Abstract
The phases of central (SCN) and peripheral circadian oscillators are held in specific relationships under LD cycles, but in the absence of external rhythmic input may damp or drift out of phase with each other. Rats exposed to prolonged constant light become behaviorally arrhythmic, perhaps as a consequence of dissociation of phases among SCN cells. We asked whether individual central and peripheral circadian oscillators were rhythmic in LL treated arrhythmic rats and, if rhythmic, what were the phase relationships among them. We prepared SCN, pineal gland, pituitary and cornea cultures from transgenic Per1-luc rats whose body temperature and locomotor activity were arrhythmic, and from several groups of rhythmic rats held in LD, DD, and short-term LL. We measured mPer1 gene expression by recording light output with sensitive photomultipliers. Most of the cultures from all groups displayed circadian rhythms. This could reflect persistent rhythmicity in vivo prior to culture, or, alternatively, rhythmicity that may have been initiated by the culture procedure. To test this we cultured tissues at two different times 12 h apart and asked whether phase of the rhythm was related to culture time. The pineal, pituitary and SCN cultures showed partial or complete dependence of phase on culture time, while peak phases of the cornea cultures were independent of culture time in rhythmic rats, and were randomly distributed regardless of culture time in arrhythmic animals. These results suggest that in behaviorally arrhythmic rats oscillators in the pineal, pituitary and SCN had been arrhythmic or severely damped in vivo while the cornea oscillator was free-running. The peak phases of the SCN cultures were particularly sensitive to some aspect of the culture procedure since rhythmicity of SCN cultures from robustly rhythmic LD-entrained rats was strongly influenced when the procedure was carried out at any time except the second half of the day.
Keywords: circadian organization, Period1, constant light, suprachiasmatic nucleus, pineal, pituitary, cornea, culture
While the molecular mechanisms that generate circadian oscillations within cells have been extensively studied (Reppert and Weaver 2002; Lowrey and Takahashi, 2004), understanding of the mechanisms that connect the molecular framework to physiological function and behavior is still limited (Buijs et al., 2003). We have approached these questions using a transgenic rat model carrying a luciferase gene under the control of the mouse Period1 (Per1) promoter. SCN cultures from these animals express circadian oscillations of Per1-luc expression indefinitely, while oscillations in cultured peripheral tissues damp after several cycles (Yamazaki et al., 2000). The phases of central and peripheral circadian oscillators are held in specific relationships under light-dark (LD) cycles (Yamazaki et al., 2000; Abe et al., 2002). The phase relationship between the SCN and liver can be manipulated by the time of feeding (Davidson et al., 2003a), suggesting that coupling among an organism's circadian oscillators is not rigid and phase relationships are therefore malleable. In behaviorally arrhythmic SCN-lesioned Per2luc knockin mice (which have the luciferase reporter fused to the endogenous Per2 gene), PER2 expression rhythms in peripheral tissues are more robust but become desynchronized within individuals suggesting that the SCN, while not required to sustain circadian rhythmicity in peripheral tissues, does serve to coordinate their phases (Yoo et al., 2004).
Like SCN lesion, prolonged constant light (LL) exposure disrupts behavioral circadian rhythms in rodents, resulting first in an increase in circadian period and then in arrhythmicity (Honma et al., 1996, Benstaali et al., 2001; Canal-Corretger et al., 2000). It has been suggested that this effect of LL might be the result of disrupting the coupling among SCN cells (Mason 1991; Zlomanazuck et al., 1991; Ohta et al., 2005). Only limited information is available on the expression patterns of clock genes in the SCN and peripheral tissues of LL-induced arrhythmic animals. Results from experiments using in situ hybridization and immunocytochemistry suggest that under LL conditions Per1 mRNA (Shigeyoshi et al., 1997) and PERIOD2 protein expression (Sudo et al., 2003) in the SCN continue to oscillate for the first few days, after which the oscillations damp. Behaviorally arrhythmic animals whose arrhythmicity was induced by exposure to prolonged LL (more than one month) showed no rhythmic expression of PERIOD2 protein in SCN (Sudo et al., 2003; Beaule et al., 2003; Amir et al., 2004). The population sampling required for in situ hybridization or immunocytochemistry can indicate rhythmicity only when animals are in phase with each other. Once they become behaviorally arrhythmic there is no marker to determine sampling timing for each animal. Therefore, one would not expect to see rhythmicity even if the SCN of each animal were rhythmic (for a more comprehensive discussion of this problem see Davidson et al. 2003b). To determine whether tissue oscillators are rhythmic in arrhythmic animals it is necessary to analyze potential tissue rhythmicity in each animal separately. The importance of this approach is underlined by a recent study (Granados-Fuentes et al., 2004) in which cultured olfactory bulb from LL-treated, behaviorally arrhythmic Per1-luc rats was found to have rhythmic Per1-luc expression with phases dispersed among individuals, while Per1-luc expression in SCN cultures from the same rats were not rhythmic.
In this study, we examined Per1-luc expression in cultures of SCN, pituitary, pineal and cornea from rats exposed to long-term or short-term LL as well as LD and DD. From the behavior of these tissues in culture, we have inferred the state of the oscillators that they contain in the intact animals prior to sacrifice. Such inference is subject to at least one major reservation: the culture procedure itself may initiate or reset tissue rhythmicity. To control for this possible effect, we cultured tissues at two different times 12 h apart and asked whether the phase of the rhythm in culture was related to the time of culture preparation. In cases in which the phase of the rhythm in culture was completely determined by culture time, we assume that the oscillator had been stopped or severely damped in vivo. When the phase of the rhythm in culture was affected but not completely determined by culture time, we assume a phase-dependent response to the culture procedure, and have verified this in the case of the SCN. When the phase of the rhythm in culture was unaffected by culture time, we assume that the oscillator had been robustly rhythmic in vivo and therefore was resistant to the effects of the culture procedure.
MATERIALS AND METHODS
Animals
Per1-luciferase transgenic rats [W(per1)1] were bred and raised in animal facilities at University of Virginia under conditions approved by Animal Care and Use Committee at University of Virginia (light 5:00-17:00, dark 17:00-5:00). Heterozygous males and females were used for these experiments. Animals were between 2 and 6 months old at the time of the experiments.
As shown in Fig. 1, five groups of rats were kept in different lighting conditions before sacrifice. Locomotor activity and body temperature of each rat was continuously recorded by implanted transmitters (VM-FH, Mini Mitter). 1) LD; LD entrained rats. Rats were held in LD until sacrifice. 2) DD (5d); rhythmic rats in DD. Rats were transferred from LD to DD 5-days before sacrifice. 3) LL (1d); rhythmic rats in LL. Rats were transferred from LD to LL 1-day before sacrifice. 4) LL (5d); rhythmic rats in LL. Rats were transferred from LD to LL 5-days before sacrifice. 5) LL (long term) ; LL-induced arrhythmic rats. The rats in groups 1)-4) all showed rhythmic locomotor activity and body temperature at the time of sacrifice. The rats in goup 5 were transferred to LL at the 1-2 months old, and were considered arrhythmic when both locomotor activity and body temperature lost rhythmicity. Rats were anesthetized with CO2 and killed by decapitation at either ZT 11 or ZT 23 of the LD (or previous LD) cycle. LD entrained rats were killed without anesthesia at ZT 1, 2, 5, 11, 14, 17 and 23 and SCN cultures were prepared in order to define carefully the effects of culture time on the phase of this important pacemaker. Six-h light cycle phase shifts were performed as described in Yamazaki et al. (2000) and Abe et al. (2002). Briefly, the 6-h advance in the light schedule was accomplished by advancing the time of lights-on, leading to one short, 6-h night. The 6-h phase delay was accomplished by delaying the time of lights-off, resulting in one long, 18-h day. After one complete new cycle, SCN cultures were prepared at 2 different time points [one corresponding to ZT 11 of the previous LD cycle, and the other to ZT 11 of the shifted cycle (for details see Fig. 8)].
Fig. 1.
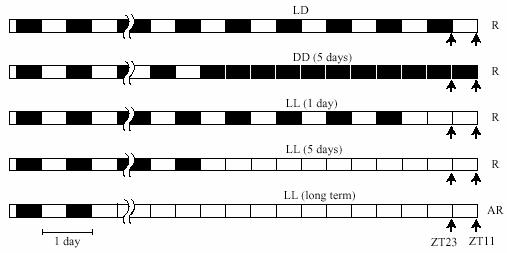
Treatment schedules and timing of culture preparation. Cultures were prepared at ZT 11 or ZT 23 of the rats' LD cycle (LD) or their previous LD cycle (LL, DD) (arrows). Rhythmicity of activity and body temperature of rats at the time of sacrifice are shown on the right (R, rhythmic; AR, arrhythmic).
Fig. 8.
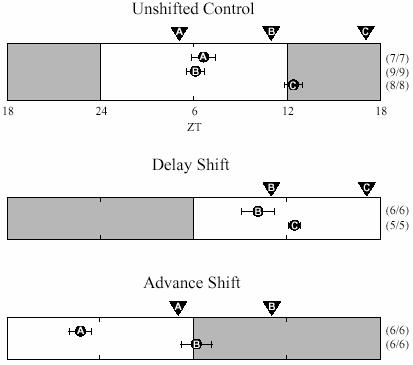
How rapidly does the SCN reset after a phase shift? In the unshifted control experiments cultures were prepared at 3 different times ( ,
,  and
and  ) as indicated above the bar. Experimentally determined phases for each culture are plotted in circles with corresponding letters. Cultures prepared at
) as indicated above the bar. Experimentally determined phases for each culture are plotted in circles with corresponding letters. Cultures prepared at  and
and  (ZT 5 and 11) peaked around ZT 6 and therefore were not shifted by the culture procedure (c.f. in situ data, Miyake et al., 2000; Asai et al., 2001). Cultures prepared at
(ZT 5 and 11) peaked around ZT 6 and therefore were not shifted by the culture procedure (c.f. in situ data, Miyake et al., 2000; Asai et al., 2001). Cultures prepared at  were delayed about 6 h. On the first day following the 6 h delay of the light cycle cultures were prepared at times
were delayed about 6 h. On the first day following the 6 h delay of the light cycle cultures were prepared at times  and
and  as indicated. Cultures prepared at
as indicated. Cultures prepared at  were significantly delayed relative to the controls (p<0.02, Students' t-test), indicating that the SCN had already been delayed. Cultures prepared at
were significantly delayed relative to the controls (p<0.02, Students' t-test), indicating that the SCN had already been delayed. Cultures prepared at  were not shifted relative to the controls, but that result does not test the hypothesis of rapid SCN phase shift because the phase of those cultures is predicted to be the same whether or not the SCN has been delayed 6 h. On the first day following the 6 h advance of the light cycle, cultures were prepared at times
were not shifted relative to the controls, but that result does not test the hypothesis of rapid SCN phase shift because the phase of those cultures is predicted to be the same whether or not the SCN has been delayed 6 h. On the first day following the 6 h advance of the light cycle, cultures were prepared at times  and
and  as indicated. Cultures prepared at
as indicated. Cultures prepared at  were significantly advanced relative to the controls (p<0.01, Students' t-test). Cultures prepared at
were significantly advanced relative to the controls (p<0.01, Students' t-test). Cultures prepared at  were not shifted relative to the controls, but their phase would be the same whether or not the SCN has been advanced 6 h. Taken together, these results support the interpretation that Per1 expression in the SCN shifts rapidly following shifts of the light cycle in either direction.
were not shifted relative to the controls, but their phase would be the same whether or not the SCN has been advanced 6 h. Taken together, these results support the interpretation that Per1 expression in the SCN shifts rapidly following shifts of the light cycle in either direction.
Tissue collections scheduled during the dark period were initiated in darkness with the aid of a infrared lamp and infrared viewer. The animals were not exposed to light before they were decapitated and the eyes were removed. Others were done under normal room lighting. Dissection procedures in light and dark were exactly same except for the lights.
Tissue cultures
Tissues were removed quickly and chilled in HBSS at 4°C. The SCN, pineal gland, pituitary, and cornea cultures were prepared, and bioluminescence was measured as previously described (Yamazaki et al., 2000, Abe et al., 2002). Briefly, the SCN containing region of the brain was dissected out from a 300-μm-thick coronal slice made with a vibratome. Pineals were cut halfway through and flattened, pituitaries were hand-sliced, and whole corneas were dissected out from eyes. The SCN, pineal and pituitary pieces were placed on Millicell culture inserts in 35-mm culture dishes with 1.2 ml of culture medium, DMEM (D2902, Sigma), supplemented with B27 (Gibco), 10 mM HEPES, 352.5 μg/ml NaHCO3, 3.5 mg/ml D- glucose, 25 U/ml penicillin, 25 μg/ml streptomycin and 0.1 mM beetle luciferin (Promega). Corneas were allowed to float free in the medium. Sealed cultures were maintained at 36 °C in darkness, and their bioluminescence was continuously monitored with photomultiplier tubes (Hamamatsu).
Data analysis
The average of bioluminescence counts from the first 3 days of culture was calculated, each count was normalized against the average and expressed as percentage relative bioluminescence. The normalized data sets were detrended (Abe et al. 2002) by subtracting the 24 h running average from the normalized data. The detrended data sets were smoothed by taking 3 h running averages. From the smoothed data set the highest number in each circadian cycle was designated as the peak value and its time of occurrence relative to the previous LD cycle was considered its phase. Cultures that expressed more than two circadian cycles were considered rhythmic (see Fig. 3). Peak phase is plotted for peaks that occur between 24 and 48 h in culture. An average vector was calculated based on the distribution of phase points around the unit circle. The angle of the vector corresponds to the mean phase for the group of points, and the magnitude of the vector represents the variability in the phase (see Fig. 4 and 6).
Fig. 3.
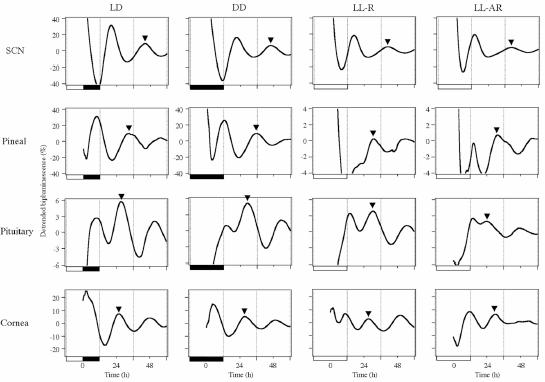
Representative detrended bioluminescence rhythm of cultures prepared at ZT 11. Plots in the same column are from tissues obtained from a single animal. Animals' previous LD conditions are labeled on the top and are also shown by black and white bars on the bottom of each graph. DD and LL-R animals were entrained to LD cycles and transferred to DD or LL for 5 days prior to culture preparation. Peaks that were used to determine phases (between 24 and 48 h in culture) are marked by arrowheads.
Fig. 4.
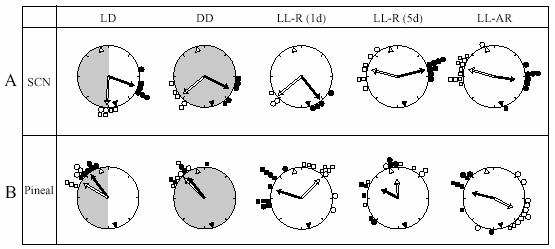
Peak phases of Per1-luc rhythms in SCN and pineal cultures. Time is indicated by position on the circle. The peak phase of each culture is represented by small circles [filled circles: cultures prepared at ZT 11 (filled triangle within the large circle); open circles: cultures prepared at ZT 23 (open triangle within the large circle)]. Arrows inside the large circle indicate the average phase of each group. The degree of synchrony within each group is indicated by the length of the arrow. The distribution within all groups was significantly non-random (Rao's spacing test, p<0.05). Light conditions (LD, DD or LL) and rhythmicity of rats (arrhythmic, AR or rhythmic, R) are shown at the top of the figure and by shading.
Fig. 6.
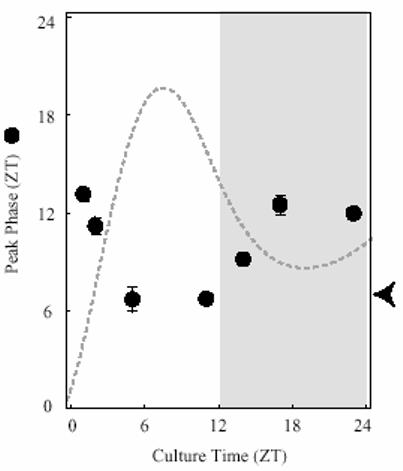
Peak phases of Per1-luc expression rhythms in SCN cultures prepared at different times from LD entrained rats. Average phases (±SEM) are plotted against time (ZT) of SCN slice preparation (n=6-14/group). Arrowhead on the Y-axis indicates the peak phase of SCN Per1 expression obtained by in situ measurements (Miyake et al., 2000; Asai et al., 2001). Broken line indicates the general shape of the Per1-luc expression rhythm from cultures (n=6) made at ZT 11 in arbitrary units [Note that when Per1 expression is high the culture procedure does not reset peak phase (c.f. with in situ data)].
The Rao's spacing test (Russel and Levin, 1996) was applied to all data sets to determine whether the distribution of phases was random (as would be expected in the case of the arrhythmic rats if their tissue clocks continued to run and were not influenced by the culture procedure).
RESULTS
Period1-luciferase (Per1-luc) rats entrained to LD cycles and showed free-running rhythms of locomotor activity and body temperature under short term constant conditions [5 days of either DD (free running period of locomotor activity 23.5±0.05 h) or LL(25.3±0.3 h); Fig. 2A and C]. Under prolonged constant light the rats gradually lost rhythmicity (Fig. 2B and D). Activity became arrhythmic first, followed days or occasionally weeks later by body temperature. Most rats became completely arrhythmic within 4 months.
Fig. 2.
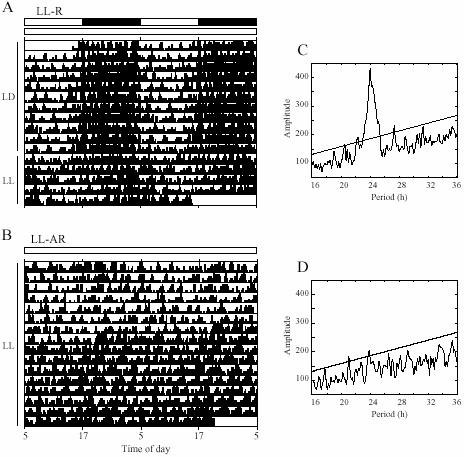
Locomotor activity rhythms of rats in LL. Activity record of rhythmic (A) and arrhythmic (B) rats. Periodograms of LL portions of A (5 days) and B (last 16 days) are shown in C and D.
Expression levels of the transgene in tissue cultures were monitored as luciferase activity (bioluminescence). We measured Per1-luc activity of 4 different tissues (Fig. 3), SCN and three peripheral oscillators known to show robust Per1-luc oscillations [pineal, pituitary, and cornea (Abe et al., 2002; Yamazaki et al., 2002)]. No sex dependent difference in Per1-luc rhythm (phase distribution and amplitude) were observed. Since the results from different tissues were quite different, we have described them separately below.
SCN
Somewhat surprisingly, peak phase of Per1-luc expression in the SCN cultures was influenced by culture preparation time in all groups regardless of prior conditions (Fig. 4A). Cultures from rats either still behaviorally rhythmic after 5 days in LL [LL-R (5d)] or behaviorally arrhythmic after long exposure (LL-AR), showed peak phases completely determined by the time of culture preparation. Preparation time also had an effect on the peak phase of SCN cultures from the other groups of rhythmic rats. Average phase differences between cultures prepared at the two different culture times were 6.0 h [LL-R (1d)], 7.7 h (DD) and 4.9 h (LD). Since it can be assumed that in all the behaviorally rhythmic rats the SCN was rhythmic in vivo, some aspect of the culture procedure must have reset its phase at least at one of the preparation times. A comparison of the effects of culture time on peak phase in DD, LL-R (1 d) and LL-R (5 d) suggests that the SCN oscillator in vivo is unaffected by one day in LL, but after 5 days in LL has damped sufficiently to be completely reset by the procedure, although still robust enough to maintain behavioral rhythmicity. Peak amplitudes of the first peak in culture were significantly higher than the following peaks in all groups (p<0.01), however there were no differences among the groups (Fig. 5A). There were no significant differences in free-running period of the Per1-luc expression rhythm among the groups, which is consistent with Yoo et al. (2004) [but see Granados-Fuentes et al., (2004)].
Fig. 5.
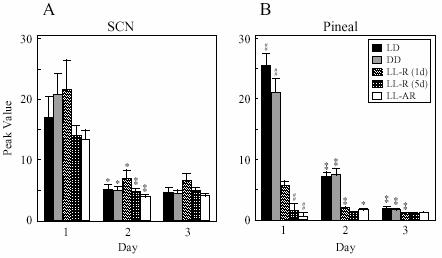
Amplitude of peak values (relative bioluminescence determined from detrended data sets) of SCN and pineal cultures (mean ± SEM). Students' t-test, ## P<0.001, L-R (1d) vs other groups within each day. * p<0.01; ** P<0.001, day 1 vs day 2 or day 2 vs day 3 within each group.
Because of the unexpectedly large effect of preparation time on SCN phase even in animals held in LD cycles, we decided to investigate this effect in more detail. SCN slices from rats in LD12:12 were prepared at 7 different times of day (ZT 1, 2, 5, 11, 14, 17 and 23) and their peak phases in culture were determined (Fig. 6). The cultures prepared at ZT 5 and ZT 11 showed Per1-luc expression rhythms with peaks around ZT 6, which corresponds to the peak phase of endogenous Per1 expression determined by in situ hybridization in rat SCN (Miyake et al., 2000; Asai et al., 2001). Therefore, cultures prepared at these times were not reset by the procedure. When cultures were prepared at ZT 1, ZT 17 or ZT 23, the peak phases were delayed by 6 h. Cultures prepared between those times (ZT 2 and ZT 14) peaked with intermediate delays.
Pineal
Pineal cultures from both rhythmic and arrhythmic rats showed rhythmic Per1-luc expression (Fig. 3). Pineal cultures from rhythmic rats in LD or DD showed similar patterns of Per1-luc expression, with nighttime peak phases that were independent of culture preparation time (Fig. 4B) and corresponded with those obtained in our previous study (Abe et al., 2002). In the cultures from LL-AR rats, the expression levels peaked almost 11 h apart, suggesting strongly that phase was completely reset at least at one of the times at which the cultures were prepared. Short term LL (1d or 5 d) did not reset the peak phase of Per1-luc expression completely. However, peak phases were clearly influenced by the time of culture preparation. One day of LL already had an effect on the lability of the pineal circadian oscillator which is consistent with its effect on peak amplitude (see below). The phases of LL-R (5 d) cultures were dispersed compared to those from LL-R (1 d) cultures, suggesting that the pineal oscillator becomes progressively weaker with time in LL, although after 5 days it is still capable of independent oscillation (i.e., its phase is not completely determined by culture time). In contrast, the fact that peak phase is directly related to culture time in LL-AR rats suggests that the Per1 expression rhythm is abolished or severely damped in these animals and that culture preparation either initiates or completely resets the oscillation.
Pineal cultures from rhythmic rats in DD and LD show distinct Per1-luc expression peaks on the first day in culture, while peaks of the cultures from LL-housed rats [LL-AR and LL-R (5d)] were less clear. The peak amplitudes determined from the smoothed data sets were compared between groups and between days within a group. On the first day of culture, the peak amplitudes in LD and DD were significantly higher than those of any of the groups in LL, and decreased over the next two days (Fig. 5B). The first-day peak amplitudes of cultures from animals treated with a single day of LL were significantly lower than those from LD or DD rats and significantly higher than those from LL-AR or LL-R (5 d) rats. There was no difference in the peak amplitude between LL-AR and LL-R (5 d). Apparently the effect of LL on peak amplitude begins on the first day in LL and is complete by the fifth day.
Pituitary
95% of pituitary cultures showed rhythmic Per1-luc expression (Fig. 3). In the cultures from rhythmic rats in constant conditions [LL-R (5d) and DD], there were no phase differences regardless of time of culture preparation (Fig. 7A). However, the cultures from LL-R rats peaked 6 h later than the cultures from DD rats. Free-running periods of locomotor activity in LL were shorter than that in DD, suggesting that the difference in phase may have been caused by the difference in free-running periods over the 5 days in constant conditions. When the cultures from LD entrained rats were prepared at ZT 11, they showed peak phase and phase distribution comparable to cultures from rats in DD. On the other hand, cultures of LD rats prepared at ZT 23 exhibited quite a wide distribution of phases. Six out of ten cultures prepared at ZT 23 peaked at times similar to the cultures prepared at ZT 11, while the remaining four cultures peaked at roughly opposite phase. Surprisingly, the peak phases of cultures prepared at ZT 11 were more tightly clustered than those prepared at ZT 23. It is difficult to account for this since by the time the animals have been in LL for months, culture times are essentially arbitrary and should be equivalent relative to any remaining circadian oscillations.
Fig. 7.
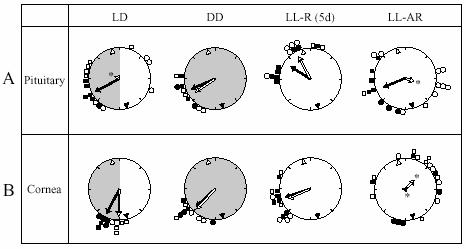
Peak phases of Per1-luc rhythms in (A) pituitary and (B) cornea cultures. Details as in Fig. 4, except that starred arrows indicate random distribution of phases by Rao's spacing test.
Cornea
The results from cultured corneas were quite different from those of the other three tissues (Fig. 7B) There was no effect of culture time in any of the groups. Cultures from rhythmic rats in all lighting conditions (LD, DD and LL) showed Per1-luc expression rhythms peaking in the early subjective night regardless of culture time and there was no difference in phase between the DD and LL-R (5d) groups (c.f. pituitary data). Twenty out of twenty one cornea cultures from arrhythmic rats (LL-AR) showed rhythmic expression of Per1-luc. Their peak phases were dispersed throughout the day; the Rao's spacing test indicated that their distribution did not differ significantly from uniformity. The data from the LL-treated, behaviorally arrhythmic rats suggest that the oscillators in their corneas continued to free run in vivo, and drifted out of phase with each other because they received no synchronizing information. Peak amplitude in culture was not significantly different within each group (LL-AR, LL-R, DD and LD). Furthermore, there were no significant differences in peak amplitude between groups except that the third peak from LL-AR cultures was lower than from the other groups (data not shown but see examples in Fig. 3).
DISCUSSION
Perhaps the most striking observation that emerges from the work reported here is the differential effect of the light environment on the several oscillators that comprise the rat's circadian system. This is particularly apparent when one compares the effects of long-term exposure to constant light on SCN with its effects on cornea. The SCN oscillator is either stopped or severely damped by this treatment, whereas those in the cornea are essentially unaffected. Intermediate effects are found in the other two peripheral oscillators that we studied. Taken together, these results imply that coupling strengths and coordinating pathways among circadian oscillators vary considerably. If that is true, the behavior of the system (as well as its individual components) as it regains steady-state following a perturbation will be complex and can be expected to interfere with normal function.
The behavior of the cultured SCN in response to prior treatment of the animal with the several different light regimens was somewhat unexpected. Even when rats were entrained to 12:12 LD cycles, peak phase of the Per1-luc expression of the SCN cultures were reset substantially by the culture procedure when it was carried out during the dark period or early in the light period (Fig. 6). The peak phase of SCN cultures were not reset when they were prepared at ZT 5 or 11 as judged by the fact that their phases in vitro corresponded to the peak phase of the Per1 expression in vivo determined by in situ hybridization (Miyake et al., 2000; Asai et al., 2001). We did not measure the transgene expression pattern in vivo. However, expression pattern of the Per1-luc was considered to represent endogenous Per1 in vivo, because we have not found any tissue which peak phase of Per1-luc expression does not match endogenous Per1 expression when tissue cultures were prepared at ZT 11. SCN cultures from animals held in DD for 5 days or LL for 1 day were reset somewhat more than those from LD animals, while those from animals held in LL for longer times were affected the most. This pattern of response suggests that SCN oscillators are less robust and therefore more easily reset in DD than in LD, and even less robust in LL of more than one day. In the 5 day LL group, the peak phase of Per1-luc in the SCN cultures was completely determined by culture time (at least at the two times studied) in spite of the fact that locomotor activity and body temperature of the animals were both rhythmic (Fig. 2A, C). It is likely that the amplitude and/or synchrony among SCN oscillators was severely affected yet its output was sufficiently robust to maintain these rhythms. The peak phases of SCN cultures from the arrhythmic animals exposed to long-term LL were determined by culture time (as in 5 day LL group), suggesting either severely damped oscillation or arrhythmicity of their SCN oscillators. Granados-Fuentes et al. (2004) reported that 9 out of 10 SCN cultures showed no circadian rhythm of the Per1-luc expression, when the tissues were taken from rats made arrhythmic by brighter light than we used. This result contrasts sharply with ours in which 20 of 21 SCN cultures from arrhythmic rats were rhythmic, although the rhythmicity was almost certainly induced by the culture procedure. This discrepancy suggests a significant but unrecognized difference in the culture techniques in use in these two laboratories, and underlines the importance of carefully specifying all techniques and conditions to meaningful interpretation of such in vitro experiments.
Using mice with a brighter reporter (Per1-GFP) Ohta et al. (2005) have shown that constant light treatment that renders the animals behaviorally arrhythmic does not abolish rhythmicity in individual SCN cells, although the rhythms recorded from SCN slices were severely damped (trough-to-peak amplitude was 1.09). The constant light treatment caused SCN cells to dissociate from one another and assume a random distribution of individual phases. Apparently, constant light weakens coupling among the cells within a tissue as well as among tissues and organs. Since we know from behavioral experiments with many different organisms that constant light changes free running period, it is tempting to speculate that uncoupling within a tissue is a consequence of driving the constituent cells to a range of periods so different that the normal mutual entrainment mechanisms fail (Nakamura et al., 2002; Honma et al., 2004). This hypothesis has the virtue of providing a single explanation for the effects of constant light on circadian period and on coupling among circadian oscillators; it leaves unexamined the mechanisms that may be involved.
In the course of normal entrainment to light cycles, the SCN responds to retinal signals conveyed by neurotransmitters, primarily glutamate (Ebling 1996). To prepare SCN cultures, animals are usually anesthetized and decapitated, which must cause uncontrolled release of neurotransmitters. Such a neurotransmitter “flood” may provide the phase-shifting stimulus connected with culture preparation. This suggestion is supported by the fact that phase shifts occur primarily when the tissue is prepared in the dark period, when exposure to light is also most effective in shifting behavioral phase. It should be possible to test this idea by administration of excitatory neurotransmitter blockers prior to decapitation.
Regardless of the nature of the resetting signal provided by the culture procedure, it may well act by inducing expression of Per1. Miyake et al. (2000) observed significant induction of Per1 expression in the rat SCN by light pulses during the subjective night at times when endogenous expression is low, but no induction during the subjective day when endogenous expression is high. The induction was observed mainly in the ventrolateral SCN which receives most of the direct retinal inputs, suggesting that light-induced release of glutamate and/or PACAP induced the Per1 expression. In our hands, when SCN slices were prepared for culture while Per1 expression in the SCN was high (ZT 5 and ZT 11), the peak phases measured in culture were not shifted. In contrast, when cultures were prepared while Per1-luc expression in the SCN was low (ZT 17, ZT 23 and ZT 1), the phases in culture were delayed up to 6 h (Fig. 6). These results suggest that when the endogenous Per1 expression level is low additional Per1 expression is induced by some aspect of culture preparation, and that such induction may cause phase shifts of the Per1-luc rhythm.
Gillette (1986) reported preparation time dependent phase shifts of the circadian rhythm of neuronal firing in the rat SCN slice. Peak phase of the firing rate delayed and advanced when brain slices were prepared during the early and middle of the night, respectively, while no major shift was seen when slices were prepared during the day. The preparation of brain slices in the subjective night did not induce phase shifts in the neural firing rhythm in the hamster SCN (Yannielli and Harrington 2000). Neither of those results coincide with the pattern we report here for the Per1-luc rhythm. Animals in Gillette's study were exposed to ambient light prior to decapitation, and dim red light was used by Yannielli and Harrington, while our animals did not see any visible light prior to decapitation. These differences in light conditions might result in different phase shift effects. Yamazaki et al. (2000) showed that locomotor activity rhythm shifted slower than Per1-luc rhythm in the SCN after LD cycles were shifted by 6 hours. This suggests that behavioral and Per1-luc expression rhythms do not behave in the same way always. Furthermore, since electrical activity and Per1-luc expression rhythms do not always behave in the same way (Vansteensel et al., 2003), it is not excluded that common mechanisms might underlie culture time dependent phase shifts of these two different SCN rhythms.
In a previously published study we described the rate of re-entrainment in cultured tissues following 6-h delays and advances of the LD cycle applied to intact animals (Yamazaki et al., 2000). After the 6-h phase shifts the cultures were prepared at ZT 11 of the shifted LD cycle. We reported that the Per1-luc expression rhythm in the SCN shifted almost completely (6-h delay or advance) after the first cycle (Yamazaki et al., 2000). Experiments in that study were not controlled for the possible phase-shifting effects of culture time which we now know, on the basis of data reported here, could play a confounding role. To clarify the interpretation of our earlier data we repeated the phase shifting experiments, but cultured SCNs at two different ZT times after the phase shifts. We reasoned that if our original interpretation was correct (i.e., that the SCN oscillation shifted rapidly following the light cycle shift) then SCNs from shifted animals should respond to culture times in the same way as those from unshifted animals. In other words, the SCNs of the shifted animals should already have reached steady state. Results of a limited test of this prediction are plotted in Fig. 8, and support our original interpretation.
Like the SCN, Per1-luc rhythm in the pineal cultures are completely reset by the culture procedure in rats made arrhythmic by long term exposure to constant light. However, in both LD- and DD-treated animals, its response to culture is strikingly different from that of the SCN. Under these conditions the peak phase of the pineal cultures were unaffected by culture time, suggesting that it is either not exposed to (or is not sensitive to) a barrage of neurotransmitters during dissection. On the other hand, its rhythmicity in vivo must be rapidly damped by constant light, since after only one day of exposure its phase is robustly affected by culture time. The nocturnal increase of Per1 mRNA expression in the rat pineal is abolished by light exposure in the similar way as AA-NAT mRNA (Fukuhara et al., 2000; Simonneaux et al., 2004). It is indicated that both expressions are regulated by norepinepherine release from the SCN (Simonneaux et al., 2004). Taken together with the results reported here, this suggests that LL suppresses Per1 expression in vivo and that this effect is maintained in culture.
The response of the pituitary is difficult to interpret. Pituitaries from LD-treated animals dissected at ZT 11 express rhythms in vitro with phases tightly clustered around ZT 16.5, however dissection at ZT 23 yields a random temporal distribution of phases. Since the random distribution was observed in males as well as in females in the ZT 23 group, estrus cycles were not responsible. Something may be occurring at this time that sensitizes the pituitary to an unknown and highly variable signal from the culture procedure. The discrepancy between the effects of the two different culture times disappears in DD, perhaps because the “signal” is not present in the dark, or, more likely, because five days of DD free run changes the phase of the animals' endogenous rhythm relative to dissection time. Even more puzzling is the response of the pituitary in LL-treated, arrhythmic animals. In these animals, there should be no difference between the effects of the two culture times: either they should both reset rhythms completely (or initiate them) as in the SCN and pineal, or not affect phase, as in the cornea (see below). However, pituitaries cultured at “ZT 23” have randomly distributed phases, while those cultured at “ZT 11” have clustered phases. Although there were only two males in this group, female rats kept in LL show persistent estrus (Hoffmann 1978), and therefore it is unlikely that females' estrus cycles caused the random phase distribution. One possible explanation for this paradoxical result (which is qualitatively similar to the result from LD animals) is that there is an uncontrolled entraining agent in the environment, but it is hard to imagine such an agent operating in LL but not in DD.
The cornea is unique among the tissues that we studied. Its phase is unaffected by the culture procedure under all conditions. Even in animals made arrhythmic by long exposure to constant light, the phases of individually cultured corneas are randomly distributed as would be expected if the cornea's circadian oscillators continued to free run in these behaviorally arrhythmic animals. Apparently rhythmicity in the cornea persists for long periods in the absence of rhythmic input from the SCN, since we know from the behavior of the SCN in this study as well as other studies that many of its outputs are arrhythmic or severely damped in LL (Granados-Fuentes et al., 2004, Ohta et al. 2005).
Yoo et al. (2004) reported that peripheral tissues from SCN-lesioned mPer2luc knockin mice lost normal synchrony within and among animals. This suggests that the SCN, while not needed to maintain rhythmicity in peripheral structures, is required to maintain normal phase relationships. In that study, the only exception was the cornea, which was tightly synchronized among SCN-lesioned animals. Although these animals were behaviorally arrhythmic, their eyes were exposed to light during dissection, and one explanation of the behavior of the cornea is that, uniquely among peripheral tissues, it may have unusually direct access to the environmental light cycle. The idea that the cornea contains a robust, self-sustained circadian oscillator that is normally synchronized more or less directly by the retina would account for both the results reported here and those in the Yoo et al. (2004) study. This is not unreasonable since the cornea receives innervation from ocular nerves and also contains melatonin receptors (Dyson et al., 1987, Meyer et al., 2002, Wiechmann and Rada, 2003).
At least one central structure, the olfactory bulb (ob) behaves in a somewhat similar way. Rhythmicity of mPer1 expression in this explanted tissue from transgenic rats persists when the tissue is taken from SCN-lesioned animals or from animals made arrhythmic by two weeks of exposure to bright constant light (Granados-Fuentes et al., 2004). The authors specifically reject the possibility that rhythmicity is induced by the culture procedure on the basis that the distribution of phases is not related to culture time.
More generally, our results support an emerging view of circadian organization in multicellular animals: most individual cells, tissues, and organs contain oscillators with the full range of circadian properties (including light sensitivity in some cases, e.g., Drosophila and zebra fish). These may be coupled more or less tightly to each other and to one or more central oscillators that influence their phase relationships. In the real world, the entire system is entrained to the environmental light cycle and under those circumstances, the component oscillators are held in functionally adaptive phase relationships to each other. This view of circadian organization is consistent with the well documented involvement of circadian rhythmicity in a wide variety of behavioral, physiological, and biochemical processes, and has an important corollary: because internal phase relationships have been fine tuned by natural selection, disrupting them is likely to have deleterious consequences.
ACKNOWLEDGMENT
This work was supported in part by NIH grant MH56647 and NSBRI grant NCC 9-58-HPF 00406 (to M.M.). T.Y. was supported by Fellowship from the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S, Lamont EW, Robinson B, Stewart J. A Circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J Neurosci. 2004;24:781–790. doi: 10.1523/JNEUROSCI.4488-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res. 2001;66:1133–1139. doi: 10.1002/jnr.10010. [DOI] [PubMed] [Google Scholar]
- Beaule C, Houle LM, Amir S. Expression profiles of PER2 immunoreactivity within the shell and core regions of the rat suprachiasmatic nucleus: lack of effect of photic entrainment and disruption by constant light. J Mol Neurosci. 2003;21:133–147. doi: 10.1385/JMN:21:2:133. [DOI] [PubMed] [Google Scholar]
- Benstaali C, Mailloux A, Bogdan A, Auzeby A, Touitou Y. Circadian rhythms of body temperature and motor activity in rodents their relationships with the light-dark cycle. Life Sci. 2001;68:2645–256. doi: 10.1016/s0024-3205(01)01081-5. [DOI] [PubMed] [Google Scholar]
- Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- Canal-Corretger MM, Cambras T, Vilaplana J, Diez-Noguera A Bright light during lactation alters the functioning of the circadian system of adult rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R201–208. doi: 10.1152/ajpregu.2000.278.1.R201. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav. 2003a;2:32–39. doi: 10.1034/j.1601-183x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found Symp. 2003b;253:110–121. discussion 121-125, 281-284. [PubMed] [Google Scholar]
- Dyson H, Shimeld C, Hill TJ, Blyth WA, Easty DL. Spread of herpes simplex virus within ocular nerves of the mouse: demonstration of viral antigen in whole mounts of eye tissue. J Gen Virol. 1987;68:2989–2995. doi: 10.1099/0022-1317-68-12-2989. [DOI] [PubMed] [Google Scholar]
- Ebling FJ. The role of glutamete in the photic regulation of the sprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Fukuhara C, Dirden JC, Tosini G. Circadian expression of period 1, period 2, and arylalkylamine N-acetyltransferase mRNA in the rat pineal gland under different light conditions. Neurosci Lett. 2000;286:167–170. doi: 10.1016/s0304-3940(00)01129-0. [DOI] [PubMed] [Google Scholar]
- Gillette MU. The suprachiasmatic nuclei: circadian phase-shifts induced at the time of hypothalamic slice preparation are preserved in vitro. Brain Res. 1986;379:176–181. doi: 10.1016/0006-8993(86)90273-8. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J Neurosci. 2004:24, 615–619. doi: 10.1523/JNEUROSCI.4002-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JC. Effects of adrenalectomy and constant light on the rat estrous cycle. Neuroendocrinology. 1978;27:247–256. doi: 10.1159/000122817. [DOI] [PubMed] [Google Scholar]
- Honma S, Kanematsu N, Katsuno Y, Honma K. Persistence of circadian oscillation while locomotor activity and plasma melatonin levels became aperiodic under prolonged continuous light in the rat. Neurosci Lett. 1996;216:49–52. doi: 10.1016/0304-3940(96)13006-8. [DOI] [PubMed] [Google Scholar]
- Honma S, Nakamura W, Shirakawa T, Honma K. Diversity in the circadian periods of single neurons of the rat suprachiasmatic nucleus depends on nuclear structure and intrinsic period. Neurosci Lett. 2004;358:173–176. doi: 10.1016/j.neulet.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annual Review of Genomics & Human Genetics. 2004;5:407–41. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. The effects of continuous light exposure on Syrian hamster suprachiasmatic (SCN) neuronal discharge activity in vitro. Neurosci Lett. 1991;123:160–163. doi: 10.1016/0304-3940(91)90920-o. [DOI] [PubMed] [Google Scholar]
- Meyer P, Pache M, Loeffler KU, Brydon L, Jockers R, Flammer J, Wirz-Justice A, Savaskan E. Melatonin MT-1-receptor immunoreactivity in the human eye. Br J Ophthalmol. 2002;86:1053–1057. doi: 10.1136/bjo.86.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S, Sumi Y, Yan L, Takekida S, Fukuyama T, Ishida Y, Yamaguchi S, Yagita K, Okamura H. Phase-dependent responses of Per1 and Per2 genes to a light-stimulus in the suprachiasmatic nucleus of the rat. Neurosci Lett. 2000;294:41–44. doi: 10.1016/s0304-3940(00)01545-7. [DOI] [PubMed] [Google Scholar]
- Nakamura W, Honma S, Shirakawa T, Honma K. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat Neurosci. 2002;5:399–400. doi: 10.1038/nn843. [DOI] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Russel GS, Levitin DJ. An expanded table of probability values for Rao's Spacing Test. Communications in Statistics: Simulation and Computation. 1996;24:879–888. [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- Simonneaux V, Poirel VJ, Garidou ML, Nguyen D, Diaz-Rodriguez E, Pevet P. Daily rhythm and regulation of clock gene expression in the rat pineal gland. Brain Res Mol Brain Res. 2004;120:164–172. doi: 10.1016/j.molbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Sudo M, Sasahara K, Moriya T, Akiyama M, Hamada T, Shibata S. Constant light housing attenuates circadian rhythms of mPer2 mRNA and mPER2 protein expression in the suprachiasmatic nucleus of mice. Neuroscience. 2003;12:493–499. doi: 10.1016/s0306-4522(03)00457-3. [DOI] [PubMed] [Google Scholar]
- Vansteensel MJ, Yamazaki S, Albus H, Deboer T, Block GD, Meijer JH. Dissociation between circadian Per1 and neuronal and behavioral rhythms following a shifted environmental cycle. Curr Biol. 2003;13:1538–1542. doi: 10.1016/s0960-9822(03)00560-8. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Rada JA. Melatonin receptor expression in the cornea and sclera. Exp Eye Res. 2003;77(2):219–225. doi: 10.1016/s0014-4835(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yannielli PC, Harrington ME. Neuropeptide Y applied in vitro can block the phase shifts induced by light in vivo. Neuroreport. 2000;11:1587–1591. [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlomanczuk P, Margraf RR, Lynch GR. In vitro electrical activity in the suprachiasmatic nucleus following splitting and masking of wheel-running behavior. Brain Res. 1991;559:94–99. doi: 10.1016/0006-8993(91)90291-3. [DOI] [PubMed] [Google Scholar]


