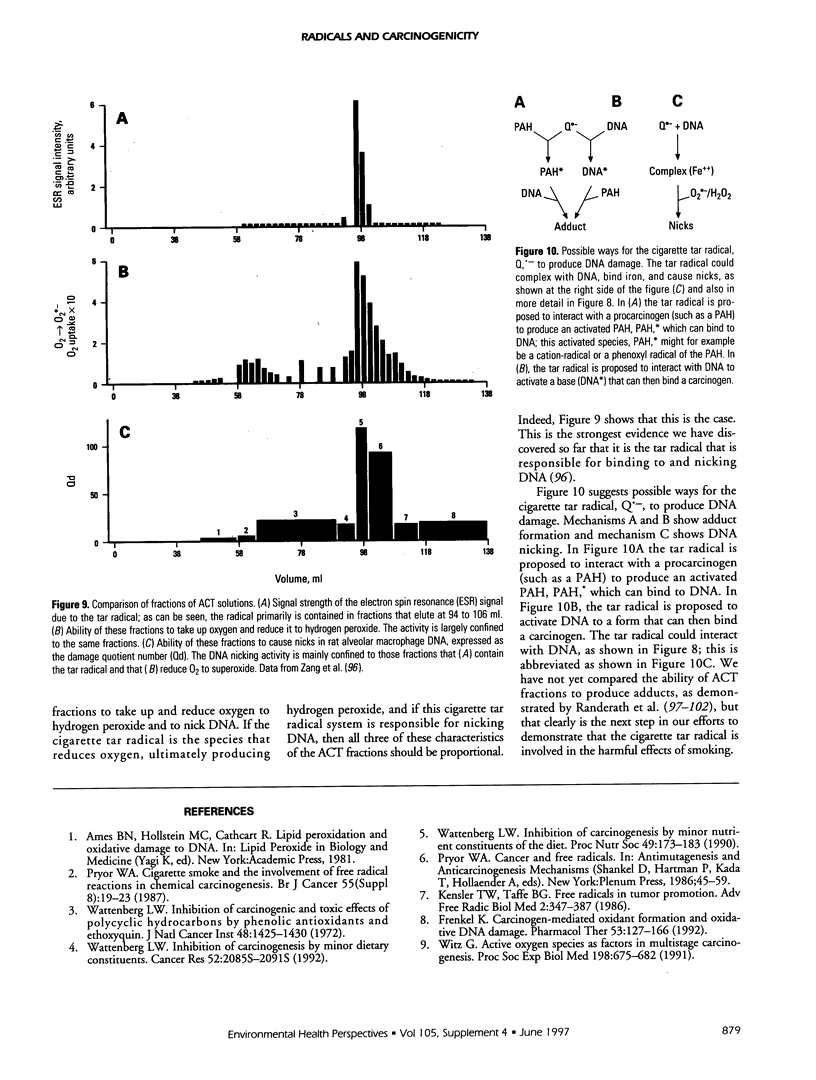



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard J. P., Royall D., Kurian R., Muggli R., Jeejeebhoy K. N. Effects of beta-carotene supplementation on lipid peroxidation in humans. Am J Clin Nutr. 1994 Apr;59(4):884–890. doi: 10.1093/ajcn/59.4.884. [DOI] [PubMed] [Google Scholar]
- Augusto O., Cavalieri E. L., Rogan E. G., RamaKrishna N. V., Kolar C. Formation of 8-methylguanine as a result of DNA alkylation by methyl radicals generated during horseradish peroxidase-catalyzed oxidation of methylhydrazine. J Biol Chem. 1990 Dec 25;265(36):22093–22096. [PubMed] [Google Scholar]
- Battista J. R., Marnett L. J. Prostaglandin H synthase-dependent epoxidation of aflatoxin B1. Carcinogenesis. 1985 Aug;6(8):1227–1229. doi: 10.1093/carcin/6.8.1227. [DOI] [PubMed] [Google Scholar]
- Benner S. E., Hong W. K. Clinical chemoprevention: developing a cancer prevention strategy. J Natl Cancer Inst. 1993 Sep 15;85(18):1446–1447. doi: 10.1093/jnci/85.18.1446. [DOI] [PubMed] [Google Scholar]
- Benner S. E., Wargovich M. J., Lippman S. M., Fisher R., Velasco M., Winn R. J., Hong W. K. Reduction in oral mucosa micronuclei frequency following alpha-tocopherol treatment of oral leukoplakia. Cancer Epidemiol Biomarkers Prev. 1994 Jan-Feb;3(1):73–76. [PubMed] [Google Scholar]
- Birnboim H. C. DNA strand breaks in human leukocytes induced by superoxide anion, hydrogen peroxide and tumor promoters are repaired slowly compared to breaks induced by ionizing radiation. Carcinogenesis. 1986 Sep;7(9):1511–1517. doi: 10.1093/carcin/7.9.1511. [DOI] [PubMed] [Google Scholar]
- Blot W. J., Li J. Y., Taylor P. R., Guo W., Dawsey S., Wang G. Q., Yang C. S., Zheng S. F., Gail M., Li G. Y. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993 Sep 15;85(18):1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- Bodell W. J., Devanesan P. D., Rogan E. G., Cavalieri E. L. 32P-postlabeling analysis of benzo[a]pyrene-DNA adducts formed in vitro and in vivo. Chem Res Toxicol. 1989 Sep-Oct;2(5):312–315. doi: 10.1021/tx00011a008. [DOI] [PubMed] [Google Scholar]
- Bond J. A., Chen B. T., Griffith W. C., Mauderly J. L. Inhaled cigarette smoke induces the formation of DNA adducts in lungs of rats. Toxicol Appl Pharmacol. 1989 Jun 1;99(1):161–172. doi: 10.1016/0041-008x(89)90121-x. [DOI] [PubMed] [Google Scholar]
- Borish E. T., Cosgrove J. P., Church D. F., Deutsch W. A., Pryor W. A. Cigarette tar causes single-strand breaks in DNA. Biochem Biophys Res Commun. 1985 Dec 17;133(2):780–786. doi: 10.1016/0006-291x(85)90972-6. [DOI] [PubMed] [Google Scholar]
- Borish E. T., Pryor W. A., Venugopal S., Deutsch W. A. DNA synthesis is blocked by cigarette tar-induced DNA single-strand breaks. Carcinogenesis. 1987 Oct;8(10):1517–1520. doi: 10.1093/carcin/8.10.1517. [DOI] [PubMed] [Google Scholar]
- Boyd N. F., McGuire V. The possible role of lipid peroxidation in breast cancer risk. Free Radic Biol Med. 1991;10(3-4):185–190. doi: 10.1016/0891-5849(91)90074-d. [DOI] [PubMed] [Google Scholar]
- Breen A. P., Murphy J. A. Reactions of oxyl radicals with DNA. Free Radic Biol Med. 1995 Jun;18(6):1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- Brown K. M., Morrice P. C., Duthie G. G. Vitamin E supplementation suppresses indexes of lipid peroxidation and platelet counts in blood of smokers and nonsmokers but plasma lipoprotein concentrations remain unchanged. Am J Clin Nutr. 1994 Sep;60(3):383–387. doi: 10.1093/ajcn/60.3.383. [DOI] [PubMed] [Google Scholar]
- Cavalieri E. L., Rogan E. G. The approach to understanding aromatic hydrocarbon carcinogenesis. The central role of radical cations in metabolic activation. Pharmacol Ther. 1992;55(2):183–199. doi: 10.1016/0163-7258(92)90015-r. [DOI] [PubMed] [Google Scholar]
- Cerutti P., Ghosh R., Oya Y., Amstad P. The role of the cellular antioxidant defense in oxidant carcinogenesis. Environ Health Perspect. 1994 Dec;102 (Suppl 10):123–129. doi: 10.1289/ehp.94102s10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A. K., Nokubo M., Reddy G. R., Yeola S. N., Morrow J. D., Blair I. A., Marnett L. J. Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science. 1994 Sep 9;265(5178):1580–1582. doi: 10.1126/science.8079172. [DOI] [PubMed] [Google Scholar]
- Church D. F., Pryor W. A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985 Dec;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churg A., Cherukupalli K. Cigarette smoke causes rapid lipid peroxidation of rat tracheal epithelium. Int J Exp Pathol. 1993 Apr;74(2):127–132. [PMC free article] [PubMed] [Google Scholar]
- Copeland E. S. A National Institutes of Health Workshop report. Free radicals in promotion--a chemical pathology study section workshop. Cancer Res. 1983 Nov;43(11):5631–5637. [PubMed] [Google Scholar]
- Cosgrove J. P., Borish E. T., Church D. F., Pryor W. A. The metal-mediated formation of hydroxyl radical by aqueous extracts of cigarette tar. Biochem Biophys Res Commun. 1985 Oct 15;132(1):390–396. doi: 10.1016/0006-291x(85)91034-4. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Diplock A. T. Dietary supplementation with antioxidants. Is there a case for exceeding the recommended dietary allowance? Free Radic Biol Med. 1987;3(3):199–201. doi: 10.1016/0891-5849(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Dix T. A., Marnett L. J. Metabolism of polycyclic aromatic hydrocarbon derivatives to ultimate carcinogens during lipid peroxidation. Science. 1983 Jul 1;221(4605):77–79. doi: 10.1126/science.6304879. [DOI] [PubMed] [Google Scholar]
- Eling T., Curtis J., Battista J., Marnett L. J. Oxidation of (+)-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by mouse keratinocytes: evidence for peroxyl radical- and monoxygenase-dependent metabolism. Carcinogenesis. 1986 Dec;7(12):1957–1963. doi: 10.1093/carcin/7.12.1957. [DOI] [PubMed] [Google Scholar]
- Evans M. D., Church D. F., Pryor W. A. Aqueous cigarette tar extracts damage human alpha-1-proteinase inhibitor. Chem Biol Interact. 1991;79(2):151–164. doi: 10.1016/0009-2797(91)90079-m. [DOI] [PubMed] [Google Scholar]
- Evans M. D., Pryor W. A. Cigarette smoking, emphysema, and damage to alpha 1-proteinase inhibitor. Am J Physiol. 1994 Jun;266(6 Pt 1):L593–L611. doi: 10.1152/ajplung.1994.266.6.L593. [DOI] [PubMed] [Google Scholar]
- Evans M. D., Pryor W. A. Damage to human alpha-1-proteinase inhibitor by aqueous cigarette tar extracts and the formation of methionine sulfoxide. Chem Res Toxicol. 1992 Sep-Oct;5(5):654–660. doi: 10.1021/tx00029a010. [DOI] [PubMed] [Google Scholar]
- Everson R. B., Randerath E., Santella R. M., Cefalo R. C., Avitts T. A., Randerath K. Detection of smoking-related covalent DNA adducts in human placenta. Science. 1986 Jan 3;231(4733):54–57. doi: 10.1126/science.3941892. [DOI] [PubMed] [Google Scholar]
- Ferro M., Marinari U. M., Poli G., Dianzani M. U., Fauler G., Zollner H., Esterbauer H. Metabolism of 4-hydroxynonenal by the rat hepatoma cell line MH1C1. Cell Biochem Funct. 1988 Oct;6(4):245–250. doi: 10.1002/cbf.290060405. [DOI] [PubMed] [Google Scholar]
- Frei B., Forte T. M., Ames B. N., Cross C. E. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochem J. 1991 Jul 1;277(Pt 1):133–138. doi: 10.1042/bj2770133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol Ther. 1992;53(1):127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]
- Goda Y., Marnett L. J. High-performance liquid chromatography with electrochemical detection for determination of the major malondialdehyde-guanine adduct. Chem Res Toxicol. 1991 Sep-Oct;4(5):520–524. doi: 10.1021/tx00023a005. [DOI] [PubMed] [Google Scholar]
- Gupta M. P., Khanduja K. L., Sharma R. R. Effect of cigarette smoke inhalation on antioxidant enzymes and lipid peroxidation in the rat. Toxicol Lett. 1988 May;41(2):107–114. doi: 10.1016/0378-4274(88)90084-7. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Sopori M. L., Gairola C. G. Formation of cigarette smoke-induced DNA adducts in the rat lung and nasal mucosa. Cancer Res. 1989 Apr 15;49(8):1916–1920. [PubMed] [Google Scholar]
- Ji C., Marnett L. J. Oxygen radical-dependent epoxidation of (7S,8S)-dihydroxy-7,8-dihydrobenzo[a]pyrene in mouse skin in vivo. Stimulation by phorbol esters and inhibition by antiinflammatory steroids. J Biol Chem. 1992 Sep 5;267(25):17842–17848. [PubMed] [Google Scholar]
- Kamp D. W., Graceffa P., Pryor W. A., Weitzman S. A. The role of free radicals in asbestos-induced diseases. Free Radic Biol Med. 1992;12(4):293–315. doi: 10.1016/0891-5849(92)90117-y. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Wislocki P. G., Levin W., Yagi H., Jerina D. M., Conney A. H. Tumorigenicity studies with diol-epoxides of benzo(a)pyrene which indicate that (+/-)-trans-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene is an ultimate carcinogen in newborn mice. Cancer Res. 1978 Feb;38(2):354–358. [PubMed] [Google Scholar]
- Koreeda M., Moore P. D., Wislocki P. G., Levin W., Yagi H., Jerina D. M. Binding of benzo[a]pyrene 7,8-diol-9,10-epoxides to DNA, RNA, and protein of mouse skin occurs with high stereoselectivity. Science. 1978 Feb 17;199(4330):778–781. doi: 10.1126/science.622566. [DOI] [PubMed] [Google Scholar]
- Labeque R., Marnett L. J. Reaction of hematin with allylic fatty acid hydroperoxides: identification of products and implications for pathways of hydroperoxide-dependent epoxidation of 7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene. Biochemistry. 1988 Sep 6;27(18):7060–7070. doi: 10.1021/bi00418a059. [DOI] [PubMed] [Google Scholar]
- Lapenna D., Mezzetti A., de Gioia S., Pierdomenico S. D., Daniele F., Cuccurullo F. Plasma copper and lipid peroxidation in cigarette smokers. Free Radic Biol Med. 1995 Dec;19(6):849–852. doi: 10.1016/0891-5849(95)00056-4. [DOI] [PubMed] [Google Scholar]
- Li J. Y., Taylor P. R., Li B., Dawsey S., Wang G. Q., Ershow A. G., Guo W., Liu S. F., Yang C. S., Shen Q. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst. 1993 Sep 15;85(18):1492–1498. doi: 10.1093/jnci/85.18.1492. [DOI] [PubMed] [Google Scholar]
- Loft S., Vistisen K., Ewertz M., Tjønneland A., Overvad K., Poulsen H. E. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992 Dec;13(12):2241–2247. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- Malins D. C., Holmes E. H., Polissar N. L., Gunselman S. J. The etiology of breast cancer. Characteristic alteration in hydroxyl radical-induced DNA base lesions during oncogenesis with potential for evaluating incidence risk. Cancer. 1993 May 15;71(10):3036–3043. doi: 10.1002/1097-0142(19930515)71:10<3036::aid-cncr2820711025>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Marnett L. J. Prostaglandin synthase-mediated metabolism of carcinogens and a potential role for peroxyl radicals as reactive intermediates. Environ Health Perspect. 1990 Aug;88:5–12. doi: 10.1289/ehp.90885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Woods L., Weston Z., Williams A. B., Das S. K. The effect of mainstream and sidestream cigarette smoke exposure on oxygen defense mechanisms of guinea pig erythrocytes. J Biochem Toxicol. 1993 Sep;8(3):119–125. doi: 10.1002/jbt.2570080303. [DOI] [PubMed] [Google Scholar]
- Pacht E. R., Kaseki H., Mohammed J. R., Cornwell D. G., Davis W. B. Deficiency of vitamin E in the alveolar fluid of cigarette smokers. Influence on alveolar macrophage cytotoxicity. J Clin Invest. 1986 Mar;77(3):789–796. doi: 10.1172/JCI112376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn A., Snyder C. A. Inhalation of sidestream cigarette smoke accelerates development of arteriosclerotic plaques. Circulation. 1993 Oct;88(4 Pt 1):1820–1825. doi: 10.1161/01.cir.88.4.1820. [DOI] [PubMed] [Google Scholar]
- Petruzzelli S., Hietanen E., Bartsch H., Camus A. M., Mussi A., Angeletti C. A., Saracci R., Giuntini C. Pulmonary lipid peroxidation in cigarette smokers and lung cancer patients. Chest. 1990 Oct;98(4):930–935. doi: 10.1378/chest.98.4.930. [DOI] [PubMed] [Google Scholar]
- Pruess-Schwartz D., Nimesheim A., Marnett L. J. Peroxyl radical- and cytochrome P-450-dependent metabolic activation of (+)-7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene in mouse skin in vitro and in vivo. Cancer Res. 1989 Apr 1;49(7):1732–1737. [PubMed] [Google Scholar]
- Pryor W. A., Arbour N. C., Upham B., Church D. F. The inhibitory effect of extracts of cigarette tar on electron transport of mitochondria and submitochondrial particles. Free Radic Biol Med. 1992;12(5):365–372. doi: 10.1016/0891-5849(92)90085-u. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. Cigarette smoke and the involvement of free radical reactions in chemical carcinogenesis. Br J Cancer Suppl. 1987 Jun;8:19–23. [PMC free article] [PubMed] [Google Scholar]
- Pryor W. A., Dooley M. M., Church D. F. Mechanisms of cigarette smoke toxicity: the inactivation of human alpha-1-proteinase inhibitor by nitric oxide/isoprene mixtures in air. Chem Biol Interact. 1985 Jul;54(2):171–183. doi: 10.1016/s0009-2797(85)80161-7. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Dooley M. M., Church D. F. The inactivation of alpha-1-proteinase inhibitor by gas-phase cigarette smoke: protection by antioxidants and reducing species. Chem Biol Interact. 1986 Mar;57(3):271–283. doi: 10.1016/0009-2797(86)90002-5. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Godber S. S. Noninvasive measures of oxidative stress status in humans. Free Radic Biol Med. 1991;10(3-4):177–184. doi: 10.1016/0891-5849(91)90073-c. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Hales B. J., Premovic P. I., Church D. F. The radicals in cigarette tar: their nature and suggested physiological implications. Science. 1983 Apr 22;220(4595):425–427. doi: 10.1126/science.6301009. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. Measurement of oxidative stress status in humans. Cancer Epidemiol Biomarkers Prev. 1993 May-Jun;2(3):289–292. [PubMed] [Google Scholar]
- Pryor W. A., Prier D. G., Church D. F. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ Health Perspect. 1983 Jan;47:345–355. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RamaKrishna N. V., Gao F., Padmavathi N. S., Cavalieri E. L., Rogan E. G., Cerny R. L., Gross M. L. Model adducts of benzo[a]pyrene and nucleosides formed from its radical cation and diol epoxide. Chem Res Toxicol. 1992 Mar-Apr;5(2):293–302. doi: 10.1021/tx00026a023. [DOI] [PubMed] [Google Scholar]
- RamaKrishna N. V., Li K. M., Rogan E. G., Cavalieri E. L., George M., Cerny R. L., Gross M. L. Adducts of 6-methylbenzo[a]pyrene and 6-fluorobenzo[a]pyrene formed by electrochemical oxidation in the presence of deoxyribonucleosides. Chem Res Toxicol. 1993 Nov-Dec;6(6):837–845. doi: 10.1021/tx00036a013. [DOI] [PubMed] [Google Scholar]
- RamaKrishna N. V., Padmavathi N. S., Cavalieri E. L., Rogan E. G., Cerny R. L., Gross M. L. Synthesis and structure determination of the adducts formed by electrochemical oxidation of the potent carcinogen dibenzo[a,I]pyrene in the presence of nucleosides. Chem Res Toxicol. 1993 Jul-Aug;6(4):554–560. doi: 10.1021/tx00034a026. [DOI] [PubMed] [Google Scholar]
- Randerath E., Avitts T. A., Reddy M. V., Miller R. H., Everson R. B., Randerath K. Comparative 32P-analysis of cigarette smoke-induced DNA damage in human tissues and mouse skin. Cancer Res. 1986 Nov;46(11):5869–5877. [PubMed] [Google Scholar]
- Randerath E., Danna T. F., Randerath K. DNA damage induced by cigarette smoke condensate in vitro as assayed by 32P-postlabeling. Comparison with cigarette smoke-associated DNA adduct profiles in vivo. Mutat Res. 1992 Jul;268(1):139–153. doi: 10.1016/0027-5107(92)90091-f. [DOI] [PubMed] [Google Scholar]
- Randerath E., Miller R. H., Mittal D., Avitts T. A., Dunsford H. A., Randerath K. Covalent DNA damage in tissues of cigarette smokers as determined by 32P-postlabeling assay. J Natl Cancer Inst. 1989 Mar 1;81(5):341–347. doi: 10.1093/jnci/81.5.341. [DOI] [PubMed] [Google Scholar]
- Randerath E., Mittal D., Randerath K. Tissue distribution of covalent DNA damage in mice treated dermally with cigarette 'tar': preference for lung and heart DNA. Carcinogenesis. 1988 Jan;9(1):75–80. doi: 10.1093/carcin/9.1.75. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Gupta R. C. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Reddy R., Danna T. F., Watson W. P., Crane A. E., Randerath E. Formation of ribonucleotides in DNA modified by oxidative damage in vitro and in vivo. Characterization by 32P-postlabeling. Mutat Res. 1992 Sep;275(3-6):355–366. doi: 10.1016/0921-8734(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Randerath K., Yang P. F., Danna T. F., Reddy R., Watson W. P., Randerath E. Bulky adducts detected by 32P-postlabeling in DNA modified by oxidative damage in vitro. Comparison with rat lung I-compounds. Mutat Res. 1991 Sep-Oct;250(1-2):135–144. doi: 10.1016/0027-5107(91)90169-o. [DOI] [PubMed] [Google Scholar]
- Reznick A. Z., Cross C. E., Hu M. L., Suzuki Y. J., Khwaja S., Safadi A., Motchnik P. A., Packer L., Halliwell B. Modification of plasma proteins by cigarette smoke as measured by protein carbonyl formation. Biochem J. 1992 Sep 1;286(Pt 2):607–611. doi: 10.1042/bj2860607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. K., Bermúdez E., Pryor W. A. Aqueous extracts of cigarette tar containing the tar free radical cause DNA nicks in mammalian cells. Environ Health Perspect. 1994 Dec;102 (Suppl 10):173–178. doi: 10.1289/ehp.94102s10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K., Bermúdez E., Zang L. Y., Carter K. M., Queenan K. E., Pryor W. A. The ESR properties, DNA nicking, and DNA association of aged solutions of catechol versus aqueous extracts of tar from cigarette smoke. Arch Biochem Biophys. 1995 May 10;319(1):196–203. doi: 10.1006/abbi.1995.1282. [DOI] [PubMed] [Google Scholar]
- Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8(6):583–599. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- Todorovic R., Devanesan P. D., Rogan E. G., Cavalieri E. L. 32P-postlabeling analysis of the DNA adducts of 6-fluorobenzo[a]pyrene and 6-methylbenzo[a]pyrene formed in vitro. Chem Res Toxicol. 1993 Jul-Aug;6(4):530–534. doi: 10.1021/tx00034a022. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of carcinogenesis by minor anutrient constituents of the diet. Proc Nutr Soc. 1990 Jul;49(2):173–183. doi: 10.1079/pns19900022. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of carcinogenesis by minor dietary constituents. Cancer Res. 1992 Apr 1;52(7 Suppl):2085s–2091s. [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of carcinogenic and toxic effects of polycyclic hydrocarbons by phenolic antioxidants and ethoxyquin. J Natl Cancer Inst. 1972 May;48(5):1425–1430. [PubMed] [Google Scholar]
- Willey J. C., Grafstrom R. C., Moser C. E., Jr, Ozanne C., Sundquvist K., Harris C. C. Biochemical and morphological effects of cigarette smoke condensate and its fractions on normal human bronchial epithelial cells in vitro. Cancer Res. 1987 Apr 15;47(8):2045–2049. [PubMed] [Google Scholar]
- Witz G. Active oxygen species as factors in multistage carcinogenesis. Proc Soc Exp Biol Med. 1991 Nov;198(2):675–682. doi: 10.3181/00379727-198-43306. [DOI] [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]
- Wurzel H., Yeh C. C., Gairola C., Chow C. K. Oxidative damage and antioxidant status in the lungs and bronchoalveolar lavage fluid of rats exposed chronically to cigarette smoke. J Biochem Toxicol. 1995 Feb;10(1):11–17. [PubMed] [Google Scholar]
- Zang L. Y., Stone K., Pryor W. A. Detection of free radicals in aqueous extracts of cigarette tar by electron spin resonance. Free Radic Biol Med. 1995 Aug;19(2):161–167. doi: 10.1016/0891-5849(94)00236-d. [DOI] [PubMed] [Google Scholar]
- Zhang L. X., Cooney R. V., Bertram J. S. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis. 1991 Nov;12(11):2109–2114. doi: 10.1093/carcin/12.11.2109. [DOI] [PubMed] [Google Scholar]
- Ziegler R. G., Subar A. F., Craft N. E., Ursin G., Patterson B. H., Graubard B. I. Does beta-carotene explain why reduced cancer risk is associated with vegetable and fruit intake? Cancer Res. 1992 Apr 1;52(7 Suppl):2060s–2066s. [PubMed] [Google Scholar]
- deRojas-Walker T., Tamir S., Ji H., Wishnok J. S., Tannenbaum S. R. Nitric oxide induces oxidative damage in addition to deamination in macrophage DNA. Chem Res Toxicol. 1995 Apr-May;8(3):473–477. doi: 10.1021/tx00045a020. [DOI] [PubMed] [Google Scholar]


