Abstract
While the faithful transmission of genetic information requires a fidelity and stability of DNA that is involved in translation into proteins, it has become evident that a large part of noncoding DNA is organized in repeated sequences, which often exhibit a pronounced instability and dynamics. This applies both to longer repeated sequences, minisatellites (about 10-100 base pairs), and microsatellites (mostly 2-4 base pairs). Although these satellite DNAs are abundantly distributed in all kinds of organisms, no clear function has been discerned for them. However, extension of trinucleotide microsatellite sequences has been associated with several severe human disorders, such as Fragile X syndrome and Huntington's disease. Rare alleles of a minisatellite sequence have been reported to be associated with the ras oncogene leading to an increased risk for several human cancers. A dynamic behavior of repeated DNA sequences also applies to telomeres, constituting the ends of the chromosomes. Repeated DNA sequences protect the chromosome ends from losing coding sequences at cell divisions. The telomeres are maintained by the enzyme telomerase. Somatic cells, however, lose telomerase function and gradually die. Cancer cells have activated telomerase and therefore they acquire immortality.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Armour J. A., Jeffreys A. J. Biology and applications of human minisatellite loci. Curr Opin Genet Dev. 1992 Dec;2(6):850–856. doi: 10.1016/s0959-437x(05)80106-6. [DOI] [PubMed] [Google Scholar]
- Armour J. A., Neumann R., Gobert S., Jeffreys A. J. Isolation of human simple repeat loci by hybridization selection. Hum Mol Genet. 1994 Apr;3(4):599–565. doi: 10.1093/hmg/3.4.599. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Selby M. J., Rutter W. J. The highly polymorphic region near the human insulin gene is composed of simple tandemly repeating sequences. Nature. 1982 Jan 7;295(5844):31–35. doi: 10.1038/295031a0. [DOI] [PubMed] [Google Scholar]
- Bruford M. W., Wayne R. K. Microsatellites and their application to population genetic studies. Curr Opin Genet Dev. 1993 Dec;3(6):939–943. doi: 10.1016/0959-437x(93)90017-j. [DOI] [PubMed] [Google Scholar]
- Burke T., Bruford M. W. DNA fingerprinting in birds. Nature. 1987 May 14;327(6118):149–152. doi: 10.1038/327149a0. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Cederberg H., Agurell E., Hedenskog M., Rannug U. Amplification and loss of repeat units of the human minisatellite MS1 integrated in chromosome III of a haploid yeast strain. Mol Gen Genet. 1993 Apr;238(1-2):38–42. doi: 10.1007/BF00279528. [DOI] [PubMed] [Google Scholar]
- Chamberlain N. L., Driver E. D., Miesfeld R. L. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994 Aug 11;22(15):3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K., Kobayashi R., Greider C. W. Purification of Tetrahymena telomerase and cloning of genes encoding the two protein components of the enzyme. Cell. 1995 Jun 2;81(5):677–686. doi: 10.1016/0092-8674(95)90529-4. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Brown W. R., Rappold G. A. Hypervariable telomeric sequences from the human sex chromosomes are pseudoautosomal. Nature. 1985 Oct 24;317(6039):687–692. doi: 10.1038/317687a0. [DOI] [PubMed] [Google Scholar]
- Dubrova Y. E., Jeffreys A. J., Malashenko A. M. Mouse minisatellite mutations induced by ionizing radiation. Nat Genet. 1993 Sep;5(1):92–94. doi: 10.1038/ng0993-92. [DOI] [PubMed] [Google Scholar]
- Eichler E. E., Holden J. J., Popovich B. W., Reiss A. L., Snow K., Thibodeau S. N., Richards C. S., Ward P. A., Nelson D. L. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994 Sep;8(1):88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
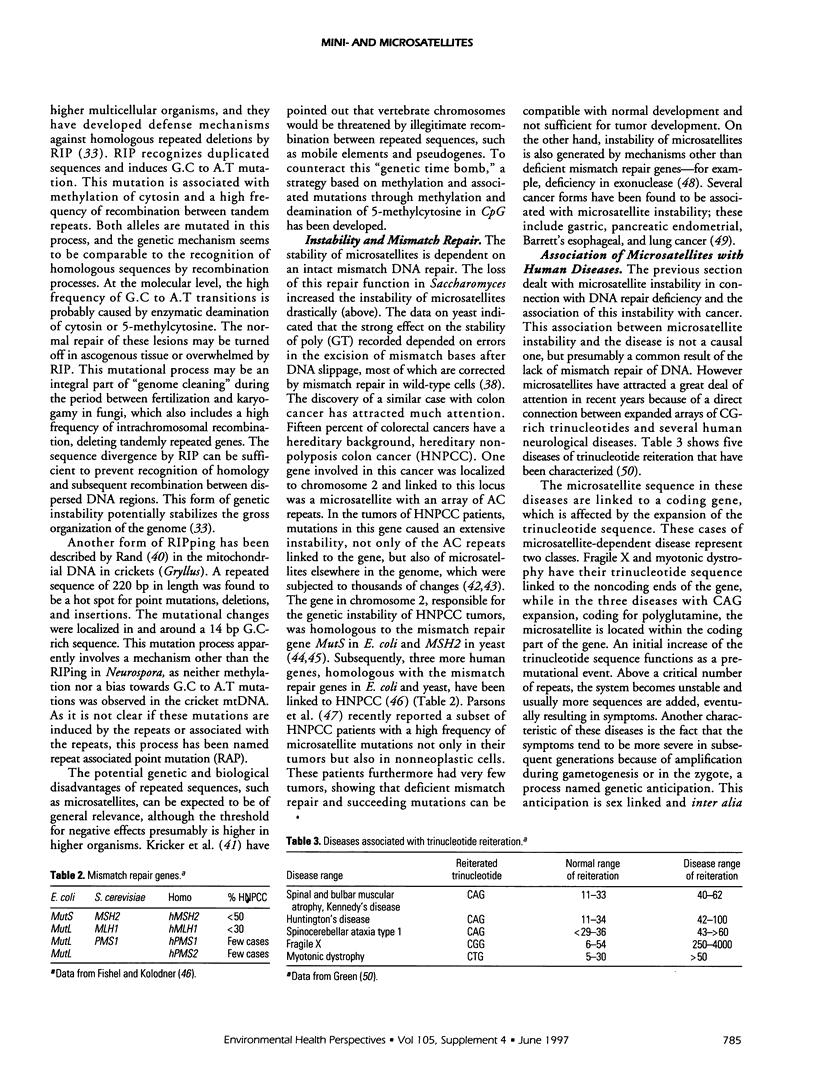
- Fishel R., Kolodner R. D. Identification of mismatch repair genes and their role in the development of cancer. Curr Opin Genet Dev. 1995 Jun;5(3):382–395. doi: 10.1016/0959-437x(95)80055-7. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gacy A. M., Goellner G., Juranić N., Macura S., McMurray C. T. Trinucleotide repeats that expand in human disease form hairpin structures in vitro. Cell. 1995 May 19;81(4):533–540. doi: 10.1016/0092-8674(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Gray I. C., Jeffreys A. J. Evolutionary transience of hypervariable minisatellites in man and the primates. Proc Biol Sci. 1991 Mar 22;243(1308):241–253. doi: 10.1098/rspb.1991.0038. [DOI] [PubMed] [Google Scholar]
- Green H. Human genetic diseases due to codon reiteration: relationship to an evolutionary mechanism. Cell. 1993 Sep 24;74(6):955–956. doi: 10.1016/0092-8674(93)90718-6. [DOI] [PubMed] [Google Scholar]
- Green M., Krontiris T. G. Allelic variation of reporter gene activation by the HRAS1 minisatellite. Genomics. 1993 Aug;17(2):429–434. doi: 10.1006/geno.1993.1343. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hultén M. Chiasma distribution at diakinesis in the normal human male. Hereditas. 1974;76(1):55–78. doi: 10.1111/j.1601-5223.1974.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J. Highly variable minisatellites and DNA fingerprints. Biochem Soc Trans. 1987 Jun;15(3):309–317. doi: 10.1042/bst0150309. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., MacLeod A., Tamaki K., Neil D. L., Monckton D. G. Minisatellite repeat coding as a digital approach to DNA typing. Nature. 1991 Nov 21;354(6350):204–209. doi: 10.1038/354204a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Tamaki K., MacLeod A., Monckton D. G., Neil D. L., Armour J. A. Complex gene conversion events in germline mutation at human minisatellites. Nat Genet. 1994 Feb;6(2):136–145. doi: 10.1038/ng0294-136. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Individual-specific 'fingerprints' of human DNA. Nature. 1985 Jul 4;316(6023):76–79. doi: 10.1038/316076a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. E., Kovvali G. K., Prakash L., Prakash S. Requirement of the yeast RTH1 5' to 3' exonuclease for the stability of simple repetitive DNA. Science. 1995 Jul 14;269(5221):238–240. doi: 10.1126/science.7618086. [DOI] [PubMed] [Google Scholar]
- Julier C., de Gouyon B., Georges M., Guénet J. L., Nakamura Y., Avner P., Lathrop G. M. Minisatellite linkage maps in the mouse by cross-hybridization with human probes containing tandem repeats. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4585–4589. doi: 10.1073/pnas.87.12.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperczyk A., DiMartino N. A., Krontiris T. G. Minisatellite allele diversification: the origin of rare alleles at the HRAS1 locus. Am J Hum Genet. 1990 Nov;47(5):854–859. [PMC free article] [PubMed] [Google Scholar]
- Kennedy G. C., German M. S., Rutter W. J. The minisatellite in the diabetes susceptibility locus IDDM2 regulates insulin transcription. Nat Genet. 1995 Mar;9(3):293–298. doi: 10.1038/ng0395-293. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Krahe R., Ashizawa T., Abbruzzese C., Roeder E., Carango P., Giacanelli M., Funanage V. L., Siciliano M. J. Effect of myotonic dystrophy trinucleotide repeat expansion on DMPK transcription and processing. Genomics. 1995 Jul 1;28(1):1–14. doi: 10.1006/geno.1995.1099. [DOI] [PubMed] [Google Scholar]
- Kricker M. C., Drake J. W., Radman M. Duplication-targeted DNA methylation and mutagenesis in the evolution of eukaryotic chromosomes. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1075–1079. doi: 10.1073/pnas.89.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Devlin B., Karp D. D., Robert N. J., Risch N. An association between the risk of cancer and mutations in the HRAS1 minisatellite locus. N Engl J Med. 1993 Aug 19;329(8):517–523. doi: 10.1056/NEJM199308193290801. [DOI] [PubMed] [Google Scholar]
- Kuhl D. P., Caskey C. T. Trinucleotide repeats and genome variation. Curr Opin Genet Dev. 1993 Jun;3(3):404–407. doi: 10.1016/0959-437x(93)90112-3. [DOI] [PubMed] [Google Scholar]
- Laurie D. A., Hultén M. A. Further studies on chiasma distribution and interference in the human male. Ann Hum Genet. 1985 Jul;49(Pt 3):203–214. doi: 10.1111/j.1469-1809.1985.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Lewontin R. C., Hartl D. L. Population genetics in forensic DNA typing. Science. 1991 Dec 20;254(5039):1745–1750. doi: 10.1126/science.1845040. [DOI] [PubMed] [Google Scholar]
- Li X. J., Li S. H., Sharp A. H., Nucifora F. C., Jr, Schilling G., Lanahan A., Worley P., Snyder S. H., Ross C. A. A huntingtin-associated protein enriched in brain with implications for pathology. Nature. 1995 Nov 23;378(6555):398–402. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- Lingner J., Cooper J. P., Cech T. R. Telomerase and DNA end replication: no longer a lagging strand problem? Science. 1995 Sep 15;269(5230):1533–1534. doi: 10.1126/science.7545310. [DOI] [PubMed] [Google Scholar]
- Mason J. M., Biessmann H. The unusual telomeres of Drosophila. Trends Genet. 1995 Feb;11(2):58–62. doi: 10.1016/s0168-9525(00)88998-2. [DOI] [PubMed] [Google Scholar]
- Meltzer S. J., Yin J., Manin B., Rhyu M. G., Cottrell J., Hudson E., Redd J. L., Krasna M. J., Abraham J. M., Reid B. J. Microsatellite instability occurs frequently and in both diploid and aneuploid cell populations of Barrett's-associated esophageal adenocarcinomas. Cancer Res. 1994 Jul 1;54(13):3379–3382. [PubMed] [Google Scholar]
- Nakamura Y., Leppert M., O'Connell P., Wolff R., Holm T., Culver M., Martin C., Fujimoto E., Hoff M., Kumlin E. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987 Mar 27;235(4796):1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- O'Donovan M. C., Guy C., Craddock N., Murphy K. C., Cardno A. G., Jones L. A., Owen M. J., McGuffin P. Expanded CAG repeats in schizophrenia and bipolar disorder. Nat Genet. 1995 Aug;10(4):380–381. doi: 10.1038/ng0895-380. [DOI] [PubMed] [Google Scholar]
- Orgel L. E., Crick F. H. Selfish DNA: the ultimate parasite. Nature. 1980 Apr 17;284(5757):604–607. doi: 10.1038/284604a0. [DOI] [PubMed] [Google Scholar]
- Parsons R., Li G. M., Longley M., Modrich P., Liu B., Berk T., Hamilton S. R., Kinzler K. W., Vogelstein B. Mismatch repair deficiency in phenotypically normal human cells. Science. 1995 May 5;268(5211):738–740. doi: 10.1126/science.7632227. [DOI] [PubMed] [Google Scholar]
- Peltomäki P., Aaltonen L. A., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Green J. S., Jass J. R., Weber J. L., Leach F. S. Genetic mapping of a locus predisposing to human colorectal cancer. Science. 1993 May 7;260(5109):810–812. doi: 10.1126/science.8484120. [DOI] [PubMed] [Google Scholar]
- Rand D. M. RIPping and RAPping at Berkeley. Genetics. 1992 Dec;132(4):1223–1224. doi: 10.1093/genetics/132.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification, drug resistance, and cancer. Cancer Res. 1984 May;44(5):1735–1742. [PubMed] [Google Scholar]
- Schlötterer C., Tautz D. Slippage synthesis of simple sequence DNA. Nucleic Acids Res. 1992 Jan 25;20(2):211–215. doi: 10.1093/nar/20.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker E. U. Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet. 1990;24:579–613. doi: 10.1146/annurev.ge.24.120190.003051. [DOI] [PubMed] [Google Scholar]
- Strand M., Prolla T. A., Liskay R. M., Petes T. D. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature. 1993 Sep 16;365(6443):274–276. doi: 10.1038/365274a0. [DOI] [PubMed] [Google Scholar]
- Tautz D., Schlötterer Simple sequences. Curr Opin Genet Dev. 1994 Dec;4(6):832–837. doi: 10.1016/0959-437x(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Trask B. J., Hamlin J. L. Early dihydrofolate reductase gene amplification events in CHO cells usually occur on the same chromosome arm as the original locus. Genes Dev. 1989 Dec;3(12A):1913–1925. doi: 10.1101/gad.3.12a.1913. [DOI] [PubMed] [Google Scholar]
- Trepicchio W. L., Krontiris T. G. IGH minisatellite suppression of USF-binding-site- and E mu-mediated transcriptional activation of the adenovirus major late promoter. Nucleic Acids Res. 1993 Feb 25;21(4):977–985. doi: 10.1093/nar/21.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepicchio W. L., Krontiris T. G. Members of the rel/NF-kappa B family of transcriptional regulatory proteins bind the HRAS1 minisatellite DNA sequence. Nucleic Acids Res. 1992 May 25;20(10):2427–2434. doi: 10.1093/nar/20.10.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier Y., Lutz Y., Stevanin G., Imbert G., Devys D., Cancel G., Saudou F., Weber C., David G., Tora L. Polyglutamine expansion as a pathological epitope in Huntington's disease and four dominant cerebellar ataxias. Nature. 1995 Nov 23;378(6555):403–406. doi: 10.1038/378403a0. [DOI] [PubMed] [Google Scholar]
- Wang Y. H., Amirhaeri S., Kang S., Wells R. D., Griffith J. D. Preferential nucleosome assembly at DNA triplet repeats from the myotonic dystrophy gene. Science. 1994 Jul 29;265(5172):669–671. doi: 10.1126/science.8036515. [DOI] [PubMed] [Google Scholar]
- Yano-Yanagisawa H., Li Y., Wang H., Kohwi Y. Single-stranded DNA binding proteins isolated from mouse brain recognize specific trinucleotide repeat sequences in vitro. Nucleic Acids Res. 1995 Jul 25;23(14):2654–2660. doi: 10.1093/nar/23.14.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A. Telomeres: beginning to understand the end. Science. 1995 Dec 8;270(5242):1601–1607. doi: 10.1126/science.270.5242.1601. [DOI] [PubMed] [Google Scholar]
- Zeitlin S., Liu J. P., Chapman D. L., Papaioannou V. E., Efstratiadis A. Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington's disease gene homologue. Nat Genet. 1995 Oct;11(2):155–163. doi: 10.1038/ng1095-155. [DOI] [PubMed] [Google Scholar]


