Abstract
Dietary restriction (DR) alters a significant environmental factor in carcinogenesis, dietary intake, thus inhibiting both spontaneous and induced tumorigenesis. Potential mechanisms for the inhibition of spontaneous cancer may include the effects of DR to do the following: decrease body weight, which decreases cellular proliferation and increases apoptosis in a number of organs that increase and decrease with body size; decrease body temperature, thereby lowering the amount of endogenous DNA damage temperature generates; decrease oxidative damage, by increasing antioxidant damage defense systems; decrease, generally, cellular proliferation; and protect the fidelity of the genome by decreasing DNA damage, increasing DNA repair, and preventing aberrant gene expression. Potential mechanisms for reducing induced tumor incidence include lowering agent activation, changing agent disposition, decreasing the adducts most associated with agent toxicity, and inhibiting tumor progression through mechanisms similar to those that can effect spontaneous tumorigenesis. As a method to control a major source of environmental cancer, and as the major modulator of the agent induction of this disease, understanding how DR works may significantly contribute to the efforts to explain how diet impacts on development of cancer in the United States, and may suggest methods to reduce the adverse impacts of other environmental agents on the disease.
Full text
PDF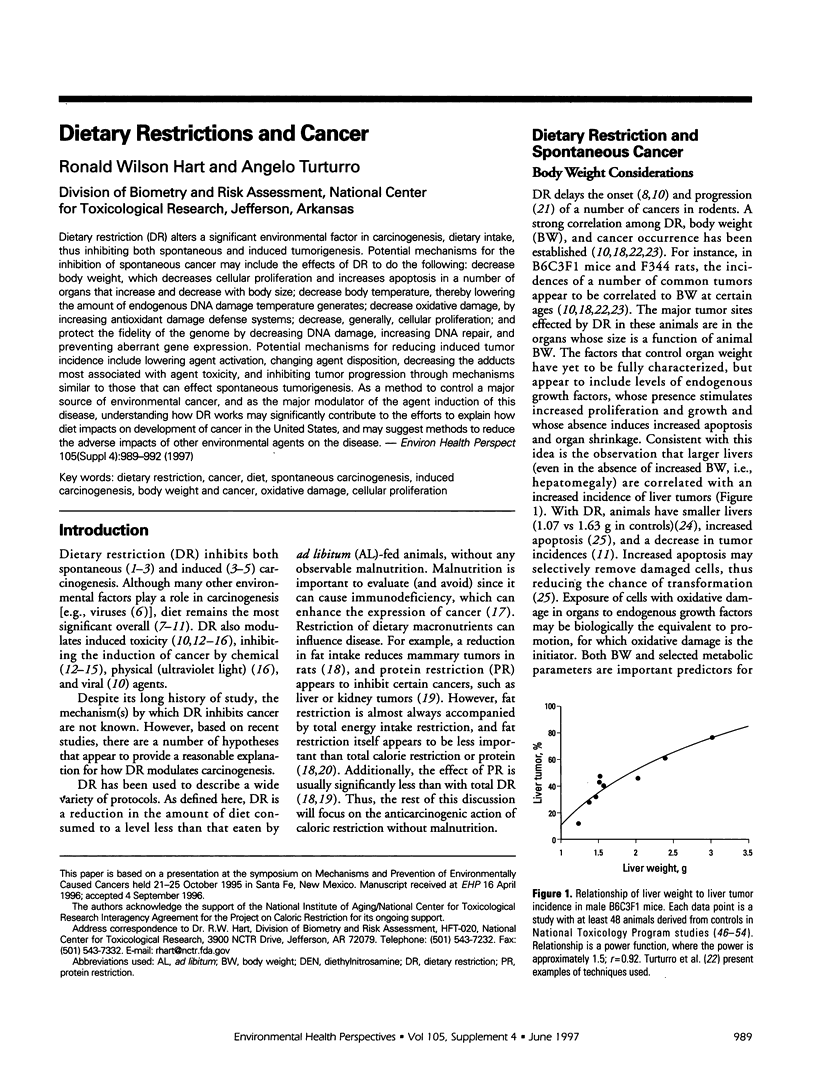



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLOUGH W. S. Mitotic activity and carcinogenesis. Br J Cancer. 1950 Sep;4(3):329–336. doi: 10.1038/bjc.1950.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou M. W., Kong J., Chung K. T., Hart R. W. Effect of caloric restriction on the metabolic activation of xenobiotics. Mutat Res. 1993 Dec;295(4-6):223–235. doi: 10.1016/0921-8734(93)90022-u. [DOI] [PubMed] [Google Scholar]
- Fu P. P., Dooley K. L., Von Tungeln L. S., Bucci T., Hart R. W., Kadlubar F. F. Caloric restriction profoundly inhibits liver tumor formation after initiation by 6-nitrochrysene in male mice. Carcinogenesis. 1994 Feb;15(2):159–161. doi: 10.1093/carcin/15.2.159. [DOI] [PubMed] [Google Scholar]
- Haley-Zitlin V., Richardson A. Effect of dietary restriction on DNA repair and DNA damage. Mutat Res. 1993 Dec;295(4-6):237–245. doi: 10.1016/0921-8734(93)90023-v. [DOI] [PubMed] [Google Scholar]
- Hart R. W., Keenan K., Turturro A., Abdo K. M., Leakey J., Lyn-Cook B. Caloric restriction and toxicity. Fundam Appl Toxicol. 1995 May;25(2):184–195. doi: 10.1006/faat.1995.1054. [DOI] [PubMed] [Google Scholar]
- Higami Y., Yu B. P., Shimokawa I., Masoro E. J., Ikeda T. Duration of dietary restriction: an important determinant for the incidence and age of onset of leukemia in male F344 rats. J Gerontol. 1994 Sep;49(5):B239–B244. doi: 10.1093/geronj/49.5.b239. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D., Klurfeld D. M. Caloric effects in experimental mammary tumorigenesis. Am J Clin Nutr. 1987 Jan;45(1 Suppl):236–242. doi: 10.1093/ajcn/45.1.236. [DOI] [PubMed] [Google Scholar]
- Lagopoulos L., Sunahara G. I., Würzner H., Dombrowsky I., Stalder R. The effects of alternating dietary restriction and ad libitum feeding of mice on the development of diethylnitrosamine-induced liver tumours and its correlation to insulinaemia. Carcinogenesis. 1991 Feb;12(2):311–315. doi: 10.1093/carcin/12.2.311. [DOI] [PubMed] [Google Scholar]
- Lipman J. M., Turturro A., Hart R. W. The influence of dietary restriction on DNA repair in rodents: a preliminary study. Mech Ageing Dev. 1989 May;48(2):135–143. doi: 10.1016/0047-6374(89)90045-6. [DOI] [PubMed] [Google Scholar]
- Lu M. H., Hinson W. G., Turturro A., Sheldon W. G., Hart R. W. Cell proliferation by cell cycle analysis in young and old dietary restricted mice. Mech Ageing Dev. 1993 May;68(1-3):151–162. doi: 10.1016/0047-6374(93)90147-j. [DOI] [PubMed] [Google Scholar]
- Maeda H., Gleiser C. A., Masoro E. J., Murata I., McMahan C. A., Yu B. P. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J Gerontol. 1985 Nov;40(6):671–688. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- Muskhelishvili L., Hart R. W., Turturro A., James S. J. Age-related changes in the intrinsic rate of apoptosis in livers of diet-restricted and ad libitum-fed B6C3F1 mice. Am J Pathol. 1995 Jul;147(1):20–24. [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. D., Duffy P. H., Lu M. H., Turturro A., Hart R. W. The effect of dietary restriction on myc protooncogene expression in mice: a preliminary study. Mech Ageing Dev. 1989 May;48(2):199–205. doi: 10.1016/0047-6374(89)90051-1. [DOI] [PubMed] [Google Scholar]
- Pariza M. W. Dietary fat, calorie restriction, ad libitum feeding, and cancer risk. Nutr Rev. 1987 Jan;45(1):1–7. doi: 10.1111/j.1753-4887.1987.tb06064.x. [DOI] [PubMed] [Google Scholar]
- Seilkop S. K. The effect of body weight on tumor incidence and carcinogenicity testing in B6C3F1 mice and F344 rats. Fundam Appl Toxicol. 1995 Feb;24(2):247–259. doi: 10.1006/faat.1995.1028. [DOI] [PubMed] [Google Scholar]
- Sheldon W. G., Bucci T. J., Hart R. W., Turturro A. Age-related neoplasia in a lifetime study of ad libitum-fed and food-restricted B6C3F1 mice. Toxicol Pathol. 1995 Jul-Aug;23(4):458–476. doi: 10.1177/019262339502300403. [DOI] [PubMed] [Google Scholar]
- Srivastava V. K., Miller S., Schroeder M. D., Hart R. W., Busbee D. Age-related changes in expression and activity of DNA polymerase alpha: some effects of dietary restriction. Mutat Res. 1993 Dec;295(4-6):265–280. doi: 10.1016/0921-8734(93)90025-x. [DOI] [PubMed] [Google Scholar]
- TANNENBAUM A., SILVERSTONE H. Failure to inhibit the formation of mammary carcinoma in mice by intermittent fasting. Cancer Res. 1950 Sep;10(9):577–579. [PubMed] [Google Scholar]
- Taylor A., Lipman R. D., Jahngen-Hodge J., Palmer V., Smith D., Padhye N., Dallal G. E., Cyr D. E., Laxman E., Shepard D. Dietary calorie restriction in the Emory mouse: effects on lifespan, eye lens cataract prevalence and progression, levels of ascorbate, glutathione, glucose, and glycohemoglobin, tail collagen breaktime, DNA and RNA oxidation, skin integrity, fecundity, and cancer. Mech Ageing Dev. 1995 Mar 31;79(1):33–57. doi: 10.1016/0047-6374(94)01541-s. [DOI] [PubMed] [Google Scholar]
- Thurman J. D., Bucci T. J., Hart R. W., Turturro A. Survival, body weight, and spontaneous neoplasms in ad Libitum-fed and food-restricted Fischer-344 rats. Toxicol Pathol. 1994 Jan-Feb;22(1):1–9. doi: 10.1177/019262339402200101. [DOI] [PubMed] [Google Scholar]
- Turturro A., Blank K., Murasko D., Hart R. Mechanisms of caloric restriction affecting aging and disease. Ann N Y Acad Sci. 1994 May 31;719:159–170. doi: 10.1111/j.1749-6632.1994.tb56827.x. [DOI] [PubMed] [Google Scholar]
- Turturro A., Duffy P. H., Hart R. W. Modulation of toxicity by diet and dietary macronutrient restriction. Mutat Res. 1993 Dec;295(4-6):151–164. doi: 10.1016/0921-8734(93)90017-w. [DOI] [PubMed] [Google Scholar]
- Turturro A., Hart R. W. Longevity-assurance mechanisms and caloric restriction. Ann N Y Acad Sci. 1991;621:363–372. doi: 10.1111/j.1749-6632.1991.tb16992.x. [DOI] [PubMed] [Google Scholar]
- Turturro A., Hart R. Dietary alteration in the rates of cancer and aging. Exp Gerontol. 1992 Sep-Dec;27(5-6):583–592. doi: 10.1016/0531-5565(92)90013-p. [DOI] [PubMed] [Google Scholar]
- Youngman L. D. Protein restriction (PR) and caloric restriction (CR) compared: effects on DNA damage, carcinogenesis, and oxidative damage. Mutat Res. 1993 Dec;295(4-6):165–179. doi: 10.1016/0921-8734(93)90018-x. [DOI] [PubMed] [Google Scholar]


