Abstract
Vitamin D status differs by latitude and race, with residents of the northeastern United States and individuals with more skin pigmentation being at increased risk of deficiency. A PubMed database search yielded 63 observational studies of vitamin D status in relation to cancer risk, including 30 of colon, 13 of breast, 26 of prostate, and 7 of ovarian cancer, and several that assessed the association of vitamin D receptor genotype with cancer risk.
The majority of studies found a protective relationship between sufficient vitamin D status and lower risk of cancer. The evidence suggests that efforts to improve vitamin D status, for example by vitamin D supplementation, could reduce cancer incidence and mortality at low cost, with few or no adverse effects.
ALTHOUGH VITAMIN D deficiency is known mainly for its association with fractures and bone disease,1–7 its newly recognized association with risk of several types of cancer is receiving considerable attention.8–11 The high prevalence of vitamin D deficiency, combined with the discovery of increased risks of certain types of cancer in those who are deficient, suggest that vitamin D deficiency may account for several thousand premature deaths from colon,12 breast,13,14 ovarian,15 and prostate16 cancer annually.17 This discovery creates a new impetus for ensuring adequate vitamin D intake in order to reduce the risk of cancer.
PREVALENCE OF VITAMIN D DEFICIENCY
A low serum level of 25(OH)D, the principal form of circulating vitamin D, is the main marker of vitamin D deficiency.18–20 High prevalence of vitamin D deficiency is present in all races, even in temperate areas,19–36 and is particularly high among Black Americans.19,21–24 A recent survey found, for example, that 42% of Black women had seriously deficient 25(OH)D levels (< 15 ng/mL).19
Residents of the northern tier of the United States receive considerably less solar ultraviolet B (UVB) radiation than those in the South, owing to the longer length and severity of northern winters.37–39 UVB is needed to make vitamin D, which cannot be photosynthesized by the skin in the Northeast from November through March.40 Although some sunscreens, such as zinc or titanium oxides, may reduce risk of some skin cancers,41–43 everyday use of sunscreens that offer a high level of protection against the sun, which currently are used periodically by about half the US population,44 completely blocks photosynthesis of vitamin D45,46 and reduces circulating vitamin D metabolites.46 This results in 25(OH)D deficiency unless there is adequate oral intake.47
A clinical laboratory test is available to identify 25(OH)D deficiency; it is most useful during the fall and winter, when deficiency is prevalent29,30 owing to the 3-week half-life of 25(OH)D.18,48 With respect to osteoporosis, the range of 25(OH)D considered deficient is less than 15 to 20 ng/mL,49 whereas serum levels below 30 ng/mL are associated with increased risk of colon cancer.50–52 Levels above 150 ng/mL suggest potential toxicity.53–55
EPIDEMIOLOGICAL EVIDENCE
Most observational studies have reported that vitamin D has a beneficial effect on risk of colon, breast, prostate, and ovarian cancer. A PubMed search (in December 2004) for epidemiological studies of vitamin D, sunlight, ultraviolet radiation, and latitude in association with these cancers yielded 63 studies, including 30 of colon cancer, 13 of breast cancer, 26 of prostate cancer, and 7 of ovarian cancer (some studies included more than one site).
Of the 30 studies of colon cancer or adenomatous polyps, 20 found a statistically significant benefit of vitamin D, its serum metabolites, sunlight exposure, or another marker of vitamin D status on cancer risk or mortality12,13,50–52,56–66 and incidence of adenomatous polyps,67–70 including 1 study in which the association was limited to men65; 5 studies reported a beneficial effect (of borderline statistical significance) of vitamin D or its markers on risk of colon or rectal cancer,71–75 and 5 reported no association.76–80
Of the 13 studies of breast cancer, 9 reported a favorable association of vitamin D markers or sunlight with cancer risk,13,14,57,64,75,81–84 including 1 where the association was limited to premenopausal women84; 1 study reported a favorable trend of borderline statistical significance85 and 3 found no association.66,80,86 None reported adverse effects.
Thirteen of the 26 studies of prostate cancer found a statistically significant favorable association,16,17,64,75,87–95 1 reported a favorable trend for serum 25(OH)D of borderline significance,96 and 11 reported no statistically significant association.66,80,97–105 One reported a U-shaped association106 and 1 reported a significant inverse correlation with latitude, with a weaker correlation with UVB.94 Five of the 7 studies of ovarian cancer found higher mortality associated with lower regional sunlight15,17,64,75 or lower vitamin D intake,107 although 2 reported no association with sunlight.66,80
The consistency of the findings of dietary and serum studies with those of geographic studies allowed triangulation on vitamin D as a common factor in risk of colon cancer,12,13,17,50–52,56–59,61–64 colonic adenomas,67–70 breast cancer,14,17,57,64,75,81,82,84 prostate cancer,16,17,64,75,87–95,108,109 and ovarian cancer.15,17,64,94,107
Dietary studies56,58,60–63,71–74,76–79,84,100–102,105,107 had certain limitations that contrasted with studies of serum.50–52,59,67,68,82,86,88,90,97,98,110 Dietary studies in the United States were somewhat limited because it was difficult to fully separate associations of vitamin D from those of calcium, because both are in milk. There are many foods, however, that contain substantial amounts of vitamin D but little calcium, including fatty ocean fish.111,112 Higher intake of fatty fish was associated with lower mortality rates of colon113,114 and breast114,115 cancer in international comparisons, and of prostate cancer in cohort studies.116,117
Although serum studies have the advantage of measuring vitamin D status regardless of source, they can be confounded by associations with physical activity, particularly in studies of colon cancer. An association between greater physical activity and lower risk of colon cancer has been reported,118–120 although this was not always found.121 A common link could be that physical activity raises serum levels of 1,25(OH)2D, the most biologically active metabolite of vitamin D.122
Six of 7 prediagnostic serum studies of colon cancer or adenomas reported significantly higher risk of colon cancer50–52 and adenomas67–69 in those with low 25(OH)D levels, whereas 1 reported a trend suggestive of higher risk in those with low serum 25(OH)D.59 Both studies of the role of vitamin D in breast cancer analyzed 1,25(OH)2D, rather than 25(OH)D.82,86 One reported that the risk of breast cancer was markedly higher in women with low prediagnostic 1,25(OH)2D,82 but the other found no association.86 Lower levels of 25(OH)D90 or 1,25(OH)2D88 were associated with higher risk of prostate cancer in 2 studies, but not in others.97,98,103,110 Some of the latter may not have detected an association with 1,25(OH)2D because its serum concentration is homeostatically regulated.123,124 On the other hand, some individuals with prolonged poor vitamin D status have below-average levels of 1,25(OH)2D,125,126 possibly accounting for the studies that found that individuals with low serum 1,25(OH)2D had high risk of breast82 and prostate88 cancer.
Vitamin D synthesis127 and serum 25(OH)D levels128–130 are inversely correlated with latitude and positively correlated with sunlight, consistent with higher incidence or mortality rates for colon12,13,17,57,75 and breast cancer,13,14,17,57,75,81 especially in areas 37° or more from the equator. There are also north–south gradients for ovarian15,17,64,75 and prostate16,17,64,75,87,92,94 cancer. Some of the gradient for breast cancer may be associated with reproductive factors.131,132
UVB exposure and vitamin D intake increase serum 25(OH)D levels in a dose-dependent manner133–135 by providing a higher concentration of 25(OH)D as substrate for synthesis of 1,25(OH)2D. Normal colon,136–138 breast,139,140 and prostate141 epithelial cells have a vitamin D receptor (VDR) that is highly sensitive to 1,25(OH)2D. This could provide a mechanism of anticarcinogenic action for either circulating or locally synthesized 1,25(OH)2D.
Because synthesis of circulating 1,25(OH)2D is regulated in the kidney by parathyroid hormone,133 increased UVB exposure usually does not elevate circulating 1,25(OH)2D. 1,25(OH)2D is the most active vitamin D metabolite, although its concentration in serum is one thousandth that of 25(OH)D.142 It is synthesized from 25(OH)D by 1-α-hydroxylase enzymes in the colon,143 prostate,144 breast,145 and other tissues146 through an autonomous mechanism not homeostatically regulated by parathyroid hormone.
The fact that 1,25(OH)2D is synthesized in colon epithelium provides a possible explanation for lower incidence rates of colon cancer50–52 and adenomatous polyps67–69 in individuals with higher levels of serum 25(OH)D. It also helps explain the association of residence at sunnier latitudes with lower mortality rates from colon,12,17,56,64 breast,13,14,17,64,85 ovary,15,17,64 and prostate16,17,64,87,90,91 cancer, because sunlight increases 25(OH)D levels, thereby providing more substrate for these tissues to make 1,25(OH)2D.
RACIAL FACTORS
Blacks have levels of 25(OH)D approximately half those of Whites.19,20,23,147–150 Blacks in northern cities with large Black populations (Chicago, Minneapolis, Detroit, Buffalo, Cleveland, and Toledo) have colon cancer mortality rates substantially higher than those of Whites.151 Case-fatality rates are higher among Blacks for colon,152–154 breast,154 prostate,154 and ovarian155 cancer. Colon cancer mortality rates are 33% higher among Blacks, and incidence rates are 19% higher.156 Breast cancer mortality rates are 28% higher among Blacks, although incidence rates are slightly lower.156
There is a possibility of confounding by stage at diagnosis, since breast cancer tends to be diagnosed in more advanced stages in Blacks than in Whites.157 However, differences in stage at diagnosis persisted after adjustment for socioeconomic status.158 Blacks have substantially poorer survival rates,159 even when mammographic screening rates are similar to those of Whites.160 Prostate cancer mortality rates are more than twice as high among Blacks as among Whites, and incidence is 1.6 times higher.156,159 Ovarian cancer mortality and incidence rates are higher among Whites, although they are rising among Blacks.156
GENETIC FACTORS
There are several VDR genotypes.161 The most important of these regarding cancer is Bsm I,162,163 which has 3 variants: BB, Bb, and bb. The bb genotype occurs in 35% of the US population164 and is associated with lower circulating concentrations of 1,25(OH)2D.162 Men with the bb genotype were found to have twice the incidence of colon cancer162 as those with the BB genotype. In men below the median serum 25(OH)D level, those with the bb genotype had more than twice the incidence of prostate cancer as other men.162,165 Risk of breast cancer in women with the bb genotype was twice that of women with the BB genotype,166,167 although breast cancer findings have been mixed.168 Women with the bb genotype were 4 times more likely to develop metastases than those with the BB genotype.169 Approximately 40% of colon and prostate cancer may be related to the bb genotype, interacting adversely with low 25(OH)D.162
VDR polymorphisms also are associated with a more severe form of malignancy. Men with the VDR Taq I TT genotype, for example, were found to be 5 times more likely to develop a severe (Gleason grade≥ 5) prostate malignancy than those with other genotypes.170 This differs from previous inconclusive studies of associations of VDR genotypes with prostate cancer.171,172 Breast cancer cases with the TT genotype were twice as likely to have lymphatic metastases.173 The population prevalence of the TT genotype is 35%.174
These studies have helped define the role of vitamin D in cancer,162,163,165,167 although most were exploratory, and only a few of the known VDR genotypes have been shown to be associated with risk of cancer.
VITAMIN D AND COLON CANCER
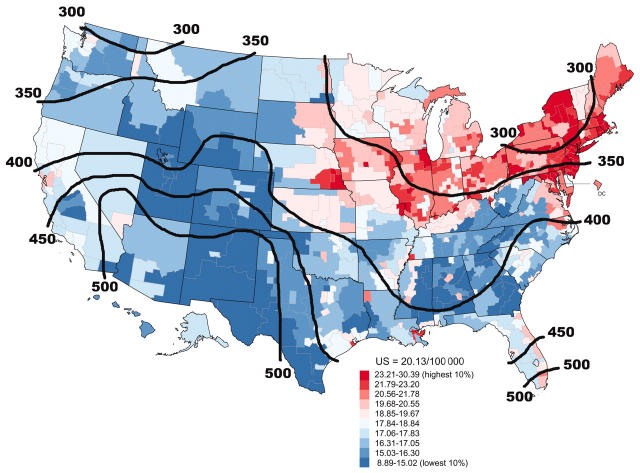
Age-adjusted death rates for colon cancer tend to be high in areas with low levels of winter sunlight and low in sunny areas (Figure 1 ▶; the contour lines show the annual mean daily solar irradiance, measured in Langleys [calories/cm2]).
FIGURE 1—
Age-adjusted colon cancer mortality rates, by county area, and contours of annual mean daily solar irradiance in Langleys (calories/cm2), United States, 1970–1994.
Source. Developed through use of National Cancer Institute and National Oceanic and Atmospheric Administration data (available at http://www3.cancer.gov/atlasplus/charts.html and http://www.noaa.gov).
Individuals with circulating 25(OH)D levels below 30 ng/mL had approximately twice the risk of colon cancer as those with higher levels in 2 studies,50,52 with doubling of incidence for those with less than 20 ng/mL in another.51 There was a consistent favorable, although non-significant, trend in a fourth.59 Individuals with 25(OH)D levels below 30 ng/mL also had higher incidence of colonic adenomas.68,69 The association of 25(OH)D with risk of colon cancer was present both early and late in follow-up,50,59 suggesting that vitamin D metabolites may have effects at all stages of carcinogenesis.175–177
Seven epidemiological studies reported higher risk of colon cancer in individuals who consumed lower amounts of vitamin D, including the Western Electric Cohort Study,56 the Nurses’ Health Study,60 the Male Health Professionals’ Follow-Up Study,62 the Iowa Women’s Health Study,71 and the American Cancer Society Cancer Prevention Study II (CPS II) Cohort Study,65 and 2 case–control studies.63,73 The association in the CPS-II Cohort was limited to men.
One study reported a trend toward higher risk of colon cancer with lower vitamin D intake,71 and another reported an inverse association of vitamin D and calcium intake with risk of rectal cancer.72 Another found that lower vitamin D intake was associated with higher risk of adenomas.70 The findings of one study of colon cancer were no longer statistically significant after multivariate analysis.71 Five studies found no association.76–79,178 Two of these were performed in sunny climates,76,178 where they could have been influenced by solar vitamin D synthesis. Although the latitude gradient helps to isolate the role of vitamin D, confounding is still possible.
VITAMIN D AND BREAST CANCER
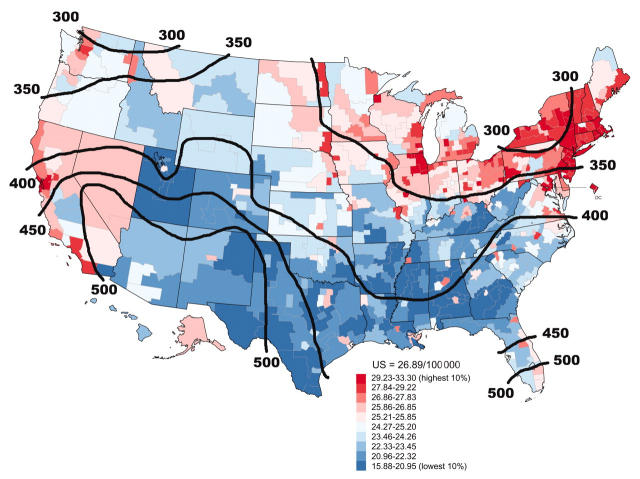
Breast cancer death rates tended to be higher in areas with low winter sunlight levels and lower in sunny areas (Figure 2 ▶).13,14 Women regularly exposed to sunlight, and consumers of above-average amounts of vitamin D, had significantly lower incidence rates of breast cancer.85 Women in the lowest quartile of serum 1,25(OH)2D had a risk of breast cancer 5 times higher than those in the highest quartile.82 Low 1,25(OH)2D levels were also associated with faster progression of metastatic breast cancer.179 Mortality rates of perimenopausal ovarian cancer also were lower in sunny regions,15,17,64,75 although one study found no geographic association within a single country.80 High intake of vitamin D and calcium markedly reduced incidence of mammary cancer in mice and rats consuming high-fat diets.9,180 Incidence of mammary cancer was only one quarter as high in rats that received high levels of vitamin D and calcium.181
FIGURE 2—
Age-adjusted breast cancer mortality rates, by county area, and contours of annual mean daily solar irradiance in Langleys (calories/cm2), United States, 1970–1994.
Source. Developed through use of National Cancer Institute and National Oceanic and Atmospheric Administration data (available at http://www3.cancer.gov/atlasplus/charts.html and http://www.noaa.gov).
VITAMIN D AND PROSTATE CANCER
Residents of sunny areas,16,87 and those with a history of exposure to high levels of sunlight,92,95,108 had lower risk of prostate cancer. In a study of 19000 men, those with 25(OH)D levels below 16 ng/mL had a 70% higher incidence rate of prostate cancer than those with levels above 16 ng/mL.90 For younger men with 25(OH)D levels below 16 ng/mL, incidence of prostate cancer was 3.5 times higher than for those with levels of 16 ng/mL or above and incidence of invasive cancer was 6.3 times higher.90 However, other studies have not found associations.80,97–102,104–106
MECHANISM OF VITAMIN D EFFECTS
Vitamin D and its metabolites reduce the incidence of many types of cancer by inhibiting tumor angiogenesis,182–185 stimulating mutual adherence of cells,186 and enhancing intercellular communication through gap junctions,187 thereby strengthening the inhibition of proliferation that results from tight physical contact with adjacent cells within a tissue (contact inhibition). Vitamin D metabolites help maintain a normal calcium gradient in the colon epithelial crypts,188 and high serum levels of 25(OH)D are associated with markedly decreased proliferation of noncancerous but high-risk epithelial calls in the colon.189 1,25(OH)2D inhibits mitosis of breast epithelial cells.190 Pulsatile release of ion-ized calcium from intracellular stores, including the endoplasmic reticulum, induces terminal differentiation and apoptosis,176 and 1,25(OH)2D enhances this release.191
RECOMMENDATIONS FOR VITAMIN D INTAKE
The National Academy of Sciences recommends the following daily intakes of vitamin D: 1 to 50 years of age, 200 international units (IU); 51 to 70 years, 400 IU; older than 71 years, 600 IU.192 In one study, 500 IU per day was associated with a 25(OH)D level of 30 ng/mL, although this included photosynthesized vitamin D.193 Sufficient vitamin D intake to achieve 30 to 35 ng/mL of 25(OH)D in serum was associated with reduced incidence of colonic adenomas,67,69 the latter in combination with adequate calcium intake. On the basis of the studies of serum 25(OH)D and risk of colorectal cancer cited in this article, the target range for serum 25(OH)D should be at least 30 ng/mL, but no more than 150 ng/mL.149,194 The National Academy of Sciences does not recommend a different intake of vitamin D by Blacks, although it suggests a need for further research on racial differences.192 On the basis of the markedly higher prevalence of 25(OH)D deficiency in Blacks,19,147 a higher level of supplementation is probably needed. Althought adverse VDR genotypes162,165–167,169 are present in a large proportion of the population,164,174 different intakes according to genotype would not be practical.
Older adults need higher amounts of vitamin D owing to decreased absorption,195 and at any age, serum 25(OH)D rises as an inverse power function of vitamin D intake.196 Intake of 800 (IU) of vitamin D3 per day, for example, would increase serum 25(OH)D by only 6 ng/mL,193 so there is no reasonable concern about inducing toxicity with daily intake of 800 to 1000 IU per day.197 The latter intake would be consistent with maintaining the serum 25(OH)D level at or above 30 ng/mL in most individuals.69,198 New vitamin D analogs have promising cellular effects, but are not currently used for prevention.199
Throughout the United States, the estimated daily solar exposure to maintain a serum 25(OH)D level of 30 ng/mL is 15 minutes in summer and 20 minutes in early fall or late spring, from 11:00 am to 2:00 pm under clear skies,18,40,200 assuming exposure of arms, shoulders, and back. Blacks require twice as long.147 During November to March, north of 37° latitude in the Northeastern and mid-Atlantic regions, no amount of solar exposure is sufficient,40 partly owing to a slightly thicker regional stratospheric ozone layer201 and denser tropospheric sulfate aerosol.202,203 In the Northwest and most other regions, some UVB is available during winter, although low ambient temperatures limit duration and area of exposure.37,38,40,127,147,200
Moderation is needed concerning sunlight exposure. Actinic changes are associated with exposure to ultraviolet radiation, and there is considerable evidence for its role in skin cancer.42,43 If sunlight is used as a source of vitamin D, exposure should be scrupulously monitored so that no reddening of the skin occurs,200,204 and intentional exposure of the face should be minimized. Individuals with skin type I or II, who tend to burn easily and tan poorly,205 should not exceed 20 minutes per day in the sun. Exposure times much longer than 20 minutes would not appreciably increase vitamin D synthesis and could increase risk of skin cancer.206 Oral vitamin D3 supplementation, rather than solar exposure, should be used by fair-skinned or sun-sensitive persons, or by individuals taking medicines causing photosensitivity.
POTENTIAL TOXICITY
Vitamin D dosages of up to 1000 IU per day have no reasonable likelihood of producing toxicity. Serum 25(OH)D levels of at least 30 ng/mL207 to 45 ng/mL143,208 are the minimum necessary to maintain normal parathyroid hormone levels, and at least 400 IU of supplemental vitamin D3 per day is needed to maintain serum 25(OH)D at a range consistent with normal parathyroid hormone levels in young and middle-aged adults; intake of at least 600 IU per day is required to maintain normal levels in adults aged older than 70 years.192 The National Academy of Sciences–Institute of Medicine has indicated that 2000 IU per day is the safe upper limit of vitamin D intake.192 Typical recommended intakes are far below this.192,209
Potential toxic effects of vitamin D overdosage, such as bone demineralization, hypercalcemia, hypercalciuria, or nephrocalcinosis with renal failure, are encountered rarely, generally only when the daily dose exceeds 10 000 IU of vitamin D on a chronic basis.55 Concerns about vitamin D toxicity in the past have been because of massive overdoses in the range of 50 000 to 150 000 IU per day on a long-term basis.54,133 According to the National Academy of Sciences, no known health risks are associated with dosages of vitamin D in the normally encountered range of intake (up to 2000 IU/day).55,192,197,198,210,211
Relatively high oral intakes of vitamin D or serum levels of 25(OH)D are not a concern from a cardiovascular viewpoint, because most studies suggest that higher levels of 25(OH)D are associated with reduced cardiovascular risk. For example, higher serum 25(OH)D,212 1,25(OH)2D,213,214 and oral vitamin D215 were associated with moderately but significantly lower blood pressure.
There also was a beneficial association between serum 25(OH)D and risk of myocardial infarction,216 ischemic heart disease mortality,217 and congestive heart failure,218 although other cardiovascular results have been mixed.219,220
Vitamin D supplementation was also associated with reduced incidence of type I diabetes221,222 and with improvement in type II diabetes.223,224 In Finland, vitamin D supplementation of infants was associated with reduction by four fifths in incidence of type I diabetes.221 Higher regional UVB levels have also been linked with lower age-adjusted death rates from endometrial and kidney cancers, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, multiple myeloma, and other malignancies.75
ADOPTION OF VITAMIN D FOR CANCER PREVENTION
Supplemental vitamin D intake could address the high prevalence of vitamin D deficiency in the United States.1,55,198,225 Strong evidence indicates that intake or synthesis of vitamin D is associated with reduced incidence and death rates of colon, breast, prostate, and ovarian cancers. More than 1000 laboratory and epidemiological studies have been published concerning the association between vitamin D and its metabolites and cancer. Long-term studies have demonstrated the efficacy of moderate intake of vitamin D in reducing cancer risk and, when administered with calcium, in reducing the incidence of fractures.226 Despite these reassuring studies, the public health and medical communities have not adopted use of vitamin D for cancer prevention.
The cost of a daily dose of vitamin D3 (1000 IU) is less than 5 cents, which could be balanced against the high human and economic costs of treating cancer attributable to insufficiency of vitamin D. Leadership from the public health community will provide the best hope for action.
Acknowledgments
This research was supported by a congressional allocation to the Hollings Cancer Center of the Medical University of South Carolina, Charleston, through the Department of the Navy, Bureau of Medicine and Surgery (Work Unit No. 60126 TR 03–1)7.
The authors thank William B. Grant of SUNARC, San Francisco, Calif, for reviewing the article and providing comments.
Note. The views expressed in this report are those of the authors and do not represent an official position of the Department of the Navy, Department of Defense, or the US Government.
Peer Reviewed
Contributors C. F. Garland, F. C. Garland, and E. D. Gorham jointly developed the plan and outline of the article, prepared the first draft, and reviewed and edited subsequent drafts. S.B. Mohr and C.F. Garland jointly performed the literature review, and S. B. Mohr edited drafts of the article. M. Lipkin, H. Newmark, and M. F. Holick reviewed and edited drafts.
References
- 1.Utiger R. The need for more vitamin D. N Engl J Med. 1998;338(12): 828–829. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Too little vitamin D in premenopausal women: why should we care? Am J Clin Nutr. 2002;76(1):3–4. [DOI] [PubMed] [Google Scholar]
- 3.Compston J. Vitamin D deficiency: time for action. Evidence supports routine supplementation for elderly people and others at risk. BMJ. 1998;317(7171): 1466–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wharton B. Low plasma vitamin D in Asian toddlers in Britain. BMJ. 1999; 318(7175):2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garabedian M, Ben-Mehkbi H. Rickets and vitamin D deficiency. In: Holick M, ed. Vitamin D: Molecular Biology, Physiology, and Clinical Applications. Totowa, NJ: Humana; 1999: 273–286.
- 6.Holick M. Vitamin D and bone health. J Nutr. 1996;126(4 suppl): 1159S–1164S. [DOI] [PubMed] [Google Scholar]
- 7.McCollum E, Simmonds N, Becker J, Shipley P. Studies on experimental rickets, XXI: an experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem. 1922;53:293–312. [PubMed] [Google Scholar]
- 8.Schwartz GG, Wang MH, Zang M, Singh RK, Siegal GP. 1 alpha,25-Dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 1997;6(9):727–732. [PubMed] [Google Scholar]
- 9.Lipkin M, Newmark HL. Vitamin D, calcium and prevention of breast cancer: a review. J Am Coll Nutr. 1999;18 (5 suppl):392S–397S. [DOI] [PubMed] [Google Scholar]
- 10.Guyton KZ, Kensler TW, Posner GH. Cancer chemoprevention using natural vitamin D and synthetic analogs. Annu Rev Pharmacol Toxicol. 2001;41: 421–442. [DOI] [PubMed] [Google Scholar]
- 11.Hansen CM, Binderup L, Hamberg KJ, Carlberg C. Vitamin D and cancer: effects of 1,25(OH)2D3 and its analogs on growth control and tumorigenesis. Front Biosci. 2001;6:D820–D848. [DOI] [PubMed] [Google Scholar]
- 12.Garland C, Garland F. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9: 227–231. [DOI] [PubMed] [Google Scholar]
- 13.Gorham E, Garland C, Garland F. Acid haze air pollution and breast and colon cancer in 20 Canadian cities. Can J Public Health. 1989;80:96–100. [PubMed] [Google Scholar]
- 14.Garland F, Garland C, Gorham E, Young J Jr. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19: 614–622. [DOI] [PubMed] [Google Scholar]
- 15.Lefkowitz ES, Garland CF. Sunlight, vitamin D, and ovarian cancer mortality rates in US women. Int J Epidemiol. 1994;23(6):1133–1136. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis). Anticancer Res. 1990;10(5A):1307–1311. [PubMed] [Google Scholar]
- 17.Grant WB. An estimate of premature cancer mortality in the US because of inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94(6): 1867–1875. [DOI] [PubMed] [Google Scholar]
- 18.Holick M. The use and interpretation of assays for vitamin D and its metabolites. J Nutr. 1990;120: 1464–1469. [DOI] [PubMed] [Google Scholar]
- 19.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002; 76(1):187–192. [DOI] [PubMed] [Google Scholar]
- 20.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. [DOI] [PubMed] [Google Scholar]
- 21.Awamey E, Hollis B, Bell N. Low serum 25-hydroxyvitamin D in blacks results from decreased production rate and not increased metabolic clearance rate [abstract]. J Bone Miner Res. 1996; 11:S165. [Google Scholar]
- 22.Mitra D, Bell N. Racial, geographic, genetic and body habitus effects on vitamin D metabolism. In: Feldman D, Glorieux FH, Pike JW, eds. Vitamin D. San Diego, Calif: Academic Press; 1997: 521–532.
- 23.Aloia JF, Mikhail M, Pagan CD, Arunachalam A, Yeh JK, Flaster E. Biochemical and hormonal variables in black and white women matched for age and weight. J Lab Clin Med. 1998; 132(5):383–389. [DOI] [PubMed] [Google Scholar]
- 24.Kyriakidou-Himonas M, Aloia JF, Yeh JK. Vitamin D supplementation in postmenopausal black women. J Clin Endocrinol Metab. 1999;84(11): 3988–3990. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child. 2002; 87(2):111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillemant J, Le HT, Maria A, Allemandou A, Peres G, Guillemant S. Wintertime vitamin D deficiency in male adolescents: effect on parathyroid function and response to vitamin D3 supplements. Osteoporos Int. 2001; 12(10):875–879. [DOI] [PubMed] [Google Scholar]
- 27.Juttmann J, Visser T, Buurman C. Seasonal fluctuations in serum concentrations of vitamin D metabolites in normal subjects. Br Med J. 1981;282: 1349–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K, Nashimoto M, Matsuyama S, Yamamoto M. Low serum concentrations of 25-hydroxyvitamin D in young adult Japanese women: a cross sectional study. Nutrition. 2001; 17(11–12):921–925. [DOI] [PubMed] [Google Scholar]
- 29.Carnevale V, Modoni S, Pileri M, et al. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: seasonal and gender differences. Osteoporos Int. 2001;12(12): 1026–1030. [DOI] [PubMed] [Google Scholar]
- 30.Vieth R, Cole DE, Hawker GA, Trang HM, Rubin LA. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr. 2001;55(12):1091–1097. [DOI] [PubMed] [Google Scholar]
- 31.Rucker D, Allan JA, Fick GH, Hanley DA. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ. 2002;166(12):1517–1524. [PMC free article] [PubMed] [Google Scholar]
- 32.Kudlacek S, Schneider B, Peterlik M, et al. Assessment of vitamin D and calcium status in healthy adult Austrians. Eur J Clin Invest. 2003;33(4):323–331. [DOI] [PubMed] [Google Scholar]
- 33.Rosen CJ, Morrison A, Zhou H, et al. Elderly women in northern New England exhibit seasonal changes in bone mineral density and calciotropic hormones. Bone Miner. 1994;25(2): 83–92. [DOI] [PubMed] [Google Scholar]
- 34.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. [DOI] [PubMed] [Google Scholar]
- 35.Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr. 2003;142(2):169–173. [DOI] [PubMed] [Google Scholar]
- 36.Arya V, Bhambri R, Godbole MM, Mithal A. Vitamin D status and its relationship with bone mineral density in healthy Asian Indians. Osteoporos Int. 2004;15(1):56–61. [DOI] [PubMed] [Google Scholar]
- 37.Frederick J, Lubin D. The budget of biologically active ultraviolet radiation in the earth-atmosphere system. J Geophys Res. 1988;93:3825–3832. [Google Scholar]
- 38.Lubin D, Jensen E, Gies P. Global surface ultraviolet radiation climatology from TOMS and ERBE data. J Geophys Res. 1998;103(D20):26061–26091. [Google Scholar]
- 39.Ainsleigh HG. Beneficial effects of sun exposure on cancer mortality. Prev Med. 1993;22(1):132–140. [DOI] [PubMed] [Google Scholar]
- 40.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–378. [DOI] [PubMed] [Google Scholar]
- 41.Garland C, Garland F, Gorham E. Could sunscreens increase melanoma risk? Am J Public Health. 1992;82: 614–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garland C, Garland F, Gorham E. Lack of efficacy of common sunscreens in melanoma prevention. In: Grob J, Stern R, MacKie R, Weinstock M, eds. Epidemiology, Causes and Prevention of Skin Disease. Oxford, England: Black-well Science; 1997:151–159.
- 43.Manson J, Rexrode K, Garland F, Garland C, Weinstock M. The case of a comprehensive national campaign to prevent melanoma and associated mortality. Epidemiology. 2000;11: 728–734. [DOI] [PubMed] [Google Scholar]
- 44.Johnson EY, Lookingbill DP. Sunscreen use and sun exposure. Trends in a white population. Arch Dermatol. 1984;120(6):727–731. [PubMed] [Google Scholar]
- 45.Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6): 1165–1168. [DOI] [PubMed] [Google Scholar]
- 46.Matsuoka LY, Wortsman J, Hollis BW. Use of topical sunscreen for the evaluation of regional synthesis of vitamin D3. J Am Acad Dermatol. 1990; 22(5 Pt 1):772–775. [DOI] [PubMed] [Google Scholar]
- 47.Matsuoka L, Wortsman J, Holick M. Chronic sunscreen use decreases the concentration of 25-hydroxyvitamin D: a preliminary study. Arch Dermatol. 1988;124:1802–1804. [PubMed] [Google Scholar]
- 48.Haddad JG Jr, Rojanasathit S. Acute administration of 25-hydroxycholecalciferol in man. J Clin Endocrinol Metab. 1976;42(2):284–290. [DOI] [PubMed] [Google Scholar]
- 49.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly. Endocrinol Rev Monogr. 2000; 22:477–501. [DOI] [PubMed] [Google Scholar]
- 50.Garland C, Comstock G, Garland F, Helsing K, Shaw E, Gorham E. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176–1178. [DOI] [PubMed] [Google Scholar]
- 51.Tangrea J, Helzlsouer K, Pietinen P, et al. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control. 1997;8(4): 615–625. [DOI] [PubMed] [Google Scholar]
- 52.Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13(9): 1502–1508. [PubMed] [Google Scholar]
- 53.Holick MF, Shao Q, Liu WW, Chen TC. The vitamin D content of fortified milk and infant formula. N Engl J Med. 1992;326(18):1178–1181. [DOI] [PubMed] [Google Scholar]
- 54.Jacobus CH, Holick MF, Shao Q, et al. Hypervitaminosis D associated with drinking milk. N Engl J Med. 1992; 326(18):1173–1177. [DOI] [PubMed] [Google Scholar]
- 55.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999; 69:842–856. [DOI] [PubMed] [Google Scholar]
- 56.Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985; 1(8424):307–309. [DOI] [PubMed] [Google Scholar]
- 57.Garland C, Garland F, Gorham E. Sunlight, sulfur dioxide and breast and colon cancer in Italy. Abstract presented at: Annual Meeting of the American Association for the Advancement of Science; February 15–20, 1990; New Orleans, La.
- 58.Ferraroni M, La Vecchia C, D’Avanzo B, Negri E, Franceschi S, Decarli A. Selected micronutrient intake and the risk of colorectal cancer. Br J Cancer. 1994; 70(6):1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. Colon cancer and serum vitamin D metabolite levels 10–17 years prior to diagnosis. Am J Epidemiol. 1995;142(6):608–611. [DOI] [PubMed] [Google Scholar]
- 60.Martinez ME, Giovannucci EL, Colditz GA, et al. Calcium, vitamin D, and the occurrence of colorectal cancer among women. J Natl Cancer Inst. 1996; 88(19):1375–1382. [DOI] [PubMed] [Google Scholar]
- 61.Pritchard RS, Baron JA, Gerhardsson de Verdier M. Dietary calcium, vitamin D, and the risk of colorectal cancer in Stockholm, Sweden. Cancer Epidemiol Biomarkers Prev. 1996;5(11): 897–900. [PubMed] [Google Scholar]
- 62.Kearney J, Giovannucci E, Rimm EB, et al. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. Am J Epidemiol. 1996; 143(9):907–917. [DOI] [PubMed] [Google Scholar]
- 63.La Vecchia C, Braga C, Negri E, et al. Intake of selected micronutrients and risk of colorectal cancer. Int J Cancer. 1997;73:525–530. [DOI] [PubMed] [Google Scholar]
- 64.Freedman D, Dosemeci M, McGlynn K. Sunlight and mortality from breast, ovarian, colon, prostate, and nonmelanoma skin cancer: a composite death certificate based case-control study. Occup Environ Med. 2002;59: 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCullough ML, Robertson AS, Rodriguez C, et al. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States). Cancer Causes Control. 2003;14(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 66.Mizoue T. Ecological studies of solar radiation and cancer mortality in Japan. Health Phys. 2004;87(5): 532–538. [DOI] [PubMed] [Google Scholar]
- 67.Platz EA, Hankinson SE, Hollis BW, et al. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and adenomatous polyps of the distal colorectum. Cancer Epidemiol Biomarkers Prev. 2000;9(10): 1059–1065. [PubMed] [Google Scholar]
- 68.Peters U, McGlynn KA, Chatterjee N, et al. Vitamin D, calcium, and vitamin D receptor polymorphism in colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2001;10(12):1267–1274. [PubMed] [Google Scholar]
- 69.Grau MV, Baron JA, Sandler RS, et al. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95(23):1765–1771. [DOI] [PubMed] [Google Scholar]
- 70.Lieberman D, Prindiville S, Weiss D, Willett W. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959–2967. [DOI] [PubMed] [Google Scholar]
- 71.Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am J Epidemiol. 1993;137(12):1302–1317. [DOI] [PubMed] [Google Scholar]
- 72.Zheng W, Anderson KE, Kushi LH, et al. A prospective cohort study of intake of calcium, vitamin D, and other micronutrients in relation to incidence of rectal cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7(3):221–225. [PubMed] [Google Scholar]
- 73.Marcus PM, Newcomb PA. The association of calcium and vitamin D, and colon and rectal cancer in Wisconsin women. Int J Epidemiol. 1998;27(5): 788–793. [DOI] [PubMed] [Google Scholar]
- 74.Pritchard RS, Baron JA, Gerhardsson de Verdier M. Dietary calcium, vitamin D and the risk of colorectal cancer. Int J Cancer. 1997;73:525–530. [PubMed] [Google Scholar]
- 75.Grant WB. Ecologic studies of solar UV-B radiation and cancer mortality rates. Recent Results Cancer Res. 2003;164:371–377. [DOI] [PubMed] [Google Scholar]
- 76.Peters RK, Pike MC, Garabrant D, Mack TM. Diet and colon cancer in Los Angeles County, California. Cancer Causes Control. 1992;3(5):457–473. [DOI] [PubMed] [Google Scholar]
- 77.Kampman E, Giovannucci E, van ‘t Veer P, et al. Calcium, vitamin D, dairy foods, and the occurrence of colorectal adenomas among men and women in two prospective studies. Am J Epidemiol. 1994;139(1):16–29. [DOI] [PubMed] [Google Scholar]
- 78.Jarvinen R, Knekt P, Hakulinen T, Aromaa A. Prospective study on milk products, calcium and cancers of the colon and rectum. Eur J Clin Nutr. 2001;55:1000–1007. [DOI] [PubMed] [Google Scholar]
- 79.Terry P, Baron JA, Bergkvist L, Holmberg L, Wolk A. Dietary calcium and vitamin D intake and risk of colorectal cancer: a prospective cohort study in women. Nutr Cancer. 2002;43(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 80.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon-and prostate cancer (Norway). Cancer Causes Control. 2004;15(2):149–158. [DOI] [PubMed] [Google Scholar]
- 81.Gorham ED, Garland FC, Garland CF. Sunlight and breast cancer incidence in the USSR. Int J Epidemiol. 1990;19(4):820–824. [DOI] [PubMed] [Google Scholar]
- 82.Janowsky EC, Lester GE, Weinberg CR, et al. Association between low levels of 1,25-dihydroxyvitamin D and breast cancer risk. Public Health Nutr. 1999;2(3):283–291. [DOI] [PubMed] [Google Scholar]
- 83.Grant WB. An ecologic study of dietary and solar ultraviolet-B links to breast carcinoma mortality rates. Cancer. 2002;94(1):272–281. [DOI] [PubMed] [Google Scholar]
- 84.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94(17):1301–1311. [DOI] [PubMed] [Google Scholar]
- 85.John E, Schwartz G, Dreon D, Koo J. Vitamin D and breast cancer risk: the NHANES I epidemiologic follow-up study, 1971–1975 to 1992. Cancer Epidemiol Biomarkers Prev. 1999;8: 399–406. [PubMed] [Google Scholar]
- 86.Hiatt R, Krieger N, Lobaugh B, Drezner M, Vogelman J, Orentreich N. Prediagnostic serum vitamin D and breast cancer. J Natl Cancer Inst. 1998; 90(6):461–463. [DOI] [PubMed] [Google Scholar]
- 87.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992; 70(12):2861–2869. [DOI] [PubMed] [Google Scholar]
- 88.Corder EH, Guess HA, Hulka BS, et al. Vitamin D and prostate cancer: a prediagnostic study with stored sera. Cancer Epidemiol Biomarkers Prev. 1993;2(5):467–472. [PubMed] [Google Scholar]
- 89.Schwartz GG. Geographic trends in prostate cancer mortality: an application of spatial smoothers and the need for adjustment. Ann Epidemiol. 1997; 7(6):430. [DOI] [PubMed] [Google Scholar]
- 90.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. 2000;11(9): 847–852. [DOI] [PubMed] [Google Scholar]
- 91.Tuohimaa P, Lyakhovich A, Aksenov N, et al. Vitamin D and prostate cancer. J Steroid Biochem Mol Biol. 2001;76(1–5):125–134. [DOI] [PubMed] [Google Scholar]
- 92.Luscombe CJ, Fryer AA, French ME, et al. Exposure to ultraviolet radiation: association with susceptibility and age at presentation with prostate cancer. Lancet. 2001;358(9282):641–642. [DOI] [PubMed] [Google Scholar]
- 93.Grant WB. A multicountry ecologic study of risk and risk reduction factors for prostate cancer mortality. Eur Urol. 2004;45(3):271–279. [DOI] [PubMed] [Google Scholar]
- 94.Grant WB. Geographic variation of prostate cancer mortality rates in the United States: implications for prostate cancer risk related to vitamin D. Int J Cancer. 2004;111(3):470–471. [DOI] [PubMed] [Google Scholar]
- 95.John EM, Dreon DM, Koo J, Schwartz GG. Residential sunlight exposure is associated with a decreased risk of prostate cancer. J Steroid Biochem Mol Biol. 2004;89–90(1–5):549–552. [DOI] [PubMed] [Google Scholar]
- 96.Nomura AM, Stemmermann GN, Lee J, et al. Serum vitamin D metabolite levels and the subsequent development of prostate cancer (Hawaii, United States). Cancer Causes Control. 1998;9: 425–432. [DOI] [PubMed] [Google Scholar]
- 97.Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. Prostate cancer and prediagnostic levels of serum vitamin D metabolites (Maryland, United States). Cancer Causes Control. 1995;6(3): 235–239. [DOI] [PubMed] [Google Scholar]
- 98.Gann P, Ma J, Hennekens C, et al. Circulating vitamin D metabolites in relation to subsequent development of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1996;5(2):121–126. [PubMed] [Google Scholar]
- 99.Andersson SO, Wolk A, Bergstrom R, et al. Energy, nutrient intake and prostate cancer risk: a population-based case-control study in Sweden. Int J Cancer. 1996;68(6):716–722. [DOI] [PubMed] [Google Scholar]
- 100.Giovannucci E, Rimm EB, Wolk A, et al. Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res. 1998;58(3):442–447. [PubMed] [Google Scholar]
- 101.Chan JM, Giovannucci E, Andersson SO, Yuen J, Adami HO, Wolk A. Dairy products, calcium, phosphorous, vitamin D, and risk of prostate cancer (Sweden). Cancer Causes Control. 1998;9(6): 559–566. [DOI] [PubMed] [Google Scholar]
- 102.Chan JM, Pietinen P, Virtanen M, et al. Diet and prostate cancer risk in a cohort of smokers, with a specific focus on calcium and phosphorus (Finland). Cancer Causes Control. 2000;11(9): 859–867. [DOI] [PubMed] [Google Scholar]
- 103.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15(3): 255–265. [DOI] [PubMed] [Google Scholar]
- 104.Kristal AR, Cohen JH, Qu P, Stanford JL. Associations of energy, fat, calcium, and vitamin D with prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11(8):719–725. [PubMed] [Google Scholar]
- 105.Rodriguez C, McCullough ML, Mondul AM, et al. Calcium, dairy products, and risk of prostate cancer in a prospective cohort of United States men. Cancer Epidemiol Biomarkers Prev. 2003;12(7):597–603. [PubMed] [Google Scholar]
- 106.Tuohimaa P, Tenkanen L, Ahonen M, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004; 108(1):104–108. [DOI] [PubMed] [Google Scholar]
- 107.Salazar-Martinez E, Lazcano-Ponce EC, Gonzalez Lira-Lira G, Escudero-De los Rios P, Hernandez-Avila M. Nutritional determinants of epithelial ovarian cancer risk: a case-control study in Mexico. Oncology. 2002;63(2):151–157. [DOI] [PubMed] [Google Scholar]
- 108.Luscombe CJ, French ME, Liu S, et al. Prostate cancer risk: associations with ultraviolet radiation, tyrosinase and melanocortin-1 receptor genotypes. Br J Cancer. 2001;85(10):1504–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luscombe CJ, French ME, Liu S, et al. Outcome in prostate cancer associations with skin type and polymorphism in pigmentation-related genes. Carcinogenesis. 2001;22(9):1343–1347. [DOI] [PubMed] [Google Scholar]
- 110.Nomura A, Stemmermann G, Lee J, et al. Serum vitamin D metabolite levels and the subsequent development of prostate cancer. Cancer Causes Control. 1998;9:425–432. [DOI] [PubMed] [Google Scholar]
- 111.Egaas E, Lambertsen G. Naturally occurring vitamin D3 in fish products analysed by HPLC, using vitamin D2 as an international standard. Int J Vitam Nutr Res. 1979;49(1):35–42. [PubMed] [Google Scholar]
- 112.US Dept of Agriculture. USDA National Nutrient Database for Standard Reference, Release 17. Available at: http://www.nal.usda.gov/fnic/foodcomp/Data/SR17/wtrank/wt_rank.html. Accessed July 10, 2005.
- 113.Caygill CP, Hill MJ. Fish, n-3 fatty acids and human colorectal and breast cancer mortality. Eur J Cancer Prev. 1995;4(4):329–332. [DOI] [PubMed] [Google Scholar]
- 114.Caygill CP, Charlett A, Hill MJ. Fat, fish, fish oil and cancer. Br J Cancer. 1996;74(1):159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaizer L, Boyd NF, Kriukov V, Tritchler D. Fish consumption and breast cancer risk: an ecological study. Nutr Cancer. 1989;12(1):61–68. [DOI] [PubMed] [Google Scholar]
- 116.Augustsson K, Michaud DS, Rimm EB, et al. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(1):64–67. [PubMed] [Google Scholar]
- 117.Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357(9270):1764–1766. [DOI] [PubMed] [Google Scholar]
- 118.Slattery ML, Schumacher MC, Smith KR, West DW, Abd-Elghany N. Physical activity, diet, and risk of colon cancer in Utah. Am J Epidemiol. 1988; 128(5):989–999. [DOI] [PubMed] [Google Scholar]
- 119.Colbert LH, Hartman TJ, Malila N, et al. Physical activity in relation to cancer of the colon and rectum in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev. 2001;10(3):265–268. [PubMed] [Google Scholar]
- 120.Fredriksson M, Bengtsson NO, Hardell L, Axelson O. Colon cancer, physical activity, and occupational exposures. A case–control study. Cancer. 1989;63(9):1838–1842. [DOI] [PubMed] [Google Scholar]
- 121.Gorham E, Garland C, Garland F. Physical activity and colon cancer risk. Int J Epidemiol. 1989;18:728–729. [DOI] [PubMed] [Google Scholar]
- 122.Yeh JK, Aloia JF. Effect of physical activity on calciotropic hormones and calcium balance in rats. Am J Physiol. 1990;258(2 Pt 1):E263–E268. [DOI] [PubMed] [Google Scholar]
- 123.Chesney RW, Rosen JF, Hamstra AJ, Smith C, Mahaffey K, DeLuca HF. Absence of seasonal variation in serum concentrations of 1,25-dihydroxyvitamin D despite a rise in 25-hydroxyvitamin D in summer. J Clin Endocrinol Metab. 1981;53(1):139–142. [DOI] [PubMed] [Google Scholar]
- 124.Overgaard K, Nilas L, Johansen JS, Christiansen C. Lack of seasonal variation in bone mass and biochemical estimates of bone turnover. Bone. 1988; 9(5):285–288. [DOI] [PubMed] [Google Scholar]
- 125.Bouillon RA, Auwerx JH, Lissens WD, Pelemans WK. Vitamin D status in the elderly: seasonal substrate deficiency causes 1,25-dihydroxycholecalciferol deficiency. Am J Clin Nutr. 1987; 45(4):755–763. [DOI] [PubMed] [Google Scholar]
- 126.Kristal-Boneh E, Froom P, Harari G, Ribak J. Seasonal changes in calcitropic hormones in Israeli men. Eur J Epidemiol. 1999;15(3):237–244. [DOI] [PubMed] [Google Scholar]
- 127.Lu Z, Chen T, Kline L, et al. Photosynthesis of previtamin D3 in cities around the world. In: Holick M, Kligman A, eds. Biologic Effects of Light. New York, NY: Walter de Gruyter; 1992:48–52.
- 128.Punnonen R, Gillespy M, Hahl M, et al. Serum 25-OHD, vitamin A and vitamin E concentrations in healthy Finnish and Floridian women. Int J Vitam Nutr Res. 1988;58:37–39. [PubMed] [Google Scholar]
- 129.Oliveri MB, Ladizesky M, Somoza J, Martinez L, Mautalen C. Winter serum levels of 25-hydroxyvitamin D in Ushuaia and Buenos Aires. Medicina (B Aires). 1990;50(4):310–314. [PubMed] [Google Scholar]
- 130.McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992;93(1):69–77. [DOI] [PubMed] [Google Scholar]
- 131.Sturgeon SR, Schairer C, Gail M, McAdams M, Brinton LA, Hoover RN. Geographic variation in mortality from breast cancer among white women in the United States. J Natl Cancer Inst. 1995;87(24):1846–1853. [DOI] [PubMed] [Google Scholar]
- 132.Prehn AW, West DW. Evaluating local differences in breast cancer incidence rates: a census-based methodology (United States). Cancer Causes Control. 1998;9(5):511–517. [DOI] [PubMed] [Google Scholar]
- 133.Adams J, Clemens T, Parrish J, Holick M. Vitamin D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med. 1982;306:722–725. [DOI] [PubMed] [Google Scholar]
- 134.Holick M. Photosynthesis of vitamin D in the skin: effect of environment and life-style variables. Fed Proc. 1987; 46:1876–1882. [PubMed] [Google Scholar]
- 135.Webb A, Pilbeam C, Hanofin N, Holick M. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr. 1990;51: 1075–1081. [DOI] [PubMed] [Google Scholar]
- 136.Wecksler WR, Mason RS, Norman AW. Specific cytosol receptors for 1,25-dihydroxyvitamin D3 in human intestine. J Clin Endocrinol Metab. 1979; 48(4):715–717. [DOI] [PubMed] [Google Scholar]
- 137.Delvin EE, Lopez V, Levy E, Menard D. Developmental expression of calcitriol receptors, 9-kilodalton calcium-binding protein, and calcidiol 24-hydroxylase in human intestine. Pediatr Res. 1996;40(5):664–670. [DOI] [PubMed] [Google Scholar]
- 138.Huerta S, Irwin RW, Heber D, et al. 1alpha,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) mouse. Cancer Res. 2002; 62(3):741–746. [PubMed] [Google Scholar]
- 139.Eisman JA, Martin TJ, MacIntyre I, Moseley JM. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979;2(8156–8157):1335–1336. [DOI] [PubMed] [Google Scholar]
- 140.Colston K, Berger U, Wilson P, et al. Mammary gland 1,25-dihydroxyvita-min D3 receptor content during pregnancy and lactation. Mol Cell Endocrinol. 1988;60:15–22. [DOI] [PubMed] [Google Scholar]
- 141.Miller GJ, Stapleton GE, Hedlund TE, Moffat KA. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1alpha,25-dihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res. 1995;1(9):997–1003. [PubMed] [Google Scholar]
- 142.Holick M. Noncalcemic actions of 1,25-dihydroxyvitamin D3 and clinical applications. Bone. 1995;17(2 suppl): 107S–111S. [DOI] [PubMed] [Google Scholar]
- 143.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev. 1998;7(5):391–395. [PubMed] [Google Scholar]
- 145.Welsh J. Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr. 2004;80(6 suppl): 1721S–1724S. [DOI] [PubMed] [Google Scholar]
- 146.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86(2): 888–894. [DOI] [PubMed] [Google Scholar]
- 147.Matsuoka LY, Wortsman J, Chen TC, Holick MF. Compensation for the interracial variance in the cutaneous synthesis of vitamin D. J Lab Clin Med. 1995; 126(5):452–457. [PubMed] [Google Scholar]
- 148.Harris S, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998;67(6):1232–1236. [DOI] [PubMed] [Google Scholar]
- 149.Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85(11):4125–4130. [DOI] [PubMed] [Google Scholar]
- 150.Bell NH, Morrison NA, Nguyen TV, Eisman J, Hollis BW. ApaI polymorphisms of the vitamin D receptor predict bone density of the lumbar spine and not racial difference in bone density in young men. J Lab Clin Med. 2001;137(2):133–140. [DOI] [PubMed] [Google Scholar]
- 151.Centers for Disease Control and Prevention. WONDER database. Available at: http//:wonder.cdc.gov. Accessed June 2004.
- 152.Cooper GS, Yuan Z, Rimm AA. Racial disparity in the incidence and case-fatality of colorectal cancer: analysis of 329 United States counties. Cancer Epidemiol Biomarkers Prev. 1997; 6(4):283–285. [PubMed] [Google Scholar]
- 153.Chen VW, Fenoglio-Preiser CM, Wu XC, et al. Aggressiveness of colon carcinoma in blacks and whites. National Cancer Institute Black/White Cancer Survival Study Group. Cancer Epidemiol Biomarkers Prev. 1997;6(12): 1087–1093. [PubMed] [Google Scholar]
- 154.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162(17):1985–1993. [DOI] [PubMed] [Google Scholar]
- 155.McGuire V, Herrinton L, Whittemore AS. Race, epithelial ovarian cancer survival, and membership in a large health maintenance organization. Epidemiology. 2002;13(2):231–234. [DOI] [PubMed] [Google Scholar]
- 156.National Cancer Institute. Surveillance, Epidemiology, and End Results Program (SEER) Web site (data for 1992–2001). Available at: http://seer.cancer.gov. Accessed August 12, 2005.
- 157.Henson DE, Chu KC, Levine PH. Histologic grade, stage, and survival in breast carcinoma: comparison of African American and Caucasian women. Cancer. 2003;98(5):908–917. [DOI] [PubMed] [Google Scholar]
- 158.Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. 2003;14(8): 761–766. [DOI] [PubMed] [Google Scholar]
- 159.Gargiulo P, Wingo P, Coates R, Thompson T. Recent trends in mortality rates for four major cancers by sex race and ethnicity—United States, 1990–1998. MMWR Morb Mortal Wkly Rep. 2002; 51(3):49–53. [PubMed] [Google Scholar]
- 160.Blackman D, Bennett E, Miller D. Trends in self-reported use of mammography (1989–1997) and Papanicolaou tests (1991–1997)—Behavioral Risk Factor Surveillance System. MMWR CDC Surveill Summ. 1999;48(6):1–22. [PubMed] [Google Scholar]
- 161.Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000;22(2):203–217. [DOI] [PubMed] [Google Scholar]
- 162.Ma J, Stampfer MJ, Gann PH, et al. Vitamin D receptor polymorphisms, circulating vitamin D metabolites, and risk of prostate cancer in United States physicians. Cancer Epidemiol Biomarkers Prev. 1998;7(5):385–390. [PubMed] [Google Scholar]
- 163.Slatter ML, Yakumo K, Hoffman M, Neuhausen S. Variants of the VDR gene and risk of colon cancer (United States). Cancer Causes Control. 2001;12(4): 359–364. [DOI] [PubMed] [Google Scholar]
- 164.Oh JY, Barrett-Connor E. Association between vitamin D receptor polymorphism and type 2 diabetes or metabolic syndrome in community-dwelling older adults: the Rancho Bernardo Study. Metabolism. 2002;51(3): 356–359. [DOI] [PubMed] [Google Scholar]
- 165.Habuchi T, Suzuki T, Sasaki R, et al. Association of vitamin D receptor gene polymorphism with prostate cancer and benign prostatic hyperplasia in a Japanese population. Cancer Res. 2000;60(2):305–308. [PubMed] [Google Scholar]
- 166.Bretherton-Watt D, Given-Wilson R, Mansi JL, Thomas V, Carter N, Colston KW. Vitamin D receptor gene polymorphisms are associated with breast cancer risk in a UK Caucasian population. Br J Cancer. 2001;85(2):171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guy M, Lowe LC, Bretherton-Watt D, Mansi JL, Colston KW. Approaches to evaluating the association of vitamin D receptor gene polymorphisms with breast cancer risk. Recent Results Cancer Res. 2003;164:43–54. [DOI] [PubMed] [Google Scholar]
- 168.Ingles SA, Garcia DG, Wang W, et al. Vitamin D receptor genotype and breast cancer in Latinas (United States). Cancer Causes Control. 2000;11(1): 25–30. [DOI] [PubMed] [Google Scholar]
- 169.Ruggiero M, Pacini S, Aterini S, Fallai C, Ruggiero C, Pacini P. Vitamin D receptor gene polymorphism is associated with metastatic breast cancer. Oncol Res. 1998;10(1):43–46. [PubMed] [Google Scholar]
- 170.Hamasaki T, Inatomi H, Katoh T, Ikuyama T, Matsumoto T. Clinical and pathological significance of vitamin D receptor gene polymorphism for prostate cancer which is associated with a higher mortality in Japanese. Endocr J. 2001; 48(5):543–549. [DOI] [PubMed] [Google Scholar]
- 171.Kibel AS, Isaacs SD, Isaacs WB, Bova GS. Vitamin D receptor polymorphisms and lethal prostate cancer. J Urol. 1998;160(4):1405–1409. [PubMed] [Google Scholar]
- 172.Blazer DG 3rd, Umbach DM, Bostick RM, Taylor JA. Vitamin D receptor polymorphisms and prostate cancer. Mol Carcinog. 2000;27(1):18–23. [DOI] [PubMed] [Google Scholar]
- 173.Lundin AC, Soderkvist P, Eriksson B, Bergman-Jungestrom M, Wingren S. Association of breast cancer progression with a vitamin D receptor gene polymorphism. South-East Sweden Breast Cancer Group. Cancer Res. 1999;59(10): 2332–2334. [PubMed] [Google Scholar]
- 174.Rees GS, Symes EK, Nicholl CG, Legon S, Chapman RS. Lack of correlation of free deoxypyridinoline excretion with Taq1 restriction length polymorphisms in the vitamin D receptor gene in males. Clin Chim Acta. 1998;272(2): 149–157. [DOI] [PubMed] [Google Scholar]
- 175.Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial cell proliferation in subjects at high risk for familial colon cancer. N Engl J Med. 1985;313:1381–1384. [DOI] [PubMed] [Google Scholar]
- 176.Brenner B, Russell N, Albrecht S, Davies R. The effect of dietary vitamin D3 on the intracellular calcium gradient in mammalian colonic crypts. Cancer Lett. 1998;12(7):43–53. [DOI] [PubMed] [Google Scholar]
- 177.Lamprecht SA, Lipkin M. Migrating colonic crypt epithelial cells: primary targets for transformation. Carcinogenesis. 2002;23(11):1777–1780. [DOI] [PubMed] [Google Scholar]
- 178.Benito E, Obrador A, Stiggelbout A, et al. A population-based case-control study of colorectal cancer in Majorca, I: dietary factors. Int J Cancer. 1990;45(1): 69–76. [DOI] [PubMed] [Google Scholar]
- 179.Mawer E, Walls J, Howell A, Davies M, Ratcliffe W, Bundred N. Serum 1,25-dihydroxyvitamin D may be related inversely to disease activity in breast cancer patients with bone metastases. J Clin Endocrinol Metab. 1997;82:118–122. [DOI] [PubMed] [Google Scholar]
- 180.Newmark HL. Vitamin D adequacy: a possible relationship to breast cancer. Adv Exp Med Biol. 1994;364: 109–114. [DOI] [PubMed] [Google Scholar]
- 181.Carroll KK, Jacobson EA, Eckel LA, Newmark HL. Calcium and carcinogenesis of the mammary gland. Am J Clin Nutr. 1991;54(1 suppl): 206S–208S. [DOI] [PubMed] [Google Scholar]
- 182.Iseki K, Tatsuta M, Uehara H, et al. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer. 1999;81(5):730–733. [DOI] [PubMed] [Google Scholar]
- 183.Majewski S, Skopinska M, Marczak M, Szmurlo A, Bollag W, Jablonska S. Vitamin D3 is a potent inhibitor of tumor cell-induced angiogenesis. J Investig Dermatol Symp Proc. 1996;1(1): 97–101. [PubMed] [Google Scholar]
- 184.Shokravi MT, Marcus DM, Alroy J, Egan K, Saornil MA, Albert DM. Vitamin D inhibits angiogenesis in transgenic murine retinoblastoma. Invest Ophthalmol Vis Sci. 1995;36(1):83–87. [PubMed] [Google Scholar]
- 185.Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87(3):214–220. [DOI] [PubMed] [Google Scholar]
- 186.Palmer HG, Gonzalez-Sancho JM, Espada J, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154(2):369–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fujioka T, Suzuki Y, Okamoto T, Mastushita N, Hasegawa M, Omori S. Prevention of renal cell carcinoma by active vitamin D3. World J Surg. 2000; 24(10):1205–1210. [DOI] [PubMed] [Google Scholar]
- 188.Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med. 1985;313(22):1381–1384. [DOI] [PubMed] [Google Scholar]
- 189.Holt P, Arber N, Halmos B, et al. Colonic epithelial cell proliferation decreases with increasing levels of serum 25-hydroxy vitamin D. Cancer Epidemiol Biomarkers Prev. 2002;11(1):113–119. [PubMed] [Google Scholar]
- 190.Campbell MJ, Reddy GS, Koeffler HP. Vitamin D3 analogs and their 24-oxo metabolites equally inhibit clonal proliferation of a variety of cancer cells but have differing molecular effects. J Cell Biochem. 1997;66(3):413–425. [PubMed] [Google Scholar]
- 191.Mathiasen IS, Sergeev IN, Bastholm L, Elling F, Norman AW, Jaattela M. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J Biol Chem. 2002;277(34):30738–30745. [DOI] [PubMed] [Google Scholar]
- 192.National Academy of Sciences, Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1997. [PubMed]
- 193.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003; 77(1):204–210. [DOI] [PubMed] [Google Scholar]
- 194.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351(9105):805–806. [DOI] [PubMed] [Google Scholar]
- 195.Ebeling P, Sandgren M, Lane A, DeLuca H, Riggs B. Evidence of an age-related decrease in intestinal responsiveness to vitamin D: relationship between serum 1,25-dihydroxyvitamin D3 and intestinal vitamin D receptor concentrations in normal women. J Clin Endocrinol Metab. 1992;75:176–182. [DOI] [PubMed] [Google Scholar]
- 196.Stamp TC, Haddad JG, Twigg CA. Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet. 1977; 1(8026):1341–1343. [DOI] [PubMed] [Google Scholar]
- 197.Vieth R. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004;20:575–579. [DOI] [PubMed] [Google Scholar]
- 198.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73(2): 288–294. [DOI] [PubMed] [Google Scholar]
- 199.Lowe L, Hansen CM, Senaratne S, Colston KW. Mechanisms implicated in the growth regulatory effects of vitamin D compounds in breast cancer cells. Recent Results Cancer Res. 2003; 164:99–110. [DOI] [PubMed] [Google Scholar]
- 200.Matsuoka LY, Wortsman J, Haddad JG, Hollis BW. In vivo threshold for cutaneous synthesis of vitamin D3. J Lab Clin Med. 1989;114(3):301–305. [PubMed] [Google Scholar]
- 201.Bowman K. A global climatology of total ozone from the Nimbus-7 total ozone mapping spectrometer. In: Zerefos CS, Ghazi A, eds. Atmospheric Ozone: Proceedings of a Quadrennial Symposium Held in Halkidiki, Greece, 3–7 September 1984. Boston, Mass: Kluwer Academic Publishers; 1985:363–367.
- 202.Waggoner A, Vanderpool A, Charlson R, et al. Sulfate light scattering as an index of the role of sulfur in tropospheric optics. Nature. 1976;261: 120–122. [Google Scholar]
- 203.Garland C, Garland F, Gorham E. Epidemiology of cancer risk and vitamin D. In: Holick M, ed. Vitamin D: Molecular Biology, Physiology, and Clinical Applications. Totowa, NJ: Humana; 1999:375–409.
- 204.Webb A, DeCosta B, Holick M. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68:882–887. [DOI] [PubMed] [Google Scholar]
- 205.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988; 124(6):869–871. [DOI] [PubMed] [Google Scholar]
- 206.Chen T. Photobiology of vitamin D. In: Holick M, ed. Vitamin D: Molecular Biology, Physiology, and Clinical Applications. Totowa, NJ: Humana; 1999: 17–37.
- 207.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439–443. [DOI] [PubMed] [Google Scholar]
- 208.Dawson-Hughes B, Harris S, Krall E, Dallal G. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. New Engl J Med. 1997;337: 670–676. [DOI] [PubMed] [Google Scholar]
- 209.Holick MF. Vitamin D requirements for humans of all ages: new increased requirements for women and men 50 years and older. Osteoporos Int. 1998;8(8):S24–S29. [DOI] [PubMed] [Google Scholar]
- 210.Davies M, Adams PH. The continuing risk of vitamin-D intoxication. Lancet. 1978;2(8090):621–623. [DOI] [PubMed] [Google Scholar]
- 211.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328(12):833–838. [DOI] [PubMed] [Google Scholar]
- 212.Muray S, Parisi E, Cardus A, Craver L, Fernandez E. Influence of vitamin D receptor gene polymorphisms and 25-hydroxyvitamin D on blood pressure in apparently healthy subjects. J Hypertens. 2003;21(11):2069–2075. [DOI] [PubMed] [Google Scholar]
- 213.Sowers MF, Wallace RB, Hollis BW, Lemke JH. Relationship between 1,25-dihydroxyvitamin D and blood pressure in a geographically defined population. Am J Clin Nutr. 1988;48(4): 1053–1056. [DOI] [PubMed] [Google Scholar]
- 214.Lind L, Hanni A, Lithell H, Hvarfner A, Sorensen OH, Ljunghall S. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens. 1995; 8(9):894–901. [DOI] [PubMed] [Google Scholar]
- 215.Sowers MR, Wallace RB, Lemke JH. The association of intakes of vitamin D and calcium with blood pressure among women. Am J Clin Nutr. 1985; 42(1):135–142. [DOI] [PubMed] [Google Scholar]
- 216.Scragg R, Jackson R, Holdaway I, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19(3):559–563. [DOI] [PubMed] [Google Scholar]
- 217.Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. 1999;149(2): 151–161. [DOI] [PubMed] [Google Scholar]
- 218.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41(1):105–112. [DOI] [PubMed] [Google Scholar]
- 219.Vik T, Try K, Thelle D, Førde O. Tromsø Heart Study: vitamin D metabolism and myocardial infarction. Br Med J. 1979;21:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rajasree S, Rajpal K, Kartha CC, et al. Serum 25-hydroxyvitamin D3 levels are elevated in South Indian patients with ischemic heart disease. Eur J Epidemiol. 2001;17(6):567–571. [DOI] [PubMed] [Google Scholar]
- 221.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001; 358(9292):1500–1503. [DOI] [PubMed] [Google Scholar]
- 222.Stene LC, Joner G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population-based, case-control study. Am J Clin Nutr. 2003;78(6):1128–1134. [DOI] [PubMed] [Google Scholar]
- 223.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5): 820–825. [DOI] [PubMed] [Google Scholar]
- 224.Borissova AM, Tankova T, Kirilov G, Dakovska L, Kovacheva R. The effect of vitamin D3 on insulin secretion and peripheral insulin sensitivity in type 2 diabetic patients. Int J Clin Pract. 2003; 57(4):258–261. [PubMed] [Google Scholar]
- 225.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998; 68(4):854–858. [DOI] [PubMed] [Google Scholar]
- 226.Lilliu H, Pamphile R, Chapuy MC, Schulten J, Arlot M, Meunier PJ. Calcium-vitamin D3 supplementation is cost-effective in hip fractures prevention. Maturitas. 2003;44(4):299–305. [DOI] [PubMed] [Google Scholar]