Abstract
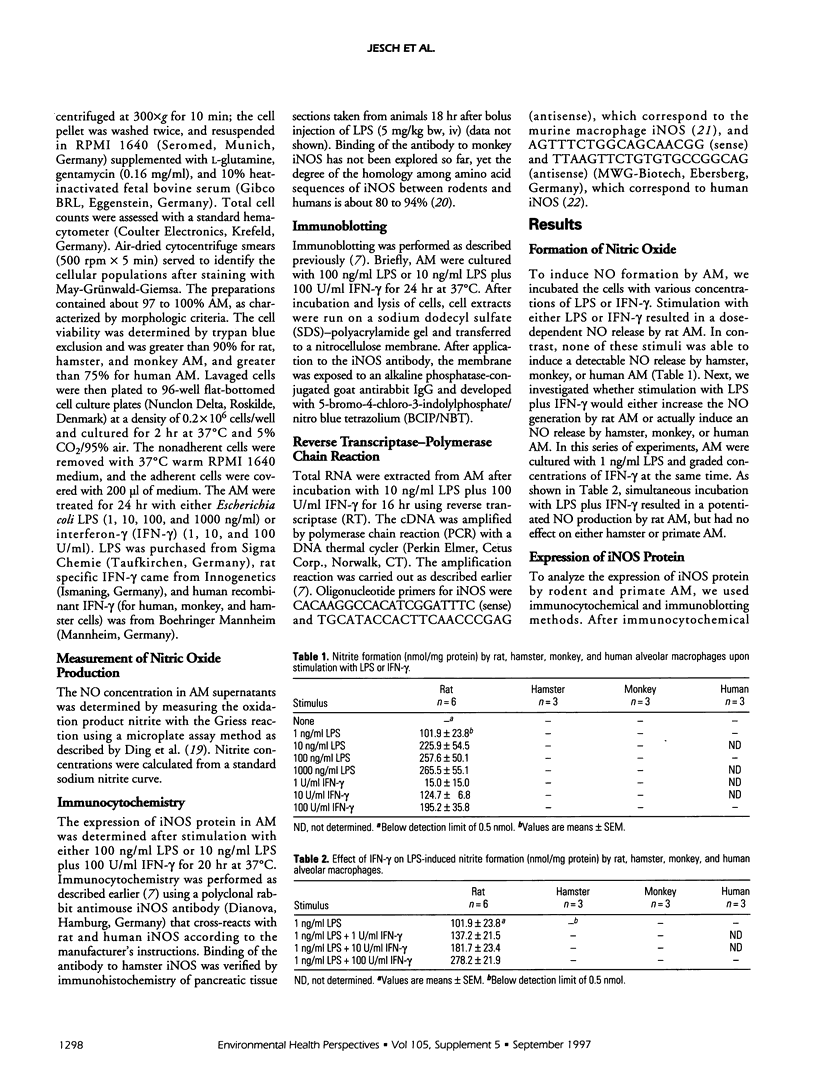
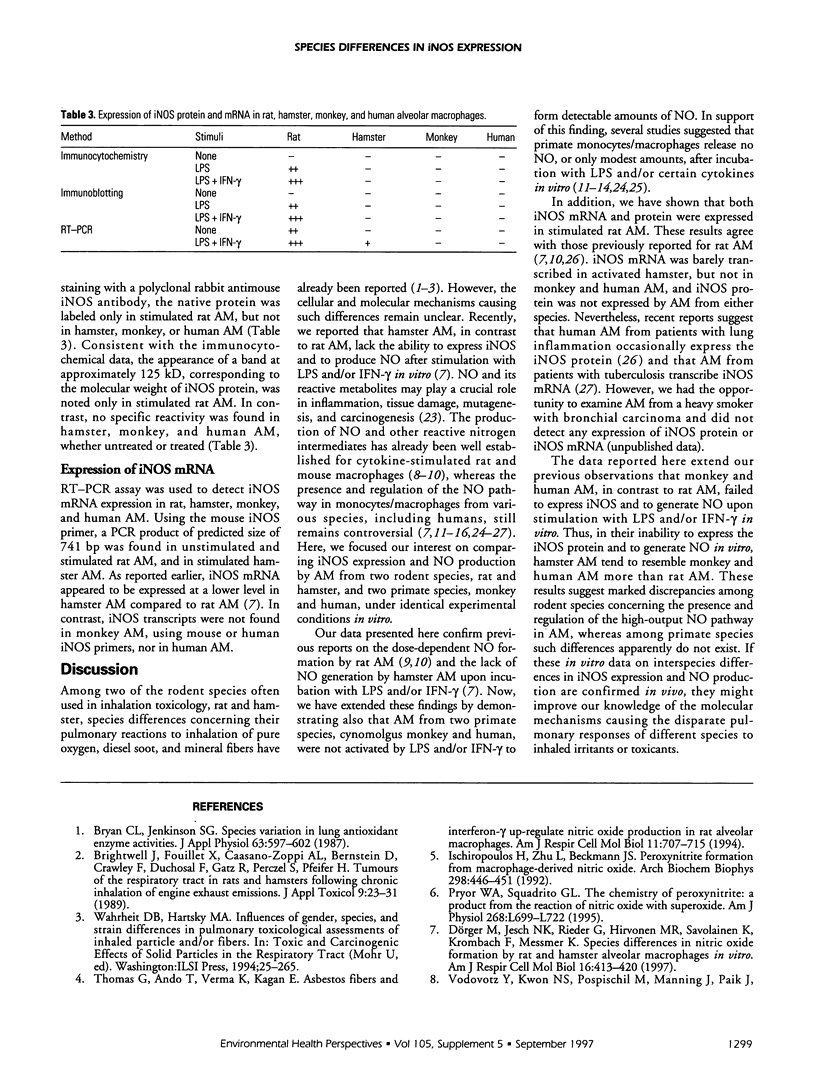
Nitric oxide (NO) is suggested to play a role in mediating pulmonary injury. However, interspecies differences appear to exist in the ability of alveolar macrophages (AM) to express the inducible nitric oxide synthase (iNOS) and to generate NO. The purpose of this study was to compare iNOS expression and NO production by rat, hamster, monkey, and human AM using the identical experimental conditions in vitro. As AM donors, CD rats, Syrian golden hamsters, cynomolgus monkeys, and nonsmoking, healthy human volunteers were used. The AM were obtained by bronchoalveolar lavage and stimulated in vitro with various concentrations and combinations of lipopolysaccharide (LPS) and interferon-gamma (IFN-gamma). The oxidation product of NO, nitrite, was measured in the AM supernatant by the Griess reaction. The expression of iNOS in AM was detected using immunocytochemistry and immunoblotting. The expression of iNOS mRNA was assessed by reverse transcriptase-polymerase chain reaction (RT-PCR). Rat AM, stimulated with either LPS or IFN-gamma, produced nitrite in a time- and dose-dependent manner. Combination of LPS and IFN-gamma resulted in a significantly enhanced nitrite formation. However, none of the treatments was able to induce hamster, monkey, or human AM to release measurable amounts of nitrite. Whereas expression of iNOS protein was only detected in stimulated rat AM, expression of iNOS mRNA was found in unstimulated and stimulated rat AM, slightly in stimulated hamster AM, but not in monkey and human AM. In conclusion, our findings point to distinct regulatory mechanisms of the NO pathway in AM from these four different species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H., Frech B., Thöny M., Pfister H., Peterhans E., Jungi T. W. Inducible nitric oxide synthase in cattle. Differential cytokine regulation of nitric oxide synthase in bovine and murine macrophages. J Immunol. 1995 May 1;154(9):4710–4718. [PubMed] [Google Scholar]
- Albina J. E. On the expression of nitric oxide synthase by human macrophages. Why no NO? J Leukoc Biol. 1995 Dec;58(6):643–649. doi: 10.1002/jlb.58.6.643. [DOI] [PubMed] [Google Scholar]
- Behr J., Vogelmeier C., Beinert T., Meurer M., Krombach F., König G., Fruhmann G. Bronchoalveolar lavage for evaluation and management of scleroderma disease of the lung. Am J Respir Crit Care Med. 1996 Aug;154(2 Pt 1):400–406. doi: 10.1164/ajrccm.154.2.8756813. [DOI] [PubMed] [Google Scholar]
- Brightwell J., Fouillet X., Cassano-Zoppi A. L., Bernstein D., Crawley F., Duchosal F., Gatz R., Perczel S., Pfeifer H. Tumours of the respiratory tract in rats and hamsters following chronic inhalation of engine exhaust emissions. J Appl Toxicol. 1989 Feb;9(1):23–31. doi: 10.1002/jat.2550090106. [DOI] [PubMed] [Google Scholar]
- Bryan C. L., Jenkinson S. G. Species variation in lung antioxidant enzyme activities. J Appl Physiol (1985) 1987 Aug;63(2):597–602. doi: 10.1152/jappl.1987.63.2.597. [DOI] [PubMed] [Google Scholar]
- Cameron M. L., Granger D. L., Weinberg J. B., Kozumbo W. J., Koren H. S. Human alveolar and peritoneal macrophages mediate fungistasis independently of L-arginine oxidation to nitrite or nitrate. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1313–1319. doi: 10.1164/ajrccm/142.6_Pt_1.1313. [DOI] [PubMed] [Google Scholar]
- Denis M. Human monocytes/macrophages: NO or no NO? J Leukoc Biol. 1994 May;55(5):682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Dörger M., Jesch N. K., Rieder G., Hirvonen M. R., Savolainen K., Krombach F., Messmer K. Species differences in NO formation by rat and hamster alveolar macrophages in vitro. Am J Respir Cell Mol Biol. 1997 Apr;16(4):413–420. doi: 10.1165/ajrcmb.16.4.9115752. [DOI] [PubMed] [Google Scholar]
- Gross S. S., Wolin M. S. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Kobzik L., Bredt D. S., Lowenstein C. J., Drazen J., Gaston B., Sugarbaker D., Stamler J. S. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993 Oct;9(4):371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- Krombach F., Fiehl E., Burkhardt D., Rienmüller R., König G., Adelmann-Grill B. C., Idel H., Rosenbruch M. Short-term and long-term effects of serial bronchoalveolar lavages in a nonhuman primate model. Am J Respir Crit Care Med. 1994 Jul;150(1):153–158. doi: 10.1164/ajrccm.150.1.8025742. [DOI] [PubMed] [Google Scholar]
- Liu S., Adcock I. M., Old R. W., Barnes P. J., Evans T. W. Lipopolysaccharide treatment in vivo induces widespread tissue expression of inducible nitric oxide synthase mRNA. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1208–1213. doi: 10.1006/bbrc.1993.2380. [DOI] [PubMed] [Google Scholar]
- Nicholson S., Bonecini-Almeida M. da G., Lapa e Silva J. R., Nathan C., Xie Q. W., Mumford R., Weidner J. R., Calaycay J., Geng J., Boechat N. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996 May 1;183(5):2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett E. L., Pruett S. B. Evaluation of nitrite production by human monocyte-derived macrophages. Biochem Biophys Res Commun. 1992 Jul 31;186(2):775–781. doi: 10.1016/0006-291x(92)90813-z. [DOI] [PubMed] [Google Scholar]
- Pendino K. J., Laskin J. D., Shuler R. L., Punjabi C. J., Laskin D. L. Enhanced production of nitric oxide by rat alveolar macrophages after inhalation of a pulmonary irritant is associated with increased expression of nitric oxide synthase. J Immunol. 1993 Dec 15;151(12):7196–7205. [PubMed] [Google Scholar]
- Prokhorova S., Lavnikova N., Laskin D. L. Functional characterization of interstitial macrophages and subpopulations of alveolar macrophages from rat lung. J Leukoc Biol. 1994 Feb;55(2):141–146. doi: 10.1002/jlb.55.2.141. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Squadrito G. L. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995 May;268(5 Pt 1):L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- Schneemann M., Schoedon G., Hofer S., Blau N., Guerrero L., Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993 Jun;167(6):1358–1363. doi: 10.1093/infdis/167.6.1358. [DOI] [PubMed] [Google Scholar]
- Sirsjö A., Karlsson M., Gidlöf A., Rollman O., Törmä H. Increased expression of inducible nitric oxide synthase in psoriatic skin and cytokine-stimulated cultured keratinocytes. Br J Dermatol. 1996 Apr;134(4):643–648. doi: 10.1111/j.1365-2133.1996.tb06963.x. [DOI] [PubMed] [Google Scholar]
- Thomas G., Ando T., Verma K., Kagan E. Asbestos fibers and interferon-gamma up-regulate nitric oxide production in rat alveolar macrophages. Am J Respir Cell Mol Biol. 1994 Dec;11(6):707–715. doi: 10.1165/ajrcmb.11.6.7524571. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y., Kwon N. S., Pospischil M., Manning J., Paik J., Nathan C. Inactivation of nitric oxide synthase after prolonged incubation of mouse macrophages with IFN-gamma and bacterial lipopolysaccharide. J Immunol. 1994 Apr 15;152(8):4110–4118. [PubMed] [Google Scholar]
- Weinberg J. B., Misukonis M. A., Shami P. J., Mason S. N., Sauls D. L., Dittman W. A., Wood E. R., Smith G. K., McDonald B., Bachus K. E. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995 Aug 1;86(3):1184–1195. [PubMed] [Google Scholar]
- Zembala M., Siedlar M., Marcinkiewicz J., Pryjma J. Human monocytes are stimulated for nitric oxide release in vitro by some tumor cells but not by cytokines and lipopolysaccharide. Eur J Immunol. 1994 Feb;24(2):435–439. doi: 10.1002/eji.1830240225. [DOI] [PubMed] [Google Scholar]



