Abstract
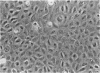
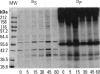
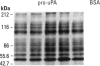
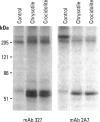
Exposure of low-passage endothelial cells in culture to nonlethal amounts of asbestos, but not refractory ceramic fiber-1, increases cell motility and gene expression. These changes may be initiated by the fibers mimicking matrix proteins as ligands for receptors on the cell surface. In the present study, 1- to 3-hr exposures of endothelial cells to 5 mg/cm2 of chrysotile asbestos caused marked cell elongation and motility. However, little morphological change was seen when chrysotile was added to cells pretreated with either mannosamine to prevent assembly of glycophosphatidylinositol (GPI)-anchored receptors or with herbimycin A to inhibit tyrosine kinase activity. Affinity purification of GPI-anchored urokinase-type plasminogen activator receptor (uPAR) from chrysotile-exposed cells demonstrated that asbestos altered the profile of proteins and phosphoproteins complexed with this receptor. Tyrosine kinase activities in the complexes were also increased by asbestos. Immunoprecipitations with selective monoclonal antibodies demonstrated that both chrysotile and crocidolite asbestos increase kinase activities associated with p60 Src or p120 focal adhesion kinase (FAK). Further, chrysotile also changed the profile of proteins and phosphoproteins associated with FAK in intact cells. These data suggest that asbestos initiates endothelial cell phenotypic change through interactions with uPAR-containing complexes and that this change is mediated through tyrosine kinase cascades.
Full text
PDF


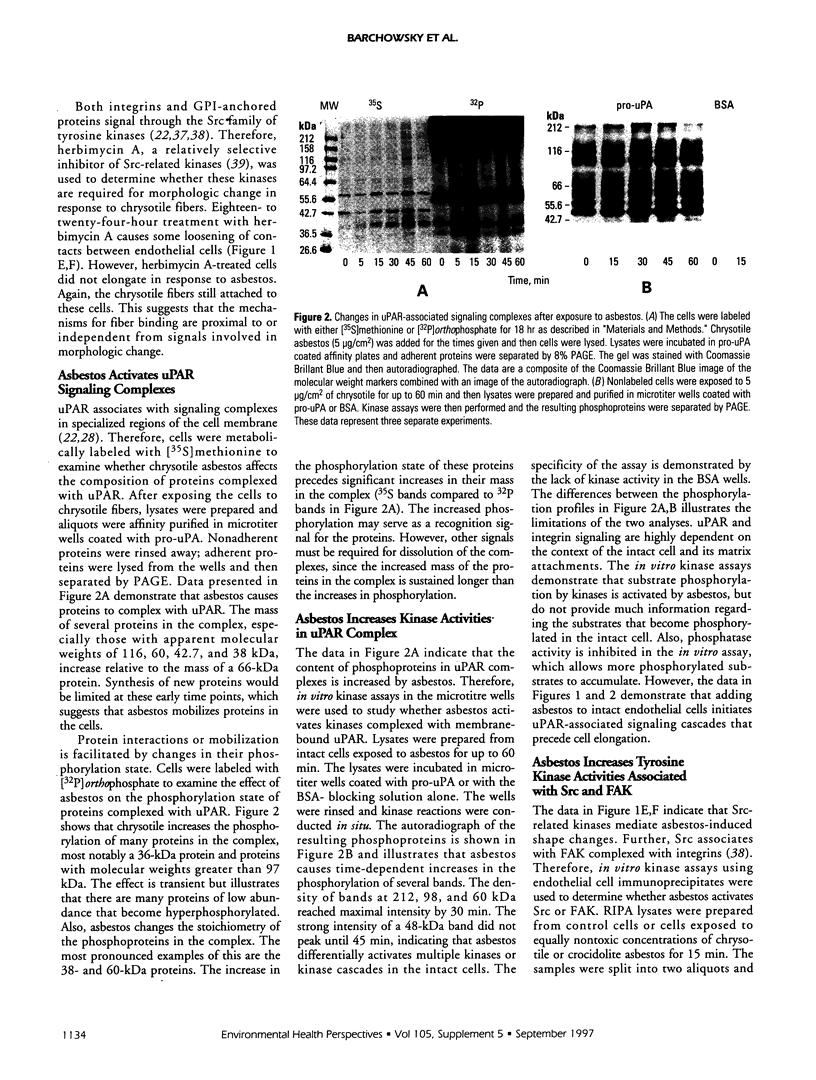
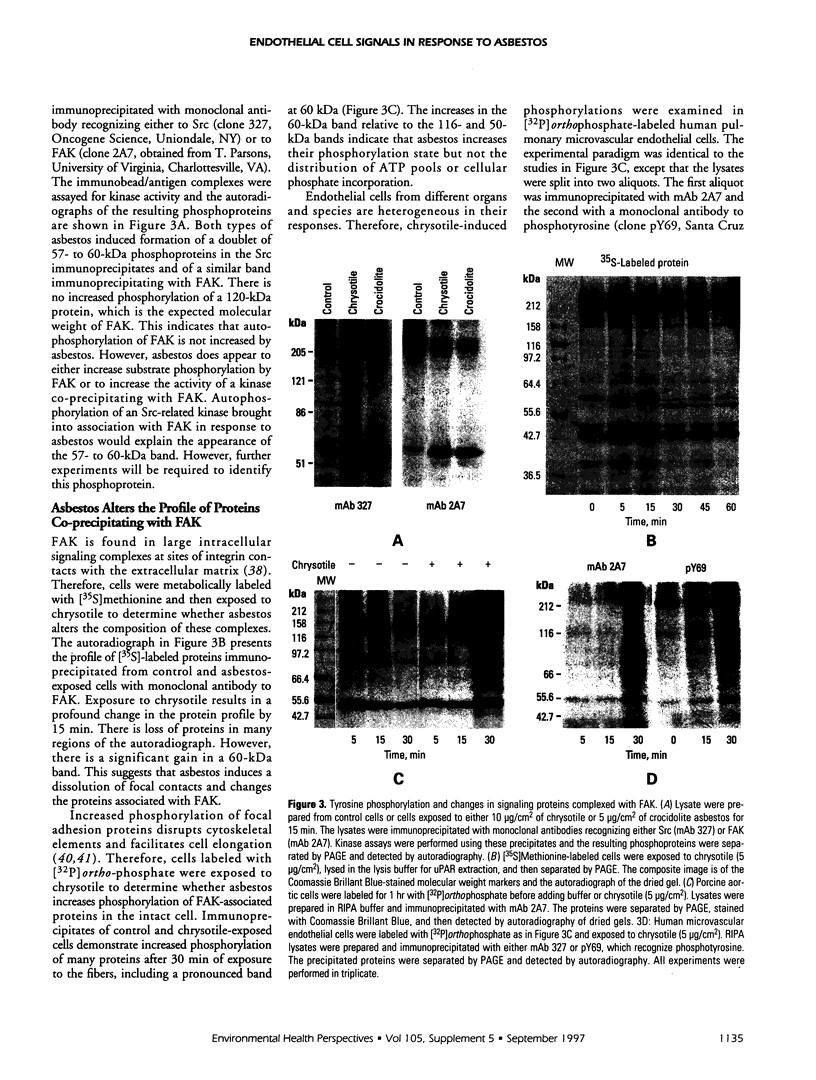


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barchowsky A., Munro S. R., Morana S. J., Vincenti M. P., Treadwell M. Oxidant-sensitive and phosphorylation-dependent activation of NF-kappa B and AP-1 in endothelial cells. Am J Physiol. 1995 Dec;269(6 Pt 1):L829–L836. doi: 10.1152/ajplung.1995.269.6.L829. [DOI] [PubMed] [Google Scholar]
- Barchowsky A., Tabrizi K., Kent R. S., Whorton A. R. Inhibition of prostaglandin synthesis after metabolism of menadione by cultured porcine endothelial cells. J Clin Invest. 1989 Apr;83(4):1153–1159. doi: 10.1172/JCI113995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchowsky A., Williams M. E., Benz C. C., Chepenik K. P. Oxidant-sensitive protein phosphorylation in endothelial cells. Free Radic Biol Med. 1994 Jun;16(6):771–777. doi: 10.1016/0891-5849(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Bohuslav J., Horejsí V., Hansmann C., Stöckl J., Weidle U. H., Majdic O., Bartke I., Knapp W., Stockinger H. Urokinase plasminogen activator receptor, beta 2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med. 1995 Apr 1;181(4):1381–1390. doi: 10.1084/jem.181.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan A. M., Sanan D. A., Sheppard D., Broaddus V. C. Vitronectin enhances internalization of crocidolite asbestos by rabbit pleural mesothelial cells via the integrin alpha v beta 5. J Clin Invest. 1995 Oct;96(4):1987–2001. doi: 10.1172/JCI118246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchaud R. M., MacDonald J. L., Kane A. B. Induction of angiogenesis by intraperitoneal injection of asbestos fibers. FASEB J. 1989 Apr;3(6):1747–1752. doi: 10.1096/fasebj.3.6.2467835. [DOI] [PubMed] [Google Scholar]
- Busso N., Masur S. K., Lazega D., Waxman S., Ossowski L. Induction of cell migration by pro-urokinase binding to its receptor: possible mechanism for signal transduction in human epithelial cells. J Cell Biol. 1994 Jul;126(1):259–270. doi: 10.1083/jcb.126.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan K. S., Griffith D. E., Garcia J. G. Asbestos exposure results in increased lung procoagulant activity in vivo and in vitro. Chest. 1990 Jul;98(1):112–119. doi: 10.1378/chest.98.1.112. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Brugge J. S. Integrins and signal transduction pathways: the road taken. Science. 1995 Apr 14;268(5208):233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Crowley C. W., Cohen R. L., Lucas B. K., Liu G., Shuman M. A., Levinson A. D. Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5021–5025. doi: 10.1073/pnas.90.11.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue J. E., Martin G. S. Phosphorylation of talin at tyrosine in Rous sarcoma virus-transformed cells. Mol Cell Biol. 1987 Jan;7(1):371–378. doi: 10.1128/mcb.7.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K., Miller B. G., Sara E., Slight J., Brown R. C. Asbestos fibre length-dependent detachment injury to alveolar epithelial cells in vitro: role of a fibronectin-binding receptor. Int J Exp Pathol. 1993 Jun;74(3):243–250. [PMC free article] [PubMed] [Google Scholar]
- Englund P. T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Dodson R. F., Callahan K. S. Effect of environmental particulates on cultured human and bovine endothelium. Cellular injury via an oxidant-dependent pathway. Lab Invest. 1989 Jul;61(1):53–61. [PubMed] [Google Scholar]
- Garcia J. G., Gray L. D., Dodson R. F., Callahan K. S. Asbestos-induced endothelial cell activation and injury. Demonstration of fiber phagocytosis and oxidant-dependent toxicity. Am Rev Respir Dis. 1988 Oct;138(4):958–964. doi: 10.1164/ajrccm/138.4.958. [DOI] [PubMed] [Google Scholar]
- Gross T. J., Cobb S. M., Gruenert D. C., Peterson M. W. Asbestos exposure increases human bronchial epithelial cell fibrinolytic activity. Am J Physiol. 1993 Mar;264(3 Pt 1):L276–L283. doi: 10.1152/ajplung.1993.264.3.L276. [DOI] [PubMed] [Google Scholar]
- Gyetko M. R., Sitrin R. G., Fuller J. A., Todd R. F., 3rd, Petty H., Standiford T. J. Function of the urokinase receptor (CD87) in neutrophil chemotaxis. J Leukoc Biol. 1995 Nov;58(5):533–538. doi: 10.1002/jlb.58.5.533. [DOI] [PubMed] [Google Scholar]
- Hauser I. A., Setter E., Bell L., Madri J. A. Fibronectin expression correlates with U937 cell adhesion to migrating bovine aortic endothelial cells in vitro. Am J Pathol. 1993 Jul;143(1):173–180. [PMC free article] [PubMed] [Google Scholar]
- Kamp D. W., Graceffa P., Pryor W. A., Weitzman S. A. The role of free radicals in asbestos-induced diseases. Free Radic Biol Med. 1992;12(4):293–315. doi: 10.1016/0891-5849(92)90117-y. [DOI] [PubMed] [Google Scholar]
- Lu H., Mabilat C., Yeh P., Guitton J. D., Li H., Pouchelet M., Shoevaert D., Legrand Y., Soria J., Soria C. Blockage of urokinase receptor reduces in vitro the motility and the deformability of endothelial cells. FEBS Lett. 1996 Feb 12;380(1-2):21–24. doi: 10.1016/0014-5793(95)01540-x. [DOI] [PubMed] [Google Scholar]
- McGavran P. D., Moore L. B., Brody A. R. Inhalation of chrysotile asbestos induces rapid cellular proliferation in small pulmonary vessels of mice and rats. Am J Pathol. 1990 Mar;136(3):695–705. [PMC free article] [PubMed] [Google Scholar]
- Mignatti P., Rifkin D. B. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993 Jan;73(1):161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Mossman B. T., Gee J. B. Asbestos-related diseases. N Engl J Med. 1989 Jun 29;320(26):1721–1730. doi: 10.1056/NEJM198906293202604. [DOI] [PubMed] [Google Scholar]
- Rao N. K., Shi G. P., Chapman H. A. Urokinase receptor is a multifunctional protein: influence of receptor occupancy on macrophage gene expression. J Clin Invest. 1995 Jul;96(1):465–474. doi: 10.1172/JCI118057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom W. N., Travis W. D., Brody A. R. Cellular and molecular basis of the asbestos-related diseases. Am Rev Respir Dis. 1991 Feb;143(2):408–422. doi: 10.1164/ajrccm/143.2.408. [DOI] [PubMed] [Google Scholar]
- Sevlever D., Rosenberry T. L. Mannosamine inhibits the synthesis of putative glycoinositol phospholipid anchor precursors in mammalian cells without incorporating into an accumulated intermediate. J Biol Chem. 1993 May 25;268(15):10938–10945. [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Dietzen D. J., Kwong J., Link D. C., Lublin D. M. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994 Jul;126(2):353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy-Scaria A. M., Gauen L. K., Kwong J., Shaw A. S., Lublin D. M. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993 Oct;13(10):6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty S., Kumar A., Johnson A., Pueblitz S., Idell S. Urokinase receptor in human malignant mesothelioma cells: role in tumor cell mitogenesis and proteolysis. Am J Physiol. 1995 Jun;268(6 Pt 1):L972–L982. doi: 10.1152/ajplung.1995.268.6.L972. [DOI] [PubMed] [Google Scholar]
- Sitrin R. G., Todd R. F., 3rd, Albrecht E., Gyetko M. R. The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. J Clin Invest. 1996 Apr 15;97(8):1942–1951. doi: 10.1172/JCI118626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A., Mueller B. M. The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J Cell Biol. 1995 Apr;129(2):335–344. doi: 10.1083/jcb.129.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Liotta L. A., Kleiner D. E., Jr Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993 Dec;7(15):1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- Treadwell M. D., Mossman B. T., Barchowsky A. Increased neutrophil adherence to endothelial cells exposed to asbestos. Toxicol Appl Pharmacol. 1996 Jul;139(1):62–70. doi: 10.1006/taap.1996.0143. [DOI] [PubMed] [Google Scholar]
- Turner C. E., Pavalko F. M., Burridge K. The role of phosphorylation and limited proteolytic cleavage of talin and vinculin in the disruption of focal adhesion integrity. J Biol Chem. 1989 Jul 15;264(20):11938–11944. [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H. Use and selectivity of herbimycin A as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:370–379. doi: 10.1016/0076-6879(91)01033-x. [DOI] [PubMed] [Google Scholar]
- Waltz D. A., Chapman H. A. Reversible cellular adhesion to vitronectin linked to urokinase receptor occupancy. J Biol Chem. 1994 May 20;269(20):14746–14750. [PubMed] [Google Scholar]
- Wei Y., Lukashev M., Simon D. I., Bodary S. C., Rosenberg S., Doyle M. V., Chapman H. A. Regulation of integrin function by the urokinase receptor. Science. 1996 Sep 13;273(5281):1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- Wei Y., Waltz D. A., Rao N., Drummond R. J., Rosenberg S., Chapman H. A. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994 Dec 23;269(51):32380–32388. [PubMed] [Google Scholar]