Abstract
The transition to pulmonary respiration following birth requires rapid alterations in the structure of the mammalian cardiovascular system. One dramatic change that occurs is the closure and remodeling of the ductus arteriosus (DA), an arterial connection in the fetus that directs blood flow away from the pulmonary circulation. A role for prostaglandins in regulating the closure of this vessel has been supported by pharmacological and genetic studies. The production of prostaglandins is dependent on two cyclooxygenases (COX-1 and COX-2), which are encoded by separate genes. We report here that the absence of either or both COX isoforms in mice does not result in premature closure of the DA in utero. However, 35% of COX-2(−/−) mice die with a patent DA within 48 h of birth. In contrast, the absence of only the COX-1 isoform does not affect closure of the DA. The mortality (35%) and patent DA incidence due to absence of COX-2 is, however, significantly increased (79%) when one copy of the gene encoding COX-1 is also inactivated. Furthermore, 100% of the mice deficient in both isoforms die with a patent DA within 12 h of birth, indicating that in COX-2-deficient mice, the contribution of COX-1 to DA closure is gene dosage-dependent. Together, these data establish roles for COX-1, and especially for COX-2, in the transition of the cardiopulmonary circulation at birth.
The ductus arteriosus (DA) is an arterial connection in the fetus between the pulmonary artery and the aorta. The DA directs deoxygenated blood away from the pulmonary circulation toward the descending aorta and to the umbilico-placental circulation where oxygenation occurs. The DA plays a critical role in the cardiovascular physiology of the fetus and newborn (for review see ref. 1). In utero patency of the DA is essential for proper fetal health, and premature DA closure causes pulmonary hypertension, congestive heart failure, and edema. In contrast, failure of the DA to close after birth, designated patent DA, compromises postnatal health by contributing to respiratory complications, including pulmonary hypertension and edema (2).
A family of lipid mediators known as prostaglandins (PGs) are among the factors that have been shown to influence the tone of the DA. The initial reaction in the synthesis of all PGs is catalyzed by prostaglandin G/H synthase, also known as cyclooxygenase (COX), two isoforms of which have been identified. Both COX-1 and COX-2 catalyze the synthesis of PGH2, a product required for the formation of the various biologically active PGs (3). Individual PGs act through specific receptors to mediate their biological effects. The role for PGs in regulation of DA tone was initially determined from the observation that nonsteroidal anti-inflammatory drugs (NSAIDs), which act by inhibiting COX (4), modulate DA tone in utero and following birth (1).
The dilation of the DA in utero is an active process maintained primarily by PGE2. The PG receptors that may have a role in dilation of the DA include the PGE2 receptors, EP2 (5) and EP4 (6), and a receptor for prostacyclin (7). The tone of the DA may also be regulated by PGs that bind to receptors in the DA and initiate contraction of the smooth muscle of the vessel wall. Contraction of the DA in vitro is induced by an agonist selective for the PGE2 receptors, EP1 and EP3, and by a stable analogue of PGH2 (8). Although mice deficient in each of the four EP receptors and in the prostacyclin receptor have been generated (9), only EP4 receptor-deficient mice show a DA phenotype, with greater than 95% of these mice dying with a patent DA within 48 h of birth (10, 11).
The disruption of the genes encoding COX-1 (Ptgs1) (12) and COX-2 (Ptgs2) (13, 14) has also been accomplished, and these mice have provided genetic models to study the functions of each COX isoform. Although no prenatal or postnatal phenotype involving the DA was originally reported in either COX-deficient line, functional compensation of one isoform for the other may have obscured a neonatal DA phenotype. To better understand the contribution of the COX isoforms and the PGs they produce to the function of the ductus, we have generated mice with reduced expression of COX-1 and/or COX-2, as well as mice deficient in both COX isoforms. Herein, we show that the deficiency of COX-2 alone is sufficient to alter normal closure of the DA after birth, and the role for COX-1 becomes evident only in the absence of COX-2. Therefore, although both COX isoforms contribute to the function of the ductus, COX-2 is the more essential isoform for initiation of DA closure.
Methods
Breedings.
Mice deficient in COX-1 and COX-2 were maintained on a mixed genetic background of C57BL/6J and 129/Ola (12, 13). Wild-type mice were generated from COX heterozygotes to ensure a similar genetic background. Because COX-2(−/−) females are infertile (15), mice lacking both COX isoforms were generated from females that expressed one allele of Ptgs2. The COX-1(+/−)/COX-2(+/−) and COX-1(−/−)/COX-2(+/−) females were fertile and produced litters of normal size, but parturition was delayed in both genotypes. Litters were therefore delivered by Cesarean section to prevent pup loss. The detection of the copulation plug the morning after pairing was designated as gestation day 0.5. The genotypes of the male mice used for breeding included COX-1(−/−)/COX-2(+/−), COX-1(+/−)/COX-2(−/−), COX-1(+/+)/COX-2(−/−), and COX-1(+/−)/COX-2(+/−).
Pathological Analysis.
Histological analyses were performed on formalin-fixed, paraffin-embedded tissues. Decapitated torsos were transected just below the rib cage before formalin fixation. For total pathological analysis, the head, upper torso, and lower torso were processed, paraffin-embedded, and serial sectioned. DA analysis was performed on transverse sections of the thorax. The entire length of the DA from the descending aorta to the pulmonary trunk was serial sectioned. The figures shown (hematoxylin and eosin-stained) are representative of the sections most central to the DA lumen.
Indomethacin-Induced Fetal DA Contraction and Postnatal Mortality.
Indomethacin-induced fetal DA contraction was determined on gestation day 18.5. Fetuses were delivered by Cesarean section 4 h after maternal indomethacin (20 mg/kg oral gavage, 5% gum arabic) administration and sacrificed immediately for histological analysis.
Indomethacin-induced postnatal mortality was determined after a normal gestation length of 19.5 days. Maternal indomethacin (20 mg/kg oral gavage, 5% gum Arabic) was administered 4 h before Cesarean delivery on day 19.5. The litters were from COX-1(+/−)/COX-2(+/−) and COX-1(−/−)/COX-2(+/−) females mated to COX-1(−/−)/COX-2(+/−), COX-1(+/−)/COX-2(−/−), or COX-1(+/−)/COX-2(+/−) males.
Immunohistochemical Analysis of COX-1 and COX-2.
Immunohistological analyses of DA tissue for detection of COX-1 and COX-2 were performed on 10% neutral buffered formalin-fixed, paraffin-embedded 10-μm-thick sections. Antigen retrieval was carried out in citrate buffer by heating to 96°C in a microwave oven, followed by cooling for 20 min at room temperature. Sections were first treated with 1% BSA, 1% nonfat dried milk, and 1% normal goat serum. Primary antibody against COX-1 (Cayman Chemical, Ann Arbor, MI) (1:2,000) or COX-2 (Cayman Chemical) (1:2,000) was then applied and incubated overnight at room temperature and then processed as recommended in the Vectastain ABC Elite kit (Vector Laboratories) for detection with 3,3-diaminobenzidine (Sigma). Slides were counterstained with Mayer's hematoxylin (Sigma). Tissue sections from COX-1(−/−) or COX-2(−/−) mice provided negative controls for the specificity of the respective antibodies.
Genotype Analysis of Ptgs1 and Ptgs2.
Tissue from pups was collected at the time of sacrifice for genotyping of Ptgs1 and Ptgs2 by PCR (16). Tissue from adult mice was collected by tail biopsy. The PCR primers used to identify genotypes were as follows: Ptgs1 targeted forward, 5′-GCAGCCTCTGTTCCACATACAC-3′; Ptgs1 wild-type forward, 5′-AGGAGATGGCTGCTGAGTTGG-3′; Ptgs1 reverse, 5′-AATCTGACTTTCTGAGTTGCC-3′; Ptgs2 targeted forward, 5′-ACGCGTCACCTTAATATGCG-3′; Ptgs2 wild-type forward, 5′-ACACCTTCAACATTGAAGACC-3′; Ptgs2 reverse, 5′-ATCCCTTCACTAAATGCCCTC-3′.
Neonatal Mortality Analysis.
Pups from COX-1(+/−)/COX-2(+/−) and COX-1(−/−)/COX-2(+/−) females that were delivered by Cesarean section after a normal gestation length of 19.5 days were maintained on pure oxygen for 1 h and then fostered with a CD-1 female. Pups were monitored for mortality during the first 48 h after delivery. Neonatal mortality for each genotype is the percentage of the number of pups that died during the first 48 h following birth compared with the number at birth.
Patent DA Incidence.
Pups obtained after natural death or following sacrifice at 5 h after birth were used for analysis of the DA. Data are expressed as the percentage of the number of pups with a patent DA compared with the total number of pups at birth.
Results
Analysis of COX-1-Deficient Mice and COX-2-Deficient Mice.
We have previously reported that absence of expression of COX-1 does not affect the survival or growth of COX-1-deficient mice (12) and that necropsy and histological analysis of these mice revealed no pathologies. In the current studies, we extend these findings to include the observation that the DA of COX-1-deficient mice closed and remodeled after natural birth and was indistinguishable from that of wild-type mice (Fig. 1A). The number of live COX-2-deficient pups at 12 h after natural birth was in agreement with Mendelian expectations but, in contrast to COX-1-deficient mice, continued observation of these litters revealed a high mortality (35%) in the COX-2(−/−) pups over the next 24 to 48 h.
Figure 1.
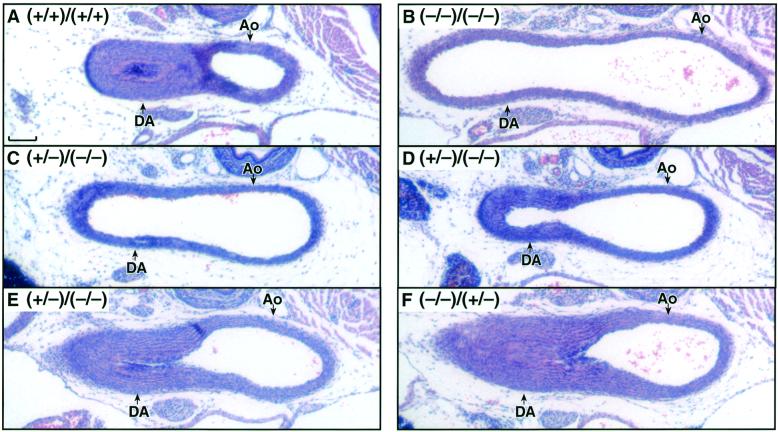
COX-dependent DA closure in neonatal mice. The DA was analyzed in pups 5 h after birth. (A) COX-1(+/+)/COX-2(+/+). (B) COX-1(−/−)/COX-2(−/−). (C) COX-1(+/−)/COX-2(−/−). (D) COX-1(+/−)/COX-2(−/−). (E) COX-1(+/−)/COX-2(−/−). (F) COX-1(−/−)/COX-2(+/−). Ao, aorta. (Scale bar, 100 μm.) Genotype figure labels, Ptgs1 genotype/Ptgs2 genotype.
Histological examination of the pups lacking COX-2 that died in the first 48 h after natural birth indicated a patent DA. Examination of the liver, particularly in pups that died on the second day after birth, revealed macrovesicular and microvesicular lipid deposits, consistent with acute ischemia, secondary to heart failure. COX-2-deficient pups that survived the perinatal period also survived to weaning. Examination of the DA in the surviving COX-2-deficient mice showed a normal transformation of the DA into the ligamentum arteriosum.
Development of Mice with Reduced Expression of Both Ptgs1 and Ptgs2.
Whereas significant mortality was observed in the COX-2-deficient population, 65% of COX-2-deficient mice survived to adulthood. This suggested that COX-1 may partially compensate for the COX-2 deficiency to allow DA closure and survival of the pups. To investigate this possibility and to better understand the role of each COX isoform in fetal and neonatal DA function, we generated mice lacking both isoforms and mice with reduced expression of one or both isoforms. The neonates with reduced COX expression were obtained by mating COX-1(+/−)/COX-2(+/−) or COX-1(−/−)/COX-2(+/−) females to COX- 1(−/−)/COX-2(+/−) males to produce pups of the various Ptgs genotypes. Previously, it has been shown that mice lacking COX-1 have delayed parturition, which results in reduced perinatal survival (17). To circumvent this problem, in the present study, pups were delivered by Cesarean section after the normal gestation length of 19.5 days. We first examined the impact of the deficiency of COX-1 and COX-2 expression on fetal development. As shown in Table 1, these matings produced live pups of all expected Ptgs genotypes, including those lacking both Ptgs1 and Ptgs2, in accordance with the Mendelian ratios. Therefore, prostanoids produced by either fetal COX-1 or COX-2 are not required for embryo implantation or for survival of the fetus.
Table 1.
Progeny genotypes agree with Mendelian expectations
| Maternal genotype: | COX-1(+/−)/COX-2(+/−) | COX-1(−/−)/COX-2(+/−) | ||
|---|---|---|---|---|
| Paternal genotype: | COX-1(−/−)/COX-2(+/−)
|
COX-1(−/−)/COX-2(+/−)
|
||
| Offspring genotype | Expected ratio | Obtained ratio* | Expected ratio | Obtained ratio† |
| COX-1(+/−)/COX-2(+/+) | 0.125 | 0.110 | NA | NA |
| COX-1(+/−)/COX-2(+/−) | 0.250 | 0.253 | NA | NA |
| COX-1(+/−)/COX-2(−/−) | 0.125 | 0.115 | NA | NA |
| COX-1(−/−)/COX-2(+/+) | 0.125 | 0.133 | 0.250 | 0.241 |
| COX-1(−/−)/COX-2(+/−) | 0.250 | 0.293 | 0.500 | 0.474 |
| COX-1(−/−)/COX-2(−/−) | 0.125 | 0.110 | 0.250 | 0.285 |
Litters were delivered after a normal gestation length of 19.5 days. Expected ratios are the Mendelian expectations. All obtained ratios are not significantly different from expected ratios (χ2 statistic, P < 0.01).
269 mice analyzed.
341 mice analyzed. NA, not applicable.
Sensitivity of the DA to NSAIDs in Mice with Reduced Expression of Both Ptgs1 and Ptgs2.
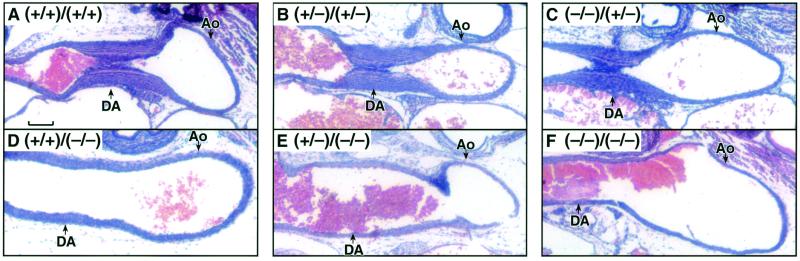
Pharmacological studies have shown that late gestation human and mouse fetuses are highly susceptible to premature contraction of the DA following acute maternal administration of NSAIDs, such as indomethacin (10, 18). Therefore, the survival to birth of the pups lacking both COX isoforms is surprising, as it suggests that prostanoids produced by the fetal COX-1 and COX-2 pathways are not required for patency of the DA in utero. Furthermore, on gestation day 18.5, the DA of the COX-deficient fetuses could not be distinguished from those of wild-type fetuses by histological examination (data not shown). To determine if premature closure of the DA on exposure to indomethacin is due to the inhibition of fetal COX-1 and/or COX-2, we administered indomethacin to the pregnant females and examined the DA in fetuses with varying expression of the two genes. In agreement with previous reports, indomethacin induced DA closure in day 18.5 wild-type fetuses (Fig. 2A). In the present study, we found DA closure in all fetuses wild-type or heterozygous (Fig. 2 B and C) for Ptgs2, irrespective of their Ptgs1 genotype (Table 2). However, indomethacin did not induce DA closure in fetuses that lacked COX-2 expression (Fig. 2 D–F). Therefore, indomethacin-induced closure of the DA requires fetal expression of COX-2, and neither maternal nor fetal expression of COX-1 is required for the DA to be sensitive to indomethacin.
Figure 2.
COX-2-dependent DA closure after maternal indomethacin treatment. Histological sections are from fetal DA on gestation day 18.5. (A) COX-1(+/+)/COX-2(+/+). (B) COX-1(+/−)/COX-2(+/−). (C) COX-1(−/−)/COX-2(+/−). (D) COX-1(+/+)/COX-2(−/−). (E) COX-1(+/−)/COX-2(−/−). (F) COX-1(−/−)/COX-2(−/−). Ao, aorta. (Scale bar, 100 μm.) Genotype figure labels, Ptgs1 genotype/Ptgs2 genotype.
Table 2.
Resistance to indomethacin-induced fetal DA closure and neonatal mortality results from the deficiency of COX-2
| Offspring genotype | Fetal DA closure,* % incidence | Neonatal survival,† % incidence |
|---|---|---|
| COX-1(+/+)/COX-2(+/+) | 100 (6/6) | 0 (0/7) |
| COX-1(+/−)/COX-2(+/+) | 100 (5/5) | 0 (0/5) |
| COX-1(−/−)/COX-2(+/+) | 100 (6/6) | 0 (0/8) |
| COX-1(+/+)/COX-2(+/−) | 100 (6/6) | 0 (0/6) |
| COX-1(+/−)/COX-2(+/−) | 100 (6/6) | 0 (0/8) |
| COX-1(−/−)/COX-2(+/−) | 100 (8/8) | 0 (0/14) |
| COX-1(+/+)/COX-2(−/−) | 0 (0/6) | 100 (5/5) |
| COX-1(+/−)/COX-2(−/−) | 0 (0/6) | 100 (7/7) |
| COX-1(−/−)/COX-2(−/−) | 0 (0/6) | 100 (12/12) |
Indomethacin-induced fetal DA closure was determined on gestation day 18.5 after maternal indomethacin administration 4 h prior to Cesarean delivery. In parentheses are number closed/number analyzed.
Neonatal survival after maternal indomethacin administration was determined after Cesarean delivery on gestation day 19.5. The surviving pups were sacrificed 5 h after birth. In parentheses are number alive/number born.
Because fetuses expressing COX-2 were sensitive to indomethacin-induced premature DA closure, we determined the susceptibility of different Ptgs genotypes to indomethacin-induced postnatal mortality. In utero closure of the fetal DA is known to produce pulmonary hypertension, which impairs postnatal respiration (19). The maternal administration of indomethacin 4 h before Cesarean delivery on day 19.5 was lethal for all of the neonates that expressed COX-2 (Table 2). These pups died within minutes after delivery and their DAs were contracted, suggesting that premature DA closure in the fetus caused the death of the neonate. In contrast, 100% of pups that were deficient in COX-2 survived until sacrifice at 5 h after birth (Table 2). Therefore, after acute maternal indomethacin administration, the absence of COX-2 prevents premature DA closure and neonatal lethality, supporting the requirement for COX-2 in indomethacin-induced DA closure.
Postnatal Mortality of Mice with Reduced Expression of Both Ptgs1 and Ptgs2.
The health of neonates lacking COX was monitored following Cesarean delivery. The newborn pups of all genotypes, including pups lacking both COX isoforms, were normal in size and had no gross deformities at birth. Consistent with our observations of naturally delivered litters, the absence of COX-1 did not reduce survival of the pups. In contrast, but consistent with the mortality observed in naturally delivered litters, loss of COX-2 also significantly decreased postnatal survival following Cesarean delivery. Thirty-five percent of the COX-2(−/−) pups delivered by Cesarean section died within 48 h (Table 3). Survival of COX-2(−/−) mice was further compromised by decreased COX-1 expression. Seventy-nine percent of the pups lacking COX-2 and having only one functional copy of the Ptgs1 gene died within 48 h of birth. Furthermore, 100% of the pups deficient in both COX-1 and COX-2 died during the postnatal period. More than 50% of these double homozygous mutant pups became cyanotic and died within 30 min of Cesarean delivery. The breathing of these pups appeared labored before cyanosis and death. Treatment of the pups with pure oxygen for the first hour improved their survival, with the majority of these pups surviving over 5 h. However, 100% of these pups died by 12 h after birth (Table 3).
Table 3.
COX-2 deficiency increases neonatal mortality and patent DA incidence with Ptgs1 gene dosage-dependent compensation
| Offspring genotype | % mortality* | Patent DA,† % incidence |
|---|---|---|
| COX-1(+/+)/COX-2(+/+) | 3 (1/31) | 0 (0/8) |
| COX-1(+/−)/COX-2(+/+) | 6 (3/47) | 0 (0/6) |
| COX-1(−/−)/COX-2(+/+) | 3 (1/40) | 0 (0/10) |
| COX-1(+/+)/COX-2(+/−) | 4 (2/49) | 0 (0/6) |
| COX-1(+/−)/COX-2(+/−) | 1 (1/99) | 0 (0/8) |
| COX-1(−/−)/COX-2(+/−) | 3 (3/88) | 0 (0/21) |
| COX-1(+/+)/COX-2(−/−) | 35‡ (20/57) | 33 (5/15) |
| COX-1(+/−)/COX-2(−/−) | 79‡ (26/33) | 74 (14/19) |
| COX-1(−/−)/COX-2(−/−) | 100‡ (38/38) | 100 (23/23) |
Neonatal mortality for each genotype is the percentage of mice that died during the 48 h following birth compared to the number at birth. Neonatal patent DA incidence was determined 5 h after Cesarean delivery or natural birth on gestation day 19.5.
In parentheses are number dead/number born.
In parentheses are number patent/number analyzed.
Significantly different (χ2 statistic, P < 0.01).
Analysis of Neonates with Reduced Expression of Both Ptgs1 and Ptgs2.
To determine the cause(s) of death of the COX-deficient pups, pathological analysis was performed on pups sacrificed 5 h after birth. A complete histological evaluation of the various organ systems was carried out with particular attention given to the COX-1(−/−)/COX-2(−/−) animals. Similar to findings in pups lacking the individual isoforms, no pathologies were apparent in the brain, urinary bladder, kidney, spleen, pancreas, intestines, or stomach in the COX-1(−/−)/COX-2(−/−) pups. Despite the observation of respiratory distress in COX-1(−/−)/COX-2(−/−) pups, their heart and lungs appeared histologically normal. The only cardiovascular pathology observed was a patent DA, and the lumen of the vessel was similar in diameter to that of the adjacent aorta (Fig. 1B).
As expected, the DA was found to be contracted in all of the wild-type pups at 5 h after birth (Table 3, Fig. 1A). The DA of pups lacking COX-1, even those with one disrupted copy of the Ptgs2 gene (Fig. 1F), could not be distinguished from the DA of the wild-type animals (Fig. 1A). The patency of the DA in mice lacking COX-2 varied between individual animals. In agreement with the survival studies, the DA was patent in 33% of the COX-2(−/−) pups analyzed at 5 h after birth. Furthermore, the incidence of patent DA was increased in the COX-2(−/−) mice heterozygous for the Ptgs1 mutant allele. A 74% incidence of patent DA was observed in COX-1(+/−)/COX-2(−/−) mice 5 h after birth (Table 3). Within this group of mice, there was considerable variability in the extent to which the DA had closed. In about one-half (6 of 14) of these mice, no contraction of the vessel was apparent, and the lumen of the vessel was similar in size to the adjacent aorta (Fig. 1C). In the remaining COX-1(+/−)/COX-2(−/−) mice, the ductus remained patent, but partial contraction of the vessel resulting in a thickening of the vessel wall was apparent (Fig. 1D). The expression of a single copy of Ptgs1 did allow the DA to close (Fig. 1E) in 26% of the COX-1(+/−)/COX-2(−/−) mice (Table 3). Therefore, halving the gene dosage of Ptgs1 in the COX-2(−/−) mice increased the incidence of patent DA from 33% to 74%, in agreement with their increased mortality (79%) (Table 3).
Immunohistochemistry of COX-1 and COX-2 in the DA.
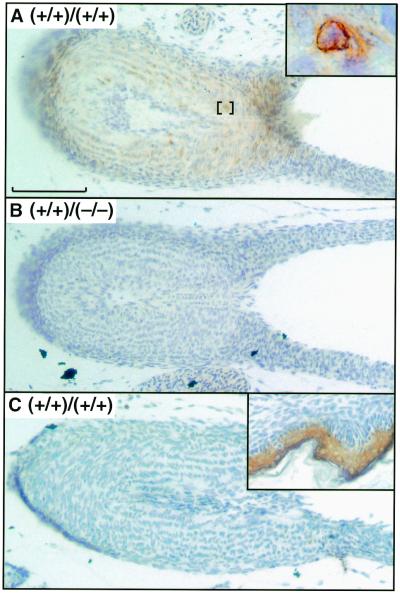
The expression of COX-1 and COX-2 in the DA was examined by immunohistochemistry to determine the contribution of localized COX expression to the physiology of DA closure. At 2.5 h after birth, COX-2 expression was evident in the smooth muscle cells of wild-type mice (Fig. 3A). Examination at higher magnification showed a concentration of staining in the perinuclear region of the smooth muscle cells (Fig. 3A, Inset). COX-2 was not detected in endothelial cells lining the DA lumen nor in fibroblasts of the tunica adventitia. As expected, COX-2 expression was not detected in the DA of COX-2-deficient mice (Fig. 3B). Analysis of wild-type mice with an antibody specific for COX-1 failed to detect expression of this isoform in the DA (Fig. 3C); although the antibody did detect COX-1 expression in the epidermis in similarly treated tissue sections (Fig. 3C, Inset). Our demonstration of a role for COX-1 in closure of the DA and our failure to detect COX-1 expression in the ductus suggest that COX-1-dependent prostanoids produced by other tissue(s) may contribute to closure of the ductus after birth.
Figure 3.
Immunohistochemical analysis of COX-1 and COX-2. DA sections obtained from neonates sacrificed 2.5 h after birth. (A) COX-1(+/+)/COX-2(+/+), anti-COX-2; (Inset) perinuclear staining. (B) COX-1(+/+)/COX-2(−/−), anti-COX-2. (C) COX-1(+/+)/COX-2(+/+), anti-COX-1; (Inset) epidermis of COX-1(+/+)/COX-2(+/+), anti-COX-1. (Scale bar, 100 μm.) Genotype figure labels, Ptgs1 genotype/Ptgs2 genotype.
Discussion
In the present study, we investigated the role of prostanoids in fetal development and survival in the perinatal period by generating mice with various homozygous and heterozygous mutations in the genes encoding COX-1 and COX-2. When examined just before the time of normal delivery, no developmental abnormalities were detected in pups deficient in both COX isoforms, indicating that if prostanoids are essential for in utero development, they are supplied maternally. However, survival in the postnatal period was dramatically reduced in mice with decreased COX expression.
We first examined survival of mice deficient in either the COX-1 isoform or the COX-2 isoform. Previously, it has been reported that mice lacking COX-2 showed increased neonatal mortality, the cause of which was not established (14). The present data show that COX-2 deficiency alone caused a patent DA and death in approximately 35% of the pups, which likely accounts for the previously reported neonatal death. Furthermore, necropsy of these pups revealed secondary pathologies consistent with a left to right shunt of blood through the DA. In contrast to mice deficient in COX-2, the DA closed normally in 100% of mice deficient in COX-1 alone.
To elucidate the combined contribution of the two COX isoforms to DA closure and neonatal survival, we also determined the status of the DA and the survival of pups with reduced expression of both COX isoforms. Although closure of the DA and survival of COX-2-deficient mice were compromised, the majority (65%) of these mice survived to adulthood. This suggests that in surviving mice deficient in COX-2, the PGs required for closure of the DA may be provided by COX-1. In support of this hypothesis, we found that reducing the functional gene copy number of Ptgs1 significantly increased both mortality and the incidence of patent DA in COX-2-deficient mice (Table 3). Therefore, in COX-2-deficient mice, the expression of COX-1 provides partial compensation to close the DA and increase postnatal survival.
Previously, it has been shown that mice deficient in the PGE2 receptor, EP4, also have an increased incidence of patent DA (10, 11). The deficiency of the EP4 receptor leads to the death of greater than 95% of the pups within 48 h of birth (10). In pups lacking either COX-2 or the EP4 receptor, survival in the early perinatal period is normal, but death is observed primarily between 24 and 48 h after birth. Secondary lesions in both the EP4 receptor-deficient and the COX-2-deficient pups are consistent with a left to right shunt leading to pulmonary congestion, decreased systemic blood flow, and death due to congestive heart failure. In contrast, the majority of pups lacking both COX isoforms died within minutes of birth, although survival was extended by treatment with oxygen. Thus, although both the EP4 receptor-deficient and COX-1/COX-2-deficient pups die in the postnatal period, differences in survival suggest that the mechanisms are not identical. We therefore propose that while COX-2, and to some extent perhaps COX-1, produces PGE2 essential for EP4 activation and remodeling of the DA after birth, additional prostanoids may play a role in closure of the DA.
Our observation of a patent DA in COX-1/COX-2-deficient mice suggests that COX-dependent metabolite(s) may initiate DA contraction immediately following birth. In addition, the expression of COX-2 in smooth muscle cells of the DA suggests that synthesis of contractile PG(s) may occur in the DA itself. Previous studies have shown that DA smooth muscle contracts in response to stable analogues of endogenous COX-dependent metabolites. DA tissue in vitro contracts in response to treatment with an EP1/EP3-selective PGE2 analogue (sulprostone) or a stable analogue of PGH2 (U46619) (8). However, because the predominant effect of PGE2 on the DA is to induce dilation, the importance of EP1 or EP3 receptors in DA contraction in vivo is uncertain. Therefore, we propose that COX-2 expressed in the DA smooth muscle produces a metabolite, possibly PGH2, that contributes to DA contraction.
Numerous observations in humans and rodents indicate that maternal administration of NSAIDs contract the DA in late-term fetuses (18, 20–22). Therefore, the finding of patent DA in the COX-deficient fetuses was surprising because the lack of PG production in COX-deficient fetuses was expected to cause premature DA contraction similar to the effects of NSAIDs. PGE2 in the fetal circulation maintains dilation of the DA in utero, and the placenta, in part, supplies the fetus with this dilatory PGE2 (1). Our findings suggest that COX-2 in the DA produces constrictor PG(s) important for DA contraction. Thus, the contraction of the DA following maternal indomethacin administration may result from the inhibition of dilatory PGE2 synthesis in the placenta without sufficient inhibition of ductal COX-2 to attenuate DA contraction.
Although NSAIDs are effective treatment for premature labor, their clinical use is limited, in part, by the adverse effect of inducing fetal DA contraction. COX-2-selective inhibitors have been suggested to provide a therapeutic advance for the treatment of premature labor with fewer fetal adverse effects than nonselective COX inhibitors (23–25). NSAIDs cross the placenta to enter the fetal circulation in concentrations similar to the maternal circulation (26, 27). The experiments reported here show that COX-2 expression in the DA is important for DA closure in mice. The maternal use of COX-2-selective inhibitors that enter the fetal circulation may inhibit COX-2 activity in the DA. If the contributions of COX-2 to the physiology of the DA are conserved across species, maternal use of COX-2-selective inhibitors near the time of delivery has the potential to increase the incidence of patent DA after birth. Therefore, our findings suggest that further investigation is required before COX-2-selective inhibitors should be considered safe for human use during pregnancy.
Acknowledgments
We thank Drs. Page A. W. Anderson and Thomas M. Coffman of Duke University for helpful discussions. Work by B.H.K. was supported in part by National Institutes of Health Grant HL66537.
Abbreviations
- DA
ductus arteriosus
- PG
prostaglandin
- NSAIDs
nonsteroidal anti-inflammatory drugs
- COX
cyclooxygenase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031573498.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031573498
References
- 1.Smith G C S. Pharmacol Rev. 1998;50:35–58. [PubMed] [Google Scholar]
- 2.Gersony W M. Pediatr Clin North Am. 1986;33:545–560. doi: 10.1016/s0031-3955(16)36042-4. [DOI] [PubMed] [Google Scholar]
- 3.Smith W L, DeWitt D L. Adv Immunol. 1996;62:167–215. doi: 10.1016/s0065-2776(08)60430-7. [DOI] [PubMed] [Google Scholar]
- 4.Vane J R. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya M, Asselin P, Hardy P, Guerguerian A M, Shichi H, Hou X, Varma D R, Bouayad A, Fouron J C, Clyman R I, et al. Circulation. 1999;100:1751–1756. doi: 10.1161/01.cir.100.16.1751. [DOI] [PubMed] [Google Scholar]
- 6.Smith G C, Coleman R A, McGrath J C. J Pharmacol Exp Ther. 1994;271:390–396. [PubMed] [Google Scholar]
- 7.Smith G C, McGrath J C. Cardiovasc Res. 1993;27:2205–2211. doi: 10.1093/cvr/27.12.2205. [DOI] [PubMed] [Google Scholar]
- 8.Smith G C, McGrath J C. J Cardiovasc Pharmacol. 1995;25:113–118. doi: 10.1097/00005344-199501000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Austin S C, Funk C D. Prostaglandins Lipid Mediat. 1999;58:231–252. doi: 10.1016/s0090-6980(99)00041-6. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen M, Camenisch T, Snouwaert J N, Hicks E, Coffman T M, Anderson P A, Malouf N N, Koller B H. Nature (London) 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 11.Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, Nishimura T, Murata T, Matsuoka T, Ushikubi F, Hirose M, et al. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 12.Langenbach R, Morham S G, Tiano H F, Loftin C D, Ghanayem B I, Chulada P C, Mahler J F, Lee C A, Goulding E H, Kluckman K D, et al. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 13.Morham S G, Langenbach R, Loftin C D, Tiano H F, Vouloumanos N, Jennette J C, Mahler J F, Kluckman K D, Ledford A, Lee C A, et al. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 14.Dinchuk J E, Car B D, Focht R J, Johnston J J, Jaffee B D, Covington M B, Contel N R, Eng V M, Collins R J, Czerniak P M, et al. Nature (London) 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 15.Davis B J, Lennard D E, Lee C A, Tiano H F, Morham S G, Wetsel W C, Langenbach R. Endocrinology. 1999;140:2685–2695. doi: 10.1210/endo.140.6.6715. [DOI] [PubMed] [Google Scholar]
- 16.Gavett S H, Madison S L, Chulada P C, Scarborough P E, Qu W, Boyle J E, Tiano H F, Lee C A, Langenbach R, Roggli V L, et al. J Clin Invest. 1999;104:721–732. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross G A, Imamura T, Luedke C, Vogt S K, Olson L M, Nelson D M, Sadovsky Y, Muglia L J. Proc Natl Acad Sci USA. 1998;95:11875–11879. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moise K J J. Am J Obstet Gynecol. 1993;168:1350–1353. doi: 10.1016/s0002-9378(11)90763-7. [DOI] [PubMed] [Google Scholar]
- 19.Van Den Veyver I B, Moise K J., Jr Obstet Gynecol. 1993;48:493–502. doi: 10.1097/00006254-199307000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Moise K J J, Huhta J C, Sharif D S, Ou C N, Kirshon B, Wasserstrum N, Cano L. N Engl J Med. 1988;319:327–331. doi: 10.1056/NEJM198808113190602. [DOI] [PubMed] [Google Scholar]
- 21.Rasanen J, Jouppila P. Am J Obstet Gynecol. 1995;173:20–25. doi: 10.1016/0002-9378(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 22.Vermillion S T, Scardo J A, Lashus A G, Wiles H B. Am J Obstet Gynecol. 1997;177:256–259. doi: 10.1016/s0002-9378(97)70184-4. [DOI] [PubMed] [Google Scholar]
- 23.Sawdy R, Slater D, Fisk N, Edmonds D K, Bennett P. Lancet. 1997;350:265–266. doi: 10.1016/S0140-6736(05)62229-5. [DOI] [PubMed] [Google Scholar]
- 24.Vane J R, Botting R M. Inflamm Res. 1998;47, Suppl. 2:S78–S87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- 25.Gross G, Imamura T, Vogt S K, Wozniak D F, Nelson D M, Sadovsky Y, Muglia L J. Am J Physiol. 2000;278:R1415–R1423. doi: 10.1152/ajpregu.2000.278.6.R1415. [DOI] [PubMed] [Google Scholar]
- 26.Moise K J J, Ou C N, Kirshon B, Cano L E, Rognerud C, Carpenter R J J. Am J Obstet Gynecol. 1990;162:549–554. doi: 10.1016/0002-9378(90)90427-9. [DOI] [PubMed] [Google Scholar]
- 27.Higby K, Elliott B, King T S, Frasier D, Langer O. J Soc Gynecol Invest. 1995;2:526–530. doi: 10.1016/1071-5576(94)00058-9. [DOI] [PubMed] [Google Scholar]