Abstract
The implantation of a blastocyst into a receptive uterus is associated with a series of events, namely the attachment reaction followed by decidualization of the stroma. Previous studies established that the gene encoding heparin-binding EGF-like growth factor (HB-EGF) is expressed in the luminal epithelium solely at the site of blastocyst apposition preceding the attachment reaction. We report here the expression during implantation of 21 genes encoding other signaling proteins, including those belonging to the Bone morphogenetic protein (BMP), fibroblast growth factor (FGF), WNT, and Hedgehog (HH) pathways. We find that the attachment reaction is associated with a localized stromal induction of genes encoding BMP-2, FGF-2, and WNT-4. Despite efforts by many investigators, a simple in vitro model of implantation is not yet available to study either the hierarchy of the events triggered in the uterus by the embryo or the function of individual signaling proteins. We have therefore approached these questions by introducing beads loaded with purified factors into the receptive uterus. We show that beads soaked in HB-EGF or insulin-like growth factor-1 (IGF-1), but not other proteins, induce many of the same discrete local responses elicited by the blastocyst, including increased localized vascular permeability, decidualization, and expression of Bmp2 at the sites of the beads. By contrast, the expression domains of Indian hedgehog (Ihh), patched, and noggin become restricted as decidualization proceeds. Significantly, beads containing BMP-2 do not themselves elicit an implantation response but affect the spacing of implantation sites induced by blastocysts cotransferred with the beads.
One of the prerequisites for mammalian reproduction is an effective reciprocal interaction between the implanting blastocyst and the receptive uterus. Unless this molecular dialogue is established, the embryo will not implant. Furthermore, failure to sustain the growth and differentiation of the uterus after blastocyst attachment results in spontaneous abortion. However, comparatively little is known about the hierarchy of events triggered by the simple apposition of an active blastocyst to the receptive uterine luminal epithelium (LE) (for reviews see refs. 1–3).
In the mouse, the initial attachment reaction between the blastocyst and the uterus occurs approximately between 2200–2300 h on Day 4 of pregnancy (Day 1 = day of plug). For unknown reasons, it always takes place on the LE of the antimesometrial (ventral) half of the uterus. The earliest change in gene expression so far reported during implantation is an increase in transcription of Hegf1, the gene encoding heparin-binding EGF-like growth factor (HB-EGF), in the LE immediately adjacent to the blastocyst. Transcription begins around 1600 h on Day 4, which is 6–7 h before the increase in vascular permeability (4). Hegf1 induction occurs in response to factor(s) secreted by an active blastocyst and cannot be triggered by a dormant blastocyst during delayed implantation unless the embryo is first activated by catechol-estrogen (4, 5). However, the crucial identity of this embryo-derived factor(s) is not yet known. Moreover, the precise function of HB-EGF itself is still unclear. There is evidence that it can act as a paracrine factor to promote blastocyst growth in vitro (4, 6), but the possibility that it induces changes in gene expression in the uterine epithelium and/or stroma associated with decidualization has not been explored.
Decidualization involves multiple changes in stromal cells adjacent to the implantation site, beginning on the antimesometrial side and spreading dorsally. Initially, there is an increased localized proliferation of mesenchymal cells, which then undergo postmitotic differentiation, forming the primary decidual zone (pdz) late on Day 5. Subsequently, the mesenchymal cells next to the pdz proliferate, forming the secondary decidual zone (sdz). These cells in turn differentiate and undergo polyploidization and finally apoptose, creating room for the growing embryo starting from Day 8 (for review see ref. 3). Characteristically, decidualizing cells hypertrophy, express high levels of alkaline phosphatase (7, 8), and synthesize extracellular matrix material, including type IV collagen, laminin, entactin, and fibronectin (9–11). Analysis of mouse mutants in which decidualization (as opposed to attachment) fails to occur has identified a requirement for genes expressed in the uterine epithelium and/or stromal cells of the implantation site. These include Hoxa10 and Hoxa11 (12, 13) and Cox2, the rate-limiting enzyme in prostaglandin synthesis (14–16). In addition, the gene encoding insulin-like growth factor-1 (Igf1) is normally expressed abundantly in decidual cells at the site of implantation (17), and the reduced fertility of Igf1 null mutant mice is consistent with a role for the protein in implantation and decidualization (18). The end result of decidualization is the accumulation of a spongy mass of tissue around each embryo. By Day 6, these decidual swellings are relatively evenly spaced along the length of the uterine horn (see, for example, Figs. 3 and 5), but precisely how this spacing is achieved is not known.
Figure 3.
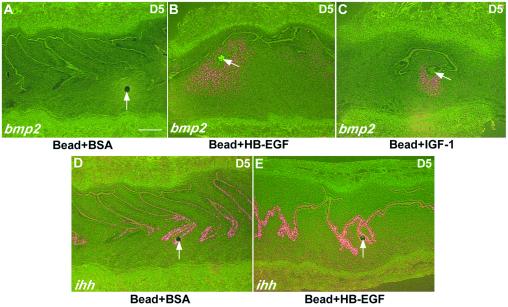
Local application of growth factors by means of beads mimics the effect of embryos on the pseudopregnant uterus. Beads preabsorbed with BSA, HB-EGF, EGF, TGFα, epiregulin (Epi), IGF-1, BMP2, BMP4, or SHH were transferred into each horn between 1000 and 1100 h on Day 4 of pseudopregnancy. Recipients were examined at 1100 h on Days 5, 6, or 8 for implantation reaction by the blue dye method. (A) Reaction to beads loaded with BSA compared with beads loaded with HB-EGF. (Bar, 0.5 cm.) (B) Quantitation of results. The numbers on top of the bars indicate the number of mice responding/total number of mice used, whereas the numbers within parentheses indicate the number of blue bands/total number of beads transferred. χ2 followed by Fisher's exact tests showed that the number of blue bands was significantly higher for beads loaded with HB-EGF or IGF-1 (P = 0.001) as compared with beads with BSA or other growth factors.
Figure 5.
Effects of BMP2- or BMP4-loaded beads on embryo spacing in the uterus. Four to five beads preadsorbed with BSA, BMP2, or BMP4 were cotransferred with 4–5 Day 4 blastocysts into each uterine horn on Day 4 of pseudopregnancy. (A) Uteri were examined and photographed on Day 9 to record embryo spacing. (Bar, 1 cm.) (B) The numbers on the top of the bars indicate the number of mice responded/total used, whereas the numbers within the bars indicate the number of implantation sites (IS)/total number of blastocysts transferred. When two or more decidual swellings touched or fused they were considered aberrantly spaced (“crowded”). χ2 followed by Fisher's exact tests showed that the number of crowded decidua was significantly higher (P = 0.001) in the uterus when blastocysts were cotransferred with BMP2 or BMP4 loaded beads as compared with BSA loaded beads. No significant difference between BMP2 and BMP4 loaded beads was noted.
The complex interplay between the embryo and uterus during implantation has many hallmarks of the reciprocal epithelial-mesenchymal interactions underlying the development of many organs, including the limb, tooth, hair follicle, lung, and kidney. Numerous studies have demonstrated the importance of a relatively small set of evolutionarily conserved signaling pathways in organogenesis. Of central importance are the hedgehog (HH), bone morphogenetic protein (BMP), fibroblast growth factor (FGF) and Wnt (WNT) families of proteins, as well as their antagonists, receptors, and components of intracellular signaling pathways (for reviews see refs. 19–21). Surprisingly, the uterine expression of many members of these important gene families has not been investigated during early implantation.
Understanding the hierarchy of events associated with implantation would be greatly facilitated by a simple system in which the response of the uterus to individual factors can be tested. To our knowledge, previous studies with organ cultures of uterine epithelium and stroma with respect to embryo implantation have not been successful. In this paper, we show that the receptive uterus responds to beads, approximately the size of blastocysts, loaded with either HB-EGF or IGF-1. Moreover, we show that this response mimics the response to a living embryo and involves the localized expression in the stroma of Bmp2. We also provide evidence that implantation involves other conserved signaling factors and their receptors or antagonists, including BMPs, WNTs, NOGGIN, and INDIAN HEDGEHOG.
Materials and Methods
Animals and Tissue Preparation.
CD-1 mice (Charles River Breeding Laboratories) were housed at the University of Kansas Medical Center in accordance with National Institutes of Health standards for the care and use of experimental animals. Adult females were mated with fertile or vasectomized males to induce pregnancy or pseudopregnancy, respectively (Day 1 = vaginal plug). Pregnancy on the mornings of Days 1–4 was confirmed by recovering embryos from the reproductive tracts. Implantation sites on Day 4 night or mornings of Days 5 and 6 were identified by the localized increase in uterine vascular permeability, detected after i.v. injections of Chicago Blue B solution (22). Richard Harland (University of California, Berkeley) kindly provided heterozygous Noglacz mice.
Preparation of Protein Carrying Beads.
Affi-Gel Blue Gel (Bio-Rad; 100–200 mesh, no. 153-7302) beads of about the size of a blastocyst were washed six times with sterile PBS and then incubated with various growth factors (100 ng/μl), or similar concentrations of BSA, in 20 μl PBS at 37°C for 1 h. Judy Abraham (Scios Nova, Sunnyvale, CA) kindly provided purified HB-EGF. For BMP2 and BMP4, beads were incubated in 10 μl of 5.5 μg/μl BMP2 or BMP4 (kindly provided by Genetics Institute, Cambridge, MA) together with 1.0 ng/μl of BSA at 37°C for 1 h. For hedgehog proteins, beads were incubated similarly with recombinant human SHH-N peptide, octyl modified (100 ng/μl, kindly provided by Curis, Cambridge, MA). After incubation, beads were washed in PBS several times and used immediately. Siliconized pipette tips and dishes were used for handling all growth factors. Loaded beads (seven beads/horn) were transferred into uteri of Day 4 pseudopregnant mice. To examine the effects of BMPs on embryo spacing, loaded beads (4–5 beads/horn) plus blastocysts (4–5 blastocysts/horn) were similarly transferred. Mice were killed between 0900 and 1000 h on Days 5–9 after blue dye injection. Uteri without blue bands were flushed to recover beads or unimplanted blastocysts. Mice without blue bands, beads, or blastocysts were excluded from the experiments.
Hybridization Probes.
Sense or anti-sense 35S-labeled cRNA probes (specific activities of 2 × 109 dpm/μg) were generated for in situ hybridization using appropriate polymerases from mouse-specific cDNAs. Ihh, Shh, Dhh, and Wnt4 probes were kindly provided by Andrew McMahon, Dan by Janet Rossant, Crim1 by Melissa Little, Fgf10 by Nobuyuki Itoh, and Tsg by Michael O'Connor.
In Situ Hybridization.
In situ hybridization was performed as previously described (4). Frozen sections (10 μm) were mounted onto poly-l-lysine-coated slides and fixed in 4% paraformaldehyde solution in PBS at 4°C. After prehybridization, sections were hybridized at 45°C for 4 h in 50% formamide buffer containing 35S-labeled sense or antisense cRNA probes. After hybridization, sections were incubated with RNase A (20 μg/ml) at 37°C for 20 min, and RNase A-resistant hybrids were detected by autoradiography using Kodak NTB-2 liquid emulsion. Sections hybridized with the sense probes served as negative controls. Sections were poststained with hematoxylin and eosin.
LacZ Staining.
The expression of β-galactosidase in uterine sections of Noglacz heterozygotes was assessed by LacZ staining as described (23). In brief, uterine pieces were fixed in 0.2% paraformaldehyde, followed by infusion in 30% sucrose at 4°C. Tissues were embedded in OCT and snap frozen in liquid Histo-Freeze (Fisher). Frozen sections (10 μm) were mounted onto glass slides and stained at 37°C in the dark using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (Life Technologies, Grand Island, NY) as a substrate. Sections were counterstained with eosin.
Results and Discussion
Expression of Bmp Genes During Implantation.
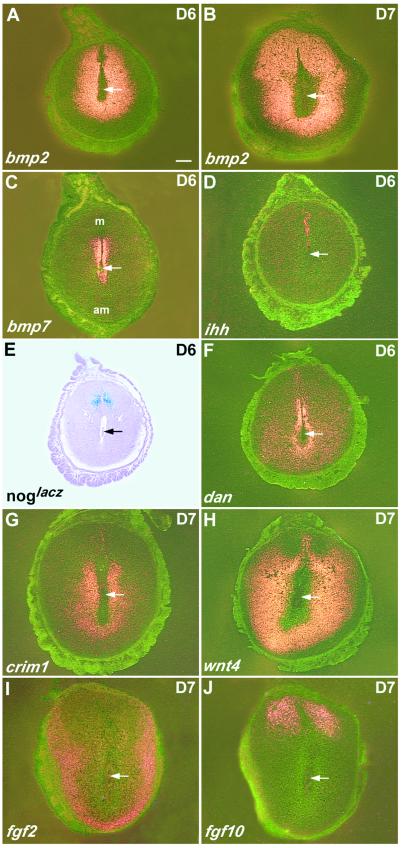
BMPs play important roles in mediating epithelial-mesenchymal interactions in many developing organs. We therefore examined the expression of a number of Bmp genes in the uterus at early stages of blastocyst implantation. We initially focused on midnight of Day 4, when the attachment reaction occurs, and there is already high, localized expression of Hegf1 in the LE (Fig. 1C). At this time, distinct accumulation of Bmp2 transcripts was detected in the stroma around the site of attachment (Fig. 1 A and B). More detailed time course studies failed to detect Bmp2 transcripts at 0900 h on Days 1–4 of pregnancy. After implantation, transcription continues in the decidua. Expression is initially highest in the proliferating stroma surrounding the implanting blastocyst, but, by Day 7, transcripts are absent in the postmitotic cells of the pdz, whereas cells in the sdz, which are still proliferating and undergoing hypertrophy, are positive (Fig. 2 A and B).
Figure 1.
Gene expression in the uterus associated with embryo implantation. Females were naturally mated and uteri collected on Day 4 (2400 h) or on Day 5 (0900 h) of pregnancy. Gene expression was assayed by in situ hybridization of uterine sections oriented with the mesometrial side uppermost. Position of blastocysts is marked with an arrow. (A) Bright- and dark-field views of Bmp2 expression. (B) Dark-field high power view of boxed region in A showing localized expression of Bmp2 in the stroma around the site of embryo attachment. (C) Section hybridized with Hegf1 probe showing localized expression in the antimesometrial LE at the site of blastocyst attachment. (D) Expression of Bmp7 throughout the endometrium excluding the muscle layer, with higher expression around the implantation sites. (E) Expression of Ihh in the LE. Note higher levels of Ihh transcripts at the antimesometrial pole. (F) Nogginlacz is expressed in the stroma underlying the LE. (G) Wnt4, (H) fgf2, and (I) fgf10 are all expressed in different patterns in the stroma. (Bars, 75 μm.) B, C, and E–I are shown at the same higher magnification, whereas A and D are at a lower magnification. am, antimesometrial; le, luminal epithelium; m, mesometrial; myo, myometrium; s, stroma.
Figure 2.
Gene expression in decidual cells on Day 6 or 7 of pregnancy. Following natural mating, uteri were collected on Day 6 or 7 of pregnancy and crosssections of the decidua assayed by in situ hybridization. (A and B) Sections hybridized with Bmp2 probe. Note change in the pattern of expression as the cells closest to the embryo terminally differentiate. (C) Restricted Bmp7 expression in the stroma around the implantation chamber. (D) Restricted expression of Ihh in the LE above the embryo. (E) Nogginlacz is expressed in the stroma underneath the remaining LE and overlaps that of Fgf10. (F) dan, (G) crim1, (H) wnt4, (I) fgf2, and (J) fgf10. (Bar, 75 μm.)
None of the other Bmp genes studied (Bmps 4, 5, 6, 7, and 8a and b) shows the same highly localized expression pattern as Bmp2. In the case of Bmp7, for example, expression is very dynamic. Transcripts are very low to undetectable in the LE on Days 1 and 2, and low levels are seen throughout the stroma on Days 3 and 4 (data not shown). At 2400 h on Day 4, Bmp7 expression is still seen in the stroma but is significantly higher around the site of implantation (Fig. 1D). On days 5–6, Bmp7 transcripts are present in a very restricted domain in the stroma underlying the intact LE adjacent to the embryo more mesometrially (Fig. 2C) and decline thereafter. Bmp5 shows low levels of expression in the stroma close to the myometrium and myometrial connective tissues on Day 4, but is undetectable from Day 5, whereas Bmp6 transcripts are not significantly above background (data not shown). A low level of Bmp4 transcripts is detected on Day 1 in the LE and from Day 4 expression shifts to the stroma, but is not localized to any specific domain (data not shown). In conclusion, of the various Bmp genes studied, only Bmp2 shows a tight correlation between the onset of high, localized expression in the stroma and the early implantation reaction.
Expression of BMP Antagonists.
In many developing systems, antagonists of growth factors, including those of BMPs and WNTs, are key components of intercellular signaling networks, binding to ligands and regulating their ability to interact productively with their receptors. The precise function of antagonists may be complex, however. For example, BMP binding proteins may facilitate dissemination/diffusion of the ligands beyond their site of production, or in the case of TWISTED GASTRULATION (TSG), either promote BMP activity or inhibit it, depending on the presence of another extracellular antagonist, CHORDIN (24). We therefore used in situ hybridization to examine the expression in the mouse uterus of a number of genes encoding known or putative growth factor antagonists.
Expression of the gene encoding NOGGIN (NOG), which binds to BMPs 2, 4, and 7, was monitored using the mouse line, Noglacz in which the gene has been replaced by lacZ (25, 26). At the time of implantation, Noglacz is expressed in the stromal layer immediately underlying the LE (Fig. 1F). However, time course studies showed that this same expression pattern is present earlier (data not shown). As the decidual region develops, transcription becomes restricted to the stroma underlying the intact mesometrial epithelium (Fig. 2E), overlapping with the Fgf10 expression domain described below (Fig. 2J).
Before and around the time of implantation, Dan, a member of the Dan/Dante BMP antagonist gene family (27), has a similar expression pattern underlying the LE to that of Nog. Decidual expression continues through Day 6, when there is somewhat higher expression in the pdz (Fig. 2F). Crim1, encoding a protein that has been suggested, but not yet shown, to bind both IGF and BMP (28), is expressed in the LE, beginning on Day 4, but before the attachment reaction has been initiated. Expression remains high in the intact epithelium during Days 5 and 6 (data not shown). On Days 7 and 8, expression is seen in the decidua, at highest levels close to the embryo in a domain partially overlapping with that of Bmp2 at this time (compare Fig. 2 B and G). The gene Tsg is initially expressed in the LE on Day 1, in the same pattern as Bmp4 and then throughout the endometrium at low levels (data not shown).
In conclusion, genes encoding growth factor antagonists show different dynamic patterns of expression before, during and after implantation. These results are consistent with the encoded proteins modulating the availability of active growth factor within the LE and stroma, and/or regulating the transfer of ligands between them. However, none of the patterns is tightly correlated with the early response of the uterus to embryo attachment.
Expression of Wnts and Fgfs.
To examine whether WNT and FGF proteins are involved in the signaling cascade during implantation, we examined the expression of Wnt 4 and Fgfs2, 4, 8, and 10 in the uterus during the periimplantation period. Wnt4 expression was undetectable in the uterus at 0900 h on Day 4, but increased in the stroma surrounding the embryo at the onset of attachment at midnight of Day 4, with a further increase on Day 5 (Fig. 1G). The expression markedly increased thereafter in the decidua (Fig. 2H). The expression of Fgf2 and Fgf10 showed unique patterns in the stroma from the time of implantation. Fgf2 transcripts were localized in the antimesometrial stroma at the site of implantation (Fig. 1H) and thereafter their expression partially overlapped with those of Bmp2 and Wnt4 (Fig. 2I). By contrast, the expression of Fgf10 was localized mesometrially (Figs. 1I and 2J). The expression of Fgf4 and Fgf8 in the uterus was insignificant during the periimplantation period.
Expression of Hedgehog Genes During Implantation.
Of the three members of the mammalian hedgehog gene family, no distinct hybridization signal was detected in the uterus with Shh or Dhh probes, at least on Days 4, 6, and 8 of pregnancy (data not shown). By contrast, Ihh is dynamically expressed at high levels in the LE and endometrial glands. As shown in Fig. 1E, there appears to be an asymmetric distribution of transcripts on Day 4, with highest levels in the antimesometrial LE. The distribution of ptc transcripts in the stroma and around the endometrial glands (data not shown) suggests that IHH protein signals to the underlying stroma where, by analogy with the mitogenic activity of hedgehog protein in other organs (reviewed in ref. 19), it may promote cell proliferation. However, there is no specific up-regulation of Ihh at the site of blastocyst attachment and Ihh expression declines in the LE as implantation proceeds, becoming restricted to the epithelium above the embryo by Day 6 (Fig. 2D). Note that this region appears adjacent to the domain of noggin expression in the stroma. The lack of temporal and spatial correlation between Ihh and Bmp2 expression suggests that the later gene is not a target of hedgehog signaling in the uterus, as found in some other organs.
Local Application of Proteins Mimics the Effect of Living Embryos.
The gene encoding HB-EGF is expressed in the LE at the site of blastocyst attachment, probably in response to an unknown, secreted factor produced by the embryo. Expression is seen at least 6–7 h before the local increase in permeability of the endometrial blood vessels (4). This raises the possibility that HB-EGF protein acts as a paracrine factor on the underlying stroma. To test this hypothesis, and to see to what extent the responses of the uterus to living embryos that we have observed can be induced by individual proteins, beads soaked in HB-EGF were transferred to the uterine lumen on Day 4 of pseudopregnancy. As shown in Fig. 3, these beads were very effective in inducing attachment reactions and decidual responses, as judged by discrete, localized increases in vessel permeability (blue bands) on Day 5, and by the formation by Day 6 of decidua that are about the same size as those induced by embryos. Histological examination also showed decidual cells with large polyploid nuclei (data not shown). Decidualization proceeded through Day 10, but by Day 12 sloughing of the decidual mass was evident, showing that the uterine response was not sustained. A similar response was also seen with IGF-1 loaded beads. Importantly, there was no significant response to beads loaded with BSA, EGF, TGFα, epiregulin, BMP2 and BMP4 (Fig. 3), or SHH (data not shown). In conclusion, the localized decidual response induced by HB-EGF- or IGF1-loaded beads is apparently similar to that produced by implanting blastocysts.
The region of the uterus around the beads loaded with HB-EGF was then examined 24 h after bead transfer for expression of genes associated with the attachment reaction. As shown in Fig. 4, beads loaded with either HB-EGF or IGF-1, but not with BSA, induce the expression of Bmp2 in the antimesometrial stroma immediately adjacent to the bead. Significantly, high-power magnification shows that the LE around the bead at this time remains intact. Moreover, there is no significant change in the expression of Ihh in the LE in response to HB-EGF beads (Fig. 4 D and E). The integrity of the LE around the HB-EGF bead at Day 5 is in contrast to the very rapid (within 10–15 min) damage and death of the LE observed in response to oil or air, agents that will induce a decidualization-like response when introduced into the receptive uterus (29). The responses to HB-EGF or IGF-1 loaded beads are thus similar to those initiated by blastocysts. These results support the hypothesis that HB-EGF secreted by the LE is a paracrine regulator of Bmp2 expression in the stroma. BMP2 protein, in combination with other factors such as FGF2, may then play a role in promoting the increased proliferation, hypertrophy, alkaline phosphatase activity and extracellular matrix synthesis associated with the process of decidualization. Numerous studies have shown that BMP2 is a potent inducer of alkaline phosphatase activity and extracellular matrix production in mesenchymal cells in culture (30, 31). In addition, a crosstalk between IGF-1 and HB-EGF in ERK activation by an autocrine loop (32) and a synergistic effect between these growth factors in wound healing (33) have been reported.
Figure 4.
Gene expression in response to growth factor loaded beads. Beads (7 beads/horn) preabsorbed either in BSA, HB-EGF, or IGF-1 (100 ng/μl) were transferred into uterine lumens of day 4 pseudopregnant mice. Mice were killed on Day 5 to examine Bmp2 and Ihh expression at the sites of beads. Arrows indicate the locations of the beads. Note localized stromal expression of Bmp2 at the sites of beads preabsorbed with HB-EGF or IGF-1. (Bar, 75 μm.)
Beads soaked with BMP2 or BMP4 do not induce an attachment reaction (Fig. 3B), showing that these proteins cannot substitute for HB-EGF. We therefore tested the effect of BMP beads on the implantation of normal blastocysts. Four to five BMP beads were transferred together with four to five Day 4 blastocysts into each uterine horn on Day 4 of pseudopregnancy and the number and distribution of decidua observed 5–6 days later. As shown in Fig. 5B, the BMP2 and BMP4 beads had no significant effect on the proportion of blastocysts implanting, compared with BSA beads (74%, 71%, and 66%, respectively). However, there was a significant effect on the spacing of the embryos. In the uteri receiving blastocysts and BSA loaded beads, the embryos are well spaced. By contrast, in the uteri receiving the BMP loaded beads, significantly more of the sites were much closer together or touching (Fig. 5 A and B). Beads preabsorbed with SHH did not alter embryo spacing (data not shown). These results show that BMP2/4 affects the overall spacing of embryos in the uterus, sometime after the transfer of the blastocysts on Day 4.
Relatively little is known about the cellular mechanisms controlling the spacing of embryos in the mouse uterus. According to one model, the distribution of embryos along the horn is effected by the peristaltic contractions of the myometrium, at least before the surfaces of the LE become tightly apposed, immobilizing the embryos between them (34). An earlier model proposed a process of serial implantation coupled with the rapid establishment of inhibitory zones around the early sites discouraging the implantation of other embryos in the vicinity. However, this model has been discounted (35). At present, we favor the possibilities that local high levels of Bmp2 in the lumen of the Day 4 pseudopregnant uterus either inhibit the mobility of the uterus or induce premature closing of the lumen, so that embryos cannot distribute evenly. Interestingly, a recent report provides genetic evidence that BMP5 and the TGFβ-related protein, NODAL, are factors important for embryo spacing in the uterus during implantation, although the precise mechanism of their action is not known (36).
In conclusion, our discovery that purified growth factors on small beads can induce many of the local changes in the uterus normally elicited by a living embryo has opened up possibilities for the in vivo functional analysis of signaling pathways during implantation. In addition, the possibility is being explored that the technique can be used to identify the crucial factor(s) secreted by a competent blastocyst which induce Hegf1 in the adjacent LE.
Acknowledgments
We thank Anne McLaren for helpful comments on the manuscript and stimulating discussion. Many investigators generously provided reagents and we thank, in particular, Melissa Little and Michael O'Connor, who made the Crim1 and Tsg probes available before publication, and Richard Harland for providing Noglacz mice. B.L.M.H. is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by National Institutes of Health Grants HD12304 and HD29968 (S. K. Dey), HD37394 (B.C.P.), and ES07814 (S. K. Das). S. K. Dey is a National Institute of Child Health and Human Development MERIT awardee.
Abbreviations
- LE
luminal epithelium
- BMP
bone morphogenetic protein
- FGF
fibroblast growth factor
- HB-EGF
heparin-binding EGF-like growth factor
- IGF-1
insulin-like growth factor-1
- HH
hedgehog
References
- 1.Ma L, Yao M, Maas R L. Semin Reprod Endocrinol. 1999;17:205–216. doi: 10.1055/s-2007-1016228. [DOI] [PubMed] [Google Scholar]
- 2.Carson D D, Bagchi I, Dey S K, Enders A C, Fazleabas A T, Lessey B A, Yoshinaga K. Dev Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 3.Dey S K. In: Reproductive Endocrinology, Surgery and Technology. Adashi E Y, Rock J A, Rosenwaks Z, editors. New York: Lippincot–Raven; 1996. pp. 421–434. [Google Scholar]
- 4.Das S K, Wand X-N, Paria B C, Damm D, Abraham J A, Klagsbrun M, Andrews G K, Dey S K. Development (Cambridge, UK) 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 5.Paria B C, Lim H, Wang X-N, Liehr J, Das S K, Dey S K. Endocrinology. 1998;139:5235–5246. doi: 10.1210/endo.139.12.6386. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Mayernik L, Schultz J F, Armant D R. Development (Cambridge, UK) 2000;127:33–44. doi: 10.1242/dev.127.1.33. [DOI] [PubMed] [Google Scholar]
- 7.Finn C A, McLaren A. J Reprod Fertil. 1967;13:259–267. doi: 10.1530/jrf.0.0130259. [DOI] [PubMed] [Google Scholar]
- 8.Pollard J W, Jahan M, Butterworth P J. J Reprod Fertil. 1990;89:735–742. doi: 10.1530/jrf.0.0890735. [DOI] [PubMed] [Google Scholar]
- 9.Wewer U M, Damjanov A, Weiss J, Liotta L A, Damjanov I. Differentiation. 1986;32:49–58. doi: 10.1111/j.1432-0436.1986.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 10.Wartiovaara J, Leivo I, Vaheri A. Dev Biol. 1979;69:247–257. doi: 10.1016/0012-1606(79)90289-6. [DOI] [PubMed] [Google Scholar]
- 11.Leivo I, Vaheri A, Timpl R, Wartiovaara J. Dev Biol. 1980;76:100–114. doi: 10.1016/0012-1606(80)90365-6. [DOI] [PubMed] [Google Scholar]
- 12.Benson G V, Lim H, Paria B C, Satokata I, Dey S K, Maas R L. Development (Cambridge, UK) 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 13.Gendron R L, Paradis H, Hsieh-Li H M, Lee D W, Potter S S, Markoff E. Biol Reprod. 1997;56:1097–1105. doi: 10.1095/biolreprod56.5.1097. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty I, Das S K, Wang J, Dey S K. J Mol Endocrinol. 1996;16:107–122. doi: 10.1677/jme.0.0160107. [DOI] [PubMed] [Google Scholar]
- 15.Lim H, Paria B C, Das S K, Dinchuk J, Langenbach R, Trzaskos J M, Dey S K. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 16.Lim H, Gupta R A, Ma W, Paria B C, Moller D E, Morrow J D, DuBois R N, Trzaskos J M, Dey S K. Genes Dev. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapur S, Tamada H, Dey S K, Andrews G K. Biol Reprod. 1992;46:208–219. doi: 10.1095/biolreprod46.2.208. [DOI] [PubMed] [Google Scholar]
- 18.Barker J, Hardy M P, Zhou J, Bondy C, Lupu F, Bellve A R, Efstratiadis A. Mol Endocrinol. 1996;10:903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- 19.Hogan B L M. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 20.Ingham P W. EMBO J. 1998;17:3505–3511. doi: 10.1093/emboj/17.13.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massague J, Chen Y G. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 22.Paria B C, Huet-Hudson Y M, Dey S K. Proc Natl Acad Sci USA. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan B L M, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo, a Laboratory Manual (Section H) Plainview, NY: Cold Spring Harbor Lab. Press; 1994. pp. 373–375. [Google Scholar]
- 24.Oelgeschlager M, Larrain J, Geissert D, De Robertis E M. Nature (London) 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerman L B, De Jesus-Escobar J M, Harland R M. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 26.Brunet L J, McMahon J A, McMahon A P, Harland R M. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 27.Pearce J J H, Penny G, Rossant J. Dev Biol. 1999;209:98–110. doi: 10.1006/dbio.1999.9240. [DOI] [PubMed] [Google Scholar]
- 28.Kolle G, Georgas K, Holmes G P, Little M H, Yamada T. Mech Dev. 2000;90:181–193. doi: 10.1016/s0925-4773(99)00248-8. [DOI] [PubMed] [Google Scholar]
- 29.Lundkvist O, Nilsson B O. Cell Tissue Res. 1982;225:355–364. doi: 10.1007/BF00214688. [DOI] [PubMed] [Google Scholar]
- 30.Thies R S, Bauduy M, Ashton B A, Kurtzberg L, Woznry J M, Rosen V. Endocrinology. 1992;130:1318–1324. doi: 10.1210/endo.130.3.1311236. [DOI] [PubMed] [Google Scholar]
- 31.Bachner D, Ahrens M, Schroder D, Hoffmann A, Lauber J, Betat N, Steinert P, Flohe L, Gross G. Dev Dyn. 1998;213:398–411. doi: 10.1002/(SICI)1097-0177(199812)213:4<398::AID-AJA5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Roudabush F L, Pierce K L, Maudsley S, Khan K D, Luttrell L M. J Biol Chem. 2000;275:22583–22589. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- 33.Marikovsky M, Vogt P, Eriksson E, Rubin J S, Taylor W G, Joachim S, Klagsbrun M. J Invest Dermatol. 1996;106:616–621. doi: 10.1111/1523-1747.ep12345413. [DOI] [PubMed] [Google Scholar]
- 34.Crane L H, Martin L. Reprod Fertil Dev. 1991;3:233–244. doi: 10.1071/rd9910233. [DOI] [PubMed] [Google Scholar]
- 35.McLaren A, Michie D. Mem Soc Endocrinol. 1959;6:65–74. [Google Scholar]
- 36.Pfendler K C, Yoon J W, Taborn G U, Kuehn M R, Iannaccone P M. Genesis. 2000;28:1–14. doi: 10.1002/1526-968x(200009)28:1<1::aid-gene10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]