Abstract
Rationale: A preventive strategy for drug addiction would benefit from being able to identify vulnerable individuals. Understanding how an individual responds during an initial drug exposure may be useful for predicting how that individual will respond to repeated drug administrations.
Objectives: This study investigated whether individual differences in initial drug sensitivity and acute tolerance can predict how chronic tolerance develops.
Methods: During an initial 3-h administration of 60% nitrous oxide (N2O), male Long-Evans rats were screened for N2O’s hypothermic effect into subsets based on being initially insensitive (II), sensitive with acute tolerance (AT), or sensitive with no intrasessional recovery (NR). Animals in each individual difference category were randomly assigned to receive six 90-min exposures of either 60% N2O or placebo gas. Core temperature was measured telemetrically.
Results: Rats that exhibited a comparable degree of hypothermia during an initial N2O exposure, but differed in acute tolerance development, developed different patterns of chronic tolerance. Specifically, the NR group did not become fully tolerant over repeated N2O exposures while the AT group developed an initial hyperthermia followed by a return of core temperature to control levels indicative of full tolerance development. By the second N2O exposure, the II group breathing N2O became hyperthermic relative to the placebo control group and this hyperthermia persisted throughout the multiple N2O exposures.
Conclusions: Individual differences in initial drug sensitivity and acute tolerance development predict different patterns of chronic tolerance. The hypothesis is suggested that individual differences in opponent adaptive responses may mediate this relationship.
Keywords: Inhalant, addictive vulnerability, addiction, drug dependence, intrasessional tolerance, allostasis, homeostasis, regulation
Introduction
Drug abuse is a major public health and economic problem worldwide (McGinnis and Foege 1999; Single et al. 1999; Fenoglio et al. 2003). While advances are being made to understand the neurobiology of addictions for devising novel pharmacological therapies (O’Brien 1997), an alternative approach is to identify especially vulnerable individuals to utilize preventive strategies (Piazza and LeMoal 1996). Individual differences in key characteristics could then be used to identify at-risk individuals before they become addicted to abusable drugs (Crabbe et al. 1999; Schuckit et al. 2001). The adaptations an individual makes during an initial drug exposure may indicate how that individual will respond to future drug exposures.
Adaptation models of drug addiction are popular because they account for central features of the drug addiction process, including tolerance, dependence and withdrawal (Jaffe 1985; Peper 2004). These phenomena are thought to be manifestations of a common underlying adaptive response that develops with repeated drug use. In brief, the drug initially perturbs a biologically regulated system, eliciting adaptive changes that reduce the perturbation (i.e., tolerance develops). Over repeated drug administrations, the individual comes to function in a drug-adapted or dependent state wherein drug-induced perturbations are effectively countered by acquired neutralizing or compensatory adaptations. If the drug effect is removed, the compensatory effects caused by these adaptations become unopposed and result in withdrawal. A premise of adaptation models is that addiction results from adaptive responses made when confronted with repeated drug-induced regulatory disturbances. Presumably, individuals who do not become addicted despite repeated drug exposures are not making those same adaptations. This suggests the hypothesis that individuals differ quantitatively or qualitatively in terms of the adaptive responses elicited by comparable drug exposures. Individuals could differ in the magnitude of the disturbance required to initiate adaptive responses; or vulnerable individuals may react to a drug-induced perturbation with rigorous adaptive responses while individuals with sluggish or weak adaptive responses would be less susceptible to becoming addicted. Investigating this hypothesis requires identifying how individuals differ in the adaptive responses that are elicited during the initial administration of a drug.
Identifying the response(s) an individual makes during an initial drug administration is not obvious. When a dependent measure is assessed before and after a drug administration, the resulting change in the measure is casually referred to as either the “drug effect” or the individual’s “response” to the drug. Yet, neither description is accurate. What is typically being measured represents an integral of many ongoing processes that include both the direct pharmacological effect of the drug as well as the adaptive responses that are elicited by the drug-induced regulatory disturbance (Dworkin 1993; Ramsay and Woods 1997; Kaiyala and Ramsay in press). The challenge is to distinguish the individual’s response from the direct effect of the drug and from the effects of other co-occurring processes (e.g., handling, circadian rhythms) that influence that same measure (Brick et al. 1984; Peris and Cunningham 1986). Proposed methods for evaluating the response an individual makes during an initial drug exposure include assessing initial drug sensitivity, acute tolerance, and precipitated withdrawal (Ramsay and Woods 1997). Human and animal data suggest that these processes have predictive value for understanding how an individual will react to chronic drug exposure. Humans who appear initially insensitive to alcohol are at greater risk of becoming addicted than are more drug sensitive individuals (Schuckit and Smith 1996; Heath et al. 1999). Schuckit (1994) has described initial insensitivity to alcohol as “... a potent predictor of future alcohol abuse or dependence” (p. 341). McBride and Li (1998) reported that “initial sensitivity and acute (within-session) tolerance are by far the most generalizable and robust responses to ethanol found in association with ethanol preference in rodents” (p. 346). Kest and colleagues (2002) quantified naloxone-precipitated withdrawal during an initial administration of morphine in 11 inbred mouse strains and found that “.... the differential genetic liability to morphine physical dependence begins with, and is predicted by, the first morphine exposure” (p. 463).
The goal of the present study was to investigate the relationship between individual differences in acute tolerance and sensitivity during an initial drug administration and the development of chronic tolerance. Core body temperature was selected as the dependent variable because it can be measured accurately, continuously and non-invasively and because it reflects the operation of a well studied, centrally regulated control system. This is important because regulatory principles are at the foundation of numerous influential theories relevant to drug addiction (e.g., Solomon 1980; Poulos and Cappell 1991; Koob and LeMoal 1997; Siegel et al. 2000). Our drug choice was influenced by the ease, speed and accuracy with which steady-state drug concentrations could be achieved and maintained. The pharmacologically active gas, nitrous oxide (N2O), is well suited for this purpose. N2O reaches steady state concentrations quickly (Eger II 1985) and its concentration can be measured accurately and continuously using infrared spectroscopy. N2O causes a pronounced hypothermia at typical ambient temperatures (Quock et al. 1987; Pertwee et al. 1990). Outbred Long-Evans rats exhibit wide between-subject variation in initial sensitivity and acute tolerance to N2O-induced hypothermia (Ramsay et al. 1999) and these differences are reliable characteristics of an individual (Kaiyala et al. 2001). Chronic tolerance also develops to N2O hypothermia (Ramsay et al. 1999).
This research addresses a long-standing question concerning the relationship between acute and chronic tolerance (Kalant et al. 1971; Tabakoff and Rothstein 1983; Wu et al. 2001). Based on the premise that adaptive responses play a role in tolerance development, two primary hypotheses were evaluated. The first was that sensitive individuals who have significant acute tolerance development are likely to develop chronic tolerance more quickly and to a greater degree than sensitive individuals who develop little acute tolerance. The second was that initially insensitive individuals will quickly become fully tolerant to a small initial hypothermic effect and then remain insensitive / tolerant over repeated drug administrations. This hypothesis is consistent with contemporary explanations for initial insensitivity. If individuals are insensitive because the drug has little or no pharmacological effect on their tissues, it seems unlikely that repeated drug exposure would cause a drug effect to develop. Alternatively, if some individuals are insensitive because of an effective or powerful adaptive response that effectively counters the drug’s hypothermic effect within an initial exposure, effective regulation of core temperature should continue over repeated drug exposures.
METHODS
Subjects. Male Long-Evans rats (10 squads of 40 rats each, Simonsen Laboratory, Gilroy, CA) weighing 200 - 250 g were housed in groups of 2-3 per tub with free access to pelleted chow and water. The housing room had a 12 h:12 h light/dark cycle (0700-1900 h) and an ambient temperature of 22 ± 1 °C. The animal procedures used in this research were approved by the University of Washington’s IACUC and were conducted in accordance with the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003).
Surgery for core temperature measures. At least 7 days after arriving in the lab, each rat was anesthetized (0.5 ml/kg of a solution containing 90.9 mg/ml ketamine and 1.8 mg/ml xylazine, i.p.) and a telemetric temperature sensor (E-mitters from Mini Mitter Co., Bend, Oregon) was implanted into the peritoneal cavity. Rats recovered for ≥ 7 days before the experiment.
N2O delivery / temperature data acquisition system. A custom-built, automated system controlled gas delivery and data acquisition. Medical grade oxygen (O2), nitrogen (N2), and N2O were blended to produce an outflow of 30% O2, 10% N2 and 60% N2O (referred to as 60% N2O). N2 and O2 were blended to produce an outflow consisting of 30% O2 and 70% N2 (referred to as placebo gas). Two lines affixed to outlets in each chamber’s lid provided a low-resistance path by which exhaust gas flowed to a fume hood. Effluent gas sampling [N2O, O2, and CO2 by an infrared gas analyzer (Normocapoxy, Datex Instruments Corp., Helsinki, Finland)] and data acquisition were controlled by a program written in LabView 5.0 (National Instruments, Austin, Texas) installed on a Macintosh IIci computer. This system is described fully elsewhere (Kaiyala et al. 2001).
Each exposure chamber (Columbus Instruments, Columbus, Ohio) was made of clear Lexan, and had a removable lid secured with 4 latches and sealed with a closed-cell silicone foam rubber gasket. The floor of the chamber was covered with a layer of wood shavings. The chamber’s internal dimensions were 18 × 18 × 31 cm, yielding a volume of 10 l. With a gas inflow (and outflow) rate of 3.0 l/min, the theoretical half-time by which the 10-l chamber achieves the target gas composition is 2.3 min (Lewis et al. 1987). The chamber’s gas environment is calculated to reach 97% of the administered gas concentration in 11.6 min. Infrared gas analyses confirmed this figure, and indicated that 60% N2O steady-state gas concentrations were achieved in this study. Each test chamber was placed on a platform that energized the implanted temperature sensor and received its signal. Temperature data were acquired using VitalView software (Mini-Mitter Co.). During all sessions, a black vinyl drape visually isolated each chamber. All gas administrations occurred in the light part of the cycle and commenced at least 2 h after lights on and ended at least 2 h prior to lights off.
Experimental Design and Procedures. During the initial screening session, each rat received a 2-h administration of placebo gas followed immediately by a 3-h administration of 60% N2O. A squad of forty rats was screened over a period of 11 days after which 10 rats (25%) were retained that exhibited: 1) minimal hypothermia while receiving N2O (designated as initially insensitive, II), 2) a large hypothermic effect that persisted throughout the N2O administration (designated sensitive with no recovery, NR), or 3) a large hypothermic effect followed by a robust return of temperature toward baseline (designated as sensitive with acute tolerance, AT). These categorizations were based on Z-scores of the regression residuals (Kaiyala et al. 2001) for: 1) the size of the drug effect (degree of sensitivity) adjusted for baseline temperature, and 2) the degree of recovery from the drug effect (acute tolerance) adjusted for baseline temperature and drug effect.
Each of the 10 retained rats was randomly assigned to receive either placebo gas or 60% N2O for 6 additional exposures on an every-other-day schedule beginning 4 or 5 days after screening. Rats assigned to the 60% N2O group received 2 h of placebo gas followed by 1.5 h of 60% N2O at each gas exposure session while rats assigned to the placebo group received 3.5 h of placebo gas.
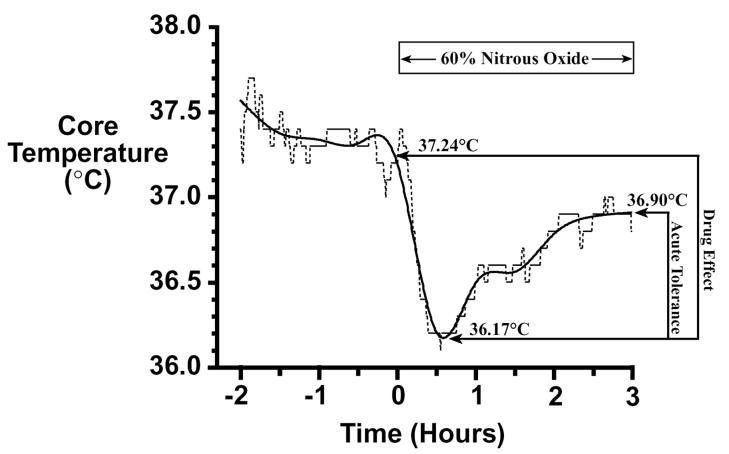
Individual Difference Group Assignment. Categorization of rats into the three individual difference groups was done using methods described previously (Kaiyala et al. 2001). Temperature values were recorded at 30-sec intervals over the 5-h screening session. These were smoothed using natural smoothing splines with 10 degrees of freedom (Hastie 1992) to minimize the influence of occasional aberrant temperature values. The smoothed temperature values were used to calculate the following three parameters (see Figure 1 for illustration): 1) baseline core temperature, equal to the average of the smoothed temperature values for the 3 min period prior to drug administration; 2) drug effect, equal to the difference between baseline temperature and the minimum smoothed temperature value after drug onset; and 3) acute tolerance (i.e., intrasessional recovery), equal to the difference between the mean of the smoothed values over the final 15 min of drug administration minus the minimum smoothed temperature. After a squad of rats completed screening, regression residuals were calculated for each rat’s drug effect and acute tolerance scores (Nagoshi et al. 1986; Kaiyala et al. 2001). The drug effect regression residual is an adjusted drug effect score that is not confounded by baseline core temperature; this adjustment is important because on average, rats with higher baseline temperatures can exhibit significantly greater N2O-induced hypothermia (Kaiyala et al. 2001). The drug effect regression residual was calculated by subtracting from the drug effect score the regression-predicted mean drug effect score given the rat’s observed baseline core temperature (T):
Based on the 387 rats that completed the screening session, the values of the β coefficients for the drug effect regression residual calculation were: β (intercept) = -1.698 and β1 (coefficient for baseline temperature) = 0.07391. Applying this formula to the data in Figure 1 yields a drug effect regression residual of 0.01637 = 1.071 - (-1.698 + 0.07391 × 37.243).
Figure 1.
Core temperature data (dashed line) from a single rat are smoothed (solid line) and calculations of baseline temperature, minimum temperature after drug onset, and the final temperature are made using the smoothed data. The drug effect equals 1.07°C (i.e., 37.24 minus 36.17), the drug effect regression residual equals 0.01637, and the drug effect Z-score equals 0.0308. Acute tolerance equals 0.73°C (i.e., 36.90 minus 36.17), the acute tolerance regression residual equals -0.01099, and the acute tolerance Z-score equals -0.0399. This rat is depicted in the scatterplot of Figure 2a as the most average rat.
The acute tolerance regression residual is an adjusted acute tolerance score that is not confounded by baseline core temperature or the drug effect score; this adjustment is important because mean acute tolerance magnitude varies directly with the drug effect which is influenced by baseline temperature. The acute tolerance regression residual was calculated by subtracting from the acute tolerance score the regression-predicted mean acute tolerance score given the rat’s observed baseline core temperature (T) and drug effect:
Based on the 387 rats that completed the screening session, the values of the β coefficients for the acute tolerance regression residual calculation were: β (intercept) = 1.790, β1 (coefficient for baseline temperature) = -0.03887 and β2 (coefficient for drug effect) = 0.03750. Applying this formula to the data provided in Figure 1 yields an acute tolerance regression residual of -0.01099 = 0.733 - (1.79 + (-0.03887) × 37.243 + 0.3750 × 1.071).
To operationally define which rats were exemplars of high / low initial sensitivity and which rats exemplified initial sensitivity with high / low acute tolerance development, Z-scores were used to identify where each rat was located within the distribution of drug effect regression residuals (i.e., initial sensitivity) and the distribution of acute tolerance regression residuals (i.e., acute tolerance development). Selection criteria for group assignment were based on critical values for independent normal distributions for drug effect and acute tolerance Z-scores, and were designed to assign 25% of the screened population into one of the three groups. Z-scores were calculated by dividing each rat’s drug effect and acute tolerance regression residuals by the corresponding standard deviation for those regression residuals. Based on the 387 rats that completed the screening session, the standard deviation of the drug effect regression residuals was 0.5316 and the standard deviation of the acute tolerance regression residuals was 0.2755. The individual rat’s data provided in Figure 1 can be converted to Z-scores as follows: the drug effect Z-score equals 0.0308 (i.e., 0.01637 / 0.5316) and the acute tolerance Z-score equals -0.0399 (i.e., -0.01099 / 0.2755). Because both Z-score values are close to zero, this rat was quite average in both the magnitude of the hypothermic drug effect and the amount of acute tolerance that developed. Thus, this rat would not be a good exemplar of high or low initial sensitivity, nor of high or low acute tolerance development (and it was not considered further).
Data Analysis. Temperatures for each of the six test sessions were summarized by the baseline temperature (mean of temperature values over the 15-min interval prior to drug administration) and the change from baseline to the mean temperature over three intervals: 1) 0-30 min after drug onset, 2) 30-60 min after drug onset, and 3) 60-90 min after drug onset. Baseline temperature values and changes from baseline temperature were analyzed by fitting linear models using Generalized Estimating Equations (GEE; Liang and Zeger 1986) to account for the correlation between repeated measures on the same animal. The individual difference group (II, AT, or NR) and the drug condition (N2O or placebo) were factors and session (2 - 7) was used as a continuous independent variable. All 2-way interactions and the 3-way interaction were also included in the models. Statistical significance was determined using empirical Wald tests (Liang and Zeger 1986). One GEE analysis was performed for baseline temperatures and three separate GEE analyses were performed for changes from baseline over each of the three time intervals (0-30 minutes, 30-60 minutes, and 60-90 minutes). The analysis of temperature changes included the baseline temperature as a precaution to protect against spurious associations due to differences between groups in baseline temperature.
RESULTS
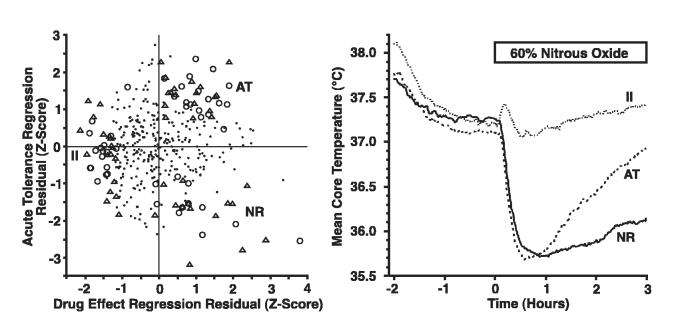
Of the 400 rats (10 squads) initially screened, 13 had unusable data resulting from emitter failure. Of the 387 remaining rats, 96 were categorized into the three individual difference groupings as follows: II = 34; AT = 37; NR = 25. These 96 rats had extreme values for the combination of drug effect and acute tolerance Z-scores (Figure 2a). The mean temperature curves during the initial screening session with 60% N2O for each of the 3 groups demonstrate that the groups fit the intended profiles (Figure 2b). The average Z-scores for each of the selected groups of rats were: -1.48 (drug effect) and -0.18 (acute tolerance) for the II group, 0.96 (drug effect) and 1.36 (acute tolerance) for the AT group, and 1.13 (drug effect) and -1.70 (acute tolerance) for the NR group. These mean Z-scores reflect highly selected sub-groups of animals; the probability of randomly selecting three groups with these characteristics is vanishingly small. The mean (SD) baseline core temperature for each individual difference group was: 37.23°C (0.45) for the II group, 37.11°C (0.48) for the AT group, and 37.27°C (0.31) for the NR group. After the onset of N2O, the mean (SD) maximal hypothermia (i.e., lowest core temperature) for each individual difference group was: 37.00°C (0.48) for the II group, 35.58°C (0.44) for the AT group, and 35.61°C (0.60) for the NR group. Since rats in each individual difference group were assigned randomly to receive placebo gas or 60% N2O during the subsequent six test sessions, there was a similar distribution of the drug effect and acute tolerance regression residual Z-scores for the placebo and N2O conditions, within each individual difference grouping (Figure 2a).
Figure 2a, b.
The scatterplot in the left panel (a) illustrates how individual rats were categorized as belonging to one of the three individual difference groups: initially insensitive (II), sensitive with acute tolerance (AT), and sensitive with no recovery (NR). Rats in each individual difference group were assigned randomly to a placebo control condition or a 60% N2O condition for the subsequent six test sessions. This assignment is indicated in the scatterplot where open circles indicate rats assigned to receive subsequent placebo exposures, open triangles indicate rats assigned to receive subsequent N2O exposures, and the small dots indicate rats that exited the study because they were not categorized into an individual difference group. Because Z-scores of regression residuals are difficult to visualize in terms of core temperature profiles, the right panel (b) illustrates the average initial temperature effect of a 3-h steady-state administration of 60% N2O for rats in the II, AT and NR groups that were selected on the basis of the Z-scores shown in the left panel (a). [Of the 400 rats screened, 387 had usable data and 96 (25%) were categorized into one of the individual difference groups as follows: II = 34 (placebo = 17, N2O = 17), AT = 37 (placebo = 18, N2O = 19), NR = 25 (placebo = 14, N2O = 11).
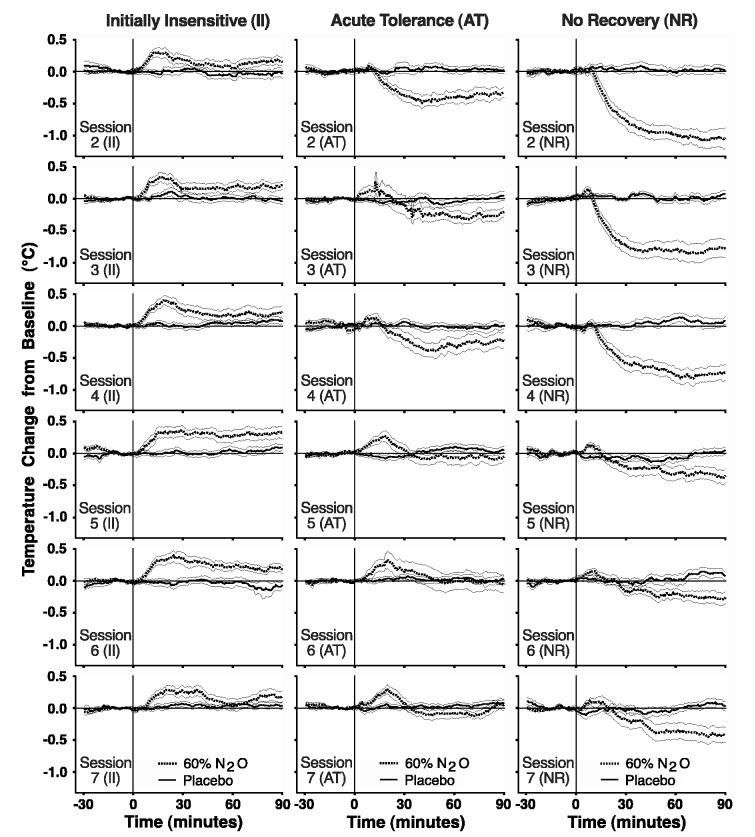
Figure 3 depicts how core temperature changed over six additional N2O exposures as a function of an individual’s initial (in)sensitivity and acute tolerance development to N2O. GEE analysis provided no evidence for differences in baseline temperature by individual difference group (p = 0.68), by drug condition (p = 0.39), or by session (p = 0.44) [see Table 1]. The 2-way interactions (Group X Session, Group X Drug, Drug X Session) and the 3-way interaction (Group X Drug X Session) were also non-significant (p = 0.32, 0.17, 0.55, and 0.45, respectively). Results from GEE analysis of temperature change from baseline over repeated N2O or placebo exposures demonstrated distinct patterns for the three individual difference groups. Results are presented separately below for each of the three 30-minute post-baseline time intervals.
Figure 3.
Mean (± SEM) change of temperature from baseline is depicted for II, AT and NR rats receiving a 90-min steady-state administration of 60% N2O or placebo on the six exposures (i.e., Sessions 2-7) following the initial screening. The three columns represent the individual difference groups and the rows represent data from Sessions 2-7.
Table 1.
Mean (SD) baseline core temperature (°C) during Sessions 2-7 for each of the individual difference groups.
| Initially Insensitive (II) Group | ||
|---|---|---|
| Session | Placebo (N=17) | N2O (N=17) |
| 2 | 37.1 (0.6) | 37.4 (0.9) |
| 3 | 37.2 (0.6) | 37.4 (1.0) |
| 4 | 37.1 (0.6) | 37.3 (0.9) |
| 5 | 37.1 (0.5) | 37.2 (0.8) |
| 6 | 37.1 (0.5) | 37.2 (0.8) |
| 7 | 37.1 (0.6) | 37.4 (0.7) |
| Acute Tolerance (AT) Group | ||
|---|---|---|
| Session | Placebo (N=19) | N2O (N=18) |
| 2 | 37.0 (0.8) | 36.6 (1.1) |
| 3 | 37.2 (0.6) | 36.7 (1.2) |
| 4 | 37.1 (0.6) | 37.0 (0.3) |
| 5 | 37.1 (0.7) | 36.9 (0.3) |
| 6 | 37.0 (0.6) | 36.9 (0.5) |
| 7 | 37.1 (0.7) | 37.0 (0.3) |
| No Recovery (NR) Group | ||
|---|---|---|
| Session | Placebo (N=11) | N2O (N=14) |
| 2 | 37.0 (0.4) | 37.1 (0.2) |
| 3 | 37.2 (0.3) | 37.1 (0.2) |
| 4 | 37.0 (0.2) | 37.1 (0.2) |
| 5 | 37.0 (0.3) | 37.0 (0.2) |
| 6 | 37.0 (0.3) | 37.0 (0.2) |
| 7 | 36.8 (0.3) | 37.1 (0.3) |
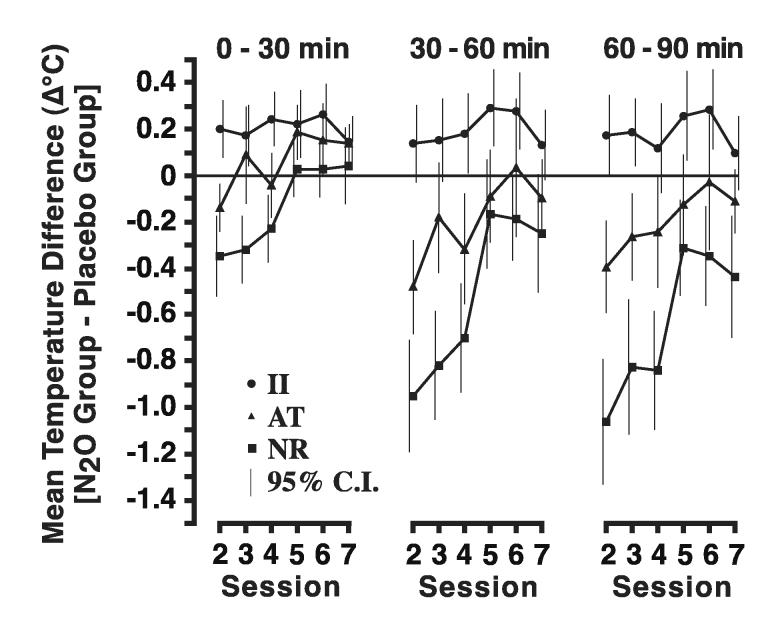
For the 0-30 minute time interval (Fig. 4), there were significant differences among individual difference groups in the effect of N2O at Session 2 (p < 0.001) and in the rate of change of core temperature across sessions (p < 0.001); however, the group differences in core temperature at Session 7 were not significant (p = 0.36). For the II group, the estimated effect of N2O at Session 2 was a hyperthermia of 0.22°C (p < 0.001), and there was no evidence for a change in the degree of hyperthermia across sessions (p = 0.94). For the AT group, there was a small non-statistically significant (p = 0.09) estimated hypothermic effect of N2O at Session 2. In subsequent sessions, the temperature difference between N2O and placebo changed significantly (p < 0.001) from hypothermia to hyperthermia such that the estimated effect of N2O at Session 7 was a statistically significant (p < 0.001) hyperthermia of 0.19°C (which was almost identical to the hyperthermia of 0.22°C in the II group). For the NR group, the estimated effect of N2O in Session 2 was a statistically significant (p < 0.001) hypothermia of 0.36°C. As in the AT group, the temperature difference between N2O and placebo changed significantly over sessions (p < 0.001); the estimated effect of N2O at Session 7 was a small non-significant (p = 0.08) hyperthermia of 0.10°C (which was approximately half of the hyperthermia in the II and AT groups).
Figure 4.
Mean (± 95% Confidence Intervals) of the difference scores between the N2O and placebo groups for each of the individual difference groups (i.e., II - Initially Insensitive, AT - Acute Tolerance, and NR - No Recovery). For purposes of statistical analysis, the continuous data presented in Figure 3 are converted to a mean difference score for three 30-minute intervals during Sessions 2-7. Full or complete tolerance development would be indicated by there being no difference (i.e., a zero change score) between the N2O and placebo groups.
For the 30-60 minute time interval (Fig. 4), as for the 0-30 minute interval, there were significant differences among individual difference groups in the effect of N2O at Session two (p < 0.001) and in the rate of change of core temperature across sessions (p < 0.001). Unlike the result for the 0-30 minute interval, the group differences in core temperature at Session 7 were significant (p = 0.01) for the 30-60 minute interval. For the II group, the estimated effect of N2O in the 30-60 minute interval was similar to the effect in the 0-30 minute interval, namely a hyperthermia of 0.18°C (compare with 0.22°C for 0-30 minutes), and there was no evidence for a change in core temperature across sessions (p = 0.44). For the AT group, there was a significant (p < 0.001) hypothermia equal to 0.43°C at Session 2. In subsequent sessions, the temperature difference between N2O and placebo changed (p < 0.001) so that the estimated effect of N2O at Session 7 was close to 0 and not significant (p = 0.94). For the NR group, the estimated effect of N2O in Session 2 was a significant (p < 0.001) hypothermia of 0.94°C. As in the AT group, the temperature difference between N2O and placebo changed over sessions (p < 0.001), so that the estimated effect of N2O at Session 7 was a non-significant (p = 0.41) hypothermia.
For the 60-90 minute time interval (Fig. 4), as for the other intervals, there were significant differences among individual difference groups in the effect of N2O at Session 2 (p < 0.001) and in the rate of change of core temperature across sessions (p < 0.001). For the 60-90 minute interval, the group differences in drug effect at Session 7 were highly significant (p < 0.001). For the II group, the estimated effect of N2O in the 60-90 minute interval of Session 2 was significant (p = 0.01) and similar to the effects in the other two intervals, namely a hyperthermia of 0.20°C (compare with 0.22°C and 0.18°C for the other two time intervals), and there was no evidence for a change in core temperature across sessions (p = 0.99). For the AT group, there was a significant (p < 0.001) hypothermia equal to 0.39°C at Session 2. In subsequent sessions, the temperature difference between N2O and placebo changed (p < 0.001) so that the estimated effect of N2O at Session 7 was small and not significant (p = 0.69). For the NR group, the estimated effect of N2O in Session 2 was a significant (p < 0.001) hypothermia of 1.00°C. As in the AT group, the temperature difference between N2O and placebo changed over sessions (p < 0.001), but unlike the AT group, the estimated effect of N2O at Session 7 for the NR group was a significant (p = 0.01) hypothermia equal to 0.26°C.
DISCUSSION
The first hypothesis tested was whether the rate and degree of chronic tolerance development for initially sensitive individuals would differ as a function of acute tolerance development during the initial screening exposure. This was clearly demonstrated as the group that developed acute tolerance (AT) subsequently developed chronic tolerance more quickly and completely than did the group that developed little acute tolerance (NR). Rats in the NR group assigned to the N2O condition continued to be hypothermic relative to NR rats receiving placebo gas during the final 30-min of gas exposure for all seven sessions (Figs 3 and 4). In contrast, during the final hour of test Sessions 5 - 7, the AT rats breathing N2O had comparable core temperatures as the AT rats receiving placebo gas (Figs 3 and 4). This difference in chronic tolerance development between the AT and NR groups did not result from differences in initial sensitivity to N2O hypothermia as the AT group had a mean temperature nadir of 35.58°C and the NR group had a mean temperature nadir of 35.61°C. Thus, differences in chronic tolerance development were predicted by reliable individual differences in acute tolerance assessed during an initial N2O exposure.
It is striking that the development of chronic tolerance in the NR group ceased to progress over the final three drug administrations (see Fig. 3 and Fig. 4, 30-60 and 60-90 min). This pattern of incomplete tolerance development is consistent with the proposal that NR rats remain relatively poor at activating anti-hypothermic heat-gaining and/or heat-conserving responses even after repeated drug-induced hypothermic challenges. We hypothesize that the thermoregulatory control system of NR rats senses and/or responds to the hypothermic challenge in a persistently less effective manner than occurs in rats that exhibit substantial acute tolerance during the initial administration. In the AT group, core temperature in the final hour of N2O administration was statistically indistinguishable from control values in each of the final three N2O administrations (Fig. 4). Moreover, Figure 4 depicts additional direct evidence for differences in thermo-adaptive responding between the NR and AT groups. Although both groups exhibited essentially identical magnitudes of peak hypothermia during the initial N2O administration, the AT group exhibited significant hyperthermia during the initial 30-min of N2O inhalation for each of the final three sessions (Fig. 4, 0-30 min). The direct observation of hyperthermia argues that a warming adaptive response is recruited during the development of chronic tolerance. This adaptive response occurs shortly after N2O onset in anticipation of the upcoming drug effect. Furthermore, the hyperthermia is of sufficient magnitude to counter N2O’s hypothermic drug effect and bring core temperature to control levels during the final hour of the last three sessions. Presumably, the NR rats also activate a warming adaptive response during chronic tolerance development. However, the less robust / effective adaptive responses made by the NR group are not sufficient to create a statistically significant hyperthermia and are thus insufficient to fully counter the hypothermic effects of N2O. Based on adaptation models of addiction, individuals with a weaker adaptive response may be expected to be less vulnerable to developing addiction.
The second hypothesis tested was whether rats that are initially insensitive to N2O hypothermia (II) would develop altered core temperature during repeated N2O administrations. We expected the II group to re-join its control group rapidly and to remain that way over the remainder of the exposure sessions. However, by the second N2O administration, the II group surpassed the temperature of the placebo control group (i.e., moved in the hyperthermic direction) and this prolonged hyperthermic effect persisted across the remainder of the sessions (Figs. 3 and 4). Based on these differences between the N2O group and placebo control group, it is inaccurate to view N2O as being relatively inert (i.e., having little impact on temperature regulatory mechanisms) in rats that appear initially insensitive to N2O’s hypothermic effects.
A provocative implication of these findings has to do with the nature of regulatory control systems in initially insensitive individuals. During the initial N2O administration, II rats may rapidly activate adaptive regulatory mechanisms that minimize the hypothermic drug perturbation such that core temperature is defended (i.e., little drug effect is observed). However, during subsequent drug administrations these warming responses are so robust that they cause core temperature to overshoot control values and the rat becomes hyperthermic. Indeed, the II rat experiences a withdrawal-like effect in the presence of the drug. This type of dysregulation could contribute to a rapid escalation of drug taking. As can occur for drug dependent individuals who take a drug to ameliorate withdrawal effects (Baker et al. 2004), II rats could attempt to restore normothermia by countering the withdrawal-like hyperthermia caused by their own dysregulated warming response by increasing the hypothermic drug effect through increased drug consumption. It may be this adaptive over-responsiveness of the regulatory system in initially insensitive individuals that puts them at increased vulnerability for addiction. Future research will need to assess whether the hyperthermia observed in II rats during repeated N2O administrations reflects a perturbation away from the defended core temperature or alternatively, represents a drug-induced allostatic (Koob and LeMoal 2001) increase in the defended core temperature. It is important to distinguish between drug-induced changes in body temperature that result from being forced away from the set point versus those that result from a change in set point temperature because these different scenarios activate quite different regulatory responses (Gordon 2001).
Direct observation of hyperthermia in the II group during subsequent N2O exposures (Sessions 2-7) suggests that an opponent regulatory response is present during those sessions. The II rats may also make an adaptive opponent response within minutes of the initial N2O exposure that is sufficiently robust to prevent significant hypothermia from developing and thereby cause the appearance of initial insensitivity. However, without direct evidence of hyperthermia during the initial exposure, this hypothesis depends upon an unobserved underlying process. To investigate possible underlying processes, our lab has begun to measure metabolic heat production and heat loss, the two proximal determinants of core temperature. We simultaneously quantify heat production using indirect calorimetry, heat loss using direct calorimetry, and core temperature via telemetry. On average, rats become hypothermic to a steady-state administration of 60% N2O because of a rapid reduction in heat production and an increase in heat loss (Kaiyala and Ramsay in press). Unpublished individual difference data from our lab indicate that it is possible for a rat to appear initially insensitive to N2O’s hypothermic effects despite exhibiting an N2O-induced increase in heat loss that is comparable to what is observed in rats that exhibit a significant hypothermia to N2O. In this case, the rat appears initially insensitive at the level of core temperature because the typical increase in heat loss is largely offset by an increase in heat production. This observation supports the hypothesis that even though a drug can exert its pharmacological effect in an initially insensitive individual, little or no change is evident in the dependent measure because the individual’s adaptive response effectively counters the drug-induced regulatory disturbance (Dworkin 1993; Ramsay and Woods 1997). If effective opponent adaptive responses are occurring in the II group during their initial N2O exposure, it is unclear why these responses become excessive (i.e., dysregulated) and result in a persistent hyperthermia with repeated exposure. Research that identifies the specific unconditioned responses that are triggered by a drug’s initial effect is likely to provide insight into the nature of the conditioned responses that develop with repeated drug exposures and modify the measured variable (Dworkin 1993; Ramsay and Woods 1997: Siegel et al. 2000). The development of hyperthermia in the II group is consistent with the possibility that increased heat production is the unconditioned response and that the “ ... conditioned reflex resembles the unconditioned reflex, and as it develops, it augments the effect of the unconditioned reflex” (Dworkin 1993, p. 38).
The findings of this study prompt a discussion about the interpretation and measurement of chronic tolerance. Chronic tolerance is a reduction in the magnitude of drug-induced change in a dependent measure that develops over repeated drug exposures. This can be seen as a shift to the right in the dose-effect curve indicating that a larger drug dose is required to elicit the same magnitude of effect in a tolerant individual as is observed in a drug naïve control subject (Lê et al. 1992). Chronic tolerance can also be measured as the degree of reduction in a drug-induced change in a dependent measure that develops when a constant drug dose is administered repeatedly. The present study used the latter method by repeatedly administering a 60% N2O concentration to evaluate chronic tolerance development. In the case of chronic tolerance development, the largest drug-induced deviation from baseline (or a control group defined baseline) typically occurs during an initial drug administration and then the magnitude of this discrepancy diminishes over repeated administrations as tolerance develops. Conceptually, there would be no tolerance development if the initial degree of drug-induced deviation did not change over repeated drug administrations while full / complete tolerance development would occur if the amount of drug-induced change in the dependent variable diminishes to the extent that it is indistinguishable from baseline or control values.
Common methods to quantify chronic tolerance development include measuring the change over sessions in either the maximum deviation from baseline (i.e., sensitivity) or the area under the curve (i.e., the total or integrated effect). The different patterns of chronic tolerance data collected for the three individual difference groups illustrate shortcomings of this analytical approach (Fig. 3). During the first 30-min of the final three drug administration sessions, the NR group is considered the most tolerant of the three groups because its core temperature is most similar to that of its control group (Fig. 4). Yet, the inability of the NR group to mount a more powerful hyperthermic response during this initial 30-min period may explain why these rats are the least tolerant during the final hour of the drug administration (Fig. 4). During the final three drug administration sessions, the AT group is the most chronically tolerant of the groups when considering the final hour of the drug exposure (Fig. 4). The AT group’s hyperthermic deviation away from control values during the first 30-min of the final drug exposure sessions, may be responsible for the full chronic tolerance that results during the following hour. How then should the II group be described in terms of tolerance development considering that they began with a high degree of initial insensitivity or “innate tolerance” (Kalant et al. 1971)? Relative to their initial exposure to N2O, the II group moved further away from their control values during subsequent exposures (i.e., Sessions 2-7) that could be described as developing more than 100% tolerance (Fig. 4). Should the II group be described as being hyper-tolerant or overly tolerant? The point is that if there are different patterns of chronic tolerance development (see Fig. 3), then simple summary measures such as area under the curve or maximum drug effect serve to mask these patterns. Further, the existence of these distinct patterns of change over repeated drug administrations illustrate the limitations of our current use of the term tolerance.
Perspectives. There is a wealth of human and animal literature, primarily from research on ethanol, that investigates whether characteristics measured during an initial drug administration predict chronic tolerance or the magnitude of subsequent drug consumption. For example, early work indicated that young men with a family history of alcoholism report being less sensitive to ethanol’s effects than do control subjects (Schuckit 1980). Schuckit and colleagues (2001) estimate that approximately 40% of children of alcoholics are relatively insensitive to ethanol while only 10% of children of non-alcoholics have this characteristic. Animal research has investigated whether these initial intrasessional factors are associated with subsequent chronic tolerance development or drug consumption by using rodents from outbred and inbred strains, as well as knock-out mice and selectively bred lines for drug consumption, drug sensitivity, and acute tolerance [e.g., outbred strains (Tabakoff and Culp 1984; San-Marina et al. 1989; Khanna et al. 1990b; Khanna et al. 1991); inbred strains (Crabbe et al. 1982; Tabakoff and Culp 1984; Khanna et al. 1990a; Crabbe et al. 1994; Gehle and Erwin 2000); knock-out mice (Naassila et al. 2002); lines selectively bred for consumption (Tampier and Mardones 1999; Tampier et al. 2000; Bell et al. 2001); lines selectively bred for sensitivity (Khanna et al. 1985; Crabbe et al. 1989; Limm and Crabbe 1992: Crabbe 1994; Browman et al. 2000; Deitrich et al. 2000; Draski et al. 2001; Palmer et al. 2002); lines selectively bred for acute tolerance (Deitrich et al. 2000; Rustay et al. 2001; Wu et al. 2001)]. While the scientific literature is in general agreement that initial sensitivity and acute tolerance are relevant to the subsequent development of chronic tolerance and drug consumption, a coherent description of this relationship has not emerged. Newlin and Thomson (1990) describe the human literature on whether offspring of alcoholics are more or less sensitive to alcohol as being “remarkably inconsistent” (p. 385). Khanna and colleagues (1990b) describe the animal literature on the relationship between sensitivity and tolerance to alcohol consumption as being “inconclusive, because of contradictory results” (p. 429). More recently, Ponomarev and Crabbe (2002) state that “It is not clear how initial sensitivity and acute tolerance individually contribute to alcohol abuse” (p. 257) and they suggest a novel method to improve animal research on the topic. Much of this literature discusses the factors that contribute to this lack of clarity. Describing some of these factors places our current study in proper context.
Too few intermittent measurements of a dependent variable can make it difficult to assess initial sensitivity (i.e., observe the maximal drug effect) and measure acute tolerance (Khanna et al. 1985; San-Marina et al. 1989). Likewise, too few measurements of the drug concentration also complicate this assessment especially when drug concentrations are rapidly changing (Lê et al. 1992; Dietrich et al. 2000; Gehle and Erwin 2000; Rustay et al. 2001). Furthermore, venous blood measurements of the drug concentration often do not reflect the drug concentration in the brain (Wu et al. 2001; Lê et al. 1992). To circumvent these methodological problems, a compelling rationale has been made for measuring acute tolerance at steady-state drug concentrations for ethanol (Ramchandani et al. 1999; Froehlich et al. 2001) and for other drugs as well (Kissin et al. 1996; Kaiyala et al. 2001). Measuring a dependent variable continuously at steady-state drug concentrations facilitates the measurement of initial sensitivity and acute tolerance.
Many dependent variables have been used to measure drug effects (Lê et al. 1992). Estimates of drug sensitivity and tolerance often do not generalize across dependent measures but instead are task specific (San-Marina et al. 1989; Deitrich et al. 2000). Interpreting the relationship between initial sensitivity, acute tolerance and chronic tolerance is facilitated when they are investigated in the same study with a consistent dependent measure. Some dependent variables are affected by the measurement procedure itself and associated handling. A good example is core temperature measurement in rodents where the act of taking a rectal temperature changes core temperature and can alter the apparent magnitude of the drug effect (Peris and Cunningham 1986). Dependent measures assessing drug effects can also be influenced by intoxicated practice (Wenger et al. 1981, Lê and Kalant 1992; Lê et al. 1992; Zack and Vogel-Sprott 1995). These are but examples of the factors that make it difficult to draw general conclusions from studies that use different methods and dependent measures.
There is often both a conceptual and an analytical confounding of initial sensitivity and acute and chronic tolerance. Numerous investigators have recognized that measures of initial sensitivity often represent a mixture of sensitivity and acute tolerance development (San-Marina et al. 1989; Khanna et al. 1990; Khanna et al. 1991; Ramsay and Woods 1997; Dietrich et al. 2000; Gehle and Erwin 2000; Ponomarev and Crabbe 2002). Research investigating how initial drug sensitivity, acute tolerance and chronic tolerance are related is susceptible to finding spurious associations because a baseline value can influence the size of the drug effect (sensitivity) that in turn can influence the amount of acute and chronic tolerance that can develop. For example, the absence of a large initial drug effect (i.e., as seen in an initially insensitive individual) prevents a large amount of acute or chronic tolerance from developing because there is little deviation to recover from when a drug effect is small (Khanna et al. 1985; San-Marina et al. 1989). The recommended strategy for testing these relationships is to use residual scores from regression analyses to quantify individual differences in initial sensitivity and tolerance rather than difference or ratio scores (Nagoshi et al. 1986; Grant et al. 1989). Unfortunately, the improper use of difference scores to relate initial sensitivity and tolerance can complicate interpretation of the literature (e.g., see Figure 1 of San-Marina et al. 1989).
The experimental paradigm selected for our current study has numerous strengths. N2O is a hypothermic drug that equilibrates quickly throughout the body and is well suited for steady-state administrations (Eger II 1985). N2O is inhaled and thus there are no surgical procedures needed to enable drug administration. This made it feasible to investigate individual differences using a large number of subjects because of the ease with which prolonged steady-state drug concentrations could be achieved over multiple sessions. Pharmacokinetic explanations for changes in the dependent variable during a steady-state N2O administration are eliminated because there are no significant metabolic pathways for N2O (Trudell 1985). Core temperature data are collected continuously and non-invasively at a known steady-state N2O concentration. This facilitates the measurement of initial sensitivity and acute and chronic tolerance. Using core temperature as the primary dependent variable also builds on an extensive body of drug tolerance research. The experimental design incorporates measures of initial sensitivity, acute tolerance and chronic tolerance in the same study. The data analytical procedures employ regression residual scores to avoid spurious findings that could result from using simple difference scores. Outbred rats were used to study individual differences so that the predictive utility of any associations could be relevant to a heterogeneous population (San-Marina et al. 1989). The ability to screen large numbers of rats efficiently permitted the use of a selective screening process to create distinct individual difference groups that enhanced our ability to visualize the effect that these differences had on chronic tolerance development. Thus, this experimental paradigm was able to address and circumvent many of the problematic factors common to this type of research.
Of course, the present study also has its limitations. It is not known whether the relationships found in the present study apply to other drugs, species, or dependent variables. It is possible to evaluate whether these findings generalize to ethanol-induced hypothermia in rats by using the steady-state ethanol infusion method described by Froehlich and colleagues (2001). It is also important to determine whether the relationships observed in this study reflect a process that is common to other regulated physiological systems or if the findings are specific to the temperature regulatory system. If unusually adaptive control systems exist in some individuals for psychophysiologically regulated variables that motivate drug taking (e.g., hedonic or affective state), then being “initially insensitive” in regard to such variables could be the result of a relatively prompt and excessive activation of adaptive responses causing withdrawal-like symptoms that could motivate an escalation of drug use. Our observation of a withdrawal-like shift at the level of core temperature in the II rats (Fig. 3) suggests a novel hypothesis to explain the documented association between initial drug (alcohol) insensitivity and addictive vulnerability. However, extending our present research paradigm to investigate the effect of individual differences in sensitivity and tolerance on drug consumption is hampered by the minimal work on N2O self-administration methods. N2O is a drug of abuse (Layzer 1985) and although self-administration methods exist for monkeys (Grubman and Woods 1982; Nemeth and Woods 1982; Wood et al. 1977), there is only a single published study investigating N2O self-administration methods in the rat (Ramsay et al. 2003). Finally, chronic tolerance development was evaluated over seven N2O exposure sessions. It is possible that the observed individual differences in chronic tolerance development could change with additional exposure sessions.
Conclusion. The present data provide new insight into the phenomenon of chronic drug tolerance. As chronic tolerance develops over repeated drug administrations, the prevailing belief has been that the perturbed variable is returning toward a baseline or control value. The among-subject variability in the speed and magnitude of chronic tolerance development typically has been viewed as error variability that obscures a single common underlying trajectory. In the present study, stringent criteria were used to select individual outbred rats for further study based on reliable individual difference characteristics in sensitivity and acute tolerance (i.e., NR, AT, II) that were assessed during an initial drug exposure. Importantly, these individual differences predict qualitatively different patterns of chronic tolerance development. We believe the effect of these individual differences on chronic tolerance has gone unnoticed because research often emphasizes group means that can obscure meaningful individual differences. The causes of these individual differences in chronic tolerance development may be relevant to understanding individual differences in addictive vulnerability and warrant further investigation.
Footnotes
REFERENCES
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bell RL, Stewart RB, Woods JE, 2nd, Lumeng L, Li TK, Murphy JM, McBride WJ. Responsivity and development of tolerance to the motor impairing effects of moderate doses of ethanol in alcohol-preferring (P) and - nonpreferring (NP) rat lines. Alcohol Clin Exp Res. 2001;25:644–650. [PubMed] [Google Scholar]
- Brick J, Pohorecky LA, Faulkner W, Adams MN. Circadian variations in behavioral and biological sensitivity to ethanol. Alcohol Clin Exp Res. 1984;8:204–211. doi: 10.1111/j.1530-0277.1984.tb05840.x. [DOI] [PubMed] [Google Scholar]
- Browman KE, Rustay NR, Nikolaidis N, Crawshaw L, Crabbe JC. Sensitivity and tolerance to ethanol in mouse lines selected for ethanol-induced hypothermia. Pharmacol Biochem Behav. 2000;67:821–829. doi: 10.1016/s0091-3057(00)00427-5. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Tolerance to ethanol hypothermia in HOT and COLD mice. Alcohol Clin Exp Res. 1994;18:42–46. doi: 10.1111/j.1530-0277.1994.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK, Mitchell SR, Crawshaw LI. Quantitative trait loci mpping genes that influence the sensitivity and tolerance to ethanol-induced hypothermia in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1994;269:184–192. [PubMed] [Google Scholar]
- Crabbe JC, Feller DJ, Dorow JS. Sensitivity and tolerance to ethanol-induced hypothermia in genetically selected mice. J Pharmacol Exp Ther. 1989;249:456–461. [PubMed] [Google Scholar]
- Crabbe JC, Janowsky TS, Young ER, Kosobud A, Stack J, Rigter H. Tolerance to ethanol hypothermia in inbred mice: Genotypic correlations with behavioral responses. Alcohol Clin Exp Res. 1982;6:446–458. doi: 10.1111/j.1530-0277.1982.tb05007.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends Neurosci. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Bludeau P, Erwin VG. Phenotypic and genotypic relationships between ethanol tolerance and sensitivity in mice selectively bred for initial sensitivity to ethanol (SS and LS) or development of acute tolerance (HAFT and LAFT) Alcohol Clin Exp Res. 2000;24:595–604. [PubMed] [Google Scholar]
- Draski LJ, Bice PJ, Deitrich RA. Developmental alterations of ethanol sensitivity in selectively bred high and low alcohol sensitive rats. Pharmacol Biochem Behav. 2001;70:387–396. doi: 10.1016/s0091-3057(01)00621-9. [DOI] [PubMed] [Google Scholar]
- Dworkin BR. Learning and physiological regulation. The University of Chicago Press; Chicago: 1993. [Google Scholar]
- Eger EI., II . MAC. In: Eger EI II, editor. Nitrous oxide / N2O. Elsevier Science Publishing Company; New York: 1985. pp. 57–67. [Google Scholar]
- Fenoglio P, Parel V, Kopp P. The social cost of alcohol, tobacco and illicit drugs in France. Eur Addict Res. 2003;9:18–28. doi: 10.1159/000067730. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Janowsky RB, Li TK, Mosemiller AK, McCullough DE, Ho MC, Kisner JM. Induction of steady-state blood alcohol levels: application to the study of within-session alcohol tolerance in rats. Alcohol Clin Exp Res. 2001;25:370–376. [PubMed] [Google Scholar]
- Gehle VM, Erwin VG. The genetics of acute functional tolerance and initial sensitivity to ethanol for an ataxia test in the LsxSS RI strains. Alcohol Clin Exp Res. 2000;24:579–587. [PubMed] [Google Scholar]
- Grant KA, Werner R, Hoffman PL, Tabakoff B. Chronic tolerance to ethanol in the N:NIH rat. Alcohol Clin Exp Res. 1989;13:402–406. doi: 10.1111/j.1530-0277.1989.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Grubman J, Woods JH. Effects of morphine and cocaine on responding under a multiple schedule of food or nitrous oxide presentation. In: Saito S, Yanagita T, editors. Learning and memory drugs as reinforcers. Vol. 620. 1982. pp. 259–74. [Google Scholar]
- Gordon CJ. The therapeutic potential of regulated hypothermia. Emerg Med J. 2001;18:81–89. doi: 10.1136/emj.18.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie TJ. Generalized additive models. In: Chambers JM, Hastie TJ, editors. Statistical models in S. Wadsworth & Brooks/Cole Advanced Books & Software; Pacific Grove, California: 1992. pp. 249–307. [Google Scholar]
- Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Jaffe JH. Drug addiction and drug abuse. In: Gilman AG, Goodman LS, Rall TW, Murad F, editors. Goodman and Gillman’s The Pharmacological Basis of Therapeutics. 7th edition. MacMillan Publishing; New York: 1985. pp. 532–581. [Google Scholar]
- Kaiyala KJ, Leroux BG, Watson CH, Prall CW, Coldwell SE, Woods SC, Ramsay DS. Reliability of individual differences in initial sensitivity and acute tolerance to nitrous oxide hypothermia. Pharmacol Biochem Behav. 2001;68:691–699. doi: 10.1016/s0091-3057(01)00488-9. [DOI] [PubMed] [Google Scholar]
- Kaiyala KJ, Ramsay DS. Assessment of heat production, heat loss, and core temperature during nitrous oxide exposure: A new paradigm for studying drug effects and opponent responses. Am J Physiol Regul Integr Comp Physiol. doi: 10.1152/ajpregu.00412.2004. [DOI] [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev. 1971;23:135–191. [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Chau AK, Sharma H. Initial sensitivity, acute tolerance and alcohol consumption in four inbred strains of rats. Psychopharmacology. 1990a;101:390–395. doi: 10.1007/BF02244059. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Sharma H, Chau AK. Initial sensitivity, acute tolerance and alcohol consumption in Fischer 344 and Long Evans rats. Psychopharmacology. 1991;105:175–180. doi: 10.1007/BF02244305. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Shah G, Sharma H. Comparison of sensitivity and alcohol consumption in four outbred strains of rats. Alcohol. 1990b;7:429–434. doi: 10.1016/0741-8329(90)90027-a. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Lê AD, LeBlanc AE, Shah G. Initial sensitivity versus acquired tolerance to ethanol in rats selectively bred for ethanol sensitivity. Psychopharmacology. 1985;86:302–306. doi: 10.1007/BF00432218. [DOI] [PubMed] [Google Scholar]
- Kissin I, Lee SS, Arthur GR, Bradley EL., Jr Time course characteristics of acute tolerance development to continuously infused alfentanil in rats. Anesth Analg. 1996;83:600–605. doi: 10.1097/00000539-199609000-00029. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Layzer RB. Nitrous oxide abuse. In: Eger EI II, editor. Nitrous oxide / N2O. Elsevier Science Publishing Company; New York: 1985. pp. 249–57. [Google Scholar]
- Lê AD, Kalant H. Influence of intoxicated practice on the development of acute tolerance to the motor impairment effect of ethanol. Psychopharmacology. 1992;106:572–576. doi: 10.1007/BF02244833. [DOI] [PubMed] [Google Scholar]
- Lê AD, Mihic SJ, Wu PH. Alcohol tolerance: methodological and experimental issues. In: Boulton A, Baker G, Wu PH, editors. Neuromethods, Vol 24: Animal Models of Drug Addiction. Humana Press; Totowa, New Jersey: 1992. pp. 95–124. [Google Scholar]
- Lewis JL, Pruhs RJ, Quock RM. Modification of an infant incubator for exposure of experimental animals to nitrous oxide. J Pharmacol Methods. 1987;18:143–150. doi: 10.1016/0160-5402(87)90007-6. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Limm M, Crabbe JC. Ethanol tolerance in a genetically insensitive selected mouse line. Alcohol Clin Exp Res. 1992;16:800–805. doi: 10.1111/j.1530-0277.1992.tb00682.x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li T K. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McGinnis JM, Foege WH. Mortality and morbidity attributable to use of addictive substances in the United States. Proc Assoc Am Physicians. 1999;111:109–118. doi: 10.1046/j.1525-1381.1999.09256.x. [DOI] [PubMed] [Google Scholar]
- Naassila M, Ledent C, Daoust M. Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi CT, Wilson JR, Plomin R. Use of regression residuals to quantify individual differences in acute sensitivity and tolerance to alcohol. Alcohol Clin Exp Res. 1986;10:343–349. doi: 10.1111/j.1530-0277.1986.tb05101.x. [DOI] [PubMed] [Google Scholar]
- Nemeth MA, Woods JH. Effects of morphine and cocaine on responding under a multiple schedule of food or nitrous oxide presentation. In: Saito S, Yanagita T, editors. Learning and memory drugs as reinforcers. Vol. 620. 1982. pp. 275–285. [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psych Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Moyer MR, Crabbe JC, Phillips TJ. Initial sensitivity, tolerance and cross-tolerance to allopregnanolone- and ethanol-induced hypothermia in selected mouse lines. Psychopharmacology. 2002;162:313–322. doi: 10.1007/s00213-002-1106-2. [DOI] [PubMed] [Google Scholar]
- Peper A. A theory of drug tolerance and dependence I: a conceptual analysis. J Theor Biol. 2004;229:477–490. doi: 10.1016/j.jtbi.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Peris J, Cunningham CL. Handling-induced enhancement of alcohol’s acute physiological effects. Life Sci. 1986;38:273–279. doi: 10.1016/0024-3205(86)90313-9. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Marshall NR, MacDonald AG. Behavioural thermoregulation in mice: Effects of low doses of general anaesthetics of different potency. Exp Physiol. 1990;75:629–637. doi: 10.1113/expphysiol.1990.sp003441. [DOI] [PubMed] [Google Scholar]
- Piazza PV, LeMoal M. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. A novel method to assess initial sensitivity and acute tolerance to hypnotic effects of ethanol. J Pharmacol Exp Ther. 2002;302:257–263. doi: 10.1124/jpet.302.1.257. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Cappell H. Homeostatic theory of drug tolerance: a general model of physiological adaptation. Psychol Rev. 1991;98:390–408. doi: 10.1037/0033-295x.98.3.390. [DOI] [PubMed] [Google Scholar]
- Quock RM, Panek RW, Kouchich FJ, Rosenthal MA. Nitrous oxide-induced hypothermia in the rat. Life Sci. 1987;41:683–690. doi: 10.1016/0024-3205(87)90447-4. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, O’Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Jr, Li TK. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcohol Clin Exp Res. 1999;23:1320–1330. [PubMed] [Google Scholar]
- Ramsay DS, Omachi K, Leroux BG, Seeley RJ, Prall C, Woods SC. Nitrous oxide-induced hypothemia in the rat: Acute and chronic tolerance. Pharmacol Biochem Behav. 1999;62:189–196. doi: 10.1016/s0091-3057(98)00156-7. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Watson C, Leroux BG, Prall CW, Kaiyala KJ. Conditioned place aversion and self-administration of nitrous oxide in rats. Pharmacol Biochem Behav. 2003;74:623–633. doi: 10.1016/s0091-3057(02)01048-1. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Woods SC. Biological consequences of a drug administration: implications for acute and chronic tolerance. Psychol Rev. 1997;104:170–193. doi: 10.1037/0033-295x.104.1.170. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Boehm SL, II, Schafer GL, Browman KE, Erwin VG, Crabbe JC. Sensitivity and tolerance to ethanol-induced incoordination and hypothermia in HAFT and LAFT mice. Pharmacol Biochem Behav. 2001;70:167–174. doi: 10.1016/s0091-3057(01)00595-0. [DOI] [PubMed] [Google Scholar]
- San-Marina A, Khanna JM, Kalant H. Relationship between initial sensitivity, acute tolerance and chronic tolerance to ethanol in a heterogeneous population of Swiss mice. Psychopharmacology. 1989;99:450–457. doi: 10.1007/BF00589891. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980;41:242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol sensitivity and dependence. In: Jansson B, Jörnvall H, Rydberg U, Terenius L, Vallee BL, editors. Toward a molecular basis of alcohol use and abuse. Birkhäuser Verlag; Basel, Switzerland: 1994. pp. 341–348. [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, Bierut L, Goate A, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res. 2001;25:323–329. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Siegel S, Baptista MA, Kim JA, McDonald RV, Weise-Kelly L. Pavlovian psychopharmacology: the associative basis of tolerance. Exp Clin Psychopharmacol. 2000;8:276–293. doi: 10.1037//1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- Single E, Robson L, Rehm J, Xie X, Xie X. Morbidity and mortality attributable to alcohol, tobacco, and illicit drug use in Canada. Am J Public Health. 1999;89:385–390. doi: 10.2105/ajph.89.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL. The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. Am Psychol. 1980;35:691–712. doi: 10.1037//0003-066x.35.8.691. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Culp SG. Studies on tolerance development in inbred and heterogeneous stock National Institutes of Health rats. Alcohol Clin Exp Res. 1984;8:495–499. doi: 10.1111/j.1530-0277.1984.tb05711.x. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Rothstein JD. Biology of tolerance and dependence. In: Tabakoff B, Sutker PB, Randall CL, editors. Medical and Social Aspects of Alcohol Abuse. Plenum Press; New York: 1983. pp. 187–220. [Google Scholar]
- Tampier L, Mardones J. Differences in ethanol sensitivity and acute tolerance between UChA and UChB rats. J Stud Alcohol. 1999;60:168–171. doi: 10.15288/jsa.1999.60.168. [DOI] [PubMed] [Google Scholar]
- Tampier L, Quintanilla ME, Mardones J. Acute tolerance, ethanol sensitivity and drinking pattern in the F2 generation of UChA and UChB rats. J Stud Alcohol. 2000;61:647–651. doi: 10.15288/jsa.2000.61.647. [DOI] [PubMed] [Google Scholar]
- Trudell JR. Metabolism of nitrous oxide. In: Eger EI II, editor. Nitrous oxide / N2O. Elsevier Science Publishing Company; New York: 1985. pp. 203–210. [Google Scholar]
- Wenger JR, Tiffany TM, Bombardier C, Nicholls K, Woods SC. Ethanol tolerance in the rat is learned. Science. 1981;213:575–577. doi: 10.1126/science.7244656. [DOI] [PubMed] [Google Scholar]
- Wood RW, Grubman J, Weiss B. Nitrous oxide self-administration in the squirrel monkey. J Pharmacol Exp Ther. 1977;202:491–499. [PubMed] [Google Scholar]
- Wu PH, Tabakoff B, Szabó G, Hoffman PL. Chronic ethanol exposure results in increased acute functional tolerance in selected lines of HAFT and LAFT mice. Psychopharmacology. 2001;155:405–412. doi: 10.1007/s002130100722. [DOI] [PubMed] [Google Scholar]
- Zack M, Vogel-Sprott M. Behavioral tolerance and sensitization to alcohol in humans: the contribution of learning. Exp Clin Psychopharmacol. 1995;3:396–401. [Google Scholar]