Abstract
Objectives. We determined the effect of diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) and measles, mumps, rubella (MMR) vaccine shortages on timeliness of the third dose of DTaP (DTaP3), the fourth dose of DTaP (DTaP4), and the first dose of MMR (MMR1) among subgroups of preschool children.
Methods. Data from the 2001 and 2002 National Immunization Surveys were analyzed. Children age-eligible to receive DTaP3, DTaP4, or MMR1 during the shortages were considered subject to the shortage, and those not age-eligible were not subject to the shortage; timeliness of vaccinations was compared.
Results. Among children vaccinated only at public clinics, children residing outside metropolitan statistical areas, and children in the Southern Census Region, those age-eligible to receive DTaP4 during the shortage were less likely to be vaccinated by 19 months of age than children not subject to the shortage.
Conclusions. There was notable disparity in the effects of the recent vaccine shortages; children vaccinated only in public clinics, in rural areas, or in the Southern United States were differentially affected by the shortages.
Between 2000 and mid-2003, the United States experienced shortages of a number of routinely recommended vaccines. The affected vaccines were diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP), measles, mumps, and rubella vaccine (MMR), varicella and pneumococcal conjugate vaccines, and tetanus and diphtheria toxoids. The shortages were of sufficient magnitude and duration that recommendations for these vaccines had to be temporarily modified, and certain doses were suspended.1–4
Specifically, if a provider had insufficient quantities of vaccine, the Advisory Committee on Immunization Practices recommended the deferral of the fourth and fifth doses of DTaP and the second dose of MMR.1–2,4 Although this strategy allowed scarce vaccine to be targeted to those in greatest need, it delayed the provision of a preventive health benefit, and in some cases an entitlement, for many children, adolescents, and adults.
Little is known about the impact of the shortages on childhood immunization coverage. An analysis of the 2002 Puerto Rico Immunization Survey, which measures vaccination coverage among children aged 24 months, showed that receipt of the third dose of DTaP was similar in 2002 to that in 2001, whereas there was a substantial drop in coverage with the fourth dose of DTaP from 95.8% in 2001 to 31.8% in 2002.5 Because substantial efforts were made by the Puerto Rico Department of Health to encourage implementation of their recommendation to suspend the fourth dose of DTaP, it is difficult to determine how much of this drop in coverage was due to actual shortage and how much to compliance with recommendations.
Earlier reports of data from the National Immunization Survey (NIS), which monitors immunization coverage in preschool children on an annual basis, showed no evidence of a national decrease in immunization coverage in 2001 and 2002 compared with previous years; however, timeliness of vaccinations and subgroup analyses were not examined.6,7
Did the vaccine shortages affect US pre-school immunization in a way not reflected in standard NIS coverage estimates? We investigated the magnitude and distribution of effects of 2 vaccine shortages on the timeliness of receipt of the third dose of DTaP (DTaP3), the fourth dose of DTaP (DTaP4), and the first dose of MMR (MMR1) among subgroups of children aged 19 to 35 months in the United States. Because vaccination with varicella and pneumococcal vaccines, also in short supply during this time, had not yet reached full implementation, coverage levels for both vaccines continued to increase. This made assessing changes in timely vaccination with these vaccines difficult. Thus, we focused on only the DTaP and MMR vaccine shortages.
METHODS
The NIS is a large, national survey conducted annually by the Centers for Disease Control and Prevention to obtain vaccination coverage estimates for noninstitutionalized children aged 19 to 35 months in the United States. The NIS is a random-digit-dialed survey of households with eligible children followed by a mail survey of vaccination providers to retrospectively obtain the child’s complete vaccination histories. Demographics of respondents’ children, such as race/ethnicity, are respondent reported. Characteristics of providers are provider reported. Methodological details of the NIS are available elsewhere.8
We combined the 2001 and 2002 NIS data sets. Children born between February 1998 and June 2000 were eligible for inclusion in the 2001 NIS. Children born between February 1999 and June 2001 were eligible for inclusion in the 2002 NIS. For DTaP3, DTaP4, and MMR1, we classified each child as being/not being in the class born at a time during which the child would be scheduled to receive a vaccine during a shortage of that vaccine; these children are hereafter referred to as subject to shortage and not subject to shortage.
Although the Advisory Committee on Immunization Practices’ interim recommendations were to defer the fourth and fifth dose of DTaP and the second dose of MMR, we examined coverage with the third dose of DTaP as well as the fourth dose because it was unknown if the shortage was so severe that it would result in delays of both doses. Similarly, for MMR, we examined receipt of the first dose; the NIS does not include children older than 35 months and thus does not collect information about MMR2.
The DTaP shortages began on March 1, 2001, and were resolved by July 31, 2002. Children born between August 1, 2000, and June 30, 2001, were age-eligible to receive the third dose of DTaP during the shortage period, that is, they were between the ages of 6 and 7 months at some time in the shortage period. We consider these children to be subject to the shortage for analyses of DTaP3 coverage. Children born between August 1, 1999, and June 30, 2001, were age-eligible to receive the fourth dose of DTaP during the shortage, that is, they were between the ages of 12 and 19 months at some time during the DTaP shortage. We consider these children subject to the DTaP shortage for analyses of fourth-dose DTaP coverage.
The MMR shortages began on October 1, 2001, and were resolved by July 31, 2002. Children born between June 1, 2000, and June 30, 2001, were age-eligible to receive their first dose of MMR during the MMR shortage period; that is, they were between the ages of 12 and 16 months at some time in the shortage period.
We compared the percentage of children who had received 3 or more doses of DTaP (3+DTaP) by 7 months of age and 4 or more doses of DTaP (4+DTaP) and 1 or more dose of MMR (1+MMR) by 19 months of age among those subject to the shortage versus those not subject to the shortage. We compared these percentages by χ2 tests. In a second type of analysis, for both visual comparison and to examine coverage at ages in addition to 7 and 19 months, we graphed cumulative vaccination coverage levels for DTaP3, DTaP4, and MMR1 by age (in months) for those children subject to the shortage versus those not subject to the shortage. Each point on the graph represents the vaccination coverage among all children at that age and younger; as one moves to the right, fewer children are added to the denominator (and numerator) because children included in the NIS are between 19 and 35 months of age.
As a control for secular trends, we performed these analyses on coverage with the third dose of polio vaccine (IPV3). There were no inactivated poliovirus shortages during the time of the survey.
In all analyses, we stratified the respondents and report results by a variety of subgroups to determine if the impact of the shortages was uniform across children in these subgroups. Categorization of site of care was based on physicians’ reports at the time vaccination data were collected and was defined as vaccinated only at public clinics, vaccinated only at private practices, or vaccinated at other or a mixture of practices (i.e., received vaccinations from hospital, military, or a mixture of several types of providers, including both public clinics and private practices).
Metropolitan statistical area (MSA) subgroups were MSA central city, MSA non–central city, and non-MSA. Census regions were Northeast, Midwest, South, and West. Race/ethnicity subgroups were Hispanic, non-Hispanic White, non-Hispanic Black, and all other non-Hispanic groups. The ratio of household income to the poverty level was calculated on the basis of reported household income, number of persons in the household, and US Census Bureau thresholds for poverty. Children were categorized into 1 of 4 groups: living above poverty for household income/poverty ratios greater than or equal to 125%, living near poverty for ratios 100% to 124%, living in intermediate poverty for ratios 50% to 99%, and living in severe poverty if the household income/poverty ratio was less than 50%.
All analyses were weighted and performed with SAS, release 8.02 (SAS Institute Inc, Cary, NC), and SUDAAN, release 8.0 (Research Triangle Institute, Research Triangle Park, NC), to take into account the complex nature of the survey. Proportions are reported along with 95% confidence intervals. All P values are 2 sided.
RESULTS
There were 45052 children in the combined 2001 and 2002 NIS with provider-verified vaccination data; 23642 in 2001 and 21410 in 2002. The overall response rate for eligible household was 63.8% in 2001 and 62.3% in 2002.6,7 More than half (58%) were vaccinated only at private practices, 15% were vaccinated only at public clinics, and 27% were vaccinated at other or a mixture of facility types. About one third (36%) resided in MSA central city regions, 46% in MSA non–central city, and 18% in non-MSA regions. More than two thirds (69%) were above poverty, 7% were near poverty, 14% were in intermediate poverty, and 10% in severe poverty. Almost one quarter (24%) were of Hispanic ethnicity, 56% were White non-Hispanic, 15% were Black non-Hispanic, and 5% were other non-Hispanic groups.
Table 1 ▶ presents vaccination coverage at the age of 19 months for those subject/not subject to the shortages for DTaP4 and MMR1 and at the age of 7 months for DTaP3 overall and among various subgroups of children. Among all children, there was a small but statistically significant difference in 4+DTaP coverage by the age of 19 months between those subject to and those not subject to the DTaP shortage (65.8% vs 67.6%, respectively, P=.02).
TABLE 1—
Vaccine Coverage Among Children Subject to and Not Subject to Shortage: United States, 2001–2002 National Immunization Survey
| Demographic Subgroups | 4+DTaP by 19 mo, % (95% CI) | 3+DTaP by 7 mo, % (95% CI) | 1+MMR by 19 mo,% (95% CI) | Control: 3+IPV by 19 mo, % (95% CI) |
| All children | ||||
| Subject to shortage | 65.8 (64.8, 66.8)* | 67.2 (65.0, 69.2) | 86.6 (85.3, 87.9) | 81.7 (80.9, 82.5)** |
| Not subject to shortage | 67.6 (66.5, 68.7)* | 66.1 (65.2, 66.9) | 85.7 (85.1, 86.4) | 79.9 (78.9, 80.9)** |
| Vaccination site | ||||
| Children vaccinated only at public clinics | ||||
| Subject to shortage | 59.4 (56.5, 62.3)** | 53.8 (47.4, 60.1) | 82.9 (78.4, 86.6) | 83.6 (81.3, 85.7) |
| Not subject to shortage | 65.4 (62.3, 68.4)** | 50.8 (48.5, 53.1) | 81.8 (79.5, 83.8) | 81.5 (79.0, 83.8) |
| Children vaccinated only at private practices | ||||
| Subject to shortage | 67.7 (66.4, 68.9) | 72.6 (70.0, 75.0) | 87.9 (86.2, 89.4) | 81.1 (80.0, 82.1) |
| Not subject to shortage | 68.8 (67.3, 70.3) | 71.5 (70.4, 72.5) | 87.3 (86.5, 88.1) | 79.6 (78.3, 80.9) |
| Children vaccinated at hospital, military, unknown, or a mixture of several practice types | ||||
| Subject to shortage | 65.2 (63.2, 67.2) | 61.6 (57.0, 66.1) | 85.7 (83.0, 88.0) | 82.0 (80.3, 83.5) |
| Not subject to shortage | 66.4 (64.3, 68.5) | 63.1 (61.5, 64.7) | 84.6 (83.4, 85.8) | 79.7 (77.9, 81.5) |
| Urbanicity | ||||
| MSA, central city | ||||
| Subject to shortage | 63.8 (62.1, 65.5) | 64.2 (60.5, 67.7) | 84.2 (81.5, 86.5) | 80.0 (78.6, 81.4) |
| Not subject to shortage | 65.2 (63.4, 67.1) | 63.3 (61.9, 64.7) | 84.9 (83.8, 86.0) | 78.5 (76.8, 80.1) |
| MSA, non–central city | ||||
| Subject to shortage | 67.8 (66.3, 69.3) | 70.7 (67.6, 73.7) | 88.8 (87.0, 90.5) | 82.1 (80.9, 83.3)* |
| Not subject to shortage | 68.6 (66.9, 70.3) | 69.8 (68.6, 71.1) | 87.2 (86.2, 88.1) | 80.0 (78.5, 81.5)* |
| Non, MSA | ||||
| Subject to shortage | 64.5 (62.3, 66.7)** | 64.0 (59.2, 68.5) | 85.7 (82.8, 88.2) | 83.9 (82.1, 85.6) |
| Not subject to shortage | 69.5 (67.2, 71.7)** | 61.9 (60.2, 63.7) | 83.6 (82.0, 85.1) | 82.4 (80.5, 84.1) |
| Census region | ||||
| Northeast | ||||
| Subject to shortage | 66.8 (64.4, 69.1) | 67.6 (62.4, 72.4) | 89.5 (86.3, 92.0) | 79.8 (77.8, 81.8)* |
| Not subject to shortage | 67.3 (64.7, 69.9) | 70.8 (68.9, 72.7) | 87.6 (86.0, 88.9) | 76.0 (73.4, 78.4)* |
| Midwest | ||||
| Subject to shortage | 67.1 (65.2, 68.9) | 69.6 (65.7, 73.2)* | 87.8 (85.4, 89.9)* | 81.3 (79.8, 82.8) |
| Not subject to shortage | 67.2 (65.2, 69.1) | 64.7 (63.2, 66.3)* | 84.7 (83.5, 85.9)* | 79.6 (77.9, 81.3) |
| South | ||||
| Subject to shortage | 64.9 (63.2, 66.6)* | 65.3 (61.7, 68.8) | 85.5 (83.1, 87.5) | 82.0 (80.6, 83.3) |
| Not subject to shortage | 67.8 (65.9, 69.7)* | 65.1 (63.7, 66.6) | 85.5 (84.3, 86.6) | 81.2 (79.5, 82.7) |
| West | ||||
| Subject to shortage | 65.3 (63.1, 67.5) | 67.5 (62.5, 72.1) | 85.5 (82.1, 88.3) | 82.8 (81.0, 84.5) |
| Not subject to shortage | 67.8 (65.2, 70.3) | 65.3 (63.4, 67.1) | 85.7 (84.2, 87.1) | 81.2 (79.0, 83.2) |
| Poverty a | ||||
| Above poverty | ||||
| Subject to shortage | 68.8 (67.6, 69.9) | 73.4 (71.1, 75.7) | 88.2 (86.6, 89.6) | 82.0 (81.1, 83.0) |
| Not subject to shortage | 70.2 (68.9, 71.5) | 72.3 (71.4, 73.2) | 87.4 (86.7, 88.1) | 80.7 (79.5, 81.8) |
| Near poverty | ||||
| Subject to shortage | 59.3 (54.5, 63.9) | 59.1 (47.9, 69.3) | 83.4 (73.1, 90.3) | 81.1 (76.6, 84.8) |
| Not subject to shortage | 64.0 (59.6, 68.3) | 54.6 (51.0, 58.2) | 82.6 (79.3, 85.4) | 77.6 (73.4, 81.2) |
| Intermediate poverty | ||||
| Subject to shortage | 59.3 (56.0, 62.5) | 55.0 (48.8, 61.0) | 83.3 (78.9, 86.9) | 81.4 (78.7, 83.8) |
| Not subject to shortage | 60.9 (57.1, 64.6) | 55.7 (53.0, 58.4) | 81.6 (79.0, 83.9) | 78.9 (75.5, 81.9) |
| Severe poverty | ||||
| Subject to shortage | 57.6 (53.9, 61.2) | 51.9 (44.2, 59.6) | 82.3 (77.1, 86.5) | 80.0 (77.0, 82.7) |
| Not subject to shortage | 59.1 (54.5, 63.5) | 50.3 (47.1, 53.6) | 82.2 (79.8, 84.3) | 78.7 (75.0, 82.0) |
| Race/ethnicity | ||||
| Hispanic | ||||
| Subject to shortage | 65.9 (63.6, 68.1) | 61.3 (56.1, 66.3) | 84.8 (81.3, 87.8) | 85.0 (83.3, 86.6) |
| Not subject to shortage | 67.9 (65.2, 70.5) | 63.3 (61.3, 65.2) | 86.5 (84.9, 88.0) | 82.6 (80.4, 84.6) |
| White, non, Hispanic | ||||
| Subject to shortage | 67.8 (66.6, 68.9) | 71.5 (68.9, 73.9) | 88.3 (86.7, 89.7)* | 81.5 (80.5, 82.5)* |
| Not subject to shortage | 69.1 (67.7, 70.4) | 70.2 (69.3, 71.2) | 86.2 (85.4, 87.0)* | 79.7 (78.5, 80.9)* |
| Black, non, Hispanic | ||||
| Subject to shortage | 57.7 (54.8, 60.6) | 60.0 (54.3, 65.5)* | 83.3 (79.5, 86.6) | 77.3 (74.8, 79.7) |
| Not subject to shortage | 60.6 (57.4, 63.7) | 53.6 (51.2, 56.1) | 82.5 (80.6, 84.4) | 76.6 (73.7, 79.2) |
| All others, non, Hispanic | ||||
| Subject to shortage | 67.3 (62.8, 71.5) | 70.9 (63.2, 77.6) | 88.3 (83.0, 92.1) | 80.6 (76.2, 84.4) |
| Not subject to shortage | 69.6 (64.6, 74.2) | 68.3 (64.6, 71.8) | 86.0 (82.6, 88.8) | 79.7 (74.9, 83.8) |
Note. 4+DTaP = 4 or more doses of diphtheria and tetanus toxoids and pertussis vaccine; 3+DTaP = 3 or more doses of diphtheria and tetanus toxoids and pertussis vaccine; 3+IPV = 3 or more doses of inactivated poliovirus vaccine; 1+MMR = 1 or more doses of measles, mumps, and rubella vaccine; CI = confidence interval; MSA = metropolitan statistical area.
aBased on US Census Bureau thresholds for poverty. Children were categorized into 1 of 4 groups: living above poverty for household income/poverty ratios ≥ 125%, living near poverty for ratios 100%–124%, living in intermediate poverty for ratios 50%–99%, and living in severe poverty if the household income/poverty ratio was < 50%.
*P< .05 for comparison of immunization coverage among those subject to the shortage versus those not subject to the shortage.
**P < .01 for comparison of immunization coverage among those subject to the shortage versus those not subject to the shortage.
Among children vaccinated only at public clinics, there was a significant difference in 4+DTaP coverage by the age of 19 months between those subject to and those not subject to the shortage (59.4% vs 65.4%, respectively, P<.01). Among children vaccinated only at private practices, there was no significant difference in 4+DTaP coverage by the age of 19 months (67.7% for those subject to the shortage, 68.8% for those not, P=.24). Among children in the Midwest and among Black non-Hispanic children, there was higher 3+DTaP coverage by 7 months of age among those subject to the shortage than among those not subject to the shortage (both P<.05).
Among children residing in non-MSA regions, the difference in 4+DTaP coverage by the age of 19 months was decreased among those subject to the shortage compared with those not subject to the shortage (64.5% vs 69.5%, respectively, P<.01). There were no differences in the other MSA regions. Likewise, among children residing in the southern census region of the United States, the difference in 4+DTaP coverage by 19 months of age was decreased among those subject to the shortage compared with those not subject to the shortage (64.9% vs 67.8%, P =.02), whereas there were no differences in the other census regions. We found no differential adverse effects of the shortages among race/ethnicity subgroups or among poverty-level subgroups.
Analysis of timeliness of vaccination, using a graphical representation and looking across a wide age interval rather than only 19 months of age (as in Table 1 ▶), revealed decreases in fourth-dose DTaP coverage among some children not only by 19 months but across a range of several months. In Figure 1 ▶, the graphs in the left column depict the age of children versus the percentage (cumulative) of children receiving the fourth dose of DTaP (DTaP4) by that age, comparing children eligible for vaccination during the shortage (solid lines) with those not eligible (dashed lines). The graphs in the right column represent the differences in vaccine coverage between the 2 groups (solid lines) and their 95% confidence intervals (dashed lines). Statistically significant differences in vaccination coverage between children eligible for DTaP4 vaccination during the shortage and children not eligible for DTaP4 vaccination during the shortage have confidence intervals that do not include 0, as seen in Figure 1b ▶ among children vaccinated only at public clinics.
FIGURE 1—
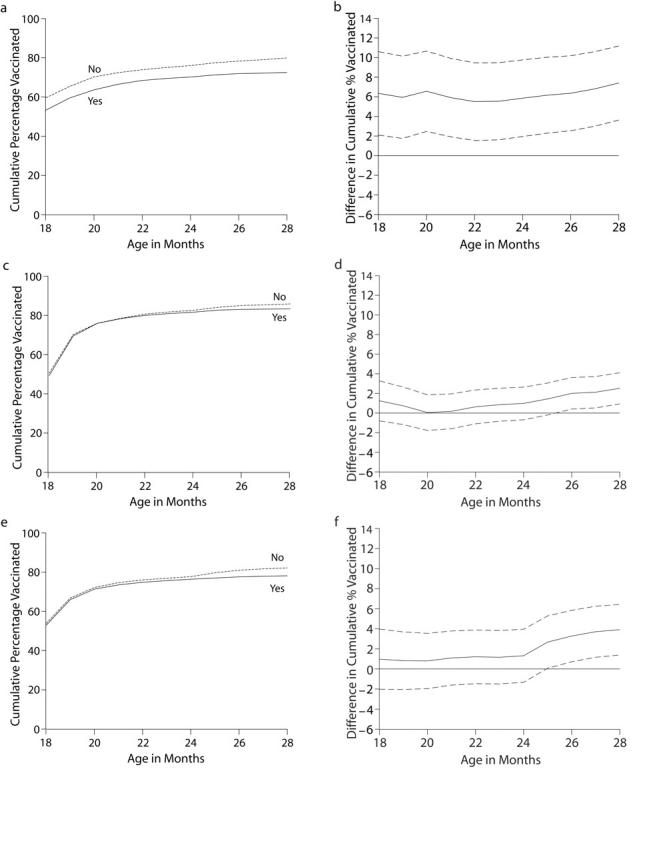
Vaccination coverage with the fourth dose of diphtheria and tetanus toxoids and pertussis vaccine (DTaP4) by eligibility for vaccination during the shortage and among children vaccinated only at public clinics (a,b), children vaccinated only at private practices (c,d), and children vaccinated at other or a mixture of practice types (e,f).
Note. The graphs in the left column depict age versus the percentage (cumulative) of children receiving DTaP4 by that age, comparing children eligible for vaccination during the shortage (solid lines) versus those not eligible dashed lines). The graphs in the right column represent the differences (solid lines) and their 95% confidence intervals (dashed lines). Statistically significant differences in vaccination coverage between children eligible for DTaP4 vaccination during the shortage and children not eligible for DTaP4 vaccination during the shortage have confidence intervals that do not include 0 (b).
Among children vaccinated only at public clinics, there was approximately a 6% or larger reduction in coverage among children subject to the shortage compared with children not subject to the shortage (Figures 1a and 1b ▶). This pattern did not occur among those who were vaccinated only at private practices or those vaccinated at other or a mixture of practice types; no adverse effects of the shortage were seen on coverage with DTaP4 up through approximately 25 months of age, after which there appeared to be a 2% reduction in coverage among those subject to the shortage compared with those not subject to the shortage (Figures 1c to 1f ▶). Graphs were produced for MSA and census region, and similar patterns were found (graphs not shown).
Similar plots of MMR1 (not shown) indicated no statistically significant differences between those subject to and those not subject to the shortage, either among all children or among those vaccinated only at public clinics or private practices. In addition, plots for IPV3 revealed no decreases in IPV3 coverage, either overall or among children vaccinated only at public clinics or only at private practices (data not shown).
DISCUSSION
In this study, we have shown that the recent DTaP shortage resulted in a differential delay in receipt of the fourth dose of DTaP among children vaccinated only at public clinics, with no similar pattern found among children vaccinated only at private practices or other practice types (Figure 1 ▶). Likewise, children in non-MSA areas and children living in the southern United States were differentially affected by the shortage (Table 1 ▶).
We found no significant adverse changes in the timeliness of receipt of the third dose of DTaP and in fact found small increases in such vaccination among children subject to the shortages, which may reflect the ability of providers to successfully prioritize their doses despite the short supply. A recent success in prioritization of influenza vaccine during the shortage of 2004–2005 has been reported.9 In addition, we found no impact on receipt of the first dose of MMR, a vaccine whose supply disruption was not as severe or long lasting as was seen with DTaP.
It is unlikely that the differences we found could be attributed to year-to-year variation in NIS coverage, because both DTaP and MMR vaccine coverage levels have been stable over the previous 3 NIS data years (1998–2000) (varying 2.2% or less for the national estimates and 1.6% or less for estimates at 19 months7). In our control analyses, we detected a small increase in coverage with 3 or more doses of IPV by 19 months of age among children subject to the DTaP shortage (Table 1 ▶), indicating a lack of downward secular trend.
Our finding of a differential vaccine delay among children served only in public clinics is of concern because public clinics often represent safety nets, serving on average the most vulnerable children.10 This differential effect is not unexpected because during the vaccine shortages, 1 of the manufacturers for DTaP vaccine made a decision to preferentially distribute available supplies of DTaP vaccine to private purchasers rather than to public purchasers (National Vaccine Advisory Committee; minutes from the October 2, 2001 meeting via teleconference and minutes from the June 4–5, 2002 meeting, Washington, DC; available on request from the committee at: http://www.hhs.gov/nvpo/nvac). Another possible contributor to the lower vaccination coverage among children served only in public clinics may be increased adherence in these clinics to the Advisory Committee on Immunization Practices’s interim recommendations. Adherence to the committee’s recommendations, however, is difficult to measure because the recommendation called for the deferral of the fourth and fifth dose of DTaP if the provider had an insufficient quantity of vaccine,1,2 a relatively subjective measure about which we did not collect information as part of this study.
In another study that focused on pneumococcal conjugate vaccine shortage, Freed and colleagues11 queried providers about their vaccine inventories and found no consistent pattern regarding the impact of the shortage on private versus public inventories of this vaccine. Some states and providers experienced shortages in their public inventories, whereas others experienced shortages in their private inventories or both inventories.
Although further study is needed to better define the reason or reasons for the decreased coverage levels found among children served in only public clinics, our results support the development of strategies, such as the use of specific language in government vaccine purchase contracts, to ensure the equitable distribution among public and private purchasers in the event of a supply disruption.
Our finding of a differential vaccine delay among children residing outside MSAs and in the southern United States are also of concern and challenging to interpret. On the basis of cross tabulations between these 3 variables (data not shown), we saw significant associations between the site of care and the MSA subgroup as well as the site of care and the census region, but it is unlikely that these associations fully explain the differential shortage effects. Therefore, increased shortage impact among children living outside MSAs and in the southern United States requires further study and consideration. It is possible that the current distribution system did not ensure an adequate supply of DTaP vaccine to those in rural areas or areas in the South during the DTaP shortage; thus, decreased vaccination coverage resulted among children in these areas compared with those in other areas of the United States. Monitoring geographic differences in vaccine distribution and supply during future shortages will be necessary to minimize the impact among the children living in these areas.
The impact of the vaccine supply shortages on immunization coverage that is documented in this study underscores the need for strategies to assist providers in the event of future vaccine supply disruptions. First, the role of federal, state, and local governments in monitoring vaccine inventories and ensuring an equitable distribution of vaccine within and among states is critical. Such activities were undertaken during the 2001–2002 shortages,12 and our findings point toward 2 geographic factors that may be worthy of particular attention in the event of future shortages. More than 50% of US preschool children currently receive public-purchased vaccines, which suggests that actions on the part of public health officials have the potential to impact a large number of US children.
Second, recent studies have shown that temporary recommendations issued during the shortages were adopted by only a minority of providers.11,13 Additional research is needed to understand the barriers to provider adoption and to develop strategies to overcome these barriers. Finally, for children whose immunization was delayed, catch-up immunization after the shortages can be facilitated by the use of recall systems. Despite their proven utility,14 such systems are infrequently used by providers. Improving the routine use of recall systems would position providers to be able to respond to future shortages.
Why do the results of this study differ from those of earlier analyses of NIS data, which did not demonstrate an impact of the DTaP shortage on coverage?6,7 There are at least 3 reasons. First, the NIS reports immunization coverage at the time of interview for children who aged 19 and 35 months at any point during a given calendar year. For both 2001 and 2002, NIS included children subject to and not subject to the DTaP shortage. Combining children both subject to and not subject to the shortages diluted the impact on overall coverage.
Second, as we have shown, the shortage’s impact was primarily on children vaccinated only in public clinics. In combined 2001–2002 NIS samples, 15% of the children saw only public providers. Thus, the group of children whom the shortage most impacted was too small to have much impact on overall NIS estimates. Third, NIS typically reports up-to-date immunization status, not timely immunization, which can be a more sensitive indicator of delay; the difference between the 2 analyses suggests that the standard NIS reporting measure of up-to-date status was not a sensitive enough measure to detect effects of the shortage.
Our study’s findings are subject to at least 3 limitations. First, because of the cohort method we used to define children as subject to the shortage, it is possible that vaccination coverage may be overestimated in this group because some children in this group were age-eligible to receive vaccination before or after the shortage occurred; however, we have found a significant reduction in coverage even in the face of such a possible overestimation of coverage. Thus, the effects of the shortage may have reduced coverage even more than what we found in this study.
Second, NIS is a telephone survey, and those without telephones or those who do not respond may have lower vaccination coverage levels than those who do respond; although statistical weights adjust for nonresponse and nontelephone households, some bias might remain.
Third, although NIS relies on provider-verified vaccination histories, incomplete records and reporting could result in underestimates of coverage if providers did not respond or did not provide all vaccination dates. The estimation procedure assumes that coverage among children whose providers do not respond is similar to that among children whose providers respond.
There was notable disparity in the effects of the recent vaccine shortages; children vaccinated only in public clinics, in rural areas, or in the southern United States were differentially affected by the shortages. This inequity of impact is troubling and needs to be addressed through monitoring of future vaccine supply disruptions and the development of strategies to manage these disruptions. These findings underscore the further need for vaccine policy that ensures the availability of vaccines and protection of the vaccine supply in order to prevent shortages.
Peer Reviewed
Contributors All the authors made substantial contributions to the conception of the study and to the analyses and interpretation of the data.
Human Participant Protection The National Immunization Survey protocol was approved by the institutional review board of the National Center for Health Statistics
References
- 1.Update on the supply of tetanus and diphtheria toxoids and of diphtheria and tetanus toxoids and acellular pertussis vaccine. MMWR Morb Mortal Wkly Rep. 2001;50:189–190. [PubMed] [Google Scholar]
- 2.Update: supply of diphtheria and tetanus toxoids and acellular pertussis vaccine. MMWR Morb Mortal Wkly Rep. 2002;50:1159. [PubMed] [Google Scholar]
- 3.Updated recommendations on the use of pneumococcal conjugate vaccine in a setting of vaccine shortage–Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2001;50:1140–1142. [Google Scholar]
- 4.Shortage of varicella and measles, mumps and rubella vaccines and interim recommendations from the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2002;51:190–197. [PubMed] [Google Scholar]
- 5.Impact of vaccine shortage on diphtheria and tetanus toxoids and acellular pertussis vaccine coverage rates among children aged 24 months—Puerto Rico, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:667–668. [PubMed] [Google Scholar]
- 6.National, state, and urban area vaccination coverage levels among children aged 19–35 months—United States, 2001. MMWR Morb Mortal Wkly Rep. 2002; 51:664–666. [PubMed] [Google Scholar]
- 7.National, state, and urban area vaccination coverage levels among children aged 19–35 months—United States, 2002. MMWR Morb Mortal Wkly Rep. 2003; 52(31):728–732. [PubMed] [Google Scholar]
- 8.Smith PJ, Hoaglin DC, Battaglia MP, Barker LE, Khare M. Statistical methodology of the National Immunization Survey: 1994–2002. Hyattsville, MD: US Department of Health and Human Services, National Center for Health Statistics; 2005. [PubMed]
- 9.Estimated influenza vaccination coverage among adults and children—United States, September 1, 2004–January 31, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:304–307. [PubMed] [Google Scholar]
- 10.Santoli JM, Barker LE, Lyons BH, Gandhi NB, Phillips C, Rodewald LE. Health department clinics as pediatric immunization providers: a national survey. Am J Prev Med. 2001;20:266–271. [DOI] [PubMed] [Google Scholar]
- 11.Freed GL, Davis MM, Clark SJ. Variation in public and private supply of pneumococcal conjugate vaccine during a shortage. JAMA. 2003;289:575–578. [DOI] [PubMed] [Google Scholar]
- 12.Childhood Vaccines: Ensuring an Adequate Supply Poses Continuing Challenges. Washington, DC: United States General Accounting Office; 2002; 1–42. GAO-02–987.
- 13.Stokley S, Santoli JM, Willis B, Kelley V, Vargas-Rosales A, Rodewald LE. Impact of vaccine shortages on immunization programs and providers. Am J Prev Med. 2004;26:15–21. [DOI] [PubMed] [Google Scholar]
- 14.Briss PA, Rodewald LE, Hinman AR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(1 suppl):97–123. [DOI] [PubMed] [Google Scholar]


