Abstract
Background: About 30% to 46% of patients with major depressive disorder (MDD) fail to fully respond to initial antidepressants. While treatment-resistant depression commonly refers to nonresponse or partial response to at least 2 adequate trials with antidepressants from different classes, due to variability in terminology, a staging system based on prior treatment response has been suggested. Aripiprazole is a novel atypical antipsychotic with partial agonism at dopamine D2 and serotonin 5-HT1A receptors and antagonism at the 5-HT2 receptors. The present study evaluated whether augmentation with aripiprazole would be beneficial and tolerable in patients with treatment-resistant MDD who had failed 1 or more trials of antidepressants.
Method: In an open-label, rater-blinded study conducted from March 2003 through December 2003, 10 patients with DSM-IV MDD without psychotic features who had failed to respond to an adequate trial of at least 1 antidepressant were prescribed aripiprazole (10–30 mg/day) for 6 weeks. The dose of preexisting antidepressants remained unchanged. Treatment response was defined as a 50% or greater reduction in score on the Hamilton Rating Scale for Depression (HAM-D) from baseline to end of treatment. Secondary efficacy measures included scores on the Clinical Global Impressions-Improvement (CGI-I) and -Severity (CGI-S) scales.
Results: Eight of 10 patients had failed 2 or more antidepressant trials. The mean daily dose of aripiprazole was 13.21 mg. Intent-to-treat analysis showed that mean ± SD HAM-D scores reduced significantly from baseline (23.0 ± 8.1) to end of treatment (8.1 ± 6.0) (p < .001). There was a significant reduction in CGI-I (p < .05) and a trend toward decrease in CGI-S (p = .06) score. Seventy percent of the subjects were responders and 30% achieved remission. Common adverse effects were akathisia (20%), nausea (20%), and restlessness (20%).
Conclusions: The study indicates the potential utility of aripiprazole as an augmenting agent in treatment-resistant depression, particularly in those who had failed 2 or more antidepressant trials. Adequately powered, randomized controlled trials are necessary to evaluate the role of aripiprazole in treatment-resistant depression.
Despite pharmacologic advances in the treatment of major depressive disorder (MDD), 30% to 46% of patients fail to respond adequately to their initial antidepressants and only 25% to 35% achieve symptom remission.1,2 Patients with MDD who show partial or no response to an adequate trial of 1 or more antidepressants are considered to have treatment-resistant depression (TRD).3 Due to the variability in terminology and definition of TRD, Thase and Rush4 proposed a staging system based on prior treatment response. According to this system, TRD has 5 stages: 1 = nonresponse to an adequate trial of 1 antidepressant; 2 = failure to respond to adequate trials of 2 antidepressants with different pharmacologic profiles; 3 = stage 2 plus 1 augmentation strategy; 4 = stage 3 plus failure of second augmentation; and 5 = stage 4 plus failure to respond to electroconvulsive therapy. Unfortunately, there is limited evidence or consensus to support the superiority of a particular strategy such as switching or augmentation in TRD.
Treatment augmentation with other psychotropic agents in depression is intuitively appealing because it enables several neurotransmitter systems to be influenced simultaneously, potentially improving therapeutic effects.5,6 Other advantages of augmentation over switching are maintenance of any partial response to the initial treatment and the potential of a rapid response. For decades, clinicians have used antipsychotic agents in combination with an antidepressant to treat psychotic or agitated depression.7
Atypical antipsychotics offer a potentially important therapeutic option in mood disorders due to their favorable tolerability profile and effects at multiple receptor systems that have been implicated in depression.8 Several mechanisms have been hypothesized to explain the benefits of augmentation with atypical antipsychotics in TRD. Atypical antipsychotics act on a variety of dopamine, serotonin (5-HT), and other receptors. The antagonism of 5-HT2A receptors is common among these drugs, and blockade of this subtype is seen with other antidepressant agents such as mirtazapine and nefazodone. Blockade of 5-HT2C receptors has been shown to enhance release of dopamine and norepinephrine in the frontal and prefrontal regions, and these effects may be synergistic with those of the selective serotonin reuptake inhibitors (SSRIs).9 Blockade of 5-HT2A and 5-HT2C receptors may also ameliorate some SSRI-induced adverse effects such as anxiety, insomnia, and sexual dysfunction.10 Preclinical studies suggest that combination of an SSRI and second generation antipsychotics may also influence immediate-early gene expression and synaptic efficacy in the prefrontal cortex and hippocampal areas.11
The evidence supporting the efficacy of atypical antipsychotic agents in treatment-resistant unipolar depression without psychotic features is surprisingly sparse. Most of the positive data have come from open-label studies and case reports and suffer from limitations inherent in uncontrolled studies.12–20 However, randomized controlled studies of olanzapine, risperidone, and ziprasidone in TRD have failed to conclusively demonstrate the superiority of any one agent.21–25 Aripiprazole, approved for the treatment of schizophrenia and bipolar mania, is the sixth atypical antipsychotic to be introduced to the market. Unlike its predecessors, aripiprazole is a partial dopamine agonist, acting on both postsynaptic D2 receptors and presynaptic autoreceptors. The partial agonism at dopaminergic neurons is believed to result in a decrease and increase in dopaminergic neurotransmission in areas of hypodopaminergic and hyperdopaminergic activity, respectively.26,27 Additionally, aripiprazole displays partial agonism at the 5-HT1A receptors with potent antagonism at the 5-HT2A receptors.28,29 At least theoretically, this binding profile has led aripiprazole to be considered as a dopamine-serotonin system stabilizer that may represent a new treatment approach in schizophrenia and possibly other psychiatric disorders including depression.28 Studies have shown that aripiprazole is superior to haloperidol in ameliorating depressive and negative symptoms in patients with schizophrenia.30 It also appears to have a favorable side effect profile.29 While unpublished data indicate that aripiprazole may hold promise in depression,31–33 to date there are no published studies evaluating aripiprazole as an augmenting agent for TRD.
The present state of evidence suggests a pharmacologic rationale for use of atypical antipsychotics in TRD. However, the data are limited by unclear definitions of treatment resistance, open-label designs, and lack of systematic efficacy ratings. The aims of the present study were (1) to evaluate whether augmentation with aripiprazole would benefit patients with MDD who did not demonstrate significant clinical improvement with adequate trial of a standard antidepressant, and (2) to assess the tolerability of aripiprazole when used in conjunction with antidepressants in TRD.
METHOD
Subjects
Study subjects were recruited through clinical referrals from the Jefferson Health Care System, Philadelphia, Pa., and through local newspaper advertisements. Eligible subjects included men and women aged 18 to 65 years who suffered from TRD. The TRD subjects were defined as those who (1) met DSM-IV criteria for MDD without psychotic features on the Mini-International Neuropsychiatric Interview (MINI),34 (2) had an entry score of ≥ 14 on the 17-item Hamilton Rating Scale for Depression (HAM-D17),35 and (3) had an adequate trial of at least 1 antidepressant at study entry, defined as a ≥ 6-week trial of an antidepressant at an acceptable therapeutic dose. A daily dose ≥ 40 mg of fluoxetine, paroxetine, or citalopram; 37.5 mg of paroxetine CR; 150 mg of sertraline; 20 mg of escitalopram; 225 mg of venlafaxine XR; 30 mg of mirtazapine; 300 mg of bupropion or bupropion XR; 400 mg of nefazodone; 100 mg of nortriptyline; 20 mg of protriptyline; or 150 mg of amitriptyline or imipramine was considered an acceptable therapeutic dose for the study. Exclusion criteria were any DSM-IV psychotic disorder including bipolar disorder, serious suicide risk, current substance abuse or history of substance abuse in the previous 12 months, history of hypersensitivity to aripiprazole, treatment with antipsychotics in the previous 3 months, start or termination of psychotherapy during the previous 12 weeks, serious or unstable medical disorders, and pregnancy or plans to become pregnant in the next 6 months.
Design
This was a prospective, 6-week, open-label, rater-blinded study that examined the clinical utility and safety of the atypical antipsychotic aripiprazole as an augmenting agent to antidepressant therapy in treatment-resistant depression. The study ran from March 2003 through December 2003.
Measures
The MINI34 was used to diagnose MDD. The HAM-D1735 and the Clinical Global Impressions-Improvement (CGI-I) and -Severity (CGI-S) scales36 were used to assess the response to the intervention. The primary efficacy measure (response) was defined as a ≥ 50% reduction in HAM-D17 score from baseline to end of treatment. Secondary efficacy measures were defined as a CGI-I score of 1 or 2 at end of treatment and a 1-point reduction in CGI-S score from baseline to end of treatment. Remission was defined as a HAM-D17 score of ≤ 7 at endpoint. Adverse effects were determined by the Systematic Assessment for Treatment Emergent Events-General Inquiry (SAFTEE-GI),37 the Simpson Angus Extrapyramidal Scale (SAES),38 and the Barnes Akathisia Scale (BAS).39
Procedures
The study protocol was approved by the Institutional Review Board of Thomas Jefferson University and was granted an exemption from an investigational new drug (IND) application by the U.S. Food and Drug Administration. Written informed consent was obtained from all subjects prior to performing any protocol. Screening procedures included a review of clinical history, establishing a DSM-IV diagnosis of MDD using the structured MINI interview, a HAM-D17 rating, physical examination, urine drug and pregnancy screen, and routine laboratory tests to examine complete blood count, liver and renal function, thyroid status, and electrolytes. The HAM-D rater was involved in rating subjects in 3 different studies and was instructed not to ask subjects about the nature of their protocol or study drug dosing.
For enrolled subjects, the dose of antidepressant remained unchanged during the study unless the patient complained of intolerable antidepressant-related adverse effects. Based on tolerability and clinical response, aripiprazole was started at 10 mg/day and increased weekly in 5-mg/day increments, the maximum dose being 30 mg/day for patients who were taking antidepressants that have no significant effect on the CYP450 3A4 enzyme system. Agents that induce CYP3A4 (e.g., carbamazepine) could cause an increase in aripiprazole clearance and lower blood levels. Inhibitors of CYP3A4 (e.g., ketoconazole) or CYP2D6 (e.g., quinidine, fluoxetine, or paroxetine) can inhibit aripiprazole elimination and cause increased blood levels.40 No other psychotropic medications were permitted during the study except for medications to alleviate treatment-emergent adverse effects. The medications were dispensed weekly and the participants were followed for 6 weeks. Participants were monitored weekly by the HAM-D17, CGI, BAS, SAES, and SAFTEE. Vital signs and weight were also taken at each in-house visit. After 6 weeks, aripiprazole was tapered over a 2-week period. Compliance was assessed at each visit by pill count.
Data Analysis
Data analysis was done using the Statistical Package for the Social Sciences (SPSS 11.0; SPSS, Inc., Chicago, Ill.). Paired t tests were employed to compare changes in HAM-D17 (primary outcome), CGI-I, and CGI-S scores from beginning to end of treatment. An intent-to-treat analysis with last observation carried forward (ITT with LOCF) examining all patients enrolled in the trial and a completer analysis for all subjects who completed the 6-week study were performed.
RESULTS
Subjects
Forty-two subjects were screened for the study and 10 eligible subjects were enrolled. The mean ± SD age was 44.9 ± 12.2 years, 6 subjects (60%) were women, and 6 (60%) were African American. The duration of current episode of MDD was 28.6 ± 35.4 months. The age at onset of MDD was 26.6 ± 15.5 years. Table 1 summarizes the antidepressant treatment history of subjects entering the study.
Table 1.
Antidepressant Treatment History of Subjects
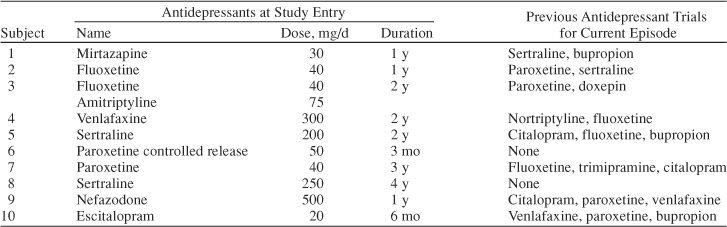
It is worth noting that all patients had failed to respond to an antidepressant trial for longer than the minimum defined duration to enroll, 50% had doses higher than the necessary doses to enroll, and 80% had failed multiple antidepressant trials for the current MDD episode. Eight subjects (80%) completed the study. One subject discontinued due to side effects, and 1 subject was lost to follow-up. Subjects who completed the study did not miss any visits. Weekly pill count showed that 1 subject had 4 visits when he/she was noncompliant; the subject dropped out of the study. The mean daily dose of aripiprazole was 13.21 mg. Although this was a flexible-dose design, 8 of 10 patients were on doses of 10 or 15 mg/day.
Efficacy Measures
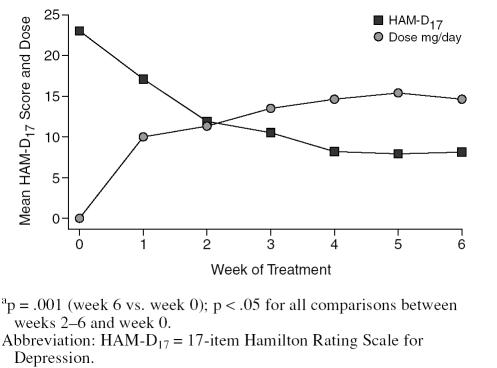
In ITT analysis, the mean ± SD HAM-D17 score reduced significantly from baseline (23.0 ± 8.1) to week 6 (8.1 ± 6.0) (t = 5.44, p < .001). Figure 1 summarizes the mean HAM-D17 scores each week.
Figure 1.
Changes in HAM-D17 Scores in Relation to Aripiprazole Dose During Study (N = 10)a
There was a trend toward reduction in HAM-D17 scores by week 1 (p = .07) that reached statistical significance by week 2 (p < .05) and continued to remain significant through weeks 3 to 6 (p < .05 to p < .001). Seven subjects (70%) were classified as responders at end of treatment (≥ 50% reduction in HAM-D17 score), and 3 (30%) achieved remission (a HAM-D17 score of ≤ 7 at week 6). Table 2 shows the number of responders at each week.
Table 2.
Number of Patients Responding at Each Week (N = 10)a

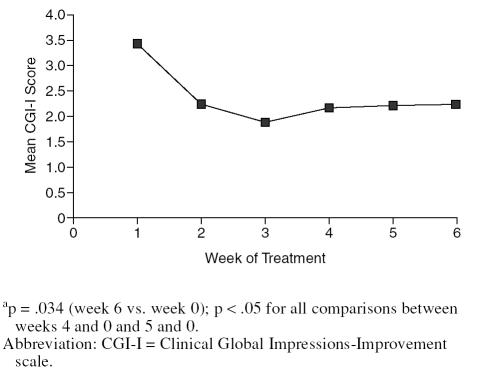
As seen from Table 2, 70% of subjects had shown a response by week 2. Mean ± SD CGI-I scores showed a corresponding decrease from week 1 (3.4 ± 0.7) to week 6 (2.2 ± 1.3) (t = 2.86, p < .05; Figure 2), with a trend toward reduction in mean ± SD CGI-S scores over the study period (3.9 ± 0.6 at baseline, 3.2 ± 0.5 at end of treatment; t = 2.44, p = .059). Based on a CGI-I score of 1 or 2, 7 subjects (70%) were considered responders at end of treatment.
Figure 2.
Change in CGI-I Scores During the Triala
Of the 2 subjects who dropped out, 1 was a responder and 1 was a nonresponder. Using a completer analysis, 7 (87.5%) were classified as responders (≥ 50% reduction in HAM-D17 scores) and 3 (37.5%) as remitters (HAM-D17 scores of ≤ 7). Consistent with ITT analyses, the mean ± SD HAM-D17 scores among completers showed a statistically significant improvement from baseline (22.2 ± 8.2) to week 6 (9.4 ± 7.2) (p < .001). Seven completers (87.5%) received a CGI-I score of 1 or 2 at end of treatment. Completers showed a significant reduction in CGI-I scores from week 1 to week 6 (p < .05) and a trend toward reduction in the CGI-S scores over the study period (p = .07).
The small number of nonresponders did not permit any meaningful comparisons of clinical variables between responders and nonresponders. Of the 3 patients who did not respond, 1 each was receiving paroxetine, venlafaxine, and fluoxetine. The mean dose of drug was similar between the responders and nonresponders.
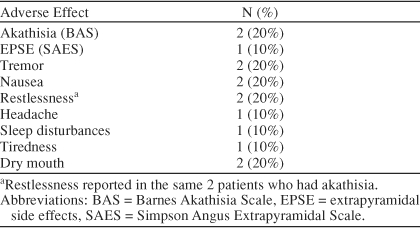
Adverse Events
Table 3 shows the adverse effects reported by patients. No patient experienced any serious adverse events. Two patients were rated to have akathisia on the BAS, leading to premature discontinuation in 1 patient. In the other patient, akathisia resolved with 1 week of benzodiazepine treatment and did not recur. The same 2 patients reported restlessness on spontaneous reports of adverse events. Patients did not significantly gain or lose weight during the study (mean ± SD weight at baseline = 213 ± 76 lb, week 6 = 210 ± 83 lb; p = .58).
Table 3.
Treatment-Emergent Adverse Effects for Aripiprazole Plus Antidepressant During the Trial (N = 10)
At the conclusion of the trial, 5 (71%) of the 7 responders chose to continue on aripiprazole therapy in the outpatient resident clinic associated with the university. Because of issues related to managed care formularies and preauthorization, 2 patients had to seek follow-up treatment elsewhere.
DISCUSSION
Our results indicate that augmentation with aripiprazole may potentially benefit patients with nonpsychotic MDD who are nonresponders or partial responders to standard antidepressant regimens. There was about a 15-point reduction in HAM-D17 scores from beginning to end of treatment. Seven patients (70%) were responders by both the HAM-D17 and CGI-I criteria, and 3 (30%) had remitted by end of the study. Of note, there was a 6-point drop in HAM-D17 scores by week 1 and an 11-point reduction by week 2, with 70% of patients having responded by week 2. The magnitude of improvement seen with aripiprazole is comparable to the rapid improvement seen in augmentation studies with risperidone, olanzapine, and ziprasidone.18,21,22 It is believed that dopamine release in the prefrontal cortex may play a role in the early response observed when second-generation antipsychotics are used as augmentation agents in TRD.18
The starting dose of aripiprazole was 10 mg/day and the mean dose was about 13 mg/day. Most patients had responded by week 2, when the dose was titrated from 10 to 15 mg/day. This finding indicates that doses of aripiprazole lower than those used in schizophrenia or bipolar disorder may be effective for augmentation in TRD. Another study has also indicated the benefit of low augmenting doses of aripiprazole in TRD.41 In this context, the doses of risperidone, olanzapine, and ziprasidone in augmentation studies in TRD have been lower than the recommended therapeutic doses in schizophrenia.18,21,22 Unfortunately, the study protocol did not permit us to examine the possibility that even lower starting doses (5 mg/day) may be efficacious for augmentation in TRD; however, clearly this issue merits further investigation.
We found that there were no major safety issues in combining aripiprazole with therapeutic doses of antidepressants. There were no serious adverse events and only 1 patient (10%) dropped out due to adverse events. From a clinical standpoint, 2 subjects (20%) experienced akathisia and restlessness leading to 1 discontinuing the study and the other requiring short-term adjunctive benzodiazepines. The other side effects did not require any clinical interventions. The rate of akathisia is consistent with rates observed during short-term placebo-controlled studies of aripiprazole in schizophrenia.42 It does not appear that combining aripiprazole with antidepressants increases the risk of extrapyramidal side effects. However, controlled studies with larger samples are needed to clarify the tolerability of aripiprazole in combination with therapeutic doses of antidepressants.
The principal limitations of this study were the small sample size, an open-label design, and lack of a placebo arm. We attempted to minimize the limitations inherent in an open-label design by having a blinded interviewer rate the primary outcome measure (HAM-D17). Without a placebo arm, the effects of augmentation cannot be reliably distinguished from improvement due to continuation of antidepressants or therapeutic effects of regularly seeing a physician. However, it is worth noting that despite the minimum 6-week requirement for adequate duration, all patients had had at least 12 weeks of treatment at study entry, over 50% had doses higher than those considered adequate, and most had failed multiple antidepressants. In such patients, it is reasonable to assume that placebo response rate will be low and that a 70% response is likely to be clinically significant. Additional limitations of the study include the retrospective definition of treatment resistance and a relatively short duration of study. All these limitations did not permit us to study whether the antidepressant efficacy of augmentation with aripiprazole is maintained following the initial improvement.
CONCLUSIONS
This study indicates that augmentation with aripiprazole may benefit patients with MDD without psychotic features who are not responsive to standard antidepressant therapy. Given the open-label design and the small sample size, adequately powered, randomized, prospective, double-blind, placebo-controlled trials are necessary to fully evaluate the efficacy and tolerability of aripiprazole in treatment-resistant depression.
Drug names: amitriptyline (Limbitrol and others), aripiprazole (Abilify), bupropion (Wellbutrin and others), carbamazepine (Carbatrol, Equetro, and others), citalopram (Celexa and others), doxepin (Sinequan and others), escitalopram (Lexapro), fluoxetine (Prozac), imipramine (Tofranil, Surmontil, and others), ketoconazole (Ketozole, Nizoral, and others), mirtazapine (Remeron and others), nortriptyline (Aventyl, Pamelor, and others), olanzapine (Zyprexa), paroxetine (Paxil, Pexeva, and others), protriptyline (Vivactil), risperidone (Risperdal), sertraline (Zoloft), trimipramine (Surmontil), venlafaxine (Effexor), ziprasidone (Geodon).
Footnotes
Supported by an investigator-initiated grant from Bristol-Myers Squibb, Inc.
Presented at the 157th annual meeting of the American Psychiatric Association, May 1–6, 2004, New York, N.Y.
The sponsor provided study medication. The authors did not receive any funding for the preparation of this article. Dr. Patkar has received research support from AstraZeneca, Bristol-Myers Squibb, Forest, GlaxoSmithKline, Janssen, McNeil Consumer and Specialty, Organon, Orphan Medical, and Pfizer and is a member of the speakers bureaus for Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, and Reckitt Benckiser. Dr. Mago has received research support from AstraZeneca and Bristol-Myers Squibb and is a member of the speakers bureaus for Bristol-Myers Squibb, Forest, and Pfizer. Dr. Masand has received research support from AstraZeneca, Forest, GlaxoSmithKline, Janssen, Ortho-McNeil, and Wyeth; is a member of the speakers bureaus for Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Novartis, Pfizer, and Wyeth; and is a shareholder in Bristol-Myers Squibb. Drs. Peindl and Mannelli report no financial relationship with any pharmaceutical companies.
REFERENCES
- Crown WH, Finkelstein S, and Berndt ER. et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002 63:963–971. [DOI] [PubMed] [Google Scholar]
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Dececco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):5–9. [PubMed] [Google Scholar]
- Thase ME, Rush AJ. Treatment-resistant depression. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Raven Press. 1995 1081–1097. [Google Scholar]
- Fava M. Augmentation and combination strategies in treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 18):4–11. [PubMed] [Google Scholar]
- Nemeroff CB. Augmentation strategies in patients with refractory depression. Depress Anxiety. 1996;4:169–181. doi: 10.1002/(SICI)1520-6394(1996)4:4<169::AID-DA3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Thase ME. What role do atypical antipsychotic drugs have in treatment-resistant depression? J Clin Psychiatry. 2002;63:95–103. doi: 10.4088/jcp.v63n0202. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Tollefson GD, and Tohen M. et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001 158:131–134. [DOI] [PubMed] [Google Scholar]
- Zhang W, Perry KW, and Wong DT. et al. Synergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine on norepinephrine and dopamine release in rat prefrontal cortex. Neuropsychopharmacology. 2000 23:250–262. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, and Amargos-Bosch M. et al. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004 29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Horowitz JM, Goyal A, and Ramdeen N. et al. Characterization of fluoxetine plus olanzapine treatment in rats: a behavior, endocrine, and immediate-early gene expression analysis. Synapse. 2003 50:353–364. [DOI] [PubMed] [Google Scholar]
- Corya SA, Andersen SW, and Detke HC. et al. Long-term antidepressant efficacy and safety of olanzapine/fluoxetine combination: a 76-week open-label study. J Clin Psychiatry. 2003 64:1349–1356. [DOI] [PubMed] [Google Scholar]
- Barbee JG, Conrad EJ, Jamhour NJ. The effectiveness of olanzapine, risperidone, quetiapine, and ziprasidone as augmentation agents in treatment-resistant major depressive disorder. J Clin Psychiatry. 2004;65:975–981. doi: 10.4088/jcp.v65n0714. [DOI] [PubMed] [Google Scholar]
- O'Connor M, Silver H. Adding risperidone to selective serotonin reup-take inhibitor improves chronic depression. J Clin Psychopharmacol. 1998;18:89–91. doi: 10.1097/00004714-199802000-00018. [DOI] [PubMed] [Google Scholar]
- Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256–259. doi: 10.4088/jcp.v60n0410. [DOI] [PubMed] [Google Scholar]
- Pitchot W, Ansseau M. Addition of olanzapine for treatment-resistant depression [letter] Am J Psychiatry. 2001;158:1737–1738. doi: 10.1176/appi.ajp.158.10.1737-a. [DOI] [PubMed] [Google Scholar]
- Stoll AL, Haura G. Tranylcypromine plus risperidone for treatment-refractory major depression [letter] J Clin Psychopharmacol. 2000;20:495–496. doi: 10.1097/00004714-200008000-00020. [DOI] [PubMed] [Google Scholar]
- Viner MW, Chen Y, and Bakshi I. et al. Low-dose risperidone augmentation of antidepressants in nonpsychotic depressive disorders with suicidal ideation. J Clin Psychopharmacol. 2003 23:104–106. [DOI] [PubMed] [Google Scholar]
- Welner M. Risperidone plus a monoamine oxidase inhibitor for agitated depression crisis [letter] J Clin Psychopharmacol. 1996;16:460–461. doi: 10.1097/00004714-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Weisler RH, Ahearn EP, and Davidson JR. et al. Adjunctive use of olanzapine in mood disorders: five case reports. Ann Clin Psychiatry. 1997 9:259–262. [DOI] [PubMed] [Google Scholar]
- Dube S, Corya SA, and Andersen SW. et al. Efficacy of olanzapine/fluoxetine combination in treatment-resistant depression. Presented at the 41st annual meeting of the American College of Neuropsychopharmacology. 8–12December2002 San Juan, Puerto Rico. [Google Scholar]
- Dube S, Paul S, and Sanger T. et al. Olanzapine-fluoxetine combination in treatment-resistant depression. Eur Psychiatry. 2002 17(suppl 1):98. [Google Scholar]
- Rapaport MH, Canuso CM, and Rouillon F. et al. Results from the augmentation with risperidone in resistant depression trial. Presented at the 157th annual meeting of the American Psychiatric Association. 1–6May2004 New York, NY. [Google Scholar]
- Papakostas GI, Petersen TJ, and Nierenberg AA. et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry. 2004 65:217–221. [DOI] [PubMed] [Google Scholar]
- Dunner DL, Amsterdam JD, and Shelton RC. et al. Adjunctive ziprasidone in treatment-resistant depression: a randomized, double-blind, 8-week, pilot study [poster]. Presented at the 43rd annual meeting of the American College of Neuropsychopharmacology. 12–16December2004 San Juan, Puerto Rico. [DOI] [PubMed] [Google Scholar]
- Burris KD, Molski TF, and Xu C. et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002 302:381–389. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, 1: “Goldilocks” actions at dopamine receptors [B Rainstorms] J Clin Psychiatry. 2001;62:841–842. doi: 10.4088/jcp.v62n1101. [DOI] [PubMed] [Google Scholar]
- DeLeon A, Patel NC, Crismon ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy and tolerability. Clin Ther. 2004;26:649–664. doi: 10.1016/s0149-2918(04)90066-5. [DOI] [PubMed] [Google Scholar]
- Taylor DM. Aripiprazole: a review of its pharmacology and clinical use. Int J Clin Pract. 2003;57:49–54. [PubMed] [Google Scholar]
- McGavin JK, Goa KL. Aripiprazole. CNS Drugs. 2002;16:779–786. doi: 10.2165/00023210-200216110-00008. [DOI] [PubMed] [Google Scholar]
- Barbee JG, Conrad EJ, and Jaber-Jamhour N. Aripiprazole augmentation in treatment-resistant depression. Presented at the 157th annual meeting of the American Psychiatric Association. 1–6May2004 New York, NY. [DOI] [PubMed] [Google Scholar]
- Worthington JW, Fava M, and Hughes ME. et al. Aripiprazole as an augmentor of SSRIs in mood and anxiety disorder patients [poster]. Presented at the 156th annual meeting of the American Psychiatric Association. 17–22May2003 San Francisco, Calif. [Google Scholar]
- Nemeroff CB, Simon JS, and Forbes A. et al. Aripiprazole augmentation of SSRIs and SNRI for the treatment of partial and non-responding patients with major depressive disorder [poster]. Presented at the 43rd annual meeting of the American College of Neuropsychopharmacology. 12–16December2004 San Juan, Puerto Rico. [Google Scholar]
- Sheehan DV, Lecrubier Y, and Sheehan KH. et al. The validity of the Mini International Neuropsychiatric Interview (M.I.N.I.) according to the SCID-P and its reliability. Eur Psychiatry. 1997 12:232–241. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–59. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76-338. Rockville, Md: National Institute of Mental Health. 1976 218–222. [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Barnes TR. The Barnes Akathisia Rating Scale: revisited. J Psychopharmacol. 2003;17:365–370. doi: 10.1177/0269881103174013. [DOI] [PubMed] [Google Scholar]
- Abilify [product insert]. Rockville, Md: Bristol-Myers Squibb Co. 2005. [Google Scholar]
- Simon JS, Nemeroff CB. Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry. 2005;66:1216–1220. doi: 10.4088/jcp.v66n1002. [DOI] [PubMed] [Google Scholar]
- Marder SR, McQuade RD, and Stock E. et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res. 2003 61:123–136. [DOI] [PubMed] [Google Scholar]