Abstract
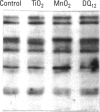
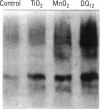
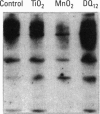
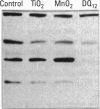
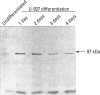
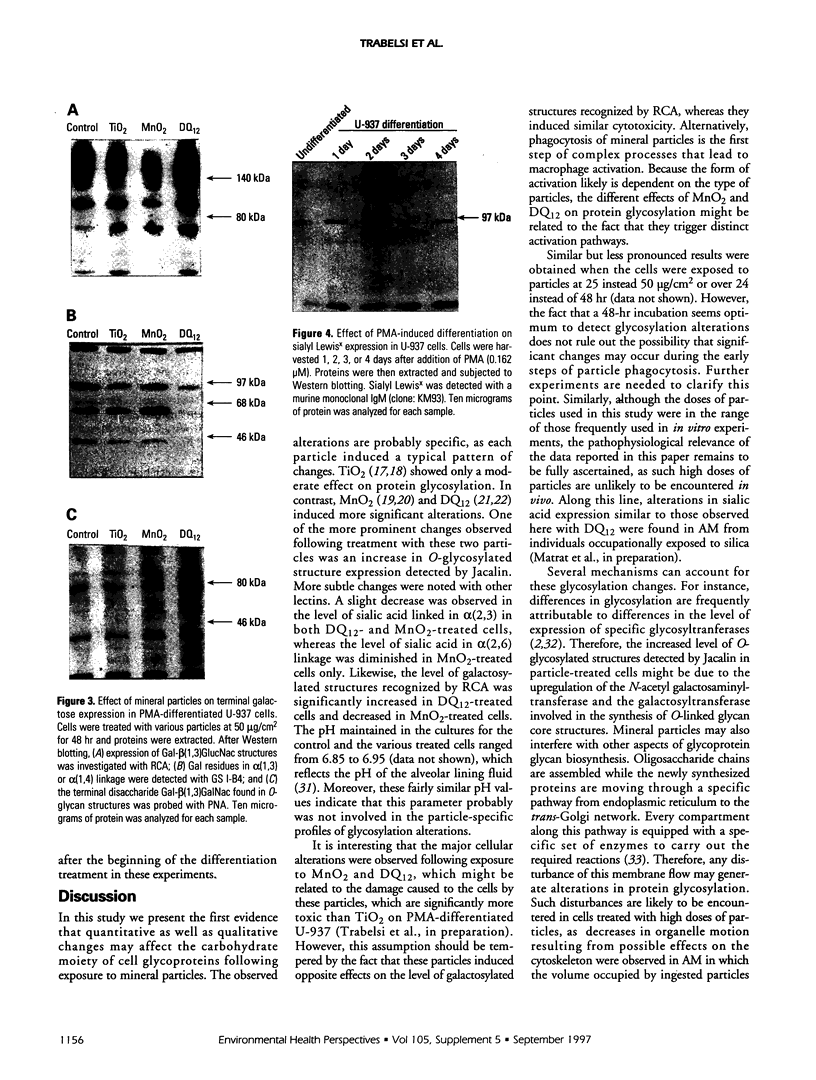
Carbohydrate moieties of cell glycoconjugates play a pivotal role in molecular recognition phenomena involved in the regulation of most biological systems and the changes observed in cell surface carbohydrates during cell activation or differentiation frequently modulate certain cell functions. Consequently, some aspects of macrophage response to particle exposure might conceivably result from alterations in glycosylation. Therefore, the effect of mineral particles on protein glycosylation was investigated in phorbol myristate acetate (PMA)-differentiated U-937. Jacalin, a lectin specific for O-glycosylated structures, showed a global increase in O-glycosylation in particle-treated cells. In contrast, no significant modifications were observed with concanavalin A, a lectin that recognizes certain N-glycosylated structures. The sialic acid-specific lectins Sambucus nigra agglutinin and Maackia amurensis agglutinin and the galactose-specific lectin Ricinus communis agglutinin revealed a complex pattern of alterations in glycoprotein glycosylation after crystalline silica or manganese dioxide treatments. Expression of sialyl Lewis(x), a glycosylated structure implicated in leukocyte trafficking, could not be detected in control or treated cells. This finding was consistent with the decrease in sialyl Lewis(x) expression observed during PMA-induced differentiation. In conclusion, various treatments used in this study induced quantitative as well as qualitative changes in protein glycosylation. Whether these changes are due to glycosidase release or to an alteration in glycosyltransferase expression remains to be determined. The potential functional implications of these changes are currently under investigation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afroun S., Tenu J. P., Lemaire G. Modifications of glycosylation patterns in macrophages upon activation. Biochim Biophys Acta. 1988 Sep 16;971(2):137–147. doi: 10.1016/0167-4889(88)90185-1. [DOI] [PubMed] [Google Scholar]
- Atsumi S., Nosaka C., Ochi Y., Iinuma H., Umezawa K. Inhibition of experimental metastasis by an alpha-glucosidase inhibitor, 1,6-epi-cyclophellitol. Cancer Res. 1993 Oct 15;53(20):4896–4899. [PubMed] [Google Scholar]
- Brown G. P., Monick M., Hunninghake G. W. Fibroblast proliferation induced by silica-exposed human alveolar macrophages. Am Rev Respir Dis. 1988 Jul;138(1):85–89. doi: 10.1164/ajrccm/138.1.85. [DOI] [PubMed] [Google Scholar]
- Brändli A. W. Mammalian glycosylation mutants as tools for the analysis and reconstitution of protein transport. Biochem J. 1991 May 15;276(Pt 1):1–12. doi: 10.1042/bj2760001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casburn-Budd R., Youinou P., Hager H., Elkington D., Baxter I., Berthelot J. M., Le Goff P., Isenberg D. A. Asialylated IgG in the serum and synovial fluid of patients with rheumatoid arthritis. J Rheumatol. 1992 Jul;19(7):1070–1074. [PubMed] [Google Scholar]
- Dorries A. M., Valberg P. A. Heterogeneity of phagocytosis for inhaled versus instilled material. Am Rev Respir Dis. 1992 Oct;146(4):831–837. doi: 10.1164/ajrccm/146.4.831. [DOI] [PubMed] [Google Scholar]
- Drickamer K., Taylor M. E. Biology of animal lectins. Annu Rev Cell Biol. 1993;9:237–264. doi: 10.1146/annurev.cb.09.110193.001321. [DOI] [PubMed] [Google Scholar]
- Driscoll K. E., Hassenbein D. G., Carter J., Poynter J., Asquith T. N., Grant R. A., Whitten J., Purdon M. P., Takigiku R. Macrophage inflammatory proteins 1 and 2: expression by rat alveolar macrophages, fibroblasts, and epithelial cells and in rat lung after mineral dust exposure. Am J Respir Cell Mol Biol. 1993 Mar;8(3):311–318. doi: 10.1165/ajrcmb/8.3.311. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Freeze H. H. A new approach to mapping co-localization of multiple glycosyl transferases in functional Golgi preparations. Glycobiology. 1996 Mar;6(2):177–189. doi: 10.1093/glycob/6.2.177. [DOI] [PubMed] [Google Scholar]
- Gossart S., Cambon C., Orfila C., Séguélas M. H., Lepert J. C., Rami J., Carré P., Pipy B. Reactive oxygen intermediates as regulators of TNF-alpha production in rat lung inflammation induced by silica. J Immunol. 1996 Feb 15;156(4):1540–1548. [PubMed] [Google Scholar]
- Hamosh A., Trapnell B. C., Zeitlin P. L., Montrose-Rafizadeh C., Rosenstein B. J., Crystal R. G., Cutting G. R. Severe deficiency of cystic fibrosis transmembrane conductance regulator messenger RNA carrying nonsense mutations R553X and W1316X in respiratory epithelial cells of patients with cystic fibrosis. J Clin Invest. 1991 Dec;88(6):1880–1885. doi: 10.1172/JCI115510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S., Weissmann G. Mechanisms of lysosomal enzyme release from leukocytes. IV. Interaction of monosodium urate crystals with dogfish and human leukocytes. Arthritis Rheum. 1975 Mar-Apr;18(2):153–165. doi: 10.1002/art.1780180213. [DOI] [PubMed] [Google Scholar]
- Kasper M., Haroske G. Alterations in the alveolar epithelium after injury leading to pulmonary fibrosis. Histol Histopathol. 1996 Apr;11(2):463–483. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Meyer K. C., Powers C., Rosenthal N., Auerbach R. Alveolar macrophage surface carbohydrate expression is altered in interstitial lung disease as determined by lectin-binding profiles. Am Rev Respir Dis. 1993 Nov;148(5):1325–1334. doi: 10.1164/ajrccm/148.5.1325. [DOI] [PubMed] [Google Scholar]
- Oberdörster G., Ferin J., Gelein R., Soderholm S. C., Finkelstein J. Role of the alveolar macrophage in lung injury: studies with ultrafine particles. Environ Health Perspect. 1992 Jul;97:193–199. doi: 10.1289/ehp.97-1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg F., Botling J., Nilsson K. Functional antagonism between vitamin D3 and retinoic acid in the regulation of CD14 and CD23 expression during monocytic differentiation of U-937 cells. J Immunol. 1993 Apr 15;150(8 Pt 1):3487–3495. [PubMed] [Google Scholar]
- Pilatte Y., Bignon J., Lambré C. R. Sialic acids as important molecules in the regulation of the immune system: pathophysiological implications of sialidases in immunity. Glycobiology. 1993 Jun;3(3):201–218. doi: 10.1093/glycob/3.3.201. [DOI] [PubMed] [Google Scholar]
- Pilatte Y., Tisserand E. M., Greffard A., Bignon J., Lambré C. R. Anticarbohydrate autoantibodies to sialidase-treated erythrocytes and thymocytes in serum from patients with pulmonary sarcoidosis. Am J Med. 1990 May;88(5):486–492. doi: 10.1016/0002-9343(90)90427-f. [DOI] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Satoh M., Handa K., Saito S., Tokuyama S., Ito A., Miyao N., Orikasa S., Hakomori S. Disialosyl galactosylgloboside as an adhesion molecule expressed on renal cell carcinoma and its relationship to metastatic potential. Cancer Res. 1996 Apr 15;56(8):1932–1938. [PubMed] [Google Scholar]
- Sawada T., Ho J. J., Sagabe T., Yoon W. H., Chung Y. S., Sowa M., Kim Y. S. Biphasic effect of cell surface sialic acids on pancreatic cancer cell adhesiveness. Biochem Biophys Res Commun. 1993 Sep 15;195(2):1096–1103. doi: 10.1006/bbrc.1993.2157. [DOI] [PubMed] [Google Scholar]
- Sedlák J., Hunáková L., Sulíková M., Chorváth B. Sialylated Lewis(x) and Lewis(a) determinants expression on human neoplastic cell lines: immunocytometric study with the 5th workshop monoclonal antibodies. Neoplasma. 1995;42(2):49–52. [PubMed] [Google Scholar]
- Seko A., Ohkura T., Kitamura H., Yonezawa S., Sato E., Yamashita K. Quantitative differences in GlcNAc:beta1-->3 and GlcNAc:beta1-->4 galactosyltransferase activities between human colonic adenocarcinomas and normal colonic mucosa. Cancer Res. 1996 Aug 1;56(15):3468–3473. [PubMed] [Google Scholar]
- Singh J., Kaw J. L., Zaidi S. H. Early biochemical response of pulmonary tissue to manganese dioxide. Toxicology. 1977 Oct;8(2):177–184. doi: 10.1016/0300-483x(77)90006-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H. Galactosyl and N-acetylgalactosaminyl homeostasis: a function for mammalian asialoglycoprotein receptors. Bioessays. 1994 Jul;16(7):519–524. doi: 10.1002/bies.950160713. [DOI] [PubMed] [Google Scholar]
- Zanetta J. P., Badache A., Maschke S., Marschal P., Kuchler S. Carbohydrates and soluble lectins in the regulation of cell adhesion and proliferation. Histol Histopathol. 1994 Apr;9(2):385–412. [PubMed] [Google Scholar]