Abstract
Objectives. We evaluated the cost-effectiveness of fee-based and free testing strategies at an HIV voluntary counseling and testing (VCT) program integrated into a community-based AIDS service organization in Moshi, Tanzania.
Methods. We waived the usual fee schedule during a 2-week free, advertised VCT campaign; analyzed the number of clients testing per day during prefree, free, and postfree testing periods; and estimated the cost-effectiveness of limited and sustained free testing strategies.
Results. The number of clients testing per day increased from 4.1 during the prefree testing interval to 15.0 during the free testing campaign (P<.0001) and remained significantly increased at 7.1 (P<.0001) after resumption of the standard fees. HIV seroprevalence (16.7%) and risk behaviors were unchanged over these intervals. Modeled over 1 year, the costs per infection averted with the standard fee schedule, with a 2-week free VCT campaign, and with sustained free VCT year-round were $170, $105, and $92, respectively, and the costs per disability-adjusted life year gained were $8.72, $5.40, and $4.72, respectively.
Conclusions. The provision of free VCT enhances both the number of clients testing per day and its cost-effectiveness in resource-limited settings.
In sub-Saharan Africa, HIV voluntary counseling and testing (VCT) is a cost-effective method of reducing high-risk sexual behavior and preventing HIV transmission. A large multicenter study conducted in Kenya, Trinidad, and Tanzania demonstrated that VCT reduced unprotected sexual contact with a nonprimary partner by 35% among men and 39% among women (vs 13% and 17% reductions, respectively, among those who received health information only).1 It has been estimated that VCT offered to 10000 Tanzanians would avert 895 HIV infections at a cost of $346 per infection averted and $17.78 per disability-adjusted life year (DALY) saved.2
Universal voluntary testing with individual informed consent and confidentiality protection in Africa has been advocated.3,4 The World Health Organization and Joint United Nations Programme on HIV/AIDS have recently endorsed moving from client-initiated requests for VCT to provider-initiated approaches.5 In addition to promoting behavior change, VCT can serve as a point of referral for preventive services, including the prevention of mother-to-child transmission and as an entry point for treatment programs for sexually transmitted infections, prophylaxis of opportunistic infections, diagnosis and treatment of tuberculosis,6 and, increasingly, initiation of highly active antiretroviral therapy,7 thereby further enhancing its cost-effectiveness. Greater access to VCT has been facilitated through cheaper, rapid, and simple HIV testing kits, which reduce the cost per test performed.8
Despite these considerations, VCT is vastly underutilized, particularly in poor countries, where the current overall coverage is estimated to be less than 1% to 10% of those at risk for HIV infection.9 In a population-based nationally representative survey in Tanzania, approximately 7% of women and 12% of men reported ever having received an HIV test.10 In the Kilimanjaro Region, even in a hospital setting, 44% of those found to be HIV infected in a systematic serosurvey were previously unaware of their infection.11
Barriers to accessing VCT services include stigmatization (with abandonment and abuse being common, particularly among women who test positive), geographic accessibility, lack of social promotion, inefficient counseling and testing practices, and cost.12 We describe a newly established VCT program in Moshi, Tanzania, designed to overcome many of these barriers and in particular focus on testing uptake before, during, and after a free VCT campaign.
METHODS
Location and Context
A new VCT program was integrated into a well-established HIV service organization, Kikundi cha Wanawake Kilimanjaro Kupambana na UKIMWI (KIWAKKUKI; Women Against AIDS in Kilimanjaro) at their easily accessible AIDS Information Centre in downtown Moshi, Tanzania. This nongovernmental organization, established in 1990 with strong community ties, supports persons living with HIV/AIDS by providing home-based care, counseling, and information about HIV infection, and orphan care and assistance. The KIWAKKUKI VCT program set charges of 1000 Tanzania shillings (TSh; US $0.95 at the 2003 exchange rate) for VCT, except for clients aged 24 years or younger and KIWAKKUKI members (the latter estimated to receive less than 5% of all tests).
The VCT program was initiated in March 2003, and data collection to analyze socio-demographic and clinical characteristics of clients began on May 19, 2003. These characteristics are described in detail elsewhere13; 52% were female, and the median age was 29 years (13 to 80 years). A stable number of clients testing per day of 4.1 was observed for 1 month before initiating a free VCT campaign from July 8 through July 21, 2003, during which KIWAKKUKI waived all VCT fees. The free VCT campaign was advertised on national radio in a series of 4 announcements. Posters advertising the availability of free testing were posted in Moshi municipality, and public announcements were made throughout the district.
VCT Procedures and Costs
Clients presenting for VCT received confidential pre- and post-test counseling with a trained counselor according to Tanzanian Ministry of Health Guidelines.14 The protocol and prevention messages of the KIWAKKUKI VCT service have been outlined previously.13 Test results were typically received within 30 to 40 minutes. Those testing positive were invited to participate in a KIWAKKUKI-sponsored peer support group and to join the KIWAKKUKI home-based care program that provided trimethoprim-sulfamethoxazole prophylaxis, weekly visits by a trained home care worker, food supplements, and treatment of some opportunistic infections. In addition, all such patients were referred to the zonal hospital HIV clinic, where antiretroviral therapy was available. Sociodemographic data, risk behaviors, and general medical information were recorded on standard questionnaires, and daily numbers of persons testing were tabulated.
Trained counselors were paid approximately $3 (all dollar amounts are in US dollars) per day. HIV testing was accomplished using Capillus (Trinity Biotech PLC, Bray, County Wicklow, Ireland) and Determine (Abbott Laboratories, Abbott Park, Ill) HIV1/ 2 rapid antibody tests. Every 20th blood sample or any blood sample yielding discrepant rapid testing results was sent to the zonal referral hospital for confirmatory testing using Vironostika HIV (Organon Teknika, Charlotte, NC).
The costs associated with testing were estimated to be $2700 per year for a laboratory technician, $1 per person for each rapid test, $5 for each validation sample sent for confirmatory testing, and $360 per year for laboratory consumables. Additional costs for the program included $500 per year for building space for the program, $600 per year for telephone, $150 per year for electricity consumption, $500 for consumables such as paper forms and copying fees, and $3700 per year for program coordination. During the free VCT campaign, additional costs included hiring a second counselor, advertising (estimated at $40), and proportional increases in consumables, including laboratory supplies and HIV test kits. No additional rental, telephone, or electricity costs were incurred.
Analysis
Data from questionnaires were entered into an electronic database constructed with Epi-Info 2002 software (Centers for Disease Control and Prevention, Atlanta, Ga). Data were validated by randomly sampling 10% of the questionnaires, with an acceptable error rate being less than 1 error per 5 forms. Data were analyzed with EpiInfo 2002 and Stata 8.0 (Stata Corp, College Station, Tex). Differences in daily number of persons testing during and after the free testing period relative to the prefree testing period were analyzed with t tests. Rates of seropositivity in the tested population, published estimates of the effectiveness of VCT in preventing HIV infections,2 and estimates of DALYs saved/ gained from prevention and treatment15 were combined with KIWAKKUKI cost data to estimate the cost per DALY saved/gained because of free testing, with and without subsequent treatment of those testing positive.
RESULTS
Observed Number of Clients Testing per Day in Relation to the Free VCT Campaign
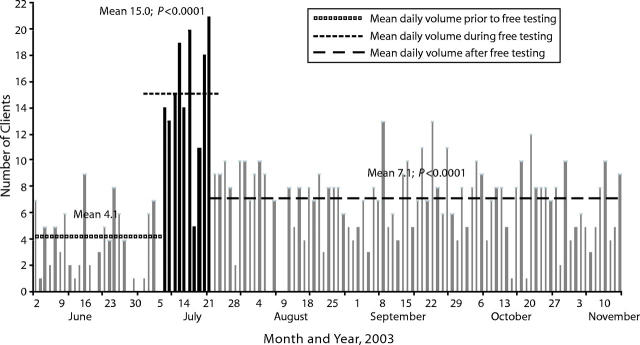
More than 99% of the 813 individuals presenting to KIWAKKUKI for testing from May 19 to November 23, 2003, consented to participate in a study describing the sociodemographic and clinical characteristics of such clients.13 The number of clients testing per day was considered in relation to the free testing campaign and divided into the prefree testing period from May 19 to July 7, 2003, during which a modest fee (1000 Tsh, approximately $0.95 at the 2003 exchange rate) was charged for clients older than 24 years; the free testing period from July 8 to July 21, 2003; and the postfree testing period from July 22 to November 13, 2003, during which the usual fee schedule was resumed. The secular trends in the number of clients testing per day for all age groups are shown in Figure 1 ▶. The periods of peak attendance corresponded to the days when testing was offered free, increasing from mean ±SD of 4.1 ±2.5 clients per day in the prefree testing period to 15.0 ±4.8 and 7.1 ±2.6 clients per day, respectively, during free and postfree testing periods (P < .0001 compared with prefree period for each).
FIGURE 1—
Number of persons testing per day before (gray bars to the left), during (black bars), and after (gray bars to the right) the free voluntary HIV counseling and testing campaign at KIWAKKUKI (n = 813).
Because fees are usually waived for clients younger than 25 years, we considered the number of clients testing per day by age strata as well. The mean daily number of tested persons aged 25 years and older quadrupled during the free testing period relative to the period before free testing (11.4 ±3.5 vs 2.7 ±2.1 clients per day; P<.0001). After free testing ended, the number of persons testing declined to 4.6 ±2.2 clients per day but remained significantly higher than before free testing (P=.0004). During the free testing period, the daily number of clients testing increased for persons younger than 25 years who received free testing throughout the study period (3.6 ±1.8 vs 1.4 ±1.2) clients per day; P= .0003). As with the older group, the daily number of clients testing per day remained at a higher level after the free VCT campaign than during the prefree testing period (2.5 ±1.8 clients per day; P=.0091). The magnitude of the differences between the 3 periods was smaller for this group of clients than for the older group. There were no significant differences among study subjects in demographic characteristics, knowledge of HIV/AIDS, prevalence of symptoms, or seropositivity between any of the 3 periods.
Annualized Models of Cost-Effectiveness
During the free VCT campaign, 109 excess clients than would have been predicted (based on the prefree testing rate of 4.1 clients per day) presented for testing, and in the following 80 days, 238 excess clients were tested. We used the HIV seroprevalence and postfree period number of persons testing per day seen over the latter 29 weeks of observation to develop a model annualizing these data in order to perform cost-effectiveness analyses. Over 1 year, without a free VCT campaign, 966 individuals would be tested at a net cost of $11 518 ($11.92 per client tested) (Table 1 ▶). With the addition of a 2-week free VCT campaign, 1864 persons would be tested for $13 771 ($7.38 per client tested) over 1 year, assuming no increases in fixed costs since the number of persons testing per day in our circumstance had not reached the capacity of the VCT center. A third scenario applied the free VCT daily client testing number to the entire year with appropriate cost increases in rent, telephone, power, testing supplies, and consumables, including adding 2 additional counselors for $6.60 per day. Under these conditions, the cost of sustained free VCT over 1 year would be reduced further to $6.45 per client.
TABLE 1—
One-Year Estimated Testing Volumes and Costs in US Dollars Without Free VCT, With a Free VCT Campaign, and With Sustained Free VCT: Moshi, Tanzania, May through November, 2003
| No Free VCT, n = 966a | Free VCT Campaign, n = 1864b | Sustained Free VCT, n = 3915c | ||||
| Economic parameters | Cost | Cost per Client | Cost | Cost per Client | Cost | Cost per Client |
| Building rent | 500.00 | 0.52 | 500.00 | 0.27 | 1000.00 | 0.26 |
| Telephone | 600.00 | 0.62 | 600.00 | 0.32 | 1200.00 | 0.31 |
| Power | 150.00 | 0.16 | 150.00 | 0.08 | 600.00 | 0.15 |
| Advertisingd | 0.00 | 0.00 | 40.00 | 0.02 | 1040.00 | 0.27 |
| Labore | 8036.80 | 8.32 | 8102.80 | 4.35 | 9759.40 | 2.49 |
| Lab supplies | 360.00 | 0.37 | 694.51 | 0.37 | 1458.16 | 0.37 |
| HIV test kits | 2021.63 | 2.09 | 3900.13 | 2.09 | 8188.46 | 2.09 |
| Other consumables | 500.00 | 0.52 | 964.60 | 0.52 | 2025.22 | 0.52 |
| Total cost | 12 168.43 | 12.59 | 14 952.05 | 8.02 | 25 271.23 | 6.45 |
| Estimated income from VCT chargesf | 650.06 | 1181.44 | 0.00 | |||
| Net cost | 11 518.37 | 11.92 | 13 770.61 | 7.38 | 25 271.23 | 6.45 |
Note. VCT = voluntary counseling and testing.
aApplies prefree VCT campaign daily client volumes to 261 testing days in a calendar year.
bApplies free VCT daily client volumes to 10 days and postfree VCT daily client volumes to 251 testing days in calendar year.
cApplies free VCT daily client volumes to 251 testing days in a calendar year.
dRadio advertisements, gasoline for car during free VCT campaign.
eLaboratory technician, VCT program director, counselors.
fAssumes fees are waived for those aged < 25 years per Tanzanian Ministry of Health guidelines.
We applied the previous estimates of Sweat et al.,2 stratified by gender and serostatus, for HIV infections averted by VCT among individuals in Tanzania. Without free VCT, we estimated that, over 1 year, 68 HIV infections would be prevented at a cost of $169.69 per infection averted and $8.72 per DALY gained (Table 2 ▶). The increased daily testing number and cost-efficiency of testing afforded by the addition of a free VCT campaign would avert 63 additional HIV infections, at a cost of $105.12 per infection averted, reducing the cost per DALY saved to $5.40, and a model of sustained free VCT program would reduce the cost per infection averted further to nearly $92 and a cost per DALY saved of $4.72.
TABLE 2—
Cost-Effectiveness and Sensitivity Analyses Without Free VCT, With a Free VCT Campaign, and With Sustained Free VCT
| No Free VCT | Free VCT Campaign | Sustained Free VCT | ||||||||||||||||
| Number Treated | New Diagnoses | Infections Averted | Cost Per Infection Averteda | DALYs Gained | Cost Per DALY, US $ | Number Treated | New Diagnoses | Infections Averted | Cost Per Infection Averted | DALYs Gained | Cost Per DALY, US $ | Number Treated | New Diagnoses | Infections Averted | Cost Per Infection Averted | DALYs Gained | Cost Per DALY, US $ | |
| Prevention | ||||||||||||||||||
| HIV prevalence | ||||||||||||||||||
| Observed (16.7%) | . . . | . . . | 67.9 | 169.69 | 1321 | 8.72 | . . . | . . . | 131.0 | 105.12 | 2549 | 5.40 | . . . | . . . | 275.0 | 91.89 | 5352 | 4.72 |
| Low (8%) | . . . | . . . | 40.0 | 288.04 | 778 | 14.80 | . . . | . . . | 77.2 | 178.47 | 1502 | 9.17 | . . . | . . . | 162.0 | 156.00 | 3152 | 8.02 |
| High (25%) | . . . | . . . | 95.0 | 121.24 | 1848 | 6.23 | . . . | . . . | 183.3 | 75.12 | 3567 | 3.86 | . . . | . . . | 384.9 | 65.66 | 7490 | 3.37 |
| New diagnoses | ||||||||||||||||||
| HIV prevalence | ||||||||||||||||||
| Observed (16.7%) | . . . | 161.4 | . . . | . . . | . . . | . . . | . . . | 311.3 | . . . | . . . | . . . | . . . | . . . | 653.8 | . . . | . . . | . . . | . . . |
| Low (8%) | . . . | 77.3 | . . . | . . . | . . . | . . . | . . . | 149.1 | . . . | . . . | . . . | . . . | . . . | 313.2 | . . . | . . . | . . . | . . . |
| High (25%) | . . . | 241.5 | . . . | . . . | . . . | . . . | . . . | 466.0 | . . . | . . . | . . . | . . . | . . . | 978.8 | . . . | . . . | . . . | . . . |
| Antiretroviral treatmentb | ||||||||||||||||||
| Rates of treatment | ||||||||||||||||||
| Medium (30%) | 48.4 | . . . | . . . | . . . | 48.4 | 420.00c | 93.4 | . . . | . . . | . . . | 93.4 | 420.00 | 196.1 | . . . | . . . | . . . | 196.1 | 420.00 |
| Low (10%) | 16.1 | . . . | . . . | . . . | 16.1 | 420.00c | 31.1 | . . . | . . . | . . . | 31.1 | 420.00 | 65.4 | . . . | . . . | . . . | 65.4 | 420.00 |
| High (50%) | 80.7 | . . . | . . . | . . . | 80.7 | 420.00c | 155.6 | . . . | . . . | . . . | 155.6 | 420.00 | 326.9 | . . . | . . . | . . . | 326.9 | 420.00 |
| Tuberculosis prophylaxisb | ||||||||||||||||||
| Rates of treatment | ||||||||||||||||||
| Medium (50%) | 80.7 | . . . | . . . | . . . | 12.1 | 166.67d | 155.6 | . . . | . . . | . . . | 23.3 | 166.67 | 326.9 | . . . | . . . | . . . | 49.0 | 166.67 |
| Low (30%) | 48.4 | . . . | . . . | . . . | 7.3 | 166.67d | 93.4 | . . . | . . . | . . . | 14.0 | 166.67 | 196.1 | . . . | . . . | . . . | 29.4 | 166.67 |
| High (70%) | 112.9 | . . . | . . . | . . . | 16.9 | 166.67d | 217.9 | . . . | . . . | . . . | 32.7 | 166.67 | 457.7 | . . . | . . . | . . . | 68.6 | 166.67 |
| Summaryb | ||||||||||||||||||
| Scenario | ||||||||||||||||||
| Medium treatment | . . . | . . . | . . . | . . . | 1381.4 | 24.52 | . . . | . . . | . . . | . . . | 2666.0 | 21.34 | . . . | . . . | . . . | . . . | 5,597.2 | 20.69 |
| Low treatment | . . . | . . . | . . . | . . . | 1344.3 | 14.51 | . . . | . . . | . . . | . . . | 2594.4 | 11.25 | . . . | . . . | . . . | . . . | 5,446.9 | 10.58 |
| High treatment | . . . | . . . | . . . | . . . | 1418.5 | 34.00 | . . . | . . . | . . . | . . . | 2737.6 | 30.90 | . . . | . . . | . . . | . . . | 5,747.6 | 30.28 |
Note. VCT = voluntary counseling and testing; DALY = disability-adjusted life year.
aBased on previous estimates in Tanzania.2
b With an observed HIV infection prevalence of 16.7%.
cAssumes cost for antiretroviral therapy of $35 per month and does not include cost for monitoring and care.
dBased on an estimated cost of $25 for 6 months of preventive therapy.15
Assuming that 30% of those presenting for testing would receive antiretroviral therapy at a cost of $420 per year (on the basis of current retail pricing for fixed dose combination stavudine/lamivudine/nevirapine in retail pharmacies in the Kilimanjaro Region) and that 50% would receive tuberculosis prophylaxis with isoniazid for 6 months at a cost of $25 (on the basis of estimates from Creese et al.15), we calculated that a total of 1381 DALYS would be gained at a cost of $24.52 per DALY without free VCT. With the free VCT campaign, 2666 DALYS would be gained at a cost per DALY of $21.34, and with the sustained free VCT program, 5597 DALYS would be gained at a cost per DALY of $20.69. Sensitivity analyses included in Table 2 ▶ varied assumptions of HIV seroprevalence and rates of treatment. At an HIV seroprevalence of 25%, there is marked improvement in the cost-effectiveness of VCT, particularly with a free VCT campaign. Holding HIV seroprevalence constant and varying rates of treatment with antiretrovirals and tuberculosis prophylaxis shows small improvements in cost-effectiveness.
DISCUSSION
We have demonstrated that a period of free VCT significantly increases the number of persons testing per day and enhances cost-effectiveness of VCT when offered as an integrated program within an existing AIDS service-oriented nongovernmental organization. Modeled over a 1-year time horizon, a policy of sustained free VCT would likely further enhance the cost-effectiveness of this intervention.
Previous work in Tanzania estimated the cost of VCT per infection prevented to be $346, and that of per DALY saved, $17.78.2 Even without a free VCT campaign or sustained free services for all clients, we have shown standard VCT practices in our setting to be nearly twice as cost-effective as these estimates.
Several factors likely explain these differences. First, our VCT program was integrated into an existing, community-based, volunteer AIDS service organization, minimizing startup costs and costs for counselors (who were motivated women from the community paid approximately $3.30/day). Second, testing costs were reduced by the use of onsite rapid HIV antibody testing, which is the method of choice in this region. Others have shown this approach to increase the number of patients receiving their results, making it both more convenient and economical in comparison with conventional enzyme-linked immunosorbent assay testing.8 Third, operating costs may be lower in Moshi municipality, compared with larger urban settings in Tanzania.
Surprisingly little research has focused on strategies to enhance testing uptake and cost-effectiveness at community-based VCT programs. Gresenguet et al.16 described increased testing volumes in Bangui, Central African Republic, at a VCT site during annual AIDS day free testing events, which attracted mostly asymptomatic students, but they did not note the effect of this campaign on subsequent testing.
A survey assessing willingness to pay among VCT clients in Kenya suggested that more people are reluctant to access VCT services as the costs approach $1 to $2.17 In our study, after the introduction of a free VCT campaign, increases in the number of clients testing per day were sustained and cost increases were limited primarily to excess testing supplies. The costs per infection averted and per DALY gained decreased by 38% to $105 and $5.40, respectively. If free VCT were offered year-round, assuming sustained client testing numbers of around 15 clients per day, the costs per infection averted and per DALY gained would decrease by 46%. The provision of free VCT, only if for a brief campaign, thus renders VCT an even more effective intervention, on the order of single-dose nevirapine for the prevention of mother-to-child transmission.15 When VCT is considered as an entry point for care of the HIV-infected and is included in the summary cost per DALY calculation, it contributes around 96% of the DALYS gained and substantially reduces the cost per DALY.
Why did more clients present for testing in the interval following the free testing campaign? The free VCT testing period was promoted soon after the initiation of VCT at KIWAKKUKI, raising the possibility that the increase in daily testing numbers reflected growing awareness of this new service by the community. Against this, the VCT program was initiated 2 months before data collection for this study, and a stable daily testing pattern was observed before the free VCT campaign. It is possible that some clients presented thinking VCT was still offered free at KIWAKKUKI, but this seems unlikely several months after the campaign, when daily testing numbers remained sustained and clients were asked to pay before testing.
It is likely that the combination of a simple, inexpensive advertising campaign helped to increase the social acceptability and awareness of VCT. Further, the provision of free testing removed a cost barrier to VCT, making it accessible to less wealthy clients. From a health policy point of view, including an inexpensive promotional campaign is necessary to create awareness of the free service.
There were limitations to this study. Although the calculations for the numbers of HIV infections averted and cost-effectiveness of this intervention were based on data from Tanzania,2 these data may not necessarily be representative of our population. In addition, our study was observational. Without formal hypothesis testing in which clinics are randomized to free VCT campaigns or their standard fee schedule, we cannot rigorously infer that free VCT campaigns lead to increased participation. Clearly a large, multisite, longer-term, randomized trial would help to refine the magnitude of the impact and cost-effectiveness of free VCT campaigns, but only after several years.
We used daily testing numbers obtained over a 2-week free testing period to estimate the cost-effectiveness of free VCT offered over a 1-year time horizon but did not demonstrate whether such daily testing numbers can be sustained. In response to these analyses, KIWAKKUKI has implemented a free VCT service and has sustained daily testing numbers of 13 persons per day over 6 months. Furthermore, there was no change in HIV seroprevalence among VCT clients over the same 6-month period. Our data suggest that VCT with free campaigns or the provision of sustained free VCT should be adopted into national HIV control policies.
In some instances, governments and nongovernmental organizations offer free VCT,18,19 but at least partial cost recovery through client charges is more typical. In resource-limited settings, sustained provision of universal free VCT may be unrealistic given the multiple demands on the resources allocated for HIV/AIDS prevention and care services.
An approach of strengthening existing health infrastructure by investing enough funds to maximize testing capacity within stable community-based organizations rather than the creation of new services would also be beneficial in terms of the relative ease of implementation. Similarly, costs could be reduced by integrating VCT into other health services, such as those for tuberculosis, sexually transmitted infections, hospital inpatient and outpatient services, and antenatal clinics.
The integration of this VCT program within a volunteer-based AIDS service organization in Moshi, Tanzania, was highly cost-effective. Our study suggests that the provision of free VCT not only results in a prolonged increase in daily testing number but also optimizes testing throughput and efficiency. The enhanced cost-effectiveness of this intervention was reflected in potential disability averted by preventing new HIV infections and facilitating access to expanding HIV treatment programs. In addition to further operational research aimed at optimizing VCT in other settings, policy-makers should support the small investment necessary to underwrite free VCT, particularly when integrated into existing community-based AIDS service organizations.
Acknowledgments
This study was supported in part by Roche Laboratories. Additional investigator support was obtained from AIDS Clinical Trials Group (U01 AI-39156, J.A. Bartlett and N.M. Thielman) and mid-career investigator (K24 AI-0744–01, J. A. Bartlett) awards from the National Institutes of Allergy and Infectious Diseases and from the US Department of State Fulbright Program (N.M. Thielman and H.Y. Chu).
We are grateful to the staff of KIWAKKUKI AIDS Information Centre for their collaboration, and in particular to the VCT counselors Beatrice Mandao, Eliakesia Shangali, Anna Msuya, Anna Mchaki, Agatha Chuwa, Alexia Mella, Awaichi Malle, B. Haule, E. Kiwla, Grace Gumbo, Lillian Mtui, Naomi Ringo, Magdalena Lyimo, Sylvia Mlay, and Yesusia Mariki.
We thank the clients of the KIWAKKUKI VCT program for their participation.
Human Participant Protection Ethical approval for the study was granted by the Kilimanjaro Christian Medical Centre Research Ethics Committee, the institutional review board of Duke University Medical Center, and the Tanzania National Institute of Medical Research National Medical Research Coordinating Committee.
Peer Reviewed
Contributors N.M. Thielman and J.A. Bartlett originated the study. N.M. Thielman wrote the final article. They received assistance in designing the survey instrument from S. Mtweve and D. Itemba. A. Mgonja enrolled clients at KIWAKKUKI. H.Y. Chu performed initial data analysis and wrote an early draft of the article. J. Ostermann oversaw statistical analyses and performed the final cost-effectiveness calculations. J.A. Crump contributed to study implementation, data management and analysis, interpretation, and article editing. J.F. Shao facilitated critical administrative support. All authors contributed to the final version of the article.
References
- 1.The Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet. 2000;356:103–112. [PubMed] [Google Scholar]
- 2.Sweat M, Gregorich S, Sangiwa G, et al. Cost-effectiveness of voluntary HIV-1 counselling and testing in reducing sexual transmission of HIV-1 in Kenya and Tanzania. Lancet. 2000;356:113–121. [DOI] [PubMed] [Google Scholar]
- 3.De Cock KM, Marum E, Mbori-Ngacha D. A serostatus-based approach to HIV/AIDS prevention and care in Africa. Lancet. 2003;362:1847–1849. [DOI] [PubMed] [Google Scholar]
- 4.Ammann AJ. Preventing HIV. BMJ. 2003;326: 1342–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS/WHO Policy Statement on HIV Testing. Geneva, Switzerland: UNAIDS/WHO; 2004.
- 6.Godfrey-Faussett P, Maher D, Mukadi YD, Nunn P, Perriens J, Raviglione M. How human immunodeficiency virus voluntary testing can contribute to tuberculosis control. Bull World Health Organ. 2002;80: 939–945. [PMC free article] [PubMed] [Google Scholar]
- 7.De Cock KM, Mbori-Ngacha D, Marum E. AIDS in Africa, V: shadow on the continent: public health and HIV/AIDS in Africa in the 21st century. Lancet. 2002;360:67–72. [DOI] [PubMed] [Google Scholar]
- 8.Ekwueme DU, Pinkerton SD, Holtgrave DR, Branson BM. Cost comparison of three HIV counseling and testing technologies. Am J Prev Med. 2003;25: 112–121. [DOI] [PubMed] [Google Scholar]
- 9.Jha P, Mills A, Hanson K, et al. Improving the health of the global poor. Science. 2002;295:2036–2039. [DOI] [PubMed] [Google Scholar]
- 10.HIV/AIDS Survey Indicators Database: Tanzania Country Report. Available at: http://www.measuredhs.com/hivdata/reports/start.cfm?L0.1&char_urban=0&char_age=0&char_ed=0&report_action=view. Accessed May 29, 2004.
- 11.Ole-Nguyaine S, Crump JA, Kibiki GS, et al. HIV-associated morbidity, mortality, and diagnostic testing opportunities among inpatients at a referral hospital in northern Tanzania. Ann Trop Med Parasitol. 2004;98: 171–179. [DOI] [PubMed] [Google Scholar]
- 12.Vermund SH, Wilson CM. Barriers to HIV testing-where next? Lancet. 2002;360:1186–1187. [DOI] [PubMed] [Google Scholar]
- 13.Chu HY, Crump JA, Osterman J, Oenga RB, Itemba DK, Mgonja A, Mtweve S, Bartlett JA, Shao JF, Thielman NM. Sociodemographic and clinical characteristics of clients presenting for HIV voluntary counseling and testing in Moshi, Tanzania. Int J STD AIDS. 2005;16:691–696. [DOI] [PubMed] [Google Scholar]
- 14.The United Republic of Tanzania Ministry of Health. National Guidelines for Clinical Management of HIV/AIDS. Dar es Salaam, Tanzania: United Republic of Tanzania Ministry of Health; 2002.
- 15.Creese A, Floyd K, Alban A, Guinness L. Cost-effectiveness of HIV/AIDS interventions in Africa: a systematic review of the evidence. Lancet. 2002;359: 1635–1642. [DOI] [PubMed] [Google Scholar]
- 16.Gresenguet G, Sehonou J, Bassirou B, et al. Voluntary HIV counseling and testing: experience among the sexually active population in Bangui, Central African Republic. J Acquir Immune Defic Syndr. 2002;31: 106–114. [DOI] [PubMed] [Google Scholar]
- 17.Forsythe S, Arthur G, Ngatia G, Mutemi R, Odhiambo J, Gilks C. Assessing the cost and willingness to pay for voluntary HIV counselling and testing in Kenya. Health Policy Plan. 2002;17:187–195. [DOI] [PubMed] [Google Scholar]
- 18.Kawichai S, Celentano DD, Chaifongsri R, et al. Profiles of HIV voluntary counseling and testing of clients at a district hospital, Chiang Mai Province, northern Thailand, from 1995 to 1999. J Acquir Immune Defic Syndr. 2002;30:493–502. [DOI] [PubMed] [Google Scholar]
- 19.Peck R, Fitzgerald DW, Liautaud B, et al. The feasibility, demand, and effect of integrating primary care services with HIV voluntary counseling and testing: evaluation of a 15-year experience in Haiti, 1985–2000. J Acquir Immune Defic Syndr. 2003;33: 470–475. [DOI] [PubMed] [Google Scholar]