Abstract
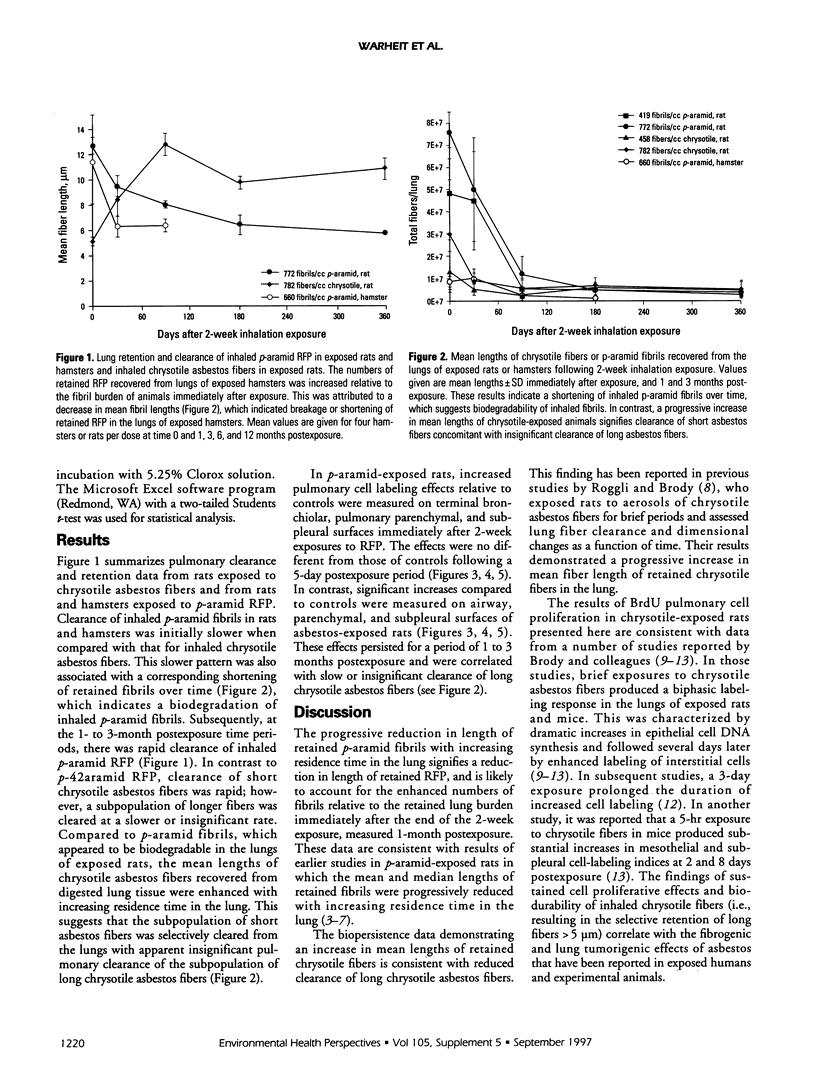
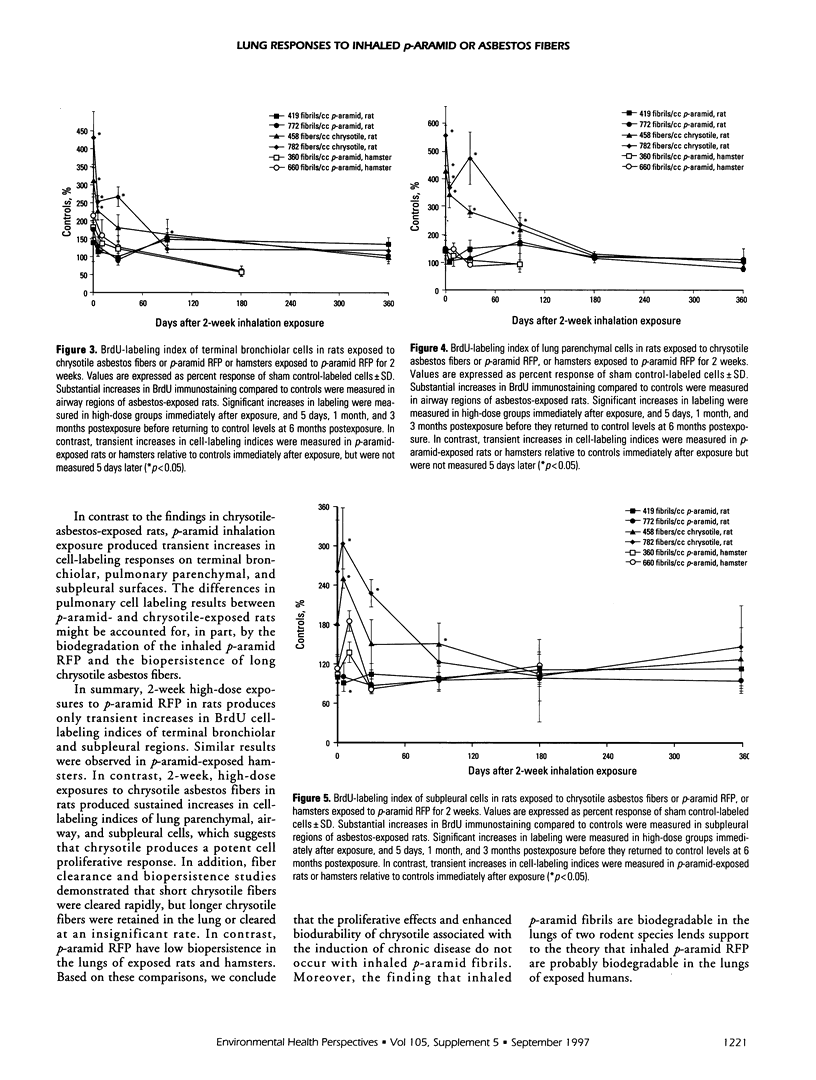
This study compared pulmonary effects of para-aramid respirable-sized, fiber-shaped particles (RFP) (p-aramid fibrils) and chrysotile asbestos fiber exposures in rats. Additional p-aramid inhalation studies were conducted in hamsters to compare species responses. The hamster results are preliminary. The parameters studied were clearance/biopersistence of inhaled p-aramid RFP or size-separated asbestos fibers as well as pulmonary cell proliferation and inflammation indices after 2-week inhalation exposures. Rats were exposed nose only to chrysotile asbestos fibers at concentrations of 459 and 782 fibers/ml or to p-aramid RFP at 419 or 772 fibrils/ml. Hamsters were exposed whole body to p-aramid RFP at concentrations of 358 and 659 fibrils/ml. Subsequently, animals were assessed immediately (time 0) as well as 5 days (10 days for hamsters), 1, 3, 6, and 12 months postexposure. Lung burdens for the p-aramid-exposed rats were 4.8 x 10(7) and 7.6 x 10(7) fibrils/lung, with similar numbers of chrysotile fibers > 5 microns recovered from the lungs of asbestos-exposed rats. In comparison, 1.4 x 10(6) fibrils/lung were recovered in the high-dose hamster group. Biopersistence studies in p-aramid-exposed rats and hamsters demonstrated an initial increase (relative to time 0) in retained p-aramid fibrils during the first month postexposure, which indicated breakage or shortening of inhaled fibrils. This result was associated with a progressive reduction, and increased residence time in the lung, in the mean lengths of the fibrils, which signified biodegradability of inhaled p-aramid fibrils in both species. In contrast, clearance of short chrysotile asbestos fibers was rapid, but clearance of the long chrysotile fibers was slow or insignificant, as evidenced by a progressive increase over time in the mean lengths of fibers recovered from the lungs of exposed rats. Two-week, high-dose exposures to p-aramid in both rats and hamsters produced transient increases in pulmonary inflammatory and cell proliferative responses. In contrast, inhalation of size-separated chrysotile asbestos fibers in rats produced persistent increases in cell labeling indices of airway, alveolar, and subpleural cells measured through a period of 1 to 3 months postexposure. These results suggest that inhaled p-aramid RFP are biodegradable in the lungs of exposed rats and hamsters. In contrast, exposures to chrysotile asbestos fibers in rats resulted in a selective pulmonary retention of long chrysotile fibers.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brody A. R., Overby L. H. Incorporation of tritiated thymidine by epithelial and interstitial cells in bronchiolar-alveolar regions of asbestos-exposed rats. Am J Pathol. 1989 Jan;134(1):133–140. [PMC free article] [PubMed] [Google Scholar]
- Coin P. G., Roggli V. L., Brody A. R. Deposition, clearance, and translocation of chrysotile asbestos from peripheral and central regions of the rat lung. Environ Res. 1992 Jun;58(1):97–116. doi: 10.1016/s0013-9351(05)80207-5. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Addison J., Bolton R. E., Donaldson K., Jones A. D., Smith T. The pathogenicity of long versus short fibre samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br J Exp Pathol. 1986 Jun;67(3):415–430. [PMC free article] [PubMed] [Google Scholar]
- Kelly D. P., Merriman E. A., Kennedy G. L., Jr, Lee K. P. Deposition, clearance, and shortening of Kevlar para-aramid fibrils in acute, subchronic, and chronic inhalation studies in rats. Fundam Appl Toxicol. 1993 Oct;21(3):345–354. doi: 10.1006/faat.1993.1107. [DOI] [PubMed] [Google Scholar]
- McGavran P. D., Butterick C. J., Brody A. R. Tritiated thymidine incorporation and the development of an interstitial lesion in the bronchiolar-alveolar regions of the lungs of normal and complement deficient mice after inhalation of chrysotile asbestos. J Environ Pathol Toxicol Oncol. 1989 Dec;9(5-6):377–391. [PubMed] [Google Scholar]
- Roggli V. L., Brody A. R. Changes in numbers and dimensions of chrysotile asbestos fibers in lungs of rats following short-term exposure. Exp Lung Res. 1984;7(2):133–147. doi: 10.3109/01902148409069674. [DOI] [PubMed] [Google Scholar]
- Warheit D. B., Hartsky M. A., Frame S. R. Pulmonary effects in rats inhaling size-separated chrysotile asbestos fibres or p-aramid fibrils: differences in cellular proliferative responses. Toxicol Lett. 1996 Nov;88(1-3):287–292. doi: 10.1016/0378-4274(96)03751-4. [DOI] [PubMed] [Google Scholar]
- Warheit D. B., Kellar K. A., Hartsky M. A. Pulmonary cellular effects in rats following aerosol exposures to ultrafine Kevlar aramid fibrils: evidence for biodegradability of inhaled fibrils. Toxicol Appl Pharmacol. 1992 Oct;116(2):225–239. doi: 10.1016/0041-008x(92)90302-9. [DOI] [PubMed] [Google Scholar]


