Abstract
Objectives. We examined the association between socioeconomic status (SES) and myocardial infarction and stroke subtypes, including the possible mediating influence of cardiovascular risk factors.
Methods. We evaluated data on 578756 Korean male public servants aged 30 to 58 years from August 1, 1990, to July 31, 2001.
Results. SES had inverse associations with mortality because of myocardial infarction and stroke subtypes, which were not changed by an adjustment for, or stratification by, cardiovascular risk factors. For nonfatal events, SES had positive, null, and inverse associations with myocardial infarction, ischemic stroke, and hemorrhagic stroke, respectively. The association between SES and nonfatal myocardial infarction depended on the presence of risk factors and was positive only among men who had cardiovascular risk factors. Case-fatality after hospital admission for cardiovascular diagnoses was significantly lower among higher SES groups, even after risk factor adjustment.
Conclusions. Inverse SES associations with cardiovascular diseases were not mediated by cardiovascular risk factors among men who were undergoing economic transition. Socioeconomically patterned access to medical care may partly explain these socioeconomic gradients.
For several decades, socioeconomic status (SES) has shown consistent inverse associations with cardiovascular diseases in most industrialized Western countries, where disadvantaged groups experience a higher risk for cardiovascular diseases.1–4 However, this inverse association is not consistently observed in developing or transitional countries. In contrast, positive associations—higher risk for cardiovascular diseases among advantaged groups—have been reported for coronary heart disease5–8 and stroke8 in Hong Kong, Puerto Rico, and Pakistan, and some sub-Saharan African countries.
Lifestyle changes associated with stage of SES development in any population may explain the varying associations between SES and cardiovascular diseases that is observed between countries.9,10 This hypothesis is supported by the reversal in the association between coronary heart disease mortality and SES observed during the 20th century in England and Wales11 and the greater decline in coronary heart disease mortality among higher SES groups during the latter part of the century, which has widened the mortality gap between different SES groups over time.12 Smoking, bad nutrition, and physical inactivity are potential mechanisms for explaining these trends,13 because earlier adoption of healthy behaviors by people who had higher SES may have caused differential declines in coronary heart disease. Some researchers have predicted that an inverse association between SES and coronary heart disease would emerge in countries where such an association is not currently seen.9,14 However, the SES inequity in risk for cardiovascular diseases is not fully accounted for by socioeconomic gradients of classical risk factors in some studies,2,15,16 which suggests additional or alternative pathways underlie the association between SES and cardiovascular diseases.
In developing countries and among recently Westernized populations, a positive association between SES and cardiovascular disease risk factors has been reported.9,17–20 It has been suggested that increases in household income resulted in greater caloric intake and physical inactivity and thus increased obesity and cardiovascular disease risk factors.21,22 Further clarification of the role of cardiovascular risk factors in the association between SES and cardiovascular diseases will have implications for public health policy in developing countries. Previous studies of non-Western populations have not comprehensively examined this issue.5–8,18
We examined the association between SES and myocardial infarction and ischemic and hemorrhagic strokes by evaluating the role of cardiovascular risk factors in explaining such associations among a large cohort of South Korean men. South Korea—a recently developed country—experienced rapid socioeconomic growth during the last half century, after the Korean War (1950–1953). There has been a 40-fold increment in the gross national income per capita for the past 30 years ($10000 US dollars in 2000).23 Because of the time lag between changes in SES and the consequent changes in risk factors and impact on cardiovascular disease occurrence, Korea is a model that may predict a future pattern of cardiovascular disease in developing countries.
METHODS
To evaluate the association between SES and cardiovascular diseases, we examined several types of data: periodic health examination, SES (determined by monthly salary), medical service use, death benefits (from the Korean National Health Service [KNHS]), and nationwide death reports (from the Korean National Statistical Office). All data were linked with each individual’s resident registration number.
Participants
Study participants were Korean male public servants aged 30 to 58 years who underwent mandatory periodic KNHS health examinations between March and July 1990. The KNHS provided all their health insurance. Public servants aged 59 years or older were excluded because, according to the law, those who have lower SES should have retired at age 59 years, and public servants aged 59 years or older who have relatively higher SES were not appropriate for an examination of socioeconomic inequality. Women were not included because the number of female public servants was small compared with male public servants, and there was a different distribution of age among female public servants.
Among the initial group of 615658 men, we excluded 36902 men who had either died or experienced myocardial infarction, ischemic stroke, or hemorrhagic stroke before August 1, 1990, (n=176) or whose monthly salary data (n = 23 593) or other study variables were missing (n=13133). Thus, 578756 men constituted the study sample.
Assessment of Socioeconomic Status
We assessed SES by monthly salary, which reflected individual income and occupational position. We used monthly salary data that were updated in 1996. We classified study participants into 4 SES levels according to the quartile distribution of their monthly salary in each 1-year age strata, because the grade of monthly salary was correlated with each participant’s age (r =0.42; P <.0001). For example, a man who was in the lowest quartile distribution of monthly salary among men his age was classified into level I SES, and a man who was in the highest quartile distribution of monthly salary among men his age was classified into level IV SES. Level I men aged 30 years earned less than $729 a month, level 1 men aged 58 years earned less than $1241 a month, level IV men aged 30 years earned less than $926 a month, and level IV men aged 58 years earned less than $2036 a month (all salaries are in US dollars).
Assessment of Cardiovascular Risk Factors and Other Variables
For each study participant, median values for height, body weight, blood pressure, fasting serum glucose, and total cholesterol levels were calculated with the periodic health examination data from 1986, 1990, 1992, and 1994. Data obtained after a subject experienced a study outcome event (myocardial infarction, ischemic stroke, or hemorrhagic stroke) were excluded from the calculation of mean values. At least 95% of study subjects had these variables evaluated 2 or more times.
We categorized blood pressure (BP) into 3 groups: normal or prehypertension (systolic BP<140 mmHg and diastolic BP<90 mmHg), stage 1 hypertension (systolic BP=140–159 mmHg or diastolic BP=90–99 mmHg), and stage 2 hypertension (systolic BP≥160 mmHg or diastolic BP≥ 100 mmHg).24 We categorized serum total cholesterol into 4 strata (<4.1, 4.1–5.1, 5.2–6.1, ≥ 6.2 mmol/L). We used 2 categories for fasting glucose level in accordance with World Health Organization criteria25 (<7.0, ≥ 7.0 mmol/L) and 4 categories for body mass index (<20.0, 20.0–24.9, 25.0–29.9, ≥ 30.0 kg/m2).
We divided study subjects into 4 categories for smoking (never smoked, ex-smoker, smoked 1–19 cigarettes per day, and smoked ≥ 20 cigarettes per day), 3 categories for alcohol intake (none, often, and frequent), and 2 categories for physical exercise (regular exercise vs. no exercise). Data on area of residence were categorized as capital, large city, and other area in accordance with the administrative district.
Mortality and Morbidity Follow-up for Myocardial Infarction, Ischemic Stroke, and Hemorrhagic Stroke
Both fatal and nonfatal events caused by myocardial infarction, ischemic stroke, and hemorrhagic stroke that occurred after July 31, 1990, were included in our analysis. We used International Classification of Diseases, 10th Revision,26 codes to identify myocardial infarction (121–124), ischemic stroke (163, 167.8), and hemorrhagic stroke (161). We obtained data on deaths of study subjects between August 1, 1990, and July 31, 2001, from the Korean National Statistical Office’s nationwide death report records and the KNHS’s death benefit records. Data on all hospital admissions because of myocardial infarction, ischemic stroke, and hemorrhagic stroke between August 1990 and July 2001 were obtained from KNHS medical service use data. Easy access to hospital care for public servants and the 11-year follow-up period made it very likely that nonfatal myocardial infarction and stroke patients were admitted to hospitals at some stage of their illness.
Statistical Analysis
Study subjects who were admitted to a hospital or who died as a result of myocardial infarction, ischemic stroke, hemorrhagic stroke, or other causes of death were censored either on the date of event or on July 31, 2001. We used the SAS statistical package (SAS Institute Inc, Cary, NC) to estimate hazard ratios (HR) for myocardial infarction and stroke subtypes by SES levels with Cox proportional hazard regression analysis. We examined the association between SES and cardiovascular disease mortality and SES and nonfatal cardiovascular disease events separately. In addition to deaths caused by cardiovascular disease, subjects who had hospital admissions for nonfatal events but later died of cardiovascular disease were considered to be cardiovascular disease deaths. Hospital admissions for nonfatal events that did not result in a cardiovascular disease death were considered to be nonfatal cardiovascular disease events.
To assess the association between SES and cardiovascular risk factors, we repeated the analysis with models that did and did not adjust for cardiovascular risk factors. Because we assessed SES with data from 1996, we repeatedly evaluated the association between SES and cardiovascular disease mortality and SES and nonfatal cardiovascular disease with both exclusion and inclusion of those subjects who were censored before SES assessment.
To evaluate the modification of the association between SES and cardiovascular disease by cardiovascular risk factor, we conducted stratified analyses with the presence and the absence of any cardiovascular risk factors (total cholesterol ≥ 6.2 mmol/L, glucose ≥ 7.0 mmol/L, blood pressure ≥ 140/90 mmHg, body mass index ≥ 25 kg/m2, and current smoking). We also examined whether SES–risk factor interaction was associated with myocardial infarction, ischemic stroke, and hemorrhagic stroke by including product interaction terms into models with the 2 variables. The product term was calculated by multiplying the presence of a risk factor and SES categorized as an ordinal variable.
To examine case-fatality of different SES groups admitted to a hospital with myocardial infarction, ischemic stroke, or hemorrhagic stroke, we performed logistic regression analysis with and without the cardiovascular risk factors included in the models. All tests were 2-sided, and the level of statistical significance was set at P =0.05.
RESULTS
The cohort comprised 6204326 person-years of follow-up. During a mean 10.7 years (SD=1.3) of follow-up, there were 3881 myocardial infarction, 5573 ischemic stroke, and 3079 hemorrhagic stroke cases, which gave us crude incidence rates of 0.63, 0.90, and 0.50 per 1000 person-years, respectively.
Table 1 ▶ shows the mixed patterns of risk factor distributions according to the SES level. Elevated blood pressure and current smoking tended to decrease with an increment in SES, and total cholesterol level, body mass index, and fasting blood glucose levels showed somewhat inconsistent patterns. Although the proportion of subjects who had any of the risk factors increased with lower SES, the proportion that had more than 2 of the risk factors was higher among men in the mid-SES level than among men in the lowest or the highest SES levels.
TABLE 1—
Cardiovascular Risk Factor Distribution, by Socioeconomic Status: Korean Male Public Servants, 1990–2001
| Level of Socioeconomic Status | ||||
| I (Lowest) | II | III | IV (Highest) | |
| Subjects, no. | 158 769 | 156 562 | 167 396 | 96 029 |
| Cholesterol (mmol/L), % | ||||
| < 4.1 | 21.4 | 19.1 | 18.8 | 18.7 |
| 4.1–5.1 | 43.7 | 43.4 | 44.2 | 43.8 |
| 5.2–6.1 | 25.4 | 26.9 | 26.8 | 27.5 |
| ≥6.2 | 9.5 | 10.6 | 10.2 | 10.0 |
| Systolic/diastolic blood pressure (mmHg), % | ||||
| < 140 and < 90 | 62.2 | 63.7 | 65.2 | 66.7 |
| 140–159 or 90–99 | 29.4 | 28.2 | 27.1 | 26.4 |
| ≥160 or ≥ 100 | 8.4 | 8.1 | 7.7 | 7.0 |
| Body mass index (kg/m2), % | ||||
| < 20 | 9.2 | 7.0 | 7.4 | 7.6 |
| 20–24 | 70.3 | 66.5 | 67.4 | 68.0 |
| 25–29 | 19.8 | 25.6 | 24.5 | 23.8 |
| ≥ 30 | 0.7 | 0.9 | 0.8 | 0.7 |
| Glucose (mmol/L), % | ||||
| < 7.0 | 96.4 | 95.8 | 96.5 | 97.2 |
| ≥ 7.0 | 3.6 | 4.2 | 3.5 | 2.8 |
| Smoking status, % | ||||
| Never | 22.0 | 25.4 | 29.5 | 31.7 |
| Former | 12.8 | 14.4 | 15.2 | 16.0 |
| Current (< 20 cigarettes/day) | 48.9 | 41.2 | 40.0 | 39.0 |
| Current (≥ 20 cigarettes/day) | 16.4 | 18.9 | 15.2 | 14.3 |
| Risk factors,a % | ||||
| No | 15.5 | 17.1 | 20.1 | 22.6 |
| Yes | 84.5 | 82.9 | 79.9 | 77.4 |
| Number of risk factorsb | ||||
| 1–2 | 87.4 | 85.2 | 86.7 | 87.7 |
| 3–5 | 12.6 | 14.8 | 13.3 | 12.3 |
a Total cholesterol ≥ 6.2 mmol/L, glucose ≥ 7.0 mmol/L, blood pressure ≥ 140/90 mmHg, body mass index ≥ 25 kg/m2, or current smoker.
bDistribution after excluding subjects who did not have risk factors.
Table 2 ▶ shows the HRs for cardiovascular disease mortality associated with SES. There was a consistent inverse association independent of cardiovascular risk factors between SES and mortality from myocardial infarction, ischemic stroke, and hemorrhagic stroke in both unstratified and stratified models. However, the inverse association between SES and mortality from ischemic stroke was stronger among men who did not have elevated cardiovascular disease risk factors, which is shown by the larger trend per increment in SES. The HR was 0.54 (95% confidence interval [CI] = 0.39, 0.74) among those who did not have risk factors versus 0.78 (95% CI = 0.72, 0.84) among those who had 1 or more risk factors. Adjustment for the cardiovascular risk factors in the unstratified analysis (model 3) did not attenuate the age-adjusted associations (model 1) or the age, height, exercise, alcohol, and area of residence adjusted HRs (model 2). Analysis after the exclusion of subjects who were censored before monthly salary data were updated (first 6 years of follow-up) did not result in materially different findings (data not shown).
TABLE 2—
Adjusted Hazard Ratios (95% Confidence Intervals) for Mortality From Myocardial Infarction, Ischemic Stroke, and Hemorrhagic Stroke in Overall and in Stratified Models, by Level of Socioeconomic Status: Korean Male Public Servants, 1990–2001
| Level of Socioeconomic Status | |||||
| I (Lowest) | II | III | IV (Highest) | Trenda | |
| Myocardial infarction (N = 1117) | |||||
| Overall modelb | |||||
| Model 1 | 1 | 0.81 (0.69, 0.94) | 0.72 (0.62, 0.84) | 0.69 (0.57, 0.83) | 0.87 (0.83, 0.93) |
| Model 2 | 1 | 0.80 (0.69, 0.93) | 0.72 (0.62, 0.85) | 0.70 (0.58, 0.85) | 0.88 (0.83, 0.93) |
| Model 3 | 1 | 0.81 (0.69, 0.94) | 0.77 (0.66, 0.89) | 0.75 (0.62, 0.91) | 0.90 (0.85, 0.96) |
| Stratified modelc by the number of risk factors (RF)d for each subject (interactioneP = 0.4360) | |||||
| RF = 0 | 1 | 0.64 (0.36, 1.11) | 0.55 (0.32, 0.95) | 0.65 (0.35, 1.20) | 0.84 (0.69, 1.02) |
| RF ≥ 1 | 1 | 0.82 (0.70, 0.97) | 0.76 (0.65, 0.89) | 0.73 (0.59, 0.89) | 0.89 (0.84, 0.95) |
| Ischemic stroke (N = 785) | |||||
| Overall modelb | |||||
| Model 1 | 1 | 0.69 (0.58, 0.82) | 0.56 (0.47, 0.67) | 0.41 (0.32, 0.54) | 0.75 (0.70, 0.80) |
| Model 2 | 1 | 0.69 (0.58, 0.83) | 0.57 (0.48, 0.68) | 0.42 (0.32, 0.55) | 0.75 (0.70, 0.81) |
| Model 3 | 1 | 0.67 (0.56, 0.80) | 0.59 (0.49, 0.71) | 0.44 (0.34, 0.58) | 0.77 (0.71, 0.83) |
| Stratified modelc by the number of risk factors (RF)d for each subject (interaction P = 0.0267 | |||||
| RF = 0 | 1 | 0.54 (0.26, 1.12) | 0.26 (0.12, 0.60) | 0.19 (0.05, 0.63) | 0.54 (0.39, 0.74) |
| RF ≥ 1 | 1 | 0.71 (0.59, 0.85) | 0.61 (0.51, 0.74) | 0.46 (0.35, 0.61) | 0.78 (0.72, 0.84) |
| Hemorrhagic stroke (N = 1312) | |||||
| Overall modelb | |||||
| Model 1 | 1 | 0.71 (0.62, 0.82) | 0.60 (0.52, 0.69) | 0.52 (0.44, 0.63) | 0.80 (0.75, 0.84) |
| Model 2 | 1 | 0.74 (0.64, 0.85) | 0.63 (0.55, 0.73) | 0.57 (0.47, 0.69) | 0.82 (0.77, 0.86) |
| Model 3 | 1 | 0.76 (0.66, 0.88) | 0.68 (0.59, 0.78) | 0.63 (0.52, 0.76) | 0.85 (0.80, 0.89) |
| Stratified modelc by the number of risk factors (RF)d for each subject (interactioneP = 0.9573) | |||||
| RF = 0 | 1 | 0.82 (0.43, 1.56) | 0.35 (0.16, 0.75) | 0.84 (0.41, 1.71) | 0.83 (0.65, 1.06) |
| RF ≥ 1 | 1 | 0.74 (0.64, 0.85) | 0.67 (0.58, 0.77) | 0.58 (0.48, 0.70) | 0.83 (0.78, 0.88) |
aFor the increment of socioeconomic status to the next higher level.
bIn model 1, age was adjusted. In model 2, age, height, physical exercise, alcohol consumption, and area of residence were adjusted. In model 3, cardiovascular risk factor profiles (smoking status, cholesterol level, glucose level, body mass index, and blood pressure level) were added to model 2.
cAge, height, physical exercise, alcohol consumption, and area of residence were adjusted.
d Total cholesterol ≥ 6.2 mmol/L, glucose ≥ 7.0 mmol/L, blood pressure ≥ 140/90 mmHg, body mass index ≥ 25 kg/m2, or current smoker.
eProduct term for the interaction test was calculated by multiplying the presence of a risk factor and socioeconomic status categorized as an ordinal variable.
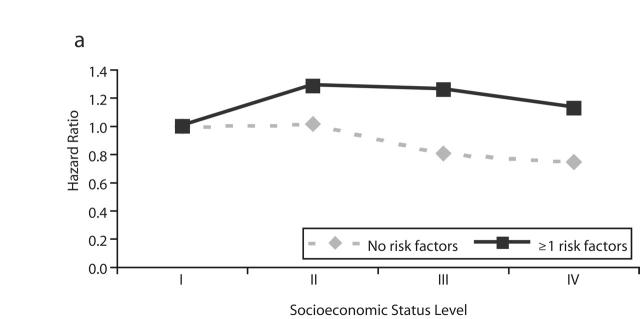
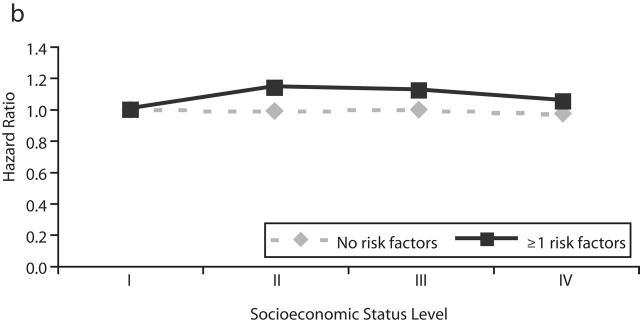
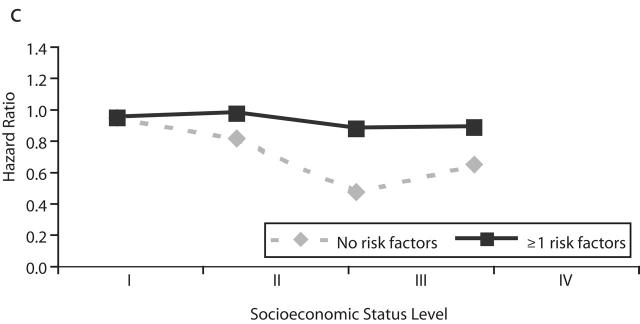
Table 3 ▶ and Figure 1 ▶ show the HRs for nonfatal cardiovascular disease events associated with SES. These associations were not consistent between the disease categories or between the overall and stratified models. In overall models, SES showed a weak positive association with myocardial infarction (trend in cardiovascular-adjusted HR=1.04; 95% CI=1.01, 1.08), and there was no association with ischemic stroke (HR=1.02; 95% CI= 0.99, 1.05) and an inverse association with hemorrhagic stroke (HR=0.96, 95% CI= 0.91, 1.00). Analysis after the exclusion of subjects who were censored before 1996 did not result in materially different findings (data not shown). In stratified models, an inverse but not significant association between SES and nonfatal myocardial infarction events was observed among men who did not have cardiovascular risk factors, and a positive association was observed among men who had risk factors. For hemorrhagic stroke, the inverse association was stronger among men who did not have cardiovascular risk factors. The SES–risk factor interaction was statistically significant for myocardial infarction and hemorrhagic stroke. Because inconsistent findings were observed for SES associations with cardiovascular disease mortality and nonfatal cardiovascular disease events, the possibility that SES was differentially associated with case-fatality and cardiovascular disease incidence was examined. In logistic regression model, the odds for case-fatality among men who had been admitted to a hospital for myocardial infarction, ischemic stroke, or hemorrhagic stroke were inversely associated with SES (Table 4 ▶, model 1) and did not change after adjustment for risk factors (model 3).
TABLE 3—
Adjusted Hazard Ratios (95% Confidence Intervals) for Nonfatal Events of Myocardial Infarction, Ischemic Stroke, and Hemorrhagic Stroke in Overall and in Stratified Models, by Level of Socioeconomic Status: Korean Male Public Servants, 1990–2001
| Level of Socioeconomic Status | ||||||
| I (Lowest) | II | III | IV (Highest) | Trenda | ||
| Myocardial infarction (N = 2764) | ||||||
| Overall modelb | ||||||
| Model 1 | 1 | 1.23 (1.11, | 1.36) | 1.16 (1.05, 1.29) | 1.02 (0.90, 1.15) | 1.01 (0.98, 1.05) |
| Model 2 | 1 | 1.25 (1.13, | 1.39) | 1.18 (1.07, 1.31) | 1.05 (0.93, 1.20) | 1.02 (0.99, 1.06) |
| Model 3 | 1 | 1.22 (1.10, | 1.35) | 1.22 (1.10, 1.35) | 1.11 (0.98, 1.26) | 1.04 (1.01, 1.08) |
| Stratified modelc by the number of risk factors (RF)d for each subject (interactioneP=0.0136) | ||||||
| RF = 0 | 1 | 1.02 (0.68, | 1.54) | 0.81 (0.54, 1.21) | 0.75 (0.46, 1.23) | 0.90 (0.77, 1.04) |
| RF ≥ 1 | 1 | 1.29 (1.16, | 1.43) | 1.26 (1.13, 1.40) | 1.13 (0.99, 1.29) | 1.05 (1.01, 1.09) |
| Ischemic stroke (N = 4849) | ||||||
| Overall modelb | ||||||
| Model 1 | 1 | 1.10 (1.02, | 1.19) | 1.05 (0.98, 1.13) | 0.95 (0.86, 1.04) | 0.99 (0.96, 1.02) |
| Model 2 | 1 | 1.12 (1.03, | 1.21) | 1.09 (1.01, 1.17) | 1.00 (0.91, 1.10) | 1.01 (0.98, 1.03) |
| Model 3 | 1 | 1.08 (1.00, | 1.17) | 1.11 (1.02, 1.19) | 1.03 (0.94, 1.14) | 1.02 (0.99, 1.05) |
| Stratified modelc by the number of risk factors (RF)d for each subject (interactioneP = 0.5434) | ||||||
| RF = 0 | 1 | 0.99 (0.71, | 1.37) | 1.00 (0.74,1.37) | 0.97 (0.67, 1.39) | 0.99 (0.89, 1.11) |
| RF ≥ 1 | 1 | 1.14 (1.05, | 1.23) | 1.12 (1.04, 1.21) | 1.05 (0.95, 1.15) | 1.02 (0.99, 1.05) |
| Hemorrhagic stroke (N = 1811) | ||||||
| Overall modelb | ||||||
| Model 1 | 1 | 0.96 (0.85, | 1.08) | 0.82 (0.72, 0.92) | 0.81 (0.70, 0.94) | 0.92 (0.88, 0.96) |
| Model 2 | 1 | 1.01 (0.89, | 1.14) | 0.87 (0.77, 0.99) | 0.89 (0.76, 1.03) | 0.95 (0.91, 0.99) |
| Model 3 | 1 | 1.00 (0.88, | 1.13) | 0.89 (0.78, 1.01) | 0.91 (0.78, 1.05) | 0.96 (0.91, 1.00) |
| Stratified modelc by the number of risk factors (RF)d for each subject (interactioneP= 0.0290) | ||||||
| RF = 0 | 1 | 0.86 (0.55, | 1.35) | 0.50 (0.31, 0.81) | 0.69 (0.41, 1.16) | 0.82 (0.69, 0.97) |
| RF ≥ 1 | 1 | 1.03 (0.91, | 1.17) | 0.93 (0.82, 1.06) | 0.94 (0.80, 1.10) | 0.97 (0.93, 1.02) |
aFor the increment of socioeconomic status to the next higher level.
bIn model 1, age was adjusted. In model 2, age, height, physical exercise, alcohol consumption, and area of residence were adjusted. In model 3, cardiovascular risk factor profiles (smoking status, cholesterol level, glucose level, body mass index, and blood pressure level) were added to model 2.
cAge, height, physical exercise, alcohol consumption, and area of residence were adjusted.
dTotal cholesterol ≥ 6.2 mmol/L, glucose ≥ 7.0 mmol/L, blood pressure ≥ 140/90 mmHg, body mass index ≥ 25 kg/m2, or current smoker.
eProduct term for the interaction test was calculated by multiplying the presence of a risk factor and socioeconomic status categorized as an ordinal variable.
FIGURE 1—
Adjusted hazard ratio for nonfatal events of (a) myocardial infarction, (b) ischemic stroke, and (c) hemorrhagic stroke by level of socioeconomic status and stratified by presence and absence of major cardiovascular risk factors.
Note. RF = risk factors.
aI = lowest; IV = highest.
TABLE 4—
Adjusted Hazard Ratios (95% Confidence Intervals [CIs]) for Risk of Subsequent Death Among Men Admitted to Hospitals for Cardiovascular Diseases, by Level of Socioeconomic Status: Korean Male Public Servants, 1990–2001
| Level of Socioeconomic Status | |||||
| Modela | I (Lowest) | II | III | IV (Highest) | Trendb |
| Myocardial infarction (n = 211/2975)c | |||||
| Model 1 | 1 | 0.85 (0.60, 1.22) | 0.64 (0.44, 0.93) | 0.64 (0.39, 1.04) | 0.83 (0.72, 0.96) |
| Model 3 | 1 | 0.86 (0.59, 1.24) | 0.65 (0.44, 0.96) | 0.67 (0.41, 1.11) | 0.85 (0.73, 0.98) |
| Ischemic stroke (n = 580/6102)c | |||||
| Model 1 | 1 | 0.74 (0.59, 0.92) | 0.57 (0.46, 0.72) | 0.49 (0.36, 0.67) | 0.77 (0.71, 0.85) |
| Model 3 | 1 | 0.73 (0.59, 0.92) | 0.57 (0.45, 0.72) | 0.48 (0.35, 0.67) | 0.77 (0.71,0.85) |
| Hemorrhagic stroke (n = 691/2911)c | |||||
| Model 1 | 1 | 0.74 (0.59, 0.92) | 0.78 (0.63, 0.98) | 0.68 (0.51, 0.91) | 0.89 (0.82, 0.97) |
| Model 3 | 1 | 0.75 (0.59, 0.94) | 0.78 (0.62, 0.98) | 0.71 (0.53, 0.96) | 0.90 (0.82, 0.98) |
aIn model 1, age was adjusted in the logistic regression model. In model 3, age, height, physical exercise, alcohol consumption, area of residence, and cardiovascular risk factor profiles (smoking status, cholesterol level, glucose level, body mass index, and blood pressure level) were adjusted.
bFor the increment of socioeconomic position to next higher level. cNumber of events, death per admission.
DISCUSSION
The associations between SES and cardiovascular risk factors among our cohort of Korean male public servants were not the same as those in Western populations, which is similar to results from previous studies conducted in less developed countries.19,20 Among Western populations, where consistent inverse associations between SES and cardiovascular disease have been found, SES also has been inversely associated with risk factor profiles,27,28 which might explain at least some of the inverse SES and cardiovascular disease gradient. In our study, body mass index and blood cholesterol were more adverse, and blood pressure and smoking habits tended to be better, among subjects who had higher SES.
However, in spite of the mixed profiles of socioeconomic gradients in cardiovascular risk factors, SES had inverse associations with mortality from myocardial infarction, ischemic stroke, and hemorrhagic stroke, and adjustment for cardiovascular risk factors and further examination of the role of risk factors by stratified analysis did not change the association of SES with cardiovascular diseases. These findings support previous reports that the association between SES and cardiovascular diseases might not be fully mediated by socioeconomic inequality in the cardiovascular risk factor profile.2,15,16
Previous studies of SES and cardiovascular diseases have not separately examined the associations of SES with cardiovascular disease mortality and nonfatal cardiovascular disease events. Although our findings of different associations between SES and cardiovascular disease mortality and SES and nonfatal cardiovascular disease events require confirmation in other studies, it is clear that there was a discrepancy between the observed association between SES and cardiovascular disease mortality and SES and cardiovascular disease morbidity, and the degree of discrepancy was different by disease categories.
Our finding of an inverse association between SES and cardiovascular disease case-fatality among men who were admitted to a hospital for cardiovascular disease diagnoses suggests that variation in quality of medical care, despite national health insurance for all SES groups, might explain the differences in the associations observed with each cardiovascular disease outcome. Some studies have suggested that the inequality in accessibility or effective use of medical services before or after the occurrence of cardiovascular disease may contribute to the socioeconomic inequality in cardiovascular diseases.29,30 Men who have higher SES might be expected to receive medical service more easily and quickly (and perhaps get better quality care) at the onset of cardiovascular disease symptoms. Presence of any risk factor might have made higher-SES men more alert to even mild symptoms of cardiovascular disease, and thus they had a higher prevalence of morbidity but better survival and lower mortality than lower-SES groups. Contrasting associations of SES with myocardial infarction mortality (inverse) and nonfatal myocardial infarction events (positive) among men who had any cardiovascular risk factors supports this explanation.
Differences between SES associations with mortality and nonfatal events were more marked for myocardial infarction and ischemic stroke than for hemorrhagic stroke. Both myocardial infarction and ischemic stroke survival may be improved by earlier diagnosis and intervention, but this is less likely for hemorrhagic stroke, which often has sudden onset and is unlikely to be markedly influenced by medical care. Consequently, it is plausible that some of the SES gradient in risk for cardiovascular disease mortality is explained by SES variation in access to and quality of medical care. However, because we could not examine the association between SES and cardiovascular diseases according to the severity of the cardiovascular disease or by the difference in recognition of cardiovascular risk factors across SES groups, further investigation is needed to determine whether medical care factors are responsible for our findings.
The similarity of ischemic stroke and myocardial infarction and their associations with SES is congruent with the similarity of ischemic stroke and coronary heart disease time trends compared with hemorrhagic stroke. We have shown that ischemic stroke time trends are very similar to coronary heart disease time trends within Britain, and hemorrhagic stroke has shown long-term consistent declines.31 Other evidence suggests that risk factors for ischemic stroke are very similar to those for coronary heart disease, whereas risk factors for hemorrhagic stroke differ. For example, blood pressure is a stronger predictor of hemorrhagic than ischemic stroke,32 and circulating cholesterol level is positively associated with ischemic stroke and coronary heart disease but shows no consistent association with hemorrhagic stroke.33 Hemorrhagic stroke shows a stronger influence of childhood SES (indexed by father’s social class or number of siblings34 or height35,36) than coronary heart disease or ischemic stroke do.
Strengths and Limitations of the Study
The major strength of our study was the wide range of risk factor data and the large number of myocardial infarction and ischemic and hemorrhagic stroke events, which made it possible to evaluate the association between SES and cardiovascular diseases with sufficient statistical power in the subgroup analyses.
However, some potential limitations need to be considered. Although we had no direct comparison data on income levels, the mean level of monthly health insurance premium per the insured, which can be a proxy of income level, was higher for public servants ($15.40) than for private-sector employees ($11.50) or the self-employed ($12.80) in 1990.37 Examining socioeconomic inequality among a cohort of male civil servants might have underestimated cardiovascular disease risk differentials compared with a study of the general population, because the poorest people were excluded from our sample. We also could not examine socioeconomic inequality among women.
We do not believe that inadequate measurement of SES explains our results, because a previous Korean cohort study that used monthly salary as an indicator of SES showed socioeconomic mortality differentials for several causes of death in accordance with other studies.38
Inaccurate diagnosis of myocardial infarction and stroke subtype might have caused misclassification bias. Although we did not perform an independent validation of morbidity data sources, a study that used the same KNHS data showed a diagnostic accuracy of 83.4% for ischemic stroke,39,40 85.7% for hemorrhagic stroke, and 85.6% for myocardial infarction.39 In those studies with the same cohort (N=15600 male public servants), 626 stroke and 258 myocardial infarction medical insurance claims filed between 1993 and 1997 were evaluated.
Because we examined outcome events with death data and hospitalization data, outpatient cases were not examined. Therefore, the generalizability of our results is limited to all myocardial infarction and stroke patients, including undiagnosed or outpatient cases. We could not adjust for hypertension, hyperlipidemia, and diabetes medicine use. However, we tried to reduce possible bias by averaging the values of blood pressure, cholesterol, glucose, and weight, which were measured repeatedly between 1986 and 1994.
Our study could not examine whether other unmeasured risk factors or mechanisms—including adult or childhood psychosocial environmental effects, conventional cardiovascular risk factors earlier in life, or other biological factors (e.g., hemostatic function)—mediated the inverse association between SES and cardiovascular diseases among men who did not have risk factors.
Conclusions
Unfavorable SES increased the risk for cardiovascular disease mortality, and the association was independent of the cardiovascular risk factor measured. However, the different associations between SES and nonfatal cardiovascular disease events, according to the presence or the absence of cardiovascular risk factors, suggests that the presence of risk factors may modify the risk for cardiovascular disease events. Significantly reduced case-fatality after admission to a hospital among men who had higher SES suggests that inequality in medical service use may have partly contributed to the socioeconomic inequality in cardiovascular disease mortality. To prevent the anticipated socioeconomic inequality in burden of cardiovascular diseases in countries where socioeconomic disparity in cardiovascular risk factor distribution is not distinct, further research is needed to elucidate and eliminate modifiable mechanisms between SES and cardiovascular disease, such as inequality in medical service use, while not neglecting the role of traditional cardiovascular risk factors.
Acknowledgments
This study was supported by a grant from the Korean Ministry of Health and Welfare (01-PJ1-PG1–01CH10–0007).
Human Participant Protection This study was approved by the institutional review board of the Samsung Medical Center.
Peer Reviewed
Contributors All the authors originated ideas, analyzed data, and wrote the article.
References
- 1.Strand BH, Tverdal A. Can cardiovascular risk factors and lifestyle explain the educational inequalities in mortality from ischaemic heart disease and from other heart diseases? 26-year follow-up of 50 000 Norwegian men and women. J Epidemiol Community Health. 2004;58:705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davey Smith G, Neaton JD, Wentworth D, Stamler R, Stamler J. Socioeconomic differentials in mortality risk among men screened for the multiple risk factor intervention trial: White men. Am J Public Health. 1996;86:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunst AE, del Rios M, Groenhof F, Mackenbach JP. For the European Union Working Group on Socioeconomic Inequalities in Health. Socioeconomic inequalities in stroke mortality among middle-aged men. An international overview. Stroke. 1998;29:2285–2291. [DOI] [PubMed] [Google Scholar]
- 4.Bennett S. Socioeconomic inequalities in coronary heartdisease and stroke mortality among Australian men, 1979–1993. Int J Epidemiol. 1996;25:266–275. [DOI] [PubMed] [Google Scholar]
- 5.Wong SL, Donnan SP. Influence of socioeconomic status on cardiovascular diseases in Hong Kong. J Epidemiol Community Health. 1992;46:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hameed K, Kadir M, Gibson T, Sultana S, Fatima Z, Syed A. The frequency of known diabetes, hypertension and ischaemic heart disease in affluent and poor urban populations of Karachi, Pakistan. Diabetes Med. 1995;12:500–503. [DOI] [PubMed] [Google Scholar]
- 7.Solie PD, Garcia-Palmieri MR. Educational status and coronary heart disease in Puerto Rico: the Puerto Rico Heart Health program. Int J Epidemiol. 1990;19:59–65. [DOI] [PubMed] [Google Scholar]
- 8.Chang CL, Marmot MG, Farley TMM, Poulter NR. The influence of economic development on the association between education and the risk of acute myocardial infarction and stroke. J Clinical Epidemiol. 2002; 55:741–747. [DOI] [PubMed] [Google Scholar]
- 9.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. [DOI] [PubMed] [Google Scholar]
- 10.Mackenbach JP, Looman CW, Kunst AF. Geographic variation in the onset of decline of male ischaemic heart disease mortality in the Netherlands. Am J Public Health. 1989;79:1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marmot MG, Adelstein AM, Robinson N, Rose G. Changing social-class distribution of heart disease. BMJ. 1978;2:1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper R, Cutler J, Desvigne-Nickens P, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States. Findings of the National Conference on Cardiovascular Disease Prevention. Circulation. 2000;102:3137–3147. [DOI] [PubMed] [Google Scholar]
- 13.Emmons KM. Health behaviors in a social context. In: Berkman LF, Kawachi I, eds. Social Epidemiology. Oxford, UK: Oxford University Press; 2000:242–266.
- 14.Marmot M. Coronary heart disease: rise and fall of a modern epidemic. In: Marmot M, Elliott P, eds. Coronary Heart Disease Epidemiology. From Aetiology to Public Health. Oxford, UK: Oxford University Press; 1992:3–19.
- 15.Davey Smith G, Wentworth D, Neaton JD, Stamler R, Stamler J. Socioeconomic differentials in mortality risk among men screened for the Multiple Risk Factor Intervention Trial: II. Black men. Am J Public Health. 1996;86:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmot MG, Shipley MJ, Rose G. Inequalities in death—specific explanations of a general pattern? Lancet. 1984;I:1003–1006. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura A, Iso H, Iida M, et al. Trends in the incidence of coronary heart disease and stroke and the prevalence of cardiovascular risk factors among Japanese men from 1963 to 1994. Am J Med. 2002;112:104–109. [DOI] [PubMed] [Google Scholar]
- 18.Gupta R, Gupta VP, Ahluwalia NS. Educational status, coronary heart disease, and coronary risk factor prevalence in a rural population of India. BMJ. 1994; 309:1332–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Symons M, Popkin BM. Contrasting socioeconomic profiles related to healthier lifestyles in China and the United States. Am J Epidemiol. 2004; 159:184–191. [DOI] [PubMed] [Google Scholar]
- 20.INCLEN Multicenter Collaborative Group. Socioeconomic status and risk factors for cardiovascular disease: a multicenter collaborative study in the international clinical epidemiology network (INCLEN). J Clin Epidemiol. 1994;47:1401–1409. [DOI] [PubMed] [Google Scholar]
- 21.McGarvey ST, Bindon J, Crews D, Schendel D. Modernization and adiposity: causes and consequences. In: Little MA, Haas JD, eds. Human Population Biology: A Trans-Disciplinary Science. New York, NY: Academic Press; 1989:263–279.
- 22.INCLEN Multicenter Collaborative Group. Body mass index and cardiovascular disease risk factors in seven Asian and five Latin American centers: data from the International Clinical Epidemiology Network. Obes Res. 1996;4:211–218. [DOI] [PubMed] [Google Scholar]
- 23.National Statistical Office of Korea. Korea Statistical Yearbook. Seoul, South Korea: National Statistical Office of Korea; 2001.
- 24.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 25.Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetes Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 26.International Classification of Diseases, 10th Revision. Geneva, Switzerland: World Health Organization; 1992.
- 27.Brunner EJ, Marmot MG, Nanchahal K, et al. Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia. 1997;40:1341–1349. [DOI] [PubMed] [Google Scholar]
- 28.Davey Smith G, Hart CL, Watt G, Hole D, Hawthorne V. Individual social class, area-based deprivation, cardiovascular disease risk factors and mortality: the Renfrew and Paisley study. J Epidemiol Community Health. 1998;52:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapral MK, Wang H, Mamdani M, Tu JV. Effect of socioeconomic status on treatment and mortality after stroke. Stroke. 2002;33:268–273. [DOI] [PubMed] [Google Scholar]
- 30.Shi L, Macinko J, Starfield B, Xu J, Politzer R. Primary care, income inequality, and stroke mortality in the United States. A longitudinal analysis, 1985–1995. Stroke. 2003;34:1958–1564. [DOI] [PubMed] [Google Scholar]
- 31.Lawlor DA, Davey Smith G, Leon DA, Sterne JAC, Ebrahim S. Secular trends in mortality by stroke subtype in the 20th century: a retrospective analysis. Lancet. 2002;360:1818–1823. [DOI] [PubMed] [Google Scholar]
- 32.Song YM, Sung J, Lawlor DA, Davey Smith G, Shin Y, Ebrahim S. Blood pressure, hemorrhagic stroke, and ischaemic stroke: the Korean national prospective occupational cohort study. BMJ. 2004;328:324–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart CL, Hole DJ, Davey Smith G. The relation between cholesterol and hemorrhagic or ischaemic stroke in the Renfrew/Paisley study. J Epidemiol Community Health. 2000;54:874–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart CL, Davey Smith G. Relation between number of siblings and adult mortality and stroke risk: 25-year follow-up of men in the Collaborative study. J Epidemiol Community Health. 2003;57:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song YM, Davey Smith G, Sung J. Adult height and cause-specific mortality: a large prospective study of Korean men. Am J Epidemiol. 2003;158:479–485. [DOI] [PubMed] [Google Scholar]
- 36.McCarron P, Hart CL, Hole D, Davey Smith G. The relation between adult height and haemorrhagic and ischaemic stroke in the Renfrew/Paisley study. J Epidemiol Community Health. 2001;55:404–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Health Insurance Corporation. 2002 National Health Insurance Statistical Yearbook. Seoul, South Korea: National Health Insurance Corporation; 2003.
- 38.Song YM, Byeon JJ. Excess mortality from avoidable and non-avoidable causes in men of low socioeconomic status: a prospective study in Korea. J Epidemiol Community Health. 2000;54:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park JK, Kim KS, Kim CB, et al. The accuracy of ICD codes for cerebrovascular diseases in medical insurance claims. Korean J Prev Med. 2000;33:76–82. [Google Scholar]
- 40.Ryu SY, Park JK, Suh I, et al. The accuracy of myocardial infarction diagnosis in medical insurance claims. Yonsei Med J. 2000;41:570–576. [DOI] [PubMed] [Google Scholar]