Abstract
Objective. We assessed the association of cigarette smoking with the effectiveness of highly active antiretroviral therapy (HAART) among low-income women.
Methods. Data were analyzed from the Women’s Interagency HIV Study, a multisite longitudinal study up to 7.9 years for 924 women representing 72% of all women who initiated HAART between July 1, 1995, and September 30, 2003.
Results. When Cox’s regression was used after control for age, race, hepatitis C infection, illicit drug use, previous antiretroviral therapy, and previous AIDS, smokers on HAART had poorer viral responses (hazard ratio [HR]=0.79; 95% confidence interval [CI]=0.67, 0.93) and poorer immunologic response (HR=0.85; 95% CI=0.73, 0.99). A greater risk of virologic rebound (HR=1.39; 95% CI=1.06, 1.69) and more frequent immunologic failure (HR=1.52; 95% CI=1.18, 1.96) were also observed among smokers. There was a higher risk of death (HR=1.53; 95% CI=1.08, 2.19) and a higher risk of developing AIDS (HR=1.36; 95% CI=1.07, 1.72) but no significant difference between smokers and nonsmokers in the risk of death due to AIDS.
Conclusions. Some of the benefits provided by HAART are negated in cigarette smokers.
HIV-positive women in the United States tend to be from lower socioeconomic strata in which smoking is common, and its adverse effects add to the burdens of HIV infection and poverty.1 Other articles have suggested that smoking may modify CD4+ lymphocyte counts, but findings have not consistently established a relation between smoking and the course of HIV/AIDS.2–5 In the Multicenter AIDS Cohort Study, no association was found between smoking and the risk of developing AIDS or dying, but the study included only gay men of middle and upper socioeconomic status.6 Also missing from this report were data on viral load, and the study was conducted before the use of highly active antiretroviral therapy (HAART) became widespread. We therefore conducted a study taking advantage of a longitudinal data set of HIV-infected women whose smoking status, viral load status, immune status, and antiretroviral use were well documented. To our knowledge, no previous studies have assessed the association of smoking with response to HAART.
METHODS
Study Design
The Women’s Interagency HIV Study (WIHS) is a multisite longitudinal cohort study of HIV infection among women enrolled between 1994 and 1995 at 6 sites in the United States (Bronx/Manhattan, NY; Brooklyn, NY; Washington, DC; San Francisco/Bay Area, Calif; Los Angeles/Southern California; and Chicago, Ill). Details of study objectives, design, and methods have been published elsewhere.7 The study was originally designed to investigate the natural course of HIV infection among women, to identify clinical and behavioral factors associated with disease progression, and to assess the effects of treatment on the clinical course of HIV/AIDS.
Study participants were screened and enrolled if they were aged at least 13 years, agreed to be tested for HIV, could complete assessments in either English or Spanish, and were able to travel to and from the study site. In order to be accepted into the study, during their initial visit, participants were required to complete a series of structured core assessments on sociodemographic characteristics, mental and physical health history, alcohol and drug use, sexual behavior, medication, and health care utilization. A physical examination and routine laboratory tests were also required. Follow-up visits were scheduled semiannually and included similar assessment tools, repeat physical examinations, and additional laboratory tests. All participants were offered remuneration for baseline and follow-up interviews consistent with local institutional review board and community advisory board guidelines. Written informed consent was obtained at the time of initial WIHS study enrollment. After 5 years, approximately 80% of the participants remained in the study.8
During each subsequent study visit, HAART compliance was reassessed. Definitions of HAART and usage patterns in the WIHS study have been described.9,10
Laboratory Methods
The HIV-1 RNA viral load in plasma was measured with the isothermal nucleic acid sequence–based amplification (Nuclisens) method (bioMérieux, Boxtel, Netherlands) and the tests were restricted to laboratories participating in the National Institutes of Health/National Institute of Allergy and Infectious Disease Virology Quality Assurance Laboratory proficiency testing program. Viral load levels less than 80 copies/mL were reported as undetectable. Lymphocyte subsets were quantified with standard flow cytometric methods, and again the tests were restricted to laboratories participating in the National Institutes of Health/National Institute of Allergy and Infectious Disease Flow Cytometry Quality Assessment Program.11
Exposure Variable and Covariates
Participants were asked about smoking habits, and anyone reporting smoking since the last study visit (i.e., past 6 months) was considered a smoker. The amount smoked and cumulative pack-year histories were also determined but did not change the results and were not included. A positive response, since the last 6-month visit, to use of either cocaine/crack or heroin was regarded as evidence for current illicit drug use. Adherence measures, previously described in detail,12 were introduced to WIHS instruments in October 1998. Participants were classified according to whether they reported taking all drugs as prescribed at least 95% of the time since the previous 6-month visit. On the basis of these self-reports, we constructed a covariate that reflected the percentage of study visits during which patients took their medications. Those individuals who reported taking HAART at least 95% of the times at every study visit were classified as compliant to treatment.
Outcome Variables
We estimated the time from HAART initiation (defined as the midpoint between the last visit without HAART and the first visit with HAART use) to both viral and immunologic marker responses to HAART and to the onset of clinical events, including the incidence of AIDS-defining conditions (ADCs) and death. Additionally, we estimated the time from viral response (the first time HIV-1 RNA dropped to ≤ 80 copies/mL) to subsequent viral failure (the first time HIV-1 RNA increased to > 1000 copies/mL after the patient achieved an initial viral response). We also tracked the time from immunologic response (the first time CD4+ lymphocyte counts increased by at least 100 cells beyond pre-HAART CD4+ nadir levels) to subsequent immunologic failure (the first time CD4+ lymphocyte counts dropped below the pre-HAART nadir level after the patient attained an immunologic response). Data were censored at the last interview date for patients who survived as well as for those who did not attain the event of interest (i.e., death). All deaths from any cause, including death from AIDS on the basis of a previous algorithm, were considered events of interest in the survival analyses.13 Data were analyzed on an intent-to-treat basis, ignoring treatment changes and interruptions.
As established by the Centers for Disease Control and Prevention (CDC) in 1993, ADCs (excluding CD4+ lymphocyte counts < 200) were ascertained through self-report or confirmed through cancer and tuberculosis registries.14 In the models measuring HAART efficacy, any ADC was recorded as incident, except for diagnoses of cervical cancer, Kaposi’s sarcoma, non-Hodgkin’s lymphoma, tuberculosis, or wasting syndrome before HAART initiation. An ADC reported during the study visit when HAART was first reported was not classified as incident.
Information regarding deaths was obtained from study participants’ friends, relatives, and medical providers, and through medical and death registries. Methods for determining cause and date of death have been described elsewhere.13
Statistical Methods
CD4+ lymphocyte count and viral load trends between smokers and nonsmokers were analyzed with a repeated measures mixed model and adjusted for age and race. Interactions between smoking status and visit number were included to estimate trends over time in CD4+ lymphocyte counts and viral load levels in smokers relative to nonsmokers. We employed a simple compound symmetrical correlation matrix that assumed equal correlation between pairs of time points. Survival time was modeled as a function of single (“fixed”) values of smoking and of the other covariates collected at the visit before the start of HAART. The models estimated the heterogeneity in the risk of each outcome by pre-HAART smoking status, with adjustment for age, race, baseline hepatitis C infection, self-reported baseline exposure category (including illicit injection drug use, male–female sexual behavior that might lead to transmission of AIDS, and transfusion history), pre-HAART CD4+ lymphocyte count, pre-HAART viral load, pre-HAART illicit injection and non-injection drug use, previous self-reported AIDS, previous antiretrovial therapy use, and adherence category. Relevant variables (shown in Table 1 ▶) were placed into the model, and those that were not significant (P > .05) were deleted in backward stepwise fashion. We also examined models that stratified patients according to their level of adherence to HAART.
TABLE 1—
Selected Characteristics in Women’s Interagency HIV Study Patients by Smoking Status Before Highly Active Antiretroviral Therapy (HAART): United States, 1995–2003
| Smoking Status Before HAARTa | |||
| Nonsmoker (n = 400), % | Smoker (n = 524), % | P | |
| Race | |||
| African American | 50.3 | 67.0 | |
| White | 21.5 | 17.2 | .001 |
| Hispanic | 28.3 | 15.8 | |
| Crack, cocaine, heroin use | 4.0 | 24.4 | .001 |
| Hepatitis C infection | 24.9 | 54.9 | .001 |
| Risk category | |||
| Illicit injection drug use | 16.1 | 45.2 | |
| Heterosexual | 52.9 | 36.3 | .001 |
| Transfusion | 4.5 | 4.1 | |
| Unknown | 26.5 | 14.5 | |
| Previously diagnosed with AIDS | 37.3 | 52.1 | .001 |
| Naive to antiretroviral therapy | 16.0 | 17.0 | .69 |
| Smoker at baseline | 8.7 | 90.3 | .001 |
| Log CD4+ cell (mean ±SE) | 5.17 (0.062) | 5.39 (0.046) | .005 |
| Log viral load (mean ±SE) | 9.51 (0.123) | 9.40 (0.112) | .42 |
aSmoking status at the visit before HAART was missing for 37 women who were excluded from this study.
SAS software version 8.02 (SAS Institute Inc, Cary, NC) was used to conduct all study analyses.
RESULTS
Cigarette Smoking in the WIHS
Smoking was very common among WIHS participants. At the time of enrollment, 56% of the women “currently smoked,” and an additional 16% had smoked previously. The average level of cumulative tobacco exposure at enrollment was 12.4 pack-years for women who were current smokers and 4.2 pack-years for women who had smoked previously. Given that the average age at enrollment was 36 years, the typical WIHS patient had smoked a pack of cigarettes per day for more than 30% of her entire life, and 50% of her life since adolescence. For eligible women on HAART (as described later) with data on smoking (n = 924), 70% were relatively consistent in their smoking habits up to 9 years after enrollment, with 31% reporting smoking during less than 10% of the follow-up visits and 39% reporting smoking during at least three quarters of the visits. Of the 909 women who reported smoking status at baseline and at the visit before HAART, 93.2% (466/500) smoked at both times.
HAART Use in the WIHS
Of the 2059 HIV-1–seropositive women enrolled, 1277 (62.0%) initiated HAART between July 1, 1995, and September 30, 2003. Among these women, 1083 (84.8%) had all post-HAART HIV-1 RNA viral load assays performed that could detect down to 80 copies/mL or consistently had detectable HIV-1 RNA. In addition, HIV-1 RNA viral loads, CD4+ lymphocyte counts, and anti-retroviral therapy data were available before HAART initiation. These same patients all had peak pre-HAART HIV-1 RNA viral levels greater than 80 copies/mL. Analysis was restricted to the 961 (88.7%) eligible HAART initiators with CD4+ lymphocyte and HIV-1 RNA viral load data available after HAART initiation whose HAART initiation date could be estimated accurately to within 1 year. Of the 961 women, 924 (96%) had data on smoking status. The median follow-up after HAART initiation for the 924 women with complete data was 5.2 years (range 0.2 to 7.9; interquartile range 3.2 to 6.3), during which time 164 deaths occurred among HAART initiators.
Table 1 ▶ reports differences between women based on smoking status before HAART initiation. Significantly more smokers were African American, used illicit drugs, or had a lifetime history of illicit injection drug use, were infected with hepatitis C virus, and had previously been diagnosed with AIDS. CD4+ lymphocyte counts and CD4+ percentages (data not shown) were significantly higher among smokers than nonsmokers, but viral loads were not significantly different.
Trends in Disease Markers After HAART
Figures 1a and 1b ▶ indicate trends in CD4+ lymphocyte counts and viral loads among smokers and nonsmokers starting at the visit before HAART and extending over a 5-year period, after adjustment for age and race. Over time, the higher CD4+ lymphocyte counts initially observed among smokers actually became lower than the mean CD4+ lymphocyte counts of nonsmokers (P = .01 for trend) (Figure 1a ▶).
FIGURE 1—

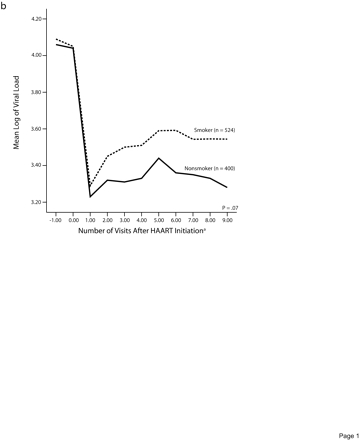
Trends in CD4+ lymphocyte counts (a) and log of viral load (b), by smoking status before highly active antiretroviral therapy initiation.
A similar pattern was observed for CD4+ percentages (P=.01 for trend; data not shown) as well as for viral load levels (P= .07 for trend) (Figure 1b ▶).
Outcomes in Smokers vs Nonsmokers
Kaplan–Meier probabilities of developing an ADC (Figure 2a ▶) or dying (Figure 2b ▶) were also higher among smokers than among nonsmokers (P< .01).
FIGURE 2—
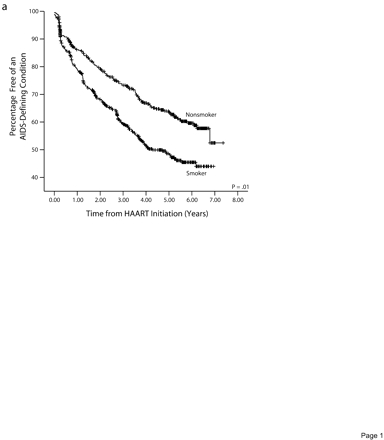
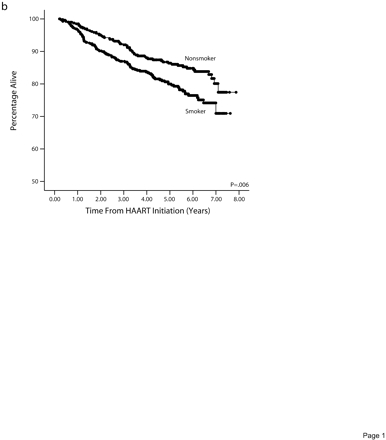
Cumulative percentage remaining free from an AIDS-defining condition (a) and surviving (b), by smoking status before highly active antiretroviral therapy initiation.
Table 2 ▶ reports the results of multivariate backward selection Cox regression analyses for each of the 7 outcomes. Each of the final models could have included age, race, and pre-HAART covariates, CD4+ lymphocyte count, viral load, illicit drug use, previous AIDS, previous ART use, baseline hepatitis C infection, and baseline exposure category. Smokers who initiated HAART had a lower chance of achieving a viral (hazard ratio [HR]=0.79; P=.006) or immunologic response (HR= 0.85; P=.041), and a greater chance of developing viral (HR=1.39; P=.013) or immunologic failure (HR=1.52; P=.001), acquiring an ADC (HR=1.36; P=.01), or dying (HR=1.53; P=.018). However, the risk of AIDS-related deaths did not differ between smokers and nonsmokers (HR=0.89 P=.68).
TABLE 2—
Backward Stepwise Proportional Hazard Models for Smoking and Prognosis after Highly Active Antiretroviral Therapy
| Hazards Ratio (95% CI) | P | |
| Viral response (n = 863; 551 events) | ||
| Age | 1.009 (1.003, 1.014) | .002 |
| Smoker | 0.79 (0.67, 0.93) | .006 |
| ART naïve | 2.18 (1.75, 2.71) | .001 |
| Log viral load | 0.84 (0.81, 0.87) | .001 |
| Viral rebound (n = 524; 333 events) | ||
| Smoker | 1.39 (1.06, 1.69) | .013 |
| Hispanic vs White | 1.29 (0.56, 0.99) | .04 |
| Illicit drug use | 1.56 (1.13, 2.07) | .007 |
| ART naïve | 0.73 (0.55, 0.99) | .034 |
| Log viral load | 1.06 (1.01, 1.11) | .01 |
| Immunologic response (n = 863; 678 events) | ||
| Smoker | 0.85 (0.73, 0.99) | .041 |
| Hispanic vs White | 0.79 (0.66, 0.96) | .016 |
| Log CD4+ count | 1.33 (1.22, 1.44) | .001 |
| Immunologic failure (n = 660; 261 events) | ||
| Smoker | 1.52 (1.18, 1.96) | .001 |
| Hispanic vs White | 1.49 (1.12, 1.97) | .006 |
| Log CD4+ count | 1.49 (1.27, 1.74) | .001 |
| AIDS incidence (n = 841; 343 events) | ||
| Smoker | 1.36 (1.07, 1.72) | .011 |
| Illicit drug use | 1.74 (1.32, 2.30) | .001 |
| Prior AIDS | 2.30 (1.83, 2.88) | .001 |
| Log CD4+ count | 0.80 (0.73, 0.87) | .001 |
| Log viral load | 1.07 (1.02, 1.13) | .004 |
| Total deaths (n = 863; 151 events) | ||
| Age | 1.02 (1.01, 1.03) | .006 |
| Smoker | 1.53 (1.08, 2.19) | .018 |
| HCV | 2.05 (1.45, 2.88) | 0.001 |
| ART naïve | 0.43 (0.25, 0.76) | .003 |
| Log CD4+ count | 0.62 (0.55, 0.70) | 0.001 |
| Log viral load | 1.09 (1.01, 1.18) | 0.023 |
| Death from AIDS (n = 863; 63 events) | ||
| HCV | 2.03 (1.23, 3.35) | 0.005 |
| ART naïve | 0.41 (0.18, 0.96) | 0.04 |
| Log CD4+ count | 0.56 (0.48, 0.67) | 0.001 |
| Log viral load | 1.28 (1.11, 1.47) | .001 |
Note. ART = antiretroviral therapy; HCV = hepatitis C virus.
The results did not change even when the 9 covariates noted previously were forced into the models.
Adherence to HAART was significantly lower among smokers than among nonsmokers. Smokers reported taking their medications less than 95% of the time during the preceding 6 months in 32.2% of their visits compared with only a 23% “noncompliance” rate among nonsmokers (P=.001). After adjustment for adherence levels, poorer responses were still more frequent among smokers (data not shown). In these models, HAART compliance was associated with higher viral response rates (HR=1.46; P=.0010), less viral (HR= 0.57; P=.001) and immunologic (HR=0.71; P=.012) failure, and fewer ADC occurrences (HR=0.65; P=.001).
To minimize the potentially confounding effects of adherence to HAART, we limited the analysis to women who reported taking their HAART medication during the previous 6 months at least 95% of the time for all study visits. The difference in response to HAART between smokers and nonsmokers in this group was similar to that for all patients, although only the differences in risk of death and ADCs were statistically significant (data not shown).
DISCUSSION
Smokers on HAART experience significantly higher morbidity (ADC=36%) and mortality rates (53%) than nonsmokers. In North America, 22% of deaths in females are attributed to smoking, and the practice remains prevalent in the United States almost 40 years after the first surgeon general’s report linking tobacco use to lung cancer.15–17 Smoking is much more common among people who have less education and income, the same individuals who are at greatest risk for acquiring HIV in the United States. Among African American women with 16 or more years of education, the smoking prevalence is only 9%, whereas 33% of African American women with fewer than 12 years of education smoke.18
Despite the widespread use of tobacco among HIV-infected women, the effect of smoking on HIV-related mortality and morbidity has never been clearly defined. The surgeon general’s 2001 report on women and smoking concluded, “The influence of smoking on progression of HIV-1 infection and on survival among women has not been examined in cohorts sufficiently large for meaningful interpretation.”16
Our study examined a large cohort of HIV-infected women, many of whom smoked. The results clearly demonstrated that HIV-positive women who smoke have a higher risk of acquiring ADCs or dying. Moreover, HAART is not as beneficial in smokers as it is in non-smokers. The decrease in HAART efficacy among smokers might be related in part to a lower level of HAART adherence. However, even after adjustment for reported compliance and illicit drug use, HAART was still less effective in smokers as measured by AIDS incidence and death. These data indicate a negative impact of smoking even while HAART may be effective in reducing AIDS-related deaths in smokers.
It is not clear whether smoking directly interferes with HAART or represents a marker for individuals at high risk for HAART failure. Other studies have shown that smoking modifies CD4+ lymphocyte counts, but they have been inconsistent in establishing a negative relation between smoking and the course of HIV/AIDS.2–6,19–21 In this study, we found that smokers initially had higher CD4+ lymphocyte counts. Over time, this relation changed with decreasing CD4+ lymphocyte counts in smokers compared with nonsmokers, who tended to increase their CD4+ lymphocyte counts and lower their viral loads. These differences may also reflect a selection bias in which healthier patients are more likely to smoke at any point in time.
Our study did not find an association between smoking and AIDS-related deaths. This is consistent with an earlier report from our group describing an algorithm for classifying death into AIDS-related or non–AIDS-related categories on the basis of data from death certificates and CD4+ lymphocyte counts.13 In that study, smokers had an adjusted relative mortality risk of 3.5 (1.2 to10) for non–AIDS-related causes and an adjusted relative mortality risk of 0.9 (0.6 to 1.2) for AIDS-related causes.
It is not clear whether the lack of association between smoking and AIDS-related deaths is because of an inability to determine the true of cause of death in this cohort setting or because competing causes of death result in smokers dying more rapidly (i.e., smokers die from acute causes such as drug overdoses, homicides/suicides/accidents before dying from AIDS-related causes) or because smoking has an impact mostly on ADCs that do not result in death or because smoking is associated with high-risk behavior, including nonadherence to medications.
However, we were able to demonstrate an association between smoking and both survival and ADC occurrence as well as with several indices of HAART effectiveness even after control for a wide array of potential confounders. Although this analysis controlled for adherence to HAART and for illicit drug use, there may still be some residual confounding factors not yet identified. Residual confounding factors are less likely to be of significance, at least as they relate to HIV-associated factors, when the analysis is restricted to HAART-compliant patients. Even in this group, smoking was consistently associated with adverse outcomes, although there were fewer significant outcomes because of the smaller sample size.
We cannot exclude potential bias among patients in poor health who may be more or less likely to smoke. For example, a patient in poor health who feels that she has nothing to lose might choose to smoke anyway, in spite of the known health risks. This study did control for immunologic, virologic, anti-retroviral use, and clinical status at each visit, but the trends in CD4+ lymphocyte counts and HIV viral loads suggested that at least initially, smokers were actually healthier. Survival bias also could have influenced the study findings, particularly if more smokers died before they could begin HAART. This seems unlikely, because the initiation of HAART usually occurs rapidly.
Finally, the assessment of smoking and adherence to treatment relied on patient self-reports. However, social desirability bias in self-reported smoking is less likely in a low-income population with a high previous use of illicit drugs. Additionally, the level of adherence to treatment was independently associated with several of the indexes of HAART efficacy, suggesting that self-reports on compliance were accurate.11
In conclusion, our data suggest that the treatment of HIV-positive women with HAART may be less effective in those who smoke cigarettes and point to a need to promote smoking cessation.
Acknowledgments
Data in this manuscript were collected by the WIHS Collaborative Study Group with centers (principal investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Alvaro Muñoz).
The WIHS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute, the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, and the National Institute of Craniofacial and Dental Research (U01-AI-35004, U01-AI-31834, U01-AI-34994, AI-34989, U01-HD-32632, U01-AI-34993, U01-AI-42590, N01-AI-35161, RO1 AI48483). Participating institutions approved this study and consent were forms provided to study participants.
Human Participant Protection Informed consent was obtained from participants in accordance with procedures and consent materials approved by the committee on human research at each of the collaborating institutions.
Peer Reviewed
Contributors J. G. Feldman originated the topic, wrote the original draft, and performed the data analyses. H. Minkoff and K. Anastos contributed to conceptualization of the article, the outcome measures, and the design of the study. M. F. Schneider and S. J. Gange contributed to the data analysis and interpretation, the study design, and data processing. M. Cohen, D. H. Watts, M. Gandhi, and R. Mocharnuk contributed to the design of the study and the data interpretation. All authors reviewed drafts and made important contributions to revisions.
References
- 1.Sepkowitz K. AIDS—the first 20 years. N Engl J Med. 2001;344:764–790. [DOI] [PubMed] [Google Scholar]
- 2.Wewers MD, Diaz PT, Wewers ML, et al. Cigarette smoking in HIV infection induces a suppressive inflammatory environment in the lung. Am J Respir Crit Care Med. 1998;158:1543–1549. [DOI] [PubMed] [Google Scholar]
- 3.Poirier CD, Inhaber N, Lalonde RG, et al. Prevalence of bronchial hyperresponsiveness among HIV-infected men. Am J Respir Crit Care Med. 2001;164:542–545. [DOI] [PubMed] [Google Scholar]
- 4.Nieman RB, Fleming J, Coker RJ, et al. The effect of cigarette smoking on the development of AIDS in HIV-1 seropositive individuals. AIDS. 1993;7:705–710. [DOI] [PubMed] [Google Scholar]
- 5.Craib KJ, Schecter MT, Montaner JS, et al. The effect of cigarette smoke on lymphocyte subsets and progression to AIDS in a cohort of homosexual men. Clin Invest Med. 1992;15:301–308. [PubMed] [Google Scholar]
- 6.Galai M, Park L, Wesch J, et al. Effect of smoking on the clinical progression of HIV-1 infection. Epidemiology. 1997;14:451–458. [DOI] [PubMed] [Google Scholar]
- 7.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 8.Hessol NA, Schneider M, Greenblatt RM, et al. Retention of women enrolled in a prospective study of human immunodeficiency virus infection: impact of race, unstable housing, and use of human immunodeficiency virus therapy. Am J Epidemiol. 2001;154:563–573. [DOI] [PubMed] [Google Scholar]
- 9.Cook J, Cohen MH, Grey D, et al. Use of highly active antiretroviral therapy in a cohort of HIV-seropositive women. Am J Public Health. 2002;92:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirstein LM, Greenblatt RM, Anastos K, et al. Women’s Interagency HIV Study Collaborative Research Group. Prevalence and correlates of highly active antiretroviral therapy switching in the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2002;29:495–503. [DOI] [PubMed] [Google Scholar]
- 11.Calvelli T, Denny T, Paxton H, Gelman R, Kagan J. Guidelines for flow cytometric immunophenotyping. Cytometry. 1993;14:702–715. [DOI] [PubMed] [Google Scholar]
- 12.Wilson T, Barrón Y, Cohen MH, et al. Adherence with antiretroviral therapy and associations with sexual behavior. Clin Infect Dis. 2002;34:529–534. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MH, French A, Benning L, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 15.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362: 847–852. [DOI] [PubMed] [Google Scholar]
- 16.Women and Smoking. A Report of the Surgeon General. Washington, DC: US Dept of Health and Human Services; 2001. DHHS publication 01–1232, 1–0237 (8/01).
- 17.Shopland DR, Burns DM, Garfinkel L, Samet JM, eds. Changes in Cigarette-Related Disease and Their Implication for Prevention and Control. Bethesda, Md: National Cancer Institute; 1997. NIH publication 97–4213 (2/97).
- 18.Tobacco Use among US Racial/Ethnic Minority Groups: A Report of the Surgeon General (1998). Available at: http://www.cdc.gov/tobacco/sgr/sgr_1998. Accessed March 2, 2006.
- 19.Royce RA, Winkelstein W Jr. HIV infection, cigarette smoking and CD4+ T-lymphocyte counts: preliminary results from the San Francisco Men’s Health Study. AIDS. 1990;4:327–333. [PubMed] [Google Scholar]
- 20.Conley LJ, Busj TJ, Buchbinder KA, Penley FN, Judson FN, Holmberg SD. The association between cigarette smoking and selected HIV-related medical conditions. AIDS. 1996;10:1121–1126. [PubMed] [Google Scholar]
- 21.Park LP, Margolick JB, Giorgi JV, et al. Influence of HIV-1 infection and cigarette smoking on leukocyte profiles in homosexual men: the Multicenter AIDS Cohort. J Aquir Immune Defic. Syndr. Hum. Retrovirol. 1992;5:1124–1130. [PubMed] [Google Scholar]


