Abstract
Inflammatory cells and their reactive oxygen metabolites can cause mutagenic effects in lung cells. The purpose of this study was to investigate the ability of activated neutrophils to modulate DNA binding of benzo[a]pyrene (B[a]P), a known carcinogen, in lung target cells. Equivalent numbers of rat lung epithelial cells (RLE-6TN cell line) and freshly isolated human blood neutrophils (PMN) were coincubated in vitro for 2 hr after addition of benzo[a]pyrene (0.5 microM) or two of its trans-diol metabolites, with or without stimulation with phorbol myristate acetate (PMA). DNA adducts of B[a]P-metabolites were determined in target cells using 32P-postlabeling; oxidative DNA damage (7-hydro-8-oxo-2'-deoxyguanosine [8-oxodG]) was evaluated by high performance liquid chromatography with electrochemical detection. Increased DNA adducts were observed in lung cells coincubated with polymorphonuclear leukocytes (PMN). Activation of PMN with PMA, or addition of more activated PMN in relation to the number of lung cells, further increased the number of adducts, the latter in a dose-response manner. Incubation with B[a]P-4,5-diol did not result in any adduct formation, while B[a]P-7,8-diol led to a significant number of adducts. Moreover, PMA-activated PMN strongly enhanced adduct formation by B[a]P-7,8-diol, but not 8-oxodG, in lung cells. The addition of antioxidants to the coincubations significantly reduced the number of adducts. Results suggest that an inflammatory response in the lung may increase the biologically effective dose of polycyclic aromatic hydrocarbons (PAHs), and may be relevant to data interpretation and risk assessment of PAH-containing particulates.
Full text
PDF

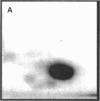
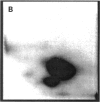
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Maidt L., Raynor T., Floyd R. A. 8-Hydroxydeoxyguanosine in DNA from TPA-stimulated human granulocytes. Free Radic Res. 1994 Feb;20(2):113–117. doi: 10.3109/10715769409147508. [DOI] [PubMed] [Google Scholar]
- Dix T. A., Marnett L. J. Metabolism of polycyclic aromatic hydrocarbon derivatives to ultimate carcinogens during lipid peroxidation. Science. 1983 Jul 1;221(4605):77–79. doi: 10.1126/science.6304879. [DOI] [PubMed] [Google Scholar]
- Dong H., Buard A., Renier A., Lévy F., Saint-Etienne L., Jaurand M. C. Role of oxygen derivatives in the cytotoxicity and DNA damage produced by asbestos on rat pleural mesothelial cells in vitro. Carcinogenesis. 1994 Jun;15(6):1251–1255. doi: 10.1093/carcin/15.6.1251. [DOI] [PubMed] [Google Scholar]
- Driscoll K. E., Carter J. M., Iype P. T., Kumari H. L., Crosby L. L., Aardema M. J., Isfort R. J., Cody D., Chestnut M. H., Burns J. L. Establishment of immortalized alveolar type II epithelial cell lines from adult rats. In Vitro Cell Dev Biol Anim. 1995 Jul-Aug;31(7):516–527. doi: 10.1007/BF02634029. [DOI] [PubMed] [Google Scholar]
- Driscoll K. E., Deyo L. C., Howard B. W., Poynter J., Carter J. M. Characterizing mutagenesis in the hprt gene of rat alveolar epithelial cells. Exp Lung Res. 1995 Nov-Dec;21(6):941–956. doi: 10.3109/01902149509031772. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Harris J., West M., Wong P. K. Formation of 8-hydroxydeoxyguanosine, hydroxyl free radical adduct of DNA in granulocytes exposed to the tumor promoter, tetradecanoylphorbolacetate. Biochem Biophys Res Commun. 1986 Jun 13;137(2):841–846. doi: 10.1016/0006-291x(86)91156-3. [DOI] [PubMed] [Google Scholar]
- Kehrer J. P., Mossman B. T., Sevanian A., Trush M. A., Smith M. T. Free radical mechanisms in chemical pathogenesis. Summary of the symposium presented at the 1988 annual meeting of the Society of Toxicology. Toxicol Appl Pharmacol. 1988 Sep 30;95(3):349–362. doi: 10.1016/0041-008x(88)90354-7. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Egner P. A., Moore K. G., Taffe B. G., Twerdok L. E., Trush M. A. Role of inflammatory cells in the metabolic activation of polycyclic aromatic hydrocarbons in mouse skin. Toxicol Appl Pharmacol. 1987 Sep 15;90(2):337–346. doi: 10.1016/0041-008x(87)90341-3. [DOI] [PubMed] [Google Scholar]
- Kwei G. Y., Bjeldanes L. F. Stimulation of binding of benzo[a]pyrene metabolites to DNA by diet-induced peroxidative stress. Food Chem Toxicol. 1990 Jul;28(7):491–495. doi: 10.1016/0278-6915(90)90119-8. [DOI] [PubMed] [Google Scholar]
- Mauthe R. J., Cook V. M., Coffing S. L., Baird W. M. Exposure of mammalian cell cultures to benzo[a]pyrene and light results in oxidative DNA damage as measured by 8-hydroxydeoxyguanosine formation. Carcinogenesis. 1995 Jan;16(1):133–137. doi: 10.1093/carcin/16.1.133. [DOI] [PubMed] [Google Scholar]
- Olin K. L., Cherr G. N., Rifkin E., Keen C. L. The effects of some redox-active metals and reactive aldehydes on DNA-protein cross-links in vitro. Toxicology. 1996 Jun 17;110(1-3):1–8. doi: 10.1016/0300-483x(96)03318-5. [DOI] [PubMed] [Google Scholar]
- Petruska J. M., Mosebrook D. R., Jakab G. J., Trush M. A. Myeloperoxidase-enhanced formation of (+-)-trans-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene-DNA adducts in lung tissue in vitro: a role of pulmonary inflammation in the bioactivation of a procarcinogen. Carcinogenesis. 1992 Jul;13(7):1075–1081. doi: 10.1093/carcin/13.7.1075. [DOI] [PubMed] [Google Scholar]
- Pruess-Schwartz D., Nimesheim A., Marnett L. J. Peroxyl radical- and cytochrome P-450-dependent metabolic activation of (+)-7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene in mouse skin in vitro and in vivo. Cancer Res. 1989 Apr 1;49(7):1732–1737. [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986 Sep;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Sagai M., Saito H., Ichinose T., Kodama M., Mori Y. Biological effects of diesel exhaust particles. I. In vitro production of superoxide and in vivo toxicity in mouse. Free Radic Biol Med. 1993 Jan;14(1):37–47. doi: 10.1016/0891-5849(93)90507-q. [DOI] [PubMed] [Google Scholar]
- Schins R. P., Schilderman P. A., Borm P. J. Oxidative DNA damage in peripheral blood lymphocytes of coal workers. Int Arch Occup Environ Health. 1995;67(3):153–157. doi: 10.1007/BF00626346. [DOI] [PubMed] [Google Scholar]
- Schraufstätter I., Hyslop P. A., Jackson J. H., Cochrane C. G. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988 Sep;82(3):1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P., Grover P. L., Swaisland A., Pal K., Hewer A. Metabolic activation of benzo(a)pyrene proceeds by a diol-epoxide. Nature. 1974 Nov 22;252(5481):326–328. doi: 10.1038/252326a0. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Nakajima M., Morimoto K. Establishment of a human system that generates O2- and induces 8-hydroxydeoxyguanosine, typical of oxidative DNA damage, by a tumor promotor. Cancer Res. 1994 Nov 15;54(22):5837–5840. [PubMed] [Google Scholar]
- Trush M. A., Seed J. L., Kensler T. W. Oxidant-dependent metabolic activation of polycyclic aromatic hydrocarbons by phorbol ester-stimulated human polymorphonuclear leukocytes: possible link between inflammation and cancer. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5194–5198. doi: 10.1073/pnas.82.15.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]
- Weitzman S. A., Gordon L. I. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990 Aug 15;76(4):655–663. [PubMed] [Google Scholar]