Abstract
Objectives. We determined the effect of national vaccine shortages on coverage with 4 doses of diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine for American Indian/Alaska Native (AIAN) children.
Methods. Data on DTaP coverage for children aged 19 to 27 months were abstracted from Indian Health Service (IHS) immunization reports. Coverage with the fourth DTaP dose (DTaP4) was compared for different periods to determine coverage levels before, during, and after the shortage. Data were stratified geographically to determine regional variation.
Results. AIAN children experienced a significant decline (14.8%) in DTaP4 coverage during the shortage. Considerable variation was seen among IHS regions (declines ranged from 4.5% to 26.5%).
Conclusions. AIAN children included in IHS immunization reports experienced a greater decline in DTaP4 coverage during the shortage than the decline reported nationally for children receiving vaccine at public clinics (14.8% vs 6%). Variations in the decline in coverage highlight possible inequities in vaccine supply and distribution and in implementation of vaccine shortage recommendations. We must identify ways to ensure more equitable vaccine distribution and consistent implementation of vaccine recommendations to protect all children from vaccine-preventable diseases.
During 2001 and 2002 in the United States, there were unprecedented shortages in 5 of the 8 routinely recommended childhood vaccines. These included diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine; varicella vaccine; measles-mumps-rubella (MMR) vaccine; and pneumococcal conjugate vaccine. Supplies of adult tetanus and diphtheria toxoids also were affected.1–4
The Advisory Committee on Immunization Practices (ACIP) is the federal advisory committee charged with providing “advice and guidance to the Secretary, the Assistant Secretary for Health, and the Centers for Disease Control and Prevention (CDC) on the most effective means to prevent vaccine-preventable diseases.”5 The shortage of DTaP, which began in January 2001 and ended in July 2002, prompted the ACIP and CDC to issue the interim recommendation that health care providers with inadequate DTaP supplies defer the fourth dose of the vaccine (DTaP4).1,2 The CDC also worked to ensure the equitable distribution of available public sector vaccine through rigorous tracking of state inventories, establishing state allocation amounts, and prioritizing vaccine shipments.6 The intent was to ensure an adequate supply of vaccine for the vaccination of infants with the first 3 doses of DTaP vaccine. These efforts, along with consistent adherence by providers to interim guidelines to defer the fourth dose, should have resulted in ample supplies of the first 3 doses and an associated uniform decline in DTaP4 coverage for all children in the United States.
Despite the CDC’s efforts, public sector DTaP vaccine supply was more severely affected by the DTaP shortage than was private sector vaccine supply.7 In addition, previous studies have shown that timely and equitable administration of vaccines during national shortages can be problematic.6–8 A survey of providers and state immunization programs found that only 16% of the providers implemented the interim ACIP recommendations to suspend the DTaP4.6
A second study assessed the effect of the vaccine shortage on coverage with data from the National Immunization Survey. This study found that children who received vaccines at public clinics and children residing in certain geographic regions experienced significantly greater declines in DTaP4 coverage than did children served by private providers or in other regions of the United States. The investigators concluded that these children were differentially affected by the shortage and that such inequities of effect should be corrected.8
The Indian Health Service (IHS) is the federal health care provider for eligible American Indian/Alaska Native (AIAN) people in the United States, with a network of hospitals and clinics serving AIAN people in 35 states. The IHS provides clinical services to 1.6 million of the 2.5 million US AIAN population.9 The IHS is divided into 12 administrative regions called “Areas.” Immunization coverage for children served by IHS, Tribal, and Urban Indian health facilities is monitored on a quarterly basis. Each of the 12 IHS Areas submits an aggregate report on all children aged 3 to 27 months who have ever been seen at an IHS, Tribal, or Urban Indian health facility and reside in a community located in the catchment area of the facility. These reports monitor age-appropriate vaccine coverage with DTaP, inactivated poliovirus (IPV) vaccine, MMR vaccine, Haemophilus influenzae type b (Hib) vaccine, hepatitis B vaccine, pneumococcal conjugate vaccine, varicella vaccine, and hepatitis A vaccine.
The IHS quarterly reporting system differs from the National Immunization Survey in 2 important ways: (1) it includes children younger than 19 months; and (2) it is not sample based—rather, it is designed to capture the entire population of AIAN children seen at participating facilities. The coverage reported by the 12 IHS Areas thus can be combined to provide an overall picture of coverage for IHS.
In September 2002, the IHS implemented more stringent reporting guidelines aimed at achieving more inclusive and more accurate reporting of childhood immunization coverage. As a result of these changes, the IHS quarterly reports now capture approximately 60% of the total 3- to 27-month-old IHS user population, compared with 40% or less in previous years (IHS, unpublished data, 2004).
All AIAN children are eligible to receive public sector vaccine through the federally funded Vaccines for Children program. Under this program, vaccine is ordered and distributed by the state to public and private clinics for eligible patients at no cost to the facility. AIAN children can receive Vaccines for Children (VFC) vaccines at IHS and tribal clinics as well as at other public and private providers that are part of the VFC program. Because all IHS, Tribal, and Urban Indian health facilities receive VFC vaccines, data from the IHS immunization coverage reports on children who receive immunizations from these sites provide insight into the effect the DTaP shortage had on DTaP4 coverage for a small group (< 3% of all VFC vaccine) of children receiving VFC vaccines.
This study analyzed changes in IHS immunization coverage; determined the effect of the DTaP shortage on DTaP4 coverage among AIAN children; and explored possible inequities in vaccine supply, distribution, and coverage during the national shortage.
METHODS
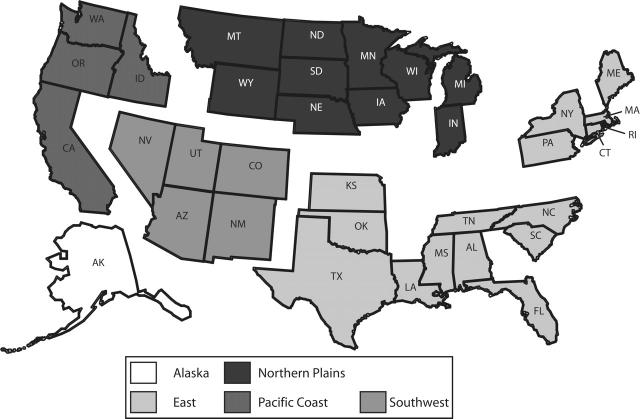
Data on children aged 19 to 27 months were abstracted from the IHS quarterly immunization reports. Data for all IHS Areas were combined to present a national picture of immunization coverage for children in this age group served by IHS. In addition, data from the IHS Areas were combined on the basis of geographic location to create 5 regional groups—Alaska, Pacific Coast, Southwest, Northern Plains, and East (Figure 1 ▶).
FIGURE 1—
Combination of Indian Health Service Areas into regional groups.
Quarterly data were aggregated and divided into 3 periods: (1) the preshortage period, (2) the shortage period, and (3) the postshortage period. Each period contained 3 quarters of data; the mean DTaP4 coverage level and the mean coverage level with the third dose of inactivated poliovirus vaccine (IPV3) were calculated for each period. The preshortage period included data from the 3 quarters prior to the onset of the shortage (April–December 2000). The shortage period contained 3 quarters of data during the national shortage in which the IHS experienced the lowest DTaP4 coverage levels (January–September 2002). The postshortage period contained data from the 3 most recent quarters (January–September 2004) and was distant enough from the shortage period to allow coverage levels to recover to preshortage levels.
To determine whether differences in DTaP4 coverage could be attributed to the DTaP shortage alone or whether other issues related to immunization delivery could have contributed to the decline, we compared DTaP4 coverage with IPV3 coverage during the same period for all IHS Areas combined and for each region. There were no IPV shortages or interim IPV guidelines, and, like DTaP4, all children should have received IPV3 by age 19 months. We used Epi Info, Version 6.04c (Centers for Disease Control and Prevention, Atlanta, Ga), to compare DTaP4 and IPV3 coverage in the preshortage period with coverage in the shortage period. We used the Mantel–Haenszel χ2 test to determine whether changes in DTaP4 and IPV3 coverage were statistically significant. A P value of less than .05 was used to determine statistical significance. Finally, we recognized that more comprehensive reporting practices could have affected coverage level estimates in the postshortage period, so we calculated the percent change in the number of children aged 19 to 27 months included in the preshortage period reports and in the postshortage period reports for IHS as a whole and for each geographical region.
RESULTS
All IHS Areas Combined
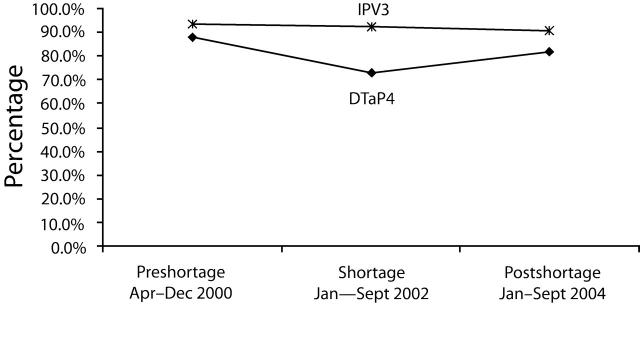
In the preshortage period, the mean DTaP4 coverage level for children aged 19 to 27 months in all IHS Areas combined was 88.0% (95% confidence interval [CI] = 87.3, 88.7). In the shortage period, DTaP4 coverage declined to a mean of 73.2% (95% CI = 72.3, 73.9). The overall 14.8% decrease (95% CI = 13.7, 15.9) in mean DTaP4 coverage from the preshortage period to the shortage period (88.0% to 73.2%) was statistically significant (P< .05).
Mean coverage with IPV3 declined 1.1% (95% CI=0.3, 1.9) overall during the DTaP shortage, from 93.1% (95% CI=92.5, 93.6) in the preshortage period to 92.0% (95% CI=91.5, 92.5) in the shortage period (Figure 2 ▶). This decline was statistically significant (P<.05).
FIGURE 2—
Coverage with fourth dose of diphtheria and tetanus toxoids and acellular pertussis (DTaP4) vaccine and third dose of inactivated poliovirus vaccine (IPV3) for all Indian Health Service Areas combined: April 2000 to September 2004.
Coverage in the postshortage period for both DTaP4 and IPV3 was lower than coverage in the preshortage period. In addition, the number of children included in the reports for each period increased; overall, 33% more children were included in the postshortage period reports compared with reports in the preshortage period.
Coverage by Region
Although all of the Areas combined experienced a significant decline in DTaP4 coverage during the shortage, considerable variation was found in the magnitude of the decline among regions (Table 1 ▶). The Southwest region experienced the greatest decline in DTaP4 coverage (26.5%), whereas the Alaska and Northern Plains regions reported the least decline (4.5%). In contrast to the significant declines in DTaP4 coverage reported by all regions, the changes in coverage with IPV3, significant in the Alaska and Pacific Coast regions, were relatively small in each region (Table 1 ▶).
TABLE 1—
Variation in Fourth Dose of Diphtheria and Tetanus Toxoids and Acellular Pertussis (DTaP4) Vaccine and Third Dose of Inactivated Poliovirus Vaccine (IPV3) Coverage for American Indian/Alaska Native Children Aged 19 to 27 Months, by Geographic Region, 2000–2004
| DTaP4 Vaccine Coverage, % | IPV3 Coverage, % | |||||||
| Preshortage (April–December 2000) | Shortage (January–September 2002) | Postshortage (January–September 2004) | % Change (Preshortage to Shortage) | Preshortage (April–December 2000) | Shortage (January–September 2002) | Postshortage (January–September 2004) | % Change (Preshortage to Shortage) | |
| Alaska | 83.3 | 78.8 | 78.1 | −4.5* | 94.5 | 91.9 | 92.7 | −2.6* |
| Northern Plains | 89.3 | 84.8 | 82.5 | −4.5* | 92.5 | 92.6 | 92.1 | 0.1 |
| Pacific Coast | 84.4 | 74.8 | 69.5 | −9.6* | 92.0 | 87.2 | 85.4 | −4.8* |
| East | 78.2 | 64.9 | 66.7 | −13.3* | 83.8 | 82.7 | 72.6 | −1.1 |
| Southwest | 93.7 | 67.2 | 91.6 | −26.5* | 96.6 | 95.9 | 97.0 | −0.7 |
| All regions | 88.0 | 73.2 | 81.7 | −14.8* | 93.1 | 92.0 | 90.7 | −1.1 |
*Changes from preshortage to shortage period were statistically significant (P < .05).
Postshortage coverage with DTaP4 was lower than preshortage coverage levels in all regions. Coverage with IPV3 was lower in the postshortage period compared with the preshortage period for all regions except the Southwest (Table 1 ▶). Changes in reporting also varied by region. Alaska experienced the greatest increase in reporting, capturing 39% more children in the postshortage period reports compared with the preshortage period; the East region reports included 6% fewer children in the postshortage period compared with the preshortage period (Table 2 ▶).
TABLE 2—
Changes by Geographic Region in Indian Health Service Quarterly Immunization Reporting System for American Indian/Alaska Native Children Aged 19 to 27 Months, 2000–2004
| Mean No. of Children in Preshortage Period | Mean No. of Children in Postshortage Period | %Change | |
| Alaska | 1259 | 1751 | 39 |
| Northern Plains | 2105 | 2403 | 30 |
| Pacific Coast | 893 | 1065 | 19 |
| East | 1591 | 1491 | −6 |
| Southwest | 3003 | 3788 | 35 |
| All regions | 8321 | 11 094 | 33 |
DISCUSSION
The 2001 to 2002 national shortage of DTaP vaccine resulted in a decline in DTaP4 coverage for AIAN children that exceeded declines seen in the US population at large. When compared with preshortage coverage levels, the decline in coverage for AIAN children was 14.8% overall. In contrast, an analysis of National Immunization Survey data from the same period found an overall decline of 1.8% for all children and a 6% decline among children receiving vaccine from public clinics.8 Because our study population was almost exclusively served by public clinics, this may explain some of the disproportionate decline experienced by AIAN children. The National Immunization Survey–based study also observed greater declines in DTaP4 coverage for children residing in rural areas; populations served by IHS reside predominantly in rural areas, and our combined findings suggest that rural-based issues, such as access to care or barriers to vaccine distribution, may contribute to the observed differential declines in coverage for recipients of public sector vaccine.
In addition to the greater declines experienced by children in rural areas, the National Immunization Survey–based analysis showed that children residing in the southern United States experienced greater declines in DTaP4 coverage than did children residing elsewhere.8 We also observed regional variation in coverage in our study. All 5 IHS regions experienced a significant decline in DTaP4 coverage during the shortage, although the magnitude of this effect varied considerably among regions, ranging from 4.5% in Alaska and the Northern Plains to more than 26% in the Southwest (Table 1 ▶).
This variation in decline in DTaP4 coverage may be a result of several factors. In the survey of providers and state immunization program managers cited earlier, the authors identified contributing factors such as providers’ problems with ordering and receiving vaccines, changes in vaccine distribution procedures, prioritizing of vaccine distribution among providers, failure of immunization program managers to distribute ACIP recommendations to providers, and failure of providers to implement interim ACIP recommendations.6 Differences in state DTaP inventory also may have played a role. Any or all of these factors may have contributed to the regional variation in DTaP4 coverage documented in our study population of AIAN children.
Although mean coverage with IPV3 also declined during the shortage period, the decline in DTaP4 coverage was 14.8 times greater. Less regional variation also was seen with IPV3 coverage (Table 1 ▶). The shortage of DTaP may have contributed indirectly to the declines in coverage with IPV3. During the DTaP shortage, the shortages with other vaccines may have caused confusion among the public and even some providers over which vaccines were in short supply. Perhaps some parents thought that all vaccines were in short supply and chose to delay visits to their providers until the shortages were resolved.
In most circumstances, declines in immunization coverage levels caused by vaccine shortages would be expected to recover to preshortage levels following resolution of the shortage. Because we did not observe such a recovery in DTaP4 in any of the geographic regions we evaluated, we sought an explanation by analyzing reporting practices across regions. When IHS implemented improved reporting practices in September 2002, it was anticipated that these changes would initially lower overall immunization coverage rates but would ultimately provide more accurate data. Because these changes were implemented after the resolution of the DTaP shortage, they could have affected estimates of postshortage recovery. Although reporting did improve in 4 of the 5 regions, with an overall increase of 33%, no consistent pattern emerged to suggest a strong relation between more comprehensive reporting and failure to recover to preshortage immunization coverage levels. Recovery approximating preshortage coverage levels was stronger for IPV3, but again, no consistent pattern was observed (Tables 1 ▶ and 2 ▶). These findings reinforce the concept that a complex combination of factors contributed to the observed differential declines in DTaP4 coverage among AIAN children.
Limitations
In this study, we did not examine other issues besides the shortage and changes in reporting practices that may have contributed to declining coverage. Although the IHS reports are designed to capture all AIAN children served by participating clinics, 100% are not currently captured. In addition, we measured vaccine coverage, not vaccine availability, making it difficult to distinguish between declines in coverage related to actual shortages in supply and declines related to better implementation of ACIP guidelines and other factors.
IHS Areas and the geographic regions used for this analysis combined multiple states and, therefore, did not allow for a state-by-state evaluation. Differences between individual states and IHS Areas may have been lost when data were aggregated, which may have led to an underestimate or overestimate of the effect of the shortages on regional vaccine coverage. In addition, only American Indians/Alaska Natives who were served by IHS, Tribal, and Urban Indian sites that regularly report to the IHS were included; findings from this study may not be applicable to AIAN children served by nonreporting IHS, Tribal, and Urban Indian health programs. Finally, although all AIAN children are eligible for VFC vaccines, there may be differences between IHS, Tribal, and Urban Indian providers and other VFC providers, and these findings are probably not generalizable to the larger VFC–using population.
Conclusions
Our findings support those of earlier studies and suggest that issues of equity in the national vaccine distribution system should be further evaluated. Clearly, inequities exist not only between private and public sector vaccine coverage but also, as our findings show, within populations receiving public sector vaccine. Further examination of variations in state and regional vaccine supply, distribution, and implementation of interim shortage recommendations is necessary.
Our study found that during the national DTaP shortage, AIAN children served by IHS, Tribal, and Urban Indian health facilities experienced a more severe decline in DTaP4 coverage compared with other populations in the United States. In the future, strategies must be identified to ensure more equitable distribution of vaccines during shortages at the national, state, tribal, and local levels. In addition, ways to ensure more consistent implementation of ACIP vaccine policy guidelines must be explored further to reduce disparities and ensure that all children are protected against vaccine-preventable diseases.
Acknowledgments
We acknowledge the contribution of the Indian Health Service and tribal immunization coordinators across the United States who provided, and continue to provide, timely and accurate reports of immunization coverage for American Indian/Alaska Native children. We thank Karen Sheff and John Redd for their assistance in reviewing the article.
Human Participant Protection This work was conducted using data routinely collected for public health practice purposes by the Indian Health Service. No human participants were involved, and no personal identifiers were included in any of the data analyzed for this study. Institutional review board approval thus was not required.
Peer Reviewed
Contributors A. V. Groom originated the study and led all aspects of its implementation. J. E. Cheek and R. T. Bryan assisted with the study and synthesis of the analyses. All authors helped to conceptualize ideas, interpret findings, and review and rewrite drafts of the article.
References
- 1.Centers for Disease Control and Prevention. Update on the supply of tetanus and diphtheria toxoids and of diphtheria and tetanus toxoids and acellular pertussis vaccine. MMWR Morb Mortal Wkly Rep. 2001;50:189–190. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Update: supply of diphtheria and tetanus toxoids and acellular pertussis vaccine. MMWR Morb Mortal Wkly Rep. 2002;50:1159. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Updated recommendations on the use of pneumococcal conjugate vaccine in a setting of vaccine shortage—Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2001;50:1140–1142. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Shortage of varicella and measles, mumps, and rubella vaccines and interim recommendations from the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2002;51:190–191. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices page. Available at: http://www.cdc.gov/nip/ACIP/default.htm. Accessed August 16, 2004.
- 6.Stokley S, Santoli J, Willis B, et al. Impact of vaccine shortages on immunization programs and providers. Am J Prev Med. 2004;26:15–21. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Impact of vaccine shortage on diphtheria and tetanus toxoids and acellular pertussis vaccine coverage rates among children aged 24 months—Puerto Rico 2002. MMWR Morb Mortal Wkly Rep. 2002;51:667–668. [PubMed] [Google Scholar]
- 8.Santibanez TA, Santoli JM, Barker LE. Differential effects of the DTaP and MMR vaccine shortages on timeliness of childhood vaccination coverage. Am J Public Health. 2006;96:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Census Bureau. The American Indian and Alaska Native population: 2000. Census 2000 Briefs page. Available at: http://www.census.gov/prod/2002pubs/c2kbr01-15.pdf. Accessed August 2, 2004.