Abstract
Endostatin, a fragment of collagen XVIII, is a potent antagonist of angiogenesis and inhibitor of tumor growth in mouse models. At present, the mechanism of action of endostatin is unknown. We show here that recombinantly produced human endostatin interacts with α5- and αv-integrins on the surface of human endothelial cells. We further demonstrate that the endostatin–integrin interaction is of functional significance in vitro, as we found that immobilized endostatin supports endothelial cell survival and migration in an integrin-dependent manner. Soluble endostatin in turn inhibits integrin-dependent endothelial cell functions, such as cell migration. Taken together, these results implicate integrins as potential targets for endostatin function and support the importance of integrins in endothelial cell biology and angiogenesis.
Angiogenesis, formation of new capillaries from preexisting blood vessels, is an important contributing factor in a variety of pathological processes, such as tumor growth and metastasis, and various inflammatory disorders (1). In particular, the expansion of solid tumors is critically dependent on angiogenesis, making cancer a clinically relevant target for anti-angiogenesis therapy (2). Endostatin, which is a 20-kDa C-terminal cleavage product of collagen XVIII, was originally identified by O'Reilly et al. (3) as a tumor-derived, highly active, and endothelial-specific angiogenic inhibitor. Recombinant endostatin has been shown to inhibit the growth of a wide variety of tumors in mice, with no toxic side effects observed (3, 4). Importantly, tumors treated with several cycles of endostatin do not develop drug resistance and become dormant, which persists even when the endostatin therapy has been discontinued (5). At present, the molecular mechanism of action of endostatin remains unknown.
Endothelial cells proliferate in an anchorage-dependent manner, suggesting that signals mediated by the integrin family of adhesion receptors are of importance in the growth of new blood vessels (6). The integrin dependence of tumor angiogenesis in vivo is evidenced by the fact that antagonists of the αvβ3 integrin, which is highly expressed in angiogenic endothelium, suppress tumor growth by inhibiting angiogenesis (7). In addition to the αvβ3 integrin, the functionally and structurally homologous αvβ5 integrin has been implicated in contributing to ocular angiogenesis (8). Recent studies have demonstrated that the α5β1 integrin also has a crucial role in angiogenesis, as antagonists of this integrin block angiogenesis induced in vivo by several different growth factors. α5β1 antagonists were also found to inhibit tumor angiogenesis, thereby causing regression of human tumors in animal models (9).
Taking into consideration that endostatin is a cleavage product of collagen XVIII, itself a normal component of the basement membranes that surround the vascular tubes (10, 11), we examined the possibility that sequences within endostatin might function as a binding site for integrins. We report here that endostatin interacts with the α5- and αv-integrins on the surface of human umbilical vein endothelial cells, and, when used in a soluble form, endostatin functions as an antagonist of integrins to inhibit endothelial cell function. Taken together, these results provide insights into the mechanisms as to how endostatin might exert its inhibitory effects on angiogenic blood vessels.
Materials and Methods
Reagents and Cell Culture.
Polylysine and gelatin were from Sigma, collagen I from Collaborative Research, plasma fibronectin from Finnish Red Cross (Helsinki, Finland), and vitronectin was purified from human plasma as described (12). GRGDSP- and GRGESP-peptides and anti-β1 integrin antibody P4C10 were from GIBCO/BRL. Purified integrins and the purified monoclonal anti-integrin antibodies P1E6 (anti-α2), P1D6 (anti-α5), NKI-SAM-1 (anti-α5), LM609 (anti-αvβ3), and P1F6 (anti-αvβ5) were from Chemicon. Purified monoclonal anti-αv antibody L230 was from American Type Culture Collection. Monoclonal anti-αv antibody VNR147 as ascites was obtained from Telios Pharmaceuticals (San Diego). Human umbilical vein endothelial cells (Huvec; Clonetics, San Diego) were cultured in EGM medium (Clonetics) supplemented with 12 μg/ml of bovine brain extract, 2 mM l-glutamine, 50 μg/ml streptomycin, and 50 units/ml penicillin (Irvine Scientific). Experimentation was carried out at cell passage number 4–12.
Expression and Characterization of Recombinant Endostatin.
A fragment of human collagen XVIII that corresponds to mouse endostatin sequences (3) was cloned to pQE-31 vector and expressed as an N-terminal His-tagged protein in Escherichia coli strain M15 according to the manufacturer's protocol (Qiagen, Chatsworth, CA). Bacterial pellets were lysed in 6 M guanidine⋅HCl, 0.5 M NaCl, 10 mM β-mercaptoethanol, 20 mM Tris⋅HCl, pH 7.9, by freeze–thaw followed by centrifugation. The supernatant was sonicated and applied to a ProBond column (Invitrogen) that had been preequilibrated with 8 M urea, 0.5 M NaCl, 20 mM Tris⋅HCl, pH 7.9. Bound protein was eluted by an imidazole gradient from 0 M to 0.5 M in the equilibrium buffer. Endostatin fractions were pooled and refolded in vitro, first by dialyzing overnight at 4°C against 4 M urea, 0.1 M NaCl, 1 mM/0.1 mM reduced/oxidized glutathione, 20 mM Tris⋅HCl, pH 7.9, then for 6 h against 1 M urea, 0.1 M NaCl, 0.1 mM/0.01 mM reduced/oxidized glutathione, 20 mM Tris⋅HCl, pH 7.9, and finally overnight against PBS, pH 6.9. Refolded soluble endostatin was separated by centrifugation and applied to a HiTrap SP cation-exchange column (Amersham Pharmacia). Endostatin fractions were eluted by a NaCl gradient from 0.1 M to 1.5 M in PBS, pH 6.9, pooled, and dialyzed against 0.1 M NaCl, 20 mM Tris⋅HCl, pH 7.4, and applied to a heparin-Sepharose CL-6B column (Amersham Pharmacia). Bound endostatin was eluted by a NaCl gradient from 0.1 M to 2 M in 20 mM Tris⋅HCl, pH 7.4. Endostatin fractions were pooled and dialyzed against PBS, pH 7.4, and passed through a Polymyxin agarose column (Sigma) by using 1× PBS buffer. Purified endostatin was concentrated by ultrafiltration to 0.5–1.5 mg/ml and stored at −20°C until use. Far UV circular dichroism (CD) spectrum was recorded on an Aviv Associates (Lakewood, NJ) model 62DS spectrometer equipped with a temperature controller. Buffer conditions in the CD analysis were 10 mM potassium phosphate, pH 8.0, and cells of 1 mm path length were used. A 5-s time constant and a 1.0-nm bandwidth were used during data acquisition over a wavelength range of 184 to 260 nm; three spectra were collected for protein or buffer and were averaged. Buffer spectra were subtracted from the protein spectra.
Cell Spreading and Immunofluorescence Analysis.
Huvec cells were washed with M199-medium (Irvine Scientific) supplemented with 10% FCS and with M199-medium supplemented with 0.5% BSA, 10 ng/ml basic fibroblast growth factor (bFGF), and 10 ng/ml epidermal growth factor (EGF). The cells were plated for 2 h on coverslips coated with endostatin (20 μg/ml), vitronectin (20 μg/ml), or polylysine (100 μg/ml). Cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and stained for vinculin (anti-vinculin antibody; Sigma) and actin (TRITC-phalloidin). Immunofluorescence analysis was carried out with Nikon inverted microscope, and the degree of cell spreading was analyzed with phase-contrast microscopy.
Immunoprecipitations and Immunoblot Analysis.
Huvec cells were washed twice with serum-free EGM medium, suspended to the same medium, and either kept in suspension at 37°C for 15 min or plated on dishes coated with endostatin (20 μg/ml), vitronectin (20 μg/ml), or polylysine (100 μg/ml) and incubated at 37°C for 45 min. Cell lysate preparations, immunoprecipitations with anti-FAK antibody (Transduction Laboratories, Lexington, KY), as well as immunoblotting with horseradish peroxidase (HRPO)-conjugated anti-phosphotyrosine py20 antibody (Transduction Laboratories) and with the anti-FAK antibody followed by enhanced chemiluminescence detection (Pierce) were carried out as in ref. 13.
Cell Attachment Assay.
Cell attachment was assayed as described previously with slight modifications (14). Microtiter wells (Linbro/Titertek; ICN) were coated overnight with the indicated concentrations of proteins. The wells were blocked for 30 min with 0.5% BSA in PBS; it is important to note that BSA binds in a nonspecific manner to endostatin and a longer blockage with BSA interferes with the assay (not shown). Huvec cells were briefly trypsinized followed by washes with serum-containing M199 medium and serum-free M199 medium containing 0.5% BSA. Cells were suspended in 3.5 × 105 cells/ml in M199 medium with growth factors as above and incubated in the presence or absence of EDTA, RGD-, or RGE-peptides or anti-integrin antibodies for 30 min at 4°C. Cell suspensions (3.5 × 104 cells/well) were added to the wells, and the plates were incubated at 37°C for 50 min. The plates were washed three times with PBS, and the cells were fixed with 20% methanol for 15 min at room temperature. Attached cells were stained with 0.5% crystal violet, the dye was eluted with 2% SDS, and the absorbance was measured at 590 nm. Background absorbance observed in the wells coated with BSA was deducted from the values obtained.
Solid-Phase Ligand-Binding Assay.
Microtiter wells were coated with 30 μg/ml of endostatin in PBS overnight at room temperature. The wells were blocked with 1% BSA in TBS⋅Ca/Mg (150 mM NaCl, 50 mM Tris⋅HCl, pH 7.4, 2 mM CaCl2, 1 mM MgCl2) at room temperature for 1 h. α5β1 integrin was overlaid in TBS⋅Ca/Mg with 4 mM octyl glucoside and incubated with rotation at +4°C overnight. To block the binding of the purified integrin to endostatin, the integrin was preincubated with 10 mM EDTA, 20 μg/ml RGD peptides, or 10 μg/ml anti-integrin antibodies for 30 min at +4°C. Unbound integrin molecules were washed three times with TBS⋅Ca/Mg, 0.05% Tween 20, and the bound ones were incubated with 1/1,000 dilution of a polyclonal anti-α5 cytoplasmic domain antibody (15) for 1 h at room temperature. After extensive washes with TBS⋅Ca/Mg, 0.05% Tween 20, the bound antibodies were detected by using biotinylated goat anti-rabbit IgG antibodies and Ultra-Sensitive ABC Peroxidase Staining reagents (Pierce). TMB liquid substrate (Sigma) was added to the wells, the reactions were stopped with 0.5 M H2SO4, and absorbance was measured at 450 nm. Background absorbance observed in the wells coated with BSA was deducted from the values obtained.
Cell Migration.
Haptotactic cell motility was measured by using a modified Boyden chamber (Neuroprobe, Cabin John, MD) as previously described (16). The undersurface of the membrane filter was precoated with 30 μg/ml of endostatin or various matrix proteins. To inhibit migration on endostatin, lower chambers were filled with serum-free EGM medium containing 10 μg/ml of anti-integrin antibodies. Huvec cells (1 × 104) were added to the upper chambers in serum-free EGM. The cells were incubated at 37°C for 3 h, after which the number of cells migrated to the lower surface was counted. Migration results are expressed in terms of the average number of cells/high-magnification microscopic field. To inhibit migration on various matrix proteins with soluble endostatin, endostatin was added to the lower chambers, as indicated in the figures.
Cell Survival.
To determine the capability of immobilized endostatin to support cell survival, Huvec cells were plated in serum-free EGM medium on dishes that had been precoated with 30 μg/ml of endostatin or LM609, or with 1% of heat denatured BSA. As indicated in the figure legend, soluble anti-integrin antibodies at a concentration of 10 μg/ml were added to cells in some of the experiments. Apoptotic cell death was monitored 6 h later by measuring DNA fragmentation using the Cell Death Detection ELISA kit (Roche Molecular Biochemicals).
Results
Purified Endostatin Immobilized on a Substrate Promotes Cell Spreading, Focal Adhesion Formation, and Focal Adhesion Kinase (FAK) Phosphorylation.
Recombinant, N-terminally His-tagged human endostatin was purified to apparent homogeneity from E. coli with a typical yield of 1.5 mg of soluble, refolded protein/liter of culture. The purified protein migrated as a discrete band at Mr 18,000 and 24,000 on SDS/PAGE under nonreducing and reducing conditions, respectively (Fig. 1A). Far-UV CD spectroscopy revealed a spectrum similar to that published earlier for mouse and human endostatin (17), with a characteristic single broad minimum from 205 to 210 nm (θ = −13,294 at 210 nm). As published before (3, 4, 18, 19), recombinant endostatin was found to potently inhibit bFGF-induced proliferation of endothelial cells with an IC50 of ≈500 ng/ml (not shown). These results demonstrate that the purified human endostatin used in these studies has very similar, if not identical, biochemical and biological properties as purified endostatin molecules used by other investigators.
Figure 1.
Immobilized endostatin promotes cell spreading, focal adhesion formation, and FAK phosphorylation. (A) SDS/PAGE analysis of recombinant human endostatin used in these studies. Five micrograms of purified endostatin was separated on SDS-gel under non-reducing (Left) and reducing (Right) conditions, and the gel was stained with Coomassie blue. Molecular weight markers in kDa are indicated. (B) Analysis of spreading, focal adhesions, and stress fibers. Huvec cells were plated for 2 h on coverslips that had been coated with 20 μg/ml of either endostatin or vitronectin. The degree of cell spreading is seen from the phase contrast micrographs (Left). Focal adhesions are visualized by vinculin staining (Middle), and actin stress fibers by phalloidin staining (Right). (C) Analysis of tyrosine phosphorylation of FAK. Huvec cells were either kept in suspension for 15 min (S), or plated for 45 min on dishes that had been coated with 20 μg/ml of endostatin (ES) or vitronectin (Vn), or 100 μg/ml of polylysine (PL). Cell lysates containing equal amounts of protein were immunoprecipitated with anti-FAK antibody, and one-half of the precipitates was analyzed by immunoblotting with anti-phosphotyrosine antibodies (Left). The other half was probed with anti-FAK antibody to confirm loading (Right).
Integrin-mediated cell attachment on cognate integrin ligands, such as extracellular matrix (ECM) proteins, results in cell spreading, focal adhesion formation, and induction of protein tyrosine phosphorylation (20). When integrin inhibitors such as antibodies are immobilized on a substrate, they act as agonists and similarly activate intracellular events (21, 22). To examine whether immobilized endostatin would function as an integrin agonist, Huvec cells were plated under serum-free conditions on dishes that had been coated with endostatin. As shown in Fig. 1, immobilized endostatin, similar to the αv-integrin ligand vitronectin, promoted endothelial cell spreading, focal adhesion formation, and tyrosine phosphorylation of the focal adhesion kinase FAK. In contrast, cell attachment to polylysine, to which cells adhere in an integrin-independent manner, does not induce these cell biological events (23). Cells on polylysine remained round, and failed to assemble focal adhesions and induce tyrosine phosphorylation of FAK (Fig. 1C, and data not shown). These results suggest that immobilized endostatin serves as an adhesive substrate for endothelial cells, possibly by interacting with integrins on the cell surface and by activating postligand binding events downstream of integrins.
Endothelial Cell Adhesion on Immobilized Endostatin Is Mediated by α5- and αv-Integrins.
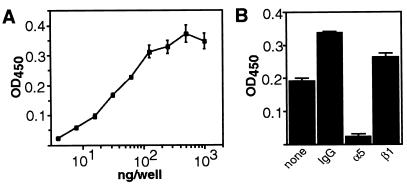
Quantitative cell attachment assays were performed to characterize the endothelial cell–endostatin interaction in more detail. As shown in Fig. 2A, immobilized endostatin supported endothelial cell adhesion in a saturable and concentration-dependent manner. Importantly, a peptide synthesized according to the N-terminal tag of the recombinant endostatin failed to support cell attachment when immobilized on a solid surface. Also, adhesion to endostatin was blocked by polyclonal affinity-purified anti-endostatin antibodies, thus confirming that the cell adhesion activity is attributable to endostatin (not shown). Further experiments demonstrated that cell attachment on immobilized endostatin is likely to be integrin-mediated. As shown in Fig. 2B, addition of 10 mM EDTA induced a significant rounding of cells plated on endostatin, suggesting that immobilized endostatin mediates cell adhesion through a divalent cation-dependent cell surface receptor, such as an integrin (20). Rounding of cells was similarly observed in the presence of RGD-peptides, suggesting that cell–endostatin interaction might be mediated by an RGD-dependent integrin (Fig. 2B). In the presence of EDTA or RGD-peptides, however, endothelial cells still remained attached to endostatin, despite the round morphology (see Discussion).
Figure 2.
Endostatin mediates endothelial cell adhesion through α5- and αv-integrins. (A) Dose dependence of cell adhesion to endostatin. Huvec cells were plated onto microtiter wells coated with the indicated concentrations of endostatin, and cell attachment was analyzed as described in Materials and Methods. (B) Effect of EDTA and RGD-peptides on cell attachment to endostatin. Huvec cells were incubated on wells coated with endostatin for 50 min in the absence (Left) or presence of 10 mM EDTA (Middle) or 0.8 mM of RGD-peptides (Right). Wells were washed with PBS, and cells were photographed. (C) Cell adhesion to endostatin is α5- and αv-integrin-dependent. Huvec cells were incubated with the indicated antibodies before plating on wells coated with 30 μg/ml of endostatin. Anti-integrin antibodies used were: anti-αv (L230, VNR147), anti-αvβ3 (LM609), anti-αvβ5 (P1F6), anti-β1 (P4C10) and anti-α5 (P1D6). Cell attachment was analyzed as above. Error bars represent SD.
To identify the integrin molecule(s) that mediate cell adhesion on immobilized endostatin, inhibitory anti-integrin antibodies were used in cell attachment assays. As shown in Fig. 2C, monoclonal antibodies L230 and VNR147 against αv-integrins slightly reduced the cell adhesion on endostatin. Reduction in the cell attachment to endostatin was also observed when antibodies LM609 and P1F6 against the αvβ3 and αvβ5 integrins, respectively, were tested. Monoclonal antibody P4C10 against the β1 integrin significantly inhibited cell attachment to endostatin, suggesting that an RGD-dependent β1 integrin, such as αvβ1 and/or α5β1, may contribute to cell–endostatin interaction. Indeed, an inhibitory P1D6 antibody against the α5 subunit reduced Huvec cell attachment to endostatin to the same extent as the anti-β1 antibody P4C10. Combination of anti-α5 and anti-αv antibodies further enhanced the inhibition of cell attachment on endostatin (Fig. 2C). Antibodies against several non-RGD-dependent β1 integrins, including α1, α2, α3, α4, and α6 integrins, failed to affect cell adhesion on endostatin. Control experiments with appropriate ECM proteins confirmed that the antibodies used in this study blocked endothelial cell adhesion in a specific manner (not shown). These results demonstrate that Huvec cell adhesion on immobilized endostatin is primarily mediated by the α5β1 integrin and, to an extent, by the αvβ3 and αvβ5 integrins.
Endostatin Binds to Purified Integrins.
To confirm the results obtained above, we studied the direct endostatin–integrin interaction in a solid-phase ligand binding assay, and used a commercially available purified α5β1 preparation for these studies. As shown in Fig. 3A, soluble α5β1 integrin demonstrates a concentration-dependent and saturable binding to immobilized endostatin. The specificity of the interaction was confirmed by inhibition of endostatin binding to the α5β1 integrin with the inhibitory α5-antibody NKI-SAM-1 (Fig. 3B). Thus, these results demonstrate a specific and direct interaction between the angiogenic inhibitor endostatin and the α5β1 integrin.
Figure 3.
Endostatin binds to purified α5β1 integrin in a concentration-dependent and specific manner in a solid-phase ligand binding assay. (A) α5β1 integrin was added to wells coated with endostatin as indicated, and incubated overnight at +4°C. Anti-α5 serum, biotinylated anti-rabbit IgG antibodies, and ABC complexes were used to detect bound integrin. (B) Soluble inhibitory anti-integrin antibodies were added to block the interaction between endostatin and α5β1 integrin, and incubated overnight at +4°C. Bound integrin was detected as above. Anti-integrin antibodies used were: anti-α5 (NKI-SAM-1) and anti-β1 (P4C10). Results are expressed as the mean +/− SD.
Immobilized Endostatin Promotes and Soluble Endostatin Inhibits Integrin-Dependent Endothelial Cell Migration and Survival.
Recent studies have shown that endostatin blocks endothelial cell migration and survival in vitro (4, 24, 25), thereby providing a cell biological explanation for endostatin function in vivo. The αvβ3 integrin is known to have a critical role in cell migration and survival (26, 27), and it has been demonstrated that αvβ3-antagonists exert their anti-angiogenic effects in vivo by blocking survival signals mediated by this integrin (7, 22). A similar crucial role in cell migration and survival has been observed for the α5β1 integrin (14, 28–30). Given our findings above, we set forth to determine whether endostatin affects αvβ3 or α5β1 integrin-dependent endothelial cell migration and survival.
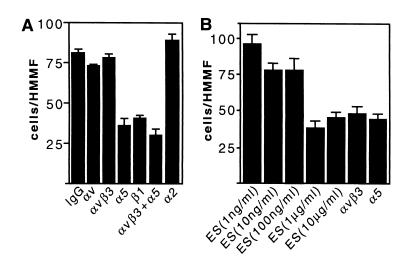
Endothelial cell contact with an increased concentration of immobilized αvβ3 or α5β1 integrin ligand is known to enhance cell migration in a haptotactic manner. On the other hand, cell migration is efficiently prevented by the same ligands when administered in solution to the cells (21, 28). Our results demonstrate that endostatin is similarly capable of modulating endothelial cell migration. Thus, Huvec cells readily migrated in a haptotactic Boyden chamber assay through a microporous membrane toward immobilized endostatin, and this migration was significantly blocked by the anti-α5 antibody P1D6, but not by the anti-α2 antibody P1E6 or the anti-αvβ3 antibody LM609 (Fig. 4A). These results are in good concordance with our finding that the anti-α5 antibody alone is an efficient inhibitor of cell attachment on immobilized endostatin (Fig. 2C). Soluble endostatin in turn was found to interfere with integrin-dependent cell migration. As shown in Fig. 4B, soluble endostatin inhibits cell migration on immobilized gelatin, a reported αvβ3 integrin ligand (31), in a concentration-dependent manner. In control experiments, blocking antibodies against both α5 and αvβ3 integrins had an inhibitory effect on endothelial cell motility on gelatin. Soluble endostatin, or antibodies against α5 and αvβ3 integrins, did not affect cell movement on collagen I, on which the cells migrate in a manner dependent on non-RGD binding β1 integrins (data not shown). Thus, immobilized endostatin promotes and soluble endostatin inhibits endothelial cell migration in an integrin-dependent and integrin-specific manner.
Figure 4.
Endostatin modulates endothelial cell migration in an integrin-dependent manner. (A) Immobilized endostatin supports endothelial cell migration in an α5β1-dependent manner. Cell motility assay was performed on immobilized endostatin in the presence or absence of inhibitory anti-integrin antibodies. The results at each time point are the mean cell number of 20 randomly selected high magnification microscopic fields. (B) Soluble endostatin inhibits migration on gelatin. Endothelial cell migration was determined on immobilized gelatin in the presence or absence of the indicated concentrations of soluble endostatin or anti-integrin antibodies. Anti-integrin antibodies used were: anti-αv (L230), anti-αvβ3 (LM609), anti-β1 (P4C10), anti-α2 (P1E6), and anti-α5 (P1D6). Relative cell migration is indicated; each value is a mean +/− SD from representative experiments.
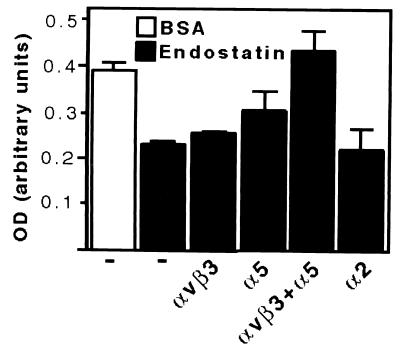
Similar results were obtained with respect to endothelial cell survival. Endothelial cells are known to undergo apoptosis upon serum withdrawal if appropriate integrin ligation is denied (6, 22). Thus, when Huvec cells were plated on immobilized BSA under serum-free conditions, significant levels of apoptosis were detected after 6 h of incubation (Fig. 5). In contrast, and similar to what was reported previously (22), plating of Huvec cells on immobilized anti-αvβ3 antibody LM609 protected cells from apoptosis (data not shown). We found that immobilized endostatin similarly promoted endothelial cell survival, as clearly decreased levels of apoptosis were detected in cells adherent on plates coated with endostatin. Administration of soluble LM609 or anti-α5 antibody P1D6, but not of the anti-α2 antibody P1E6, resulted in a slight induction in the apoptosis on cells plated on endostatin. A significant additive effect in the induction of apoptosis on endostatin was noticed when anti-α5 and anti-αvβ3 antibodies were used in combination (Fig. 5), demonstrating that immobilized endostatin supports cell survival in an α5β1 and αvβ3-dependent manner.
Figure 5.
Immobilized endostatin promotes endothelial cell survival in an integrin-dependent manner. Huvec cells were plated under serum-free conditions on BSA-coated surface or on immobilized endostatin for 6 h. Where indicated, 10 μg/ml of soluble anti-integrin antibodies were added to the cells prior plating on endostatin. DNA fragmentation was measured as an indication of apoptosis. Anti-integrin antibodies used were: anti-αvβ3 (LM609), anti-α5 (P1D6), and anti-α2 (P1E6). Data are presented as mean +/− SD.
Dhanabal et al. (24) have recently reported that treatment of pulmonary artery endothelial cells with soluble endostatin induces apoptosis of the cells up to about 19–20%. Cells were plated in these studies on fibronectin, and, taken together with our results reported here, it is plausible that endostatin might induce apoptosis by interfering with the survival signals mediated by the α5β1 integrin. We have found that, at least in Huvec cells, the levels of apoptosis induced by soluble endostatin are rather low; we typically found an induction of apoptosis at the range of 2 to 4% of the cells, and these findings are similar to what was recently reported by Dixelius et al. (32), with respect to endostatin-induced apoptosis in murine brain endothelial cells. Because of the low levels of apoptosis in endostatin-treated Huvec cells, we were unable to reliably test the hypothesis that endostatin-induced apoptosis might indeed occur as a result of interference with survival signals mediated by specific integrins.
Discussion
In the present study, we sought to assess whether the angiogenic inhibitor endostatin would function as a ligand for the integrin-family of adhesion receptors on the surface of endothelial cells. Our results demonstrate that endostatin binds to the α5β1 integrin, and to an extent also to αvβ3 and αvβ5 integrins. Our studies further show that this binding is of functional significance, as immobilized endostatin was found to promote and soluble endostatin was found to inhibit integrin-dependent endothelial cell functions. Taken together with the established importance of integrins in tumor angiogenesis, these results provide a mechanistic context for the function of endostatin as an angiogenesis inhibitor.
Several lines of cell biological and biochemical evidence demonstrated the interaction between endostatin and integrins in these studies. Thus, inhibitory anti-integrin antibodies prevented Huvec cell interaction with endostatin in a cell attachment assay, whereas endostatin interacted with purified α5β1 integrin in a specific manner in a solid-phase binding assay. Endostatin does not contain an RGD sequence, and it therefore remains to be determined what is the sequence motif in endostatin that these integrins recognize. As noted in Fig. 2, treatment of cells with RGD-peptides results in a rounding of the cells on endostatin, but the cells nevertheless remain attached to it. Similarly, RGD-peptides failed to block endostatin-α5β1 interaction in a solid-phase binding assay (data not shown). It is possible that endostatin, in addition to binding to the classical RGD-binding site in an integrin, also interacts with another, yet-to-be-determined site. If this was the case, the interaction between the α5β1 integrin and endostatin would have resemblance to the α5β1 integrin–fibronectin interaction; the central cell binding domain of fibronectin binds to the integrin in an RGD-dependent manner, whereas a second site in fibronectin recognizes another region in the N-terminal area of the α5 subunit (34). Alternatively, endostatin may bind to the integrin independent of the RGD-binding site, in which case the cell rounding effect of RGD-peptides is likely to be the result of the capability of the peptides to interfere with integrin-dependent organization of cell cytoskeleton. The function-blocking NKI-SAM-1 antibody was found to be an efficient and specific inhibitor of the α5β1-endostatin interaction in the solid phase binding assay, and fine mapping of the epitope for this antibody will provide valuable information about the mechanisms of endostatin–integrin interaction. Interestingly, a number of fragments that are derived from molecules associated with ECM have recently been found to be efficient inhibitors of angiogenesis. Further, they appear to inhibit angiogenesis in an integrin-dependent manner, despite the fact that they lack the RGD-binding site for integrins. For example, PEX is a C-terminal fragment of matrix metalloproteinase-2, which lacks an RGD-motif in the primary structure, but it nevertheless binds to the αvβ3 integrin and inhibits angiogenesis (34). Similarly, an anti-angiogenic factor derived from the α3 chain of type IV collagen termed tumstatin has been reported to bind to αvβ3 and β1 integrins, again in an RGD-independent fashion (35–37). An anti-angiogenic factor derived from the type IV collagen α1 chain, in turn, may inhibit angiogenesis by functioning through the α1β1 integrin (38). Finally, a recombinant fragment derived from the α2 chain of type IV collagen also has anti-angiogenic activity; the putative integrin connection in this case remains to be determined (39).
To study the functional significance of the endostatin–integrin interaction, we examined the capability of endostatin to modulate endothelial cell functions under conditions in which these functions are strictly dependent on integrins, and not any other agents. We found that endostatin indeed is capable of modulating cellular functions, such as migration and survival, in an integrin-dependent manner. Previous studies by others have examined the capability of endostatin to regulate biological events induced by treatment of cells with various growth factors. Yamaguchi et al. (25) have reported that endostatin inhibits vascular endothelial growth factor (VEGF)-induced endothelial cell migration. In several other cases, endostatin has been found to interfere with bFGF- rather than with VEGF-induced cellular events. Thus, Dhanabal et al. (4) have shown that endostatin inhibits bFGF-induced cell migration. Similarly, endostatin has been shown to inhibit bFGF-induced (32, 40) but not VEGF-induced angiogenesis (40). In our studies, we have found that endostatin strongly inhibits bFGF- but not VEGF-induced migration of bovine aortic endothelial cells in three-dimensional collagen (data not shown). Interestingly, it has been reported that the α5β1 integrin has an important role in regulating bFGF-induced, but not VEGF-induced angiogenesis (9). Previously, it has been shown that α5β1 expression can be up-regulated by bFGF in endothelial cells (41), whereas VEGF does not induce α5β1 expression (42). Similarly, αvβ3 has been shown to play a significant role in bFGF-induced, but not in VEGF-induced angiogenesis (8, 43). Further studies are needed to determine the potential significance of integrin-growth factor receptor cross-talk in endostatin action.
Interestingly, modulation of integrin function and signaling is emerging as a common functional theme among various angiogenesis inhibitors. In addition to the anti-angiogenic inhibitors that directly interact with integrins (see above), it was recently shown that tumor necrosis factor-α and interferon-γ disrupt tumor vasculature by reducing activation of the αvβ3 integrin (44). Angiostatin, a fragment of plasminogen and a potent inhibitor of neovascularization, in turn induces an integrin-independent, potentially flawed, activation of FAK, which appears to contribute to the induction of endothelial cell apoptosis by angiostatin (45). Thus, it is warranted to suggest that endostatin–integrin interaction might have an effect on neovascularization in vivo, e.g., by modulating integrin signaling events in processes such as cell migration and cell survival. Also, it has been demonstrated that antibodies and peptides with integrin selectivity home efficiently to tumor blood vessels (46, 47). The significance of the integrin binding function of endostatin may also be to target and concentrate endostatin at the sites of neovascularization to function as a highly efficient angiogenesis inhibitor by a currently unknown mechanism. At present, a versatile repertoire of activities for endostatin has been suggested that may contribute to its anti-angiogenic function, and, in many cases, conflicting evidence exists in the literature. In addition to our finding that endostatin binds to integrins (see also ref. 32), results both in favor and against the role of heparin binding (25, 32, 40, 48) and zinc binding (25, 49) in the function of endostatin have been published. Future additional studies are clearly required to fully understand the significance of integrin binding and other endostatin activities in the efficient inhibition of angiogenesis in vivo by endostatin.
Acknowledgments
We thank Helena Hessle and Telios Pharmaceuticals for providing integrin reagents in the initial stages of these studies. This study was supported by grants from National Institutes of Health, California Breast Cancer Research Program, Academy of Finland, European Commission, FibroGen (South San Fransisco, CA), Finnish Cultural Foundation, and Ella and Georg Ehrnrooth Foundation. M.R. is a recipient of a European Molecular Biology Organization fellowship. K.V. is a PEW scholar in biomedical sciences.
Abbreviations
- Huvec
human umbilical vein endothelial cells
- CD
circular dichroism
- bFGF
basic fibroblast growth factor
- EGF
epidermal growth factor
- FAK
focal adhesion kinase
- ECM
extracellular matrix
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031564998.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031564998
References
- 1.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.O'Reilly M S, Boehm T, Shing Y, Fukai N, Vasios G, Lane W S, Flynn E. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 4.Dhanabal M, Ramchandran R, Volk R, Stillman I E, Lombardo M, Iruela-Arispe M L, Simons M, Sukhatme V P. Cancer Res. 1999;59:189–197. [PubMed] [Google Scholar]
- 5.Boehm T, Folkman J, Browder T, O'Reilly M S. Nature (London) 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 6.Meredith J E, Jr, Fazeli B, Schwartz M A. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks P C, Montgomery A M, Rosenfeld M, Reisfeld R A, Hu T, Klier G, Cheresh D A. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 8.Friedlander M, Brooks P C, Shaffer R W, Kincaid C M, Varner J A, Cheresh D A. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Bell K, Mousa S A, Varner J A. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muragaki Y, Timmons S, Griffith C M, Oh S P, Fadel B, Quertermous T, Olsen B R. Proc Natl Acad Sci USA. 1995;92:8763–8767. doi: 10.1073/pnas.92.19.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saarela J, Rehn M, Oikarinen A, Autio-Harmainen H, Pihlajaniemi T. Am J Pathol. 1998;153:611–626. doi: 10.1016/S0002-9440(10)65603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yatohgo T, Izumi M, Kashiwagi H, Hayashi M. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- 13.Vuori K, Ruoslahti E. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- 14.Zhang Z, Morla A O, Vuori K, Bauer J S, Juliano R L, Ruoslahti E. J Cell Biol. 1993;122:235–242. doi: 10.1083/jcb.122.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giancotti F G, Ruoslahti E. Cell. 1990;6:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 16.Hayman E G, Pierschbacher M D, Ohgren Y, Ruoslahti E. Proc Natl Acad Sci USA. 1983;80:4003–4007. doi: 10.1073/pnas.80.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki T, Fukai N, Mann K, Gohring W, Olsen B R, Timpl R. EMBO J. 1998;17:4249–4256. doi: 10.1093/emboj/17.15.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen J T, Wu P, Clouse M E, Hlatky L, Terwilliger E F. Cancer Res. 1998;58:5673–5677. [PubMed] [Google Scholar]
- 19.Blezinger P, Wang J, Gondo M, Quezada A, Mehrens D, French M, Singhal A, Sullivan S, Rolland A, Ralston R, Min W. Nat Biotechnol. 1999;17:343–348. doi: 10.1038/7895. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 21.Leavesley D I, Schwartz M A, Rosenfeld M, Cheresh D A. J Cell Biol. 1993;121:163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stromblad S, Becker J C, Yebra M, Brooks P C, Cheresh D A. J Clin Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Defilippi P, Bozzo C, Volpe G, Romano G, Venturino M, Silengo L, Tarone G. Cell Adhes Commun. 1994;2:75–86. doi: 10.3109/15419069409014203. [DOI] [PubMed] [Google Scholar]
- 24.Dhanabal M, Ramchandran R, Waterman M J F, Lu L, Knebelmann B, Segal M, Sukhatme V P. J Biol Chem. 1999;274:11721–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi N, Anand-Apte B, Lee M, Sasaki T, Fukai N, Shapiro R, Que I, Lowik C, Timpl R, Olsen B R. EMBO J. 1999;18:4414–4423. doi: 10.1093/emboj/18.16.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leavesley D I, Ferguson G D, Wayner E A, Cheresh D A. J Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montgomery A M, Reisfeld R A, Cheresh D A. Proc Natl Acad Sci USA. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer J S, Schreiner C L, Giancotti F G, Ruoslahti E, Juliano R L. J Cell Biol. 1992;116:477–487. doi: 10.1083/jcb.116.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Vuori K, Reed J C, Ruoslahti E. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien V, Frisch S M, Juliano R L. Exp Cell Res. 1996;224:208–213. doi: 10.1006/excr.1996.0130. [DOI] [PubMed] [Google Scholar]
- 31.Davis G E. Biochem Biophys Res Commun. 1992;182:1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 32.Dixelius J, Larsson H, Sasaki T, Holmqvist K, Lu L, Engstrom A, Timpl R, Welsh M, Claesson-Welsh L. Blood. 2000;95:3403–3411. [PubMed] [Google Scholar]
- 33.Mould A P, Askari J A, Aota S, Yamada K M, Irie A, Takada Y, Mardon H J, Humphries M J. J Biol Chem. 1997;272:17283–17292. doi: 10.1074/jbc.272.28.17283. [DOI] [PubMed] [Google Scholar]
- 34.Brooks P C, Silletti S, von Schalscha T L, Friedlander M, Cheresh D A. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- 35.Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras M P, Jr, Hudson B G, Brooks P C. J Biol Chem. 2000;275:8051–8061. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- 36.Maeshima Y, Colorado P C, Torre A, Holthaus K A, Grunkemeyer J A, Ericksen M B, Hopfer H, Xiao Y, Stillman I E, Kalluri R. J Biol Chem. 2000;275:21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 37.Maeshima Y, Colorado P C, Kalluri R. J Biol Chem. 2000;275:23745–23750. doi: 10.1074/jbc.C000186200. [DOI] [PubMed] [Google Scholar]
- 38.Colorado P C, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky E D, Herman S, Ericksen M B, et al. Cancer Res. 2000;60:2520–2526. [PubMed] [Google Scholar]
- 39.Kamphaus G D, Colorado P C, Panka D J, Hopfer H, Ramchandran R, Torre A, Maeshima Y, Mier J W, Sukhatme V P, Kalluri R. J Biol Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki T, Larsson H, Kreuger J, Salmivirta M, Claesson-Welsh L, Lindahl U, Hohenester E, Timpl R. EMBO J. 1999;18:6240–6248. doi: 10.1093/emboj/18.22.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collo G, Pepper M S. J Cell Sci. 1997;112:569–578. doi: 10.1242/jcs.112.4.569. [DOI] [PubMed] [Google Scholar]
- 42.Senger D R, Claffey K P, Benes J E, Perruzzi C A, Sergiou A P, Detmar M. Proc Natl Acad Sci USA. 1997;94:13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eliceiri B P, Cheresh D A. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruegg C, Yilmaz A, Bieler G, Bamat J, Chaubert P, Lejeune F J. Nat Med. 1998;4:408–414. doi: 10.1038/nm0498-408. [DOI] [PubMed] [Google Scholar]
- 45.Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, O'Reilly M, Folkman J. Proc Natl Acad Sci USA. 1998;95:5579–5583. doi: 10.1073/pnas.95.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasqualini R, Koivunen E, Ruoslahti E. Nat Biotechnol. 1997;15:542–546. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- 47.Sipkins D A, Cheresh D A, Kazemi M R, Nevin L M, Bednarski M D, Li K C. Nat Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 48.Chang Z, Choon A, Friedl A. Am J Pathol. 1999;155:71–76. doi: 10.1016/S0002-9440(10)65101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boehm T, O'Reilly M S, Keough K, Shiloach J, Shapiro R, Folkman J. Biochem Biophys Res Commun. 1998;252:190–194. doi: 10.1006/bbrc.1998.9617. [DOI] [PubMed] [Google Scholar]