Abstract
The homeobox factor PDX-1 is a key regulator of pancreatic morphogenesis and glucose homeostasis; targeted disruption of the PDX-1 gene leads to pancreatic agenesis in pdx-1(−/−) homozygotes. Pdx-1 heterozygotes develop normally, but they display glucose intolerance in adulthood. Like certain other homeobox proteins, PDX-1 contains a consensus FPWMK motif that promotes heterodimer formation with the ubiquitous homeodomain protein PBX. To evaluate the importance of PDX-1:PBX complexes in pancreatic morphogenesis and glucose homeostasis, we expressed either wild-type or PBX interaction defective PDX-1 transgenes under control of the PDX-1 promoter. Both wild-type and mutant PDX-1 transgenes corrected glucose intolerance in pdx-1 heterozygotes. The wild-type PDX-1 transgene rescued the development of all pancreatic lineages in pdx-1(−/−) animals, and these mice survived to adulthood. In contrast, pancreata from pdx-1(−/−) mice expressing the mutant PDX-1 transgene were hypoplastic, and these mice died within 3 weeks of birth from pancreatic insufficiency. All pancreatic cell types were observed in pdx-1(−/−) mice expressing the mutant PDX-1 transgene; but the islets were smaller, and increased numbers of islet hormone-positive cells were noted within the ductal epithelium. These results indicate that PDX-1:PBX complexes are dispensable for glucose homeostasis and for differentiation of stem cells into ductal, endocrine, and acinar lineages; but they are essential for expansion of these populations during development.
The homeobox protein PDX-1 functions critically in pancreatic development and glucose homeostasis; murine and human pdx-1(−/−) homozygotes are apancreatic (1–3), and pdx-1 heterozygotes develop glucose intolerance in adulthood, partly because of inadequate insulin gene expression (4, 5). Further underscoring the importance of this factor for glucose homeostasis, mutations in the PDX-1 gene have been identified in patients with maturity onset diabetes of the young-type 4 (6). Consistent with this phenotype, PDX-1 stimulates the expression of certain islet-specific genes such as somatostatin (7, 8), insulin (9–11), glut-2 (12), and glucokinase (13), by binding as a monomer to promoter sites that contain a GTAATC consensus site.
Like certain homeobox factors, PDX-1 also binds as a heterodimer with the ubiquitous homeodomain protein PBX to target sites containing a consensus TGATTAAT motif. Detected in crude extracts from insulinoma cells (14), PDX-1:PBX complexes have also been observed in ductal and acinar cell lines, where they regulate the expression of exocrine target genes such as elastase (15). Compared with the PDX-1 monomer, the PDX-1:PBX heterodimer binds with 10- to 20-fold higher affinity to its sites (14), suggesting a potential mechanism by which PDX-1 target genes are hierarchically regulated during development.
To examine the requirement for PDX-1:PBX complexes in pancreatic morphogenesis, islet cell differentiation, and glucose homeostasis, we developed transgenic mice expressing either wild-type or PBX interaction defective PDX-1 transgenes under control of the PDX-1 promoter/enhancer. In the context of a pdx-1(−/−) background, the wild-type PDX-1 transgene completely rescued pancreatic development and glucose homeostasis in adult mice. The mutant PDX-1 transgene also corrected glucose intolerance in pdx-1(+/−) mice, but it was unable to fully rescue pancreatic development in pdx-1(−/−) animals. All cell types within the pancreas of pdx-1(−/−) mice expressing the mutant PDX-1 transgene were present; but their organization and overall proliferation were greatly affected. These results show that PDX-1:PBX complexes are dispensable for the generation of the various cell types within the pancreas, but they are required for expansion of these cell populations during development. Taken together, these studies demonstrate that PDX monomers and PDX:PBX heterodimers perform distinct functions in the developing pancreas, reflecting in part their capacity to regulate discrete subsets of pancreatic target genes.
Materials and Methods
Plasmids and Transgenic Mice.
Wild-type and mutant PDX-1 transgenes were constructed with the use of a 15-kb rat PDX-1 genomic clone (16) that, in addition to the 1-kb coding region, contains 6.5 kb of 5′ flanking sequence, a 4-kb intron, and 3 kb of 3′ flanking sequence. The PBX interaction defective PDX-1 clone contained mutations in sequences encoding aa 119–123 of the rat PDX-1 gene (FPWMK/AAGGQ), which were generated by PCR-based mutagenesis (14). Plasmids were injected into C57BL6 mouse oocytes. Three independent lines of mice expressing the wild-type PDX-1 transgene and two independent lines expressing the mutant PDX-1 transgene were generated. Glucose tolerance tests were performed as previously described (4).
RNase Protection and Western Blot Assays.
RNase protection assays were performed as previously reported (16), with a 450-base antisense PDX-1 riboprobe, extending from +130 to +530 of the rat PDX-1 cDNA and containing 50 nucleotides of plasmid sequence. Western blot assays were performed with the use of PDX-1-specific antiserum as reported (17).
Immunolabeling.
Double immunofluorescence labeling was performed sequentially with primary antibodies made in different species: guinea pig anti-human insulin (Linco Research Immunoassay, St. Charles, MO), rabbit anti-bovine glucagon (kindly donated by M. Appel, University of Massachusetts Medical School, Worcester, MA), and rabbit anti-synthetic somatostatin, a mixture of the three non-β cell hormone antibodies (anti-glucagon, -somatostatin and -pancreatic polypeptide). Antibodies were used on paraffin sections of 4% buffered formaldehyde-fixed pancreas, with the exception of PBX-1 antiserum (Santa Cruz Biotechnology), which was used on freshly frozen sections of 3-day-old wild-type mouse pancreas. The secondary antibodies used for immunofluorescence were Texas Red-conjugated donkey anti-guinea pig IgG, FITC-conjugated donkey anti-rabbit IgG-, and streptavidin-conjugated FITC (all 1:100 dilution; Jackson ImmunoResearch). After extensive rinsing, slides were mounted with DABCO glycerol anti-fading mounting media. Immunoperoxidase labeling was carried out with the Histomouse kit (Zymed) for insulin (1:1000) or the Vectastain ABC kit (Vector Laboratories) for glucagon (1:1000) and somatostatin (1:1000). Detection of β-galactosidase from the pdx-1lacZKO allele was performed as previously described.
Results
Mutagenesis of the PBX interaction motif in PDX-1 (aa 119–123; FPWMK/AAGGQ) has been shown to block complex formation with PBX on a heterodimer recognition site (14). Disruption of this motif in HoxA9 does not eliminate cooperativity with PBX (18), however, prompting us to evaluate functional interactions between a transcriptionally activated PBX (E2A-PBX) and wild-type or PBX interaction defective PDX proteins. Consistent with the selectivity of heterodimer binding sites for PBX:HOX complexes, wild-type and mutant PDX-1 proteins alone had marginal activity on a TSEII reporter that contains two PDX:PBX heterodimer recognition sites (Fig. 1A). After cotransfection with E2A-PBX, the wild-type PDX-1 construct induced reporter activity by 4- to 5-fold (Fig. 1A). In contrast, E2A-PBX had no effect on reporter activity in cells cotransfected with the mutant PDX-1 construct, demonstrating that disruption of the PBX interaction motif in PDX-1 is indeed sufficient to block functional cooperativity with PBX.
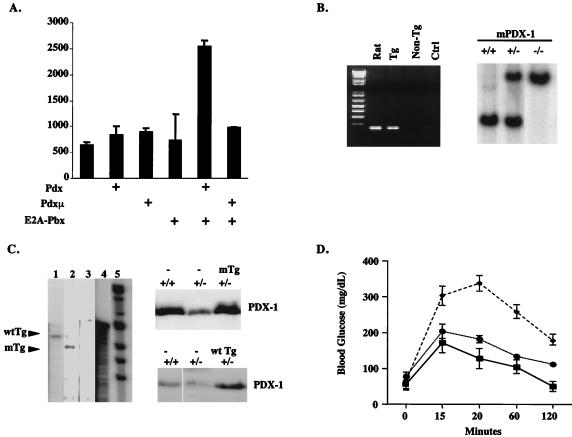
Figure 1.
Expression of wild-type or PBX interaction defective PDX-1 transgenes rescues glucose homeostasis in pdx-1 (+/−) mice. (A) Transient transfection assay of wild-type and PBX interaction defective (Pdxμ) PDX-1 constructs in 293T cells, with a TSEII luciferase reporter containing two PDX:PBX heterodimer recognition sites. Cells were transfected with wild-type or mutant PDX plus E2A-PBX expression vector where indicated. The first bar on the left represents control transfection with empty vector alone plus TSEII reporter. Luciferase activity shown after normalizing to β-galactosidase activity from cotransfected RSV-βgal plasmid. (B) Genotypic analysis of mice expressing PDX-1 transgene. (Left) Inheritance of PDX-1 transgene was evaluated by PCR assay with primers that are selective for rat PDX-1. (Right) Mice heterozygous or homozygous for targeted disruption of the murine PDX-1 gene were identified by genomic Southern blot analysis with a 32P-labeled PDX-1 cDNA probe. Insertion of the inactivating β-galactosidase gene is indicated by a 3.8-kb vs. a 3.0-kb EcoRI fragment. (C) Wild-type (Lane 1) and mutant (Lane 2) PDX-1 transgenes are comparably expressed in transgenic mice. (Left) RNase protection assay of total RNA from whole pancreas of adult mice, with the use of 32P-labeled wild-type PDX-1 antisense RNA probe. The shorter fragment in mutant mice results from RNase digestion in sequences encoding mutant FPWMK/AAGGQ motif. Lane 3, control nontransgenic littermate RNA; the rat PDX-1 probe does not recognize murine PDX-1. Lane 4, position of undigested PDX-1 antisense probe. Lane 5, molecular weight marker. (Right) Western blot assay of whole pancreas extract, showing levels of PDX-1 protein in wild-type, pdx-1(+/−), pdx-1(+/−);mTg, and pdx-1(−/−);wtTg mice. (D) Line graph of blood glucose levels in wild-type (—■—), pdx-1(+/−) heterozygous (– –⧫– –), pdx-1(+/−); wtTg, and pdx-1 (+/−);mTg mice (⋅⋅⋅●⋅⋅⋅) after i.p. glucose injection. Results for pdx-1(+/−); wtTg and pdx-1(+/−);mTg mice were indistinguishable.
To evaluate the role of PDX-1:PBX complexes in promoting pancreatic morphogenesis and glucose homeostasis, we prepared transgenic mice expressing wild-type or PBX interaction defective PDX-1 proteins under the control of the PDX-1 promoter/enhancer (Fig. 1B). In previous experiments using lacZ reporter transgenes, both rat and mouse PDX-1 gene promoter/enhancers have been found to recapitulate the spatiotemporal expression pattern of the endogenous PDX-1 gene in transgenic mice (16, 19).
When fused to the rat PDX-1 promoter/enhancer, mutant and wild-type PDX-1 transgenes were comparably expressed in pancreatic tissue from founder animals by RNase protection and Western blot assays (Fig. 1C). The size and appearance of the pancreas in animals carrying mutant (mTg) or wild-type (wtTg) transgene mice were indistinguishable from nontransgenic littermates (not shown), indicating that neither transgene interferes with normal pancreatic development in this context.
To determine whether PDX-1:PBX complexes are required for glucose homeostasis, we crossed mTg and wtTg mice with pdx-1(+/−) mice (1). PDX-1 protein levels in pancreatic extracts from pdx-1(+/−) mice were about half that of wild-type animals by Western blot analysis; and expression of either wild-type or mutant transgenes restored PDX-1 protein to wild-type levels in pdx-1 heterozygotes (Fig. 1D). After i.p. glucose injection, blood glucose levels in wild-type mice rose to 175 mg/dl within 15 min, returning to baseline after 2 h. As expected, blood glucose levels in pdx-1(+/−) mice rose to 350 mg/dl after 20 min and remained abnormally elevated for the duration of the assay (ref. 4; Fig. 1D). Consistent with the notion that monomeric PDX-1 is sufficient for the activation of genes involved in glucose homeostasis, expression of either wild-type or mutant PDX-1 transgenes corrected the impairment in glucose homeostasis in pdx-1 heterozygotes (Fig. 1D). These results suggest that PDX-1:PBX complexes are dispensable for islet cell function in the adult.
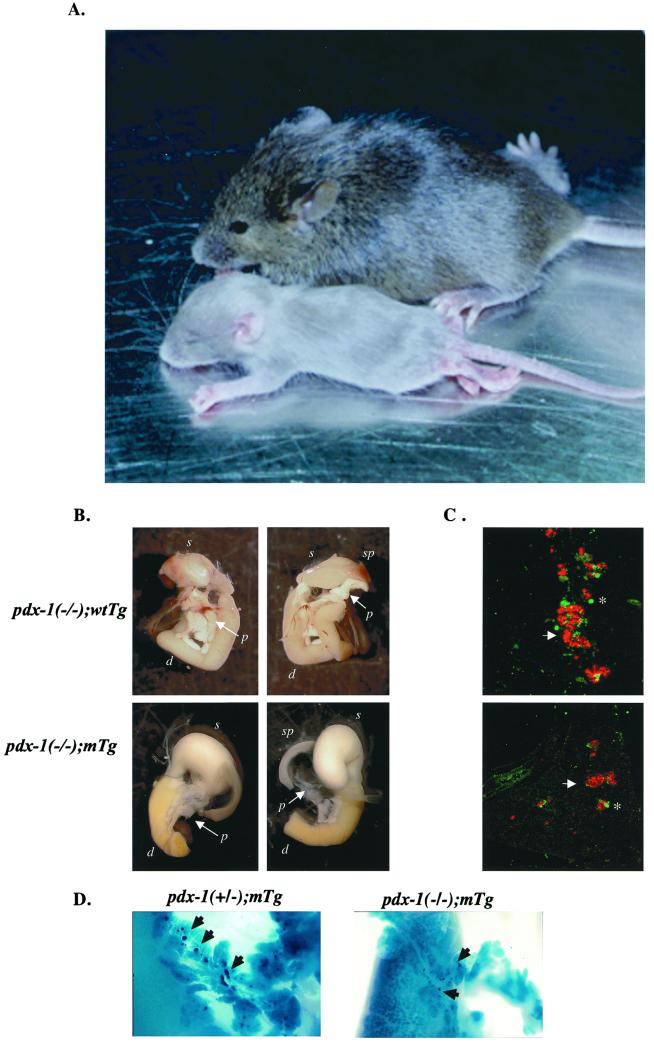
To determine the importance of PDX-1:PBX complexes in pancreatic development, we analyzed the activities of wild-type and mutant PDX-1 transgenes in the context of pdx-1(−/−) homozygous mice. pdx-1(−/−) mice were apancreatic and died 2–3 days after birth (1, 2); but pdx-1(−/−);wtTg mice developed normally and survived to adulthood. Mice expressing the mutant PDX-1 transgene (pdx-1(−/−);mTg) were comparable in size to nontransgenic mice at birth, but they were noticeably smaller after 2 weeks and failed to survive past 3 weeks (Fig. 2A). pdx-1(−/−);wtTg mice had a normal pancreas with a normal proportion of islet and exocrine cells (Fig. 2 B and C). Compared with pdx-1(−/−);wtTg mice, the overall size of the pancreas in pdx-1(−/−);mTg mice at birth was 30–40% of normal, and the islets were markedly smaller (Fig. 2 B–D).
Figure 2.
Islet hypoplasia in pdx-1(−/−);mTg neonates. (A) Comparison of pdx-1(−/−);mTg (smaller) and wild-type (larger) littermates 16 days after birth. (B) Whole mount of pdx-1(−/−); wtTg and pdx-1(−/−); mTg mice. Ventral (Left) and dorsal (Right) views are included. p, pancreas; d, duodenum; sp, spleen; s, stomach. (C) Immunocytochemical analysis of insulin (red, arrows) and glucagon + somatostatin (green, asterisks) cells in sections from pdx-1(−/−); wtTg (Upper) and pdx-1(−/−):mTg (Lower). (D) Whole mount showing smaller developing islets (dark blue; arrowheads) in pdx-1(−/−) mTg compared with pdx-1(+/−) mTgt mice. Blue color indicates expression of β-galactosidase from the endogenous pdx-1 allele pdx-1lacZKO (1).
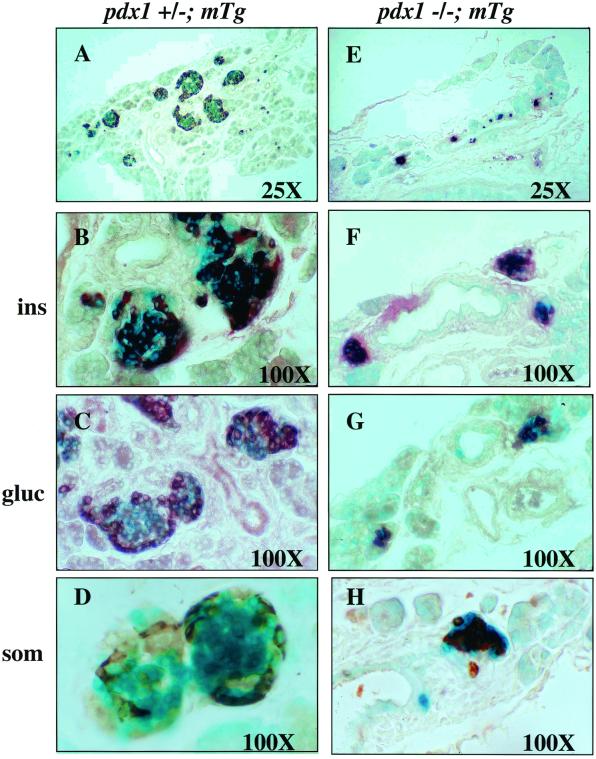
Each of the cell types evaluated immunocytochemically (insulin, somatostatin, and glucagon) was present in pdx-1(−/−);mTg mice; but the distribution of these cells within individual islets was abnormal (Fig. 3). In particular, glucagon- and somatostatin-expressing cells were located throughout the small endocrine clusters and not exclusively at the periphery, as in wild-type mice (Fig. 3, compare C, D, G, and H).
Figure 3.
Pancreatic islets of neonatal pdx-1(−/−);mTg mice are smaller and have disrupted architecture. (A–D) Sections of pdx-1(+/−); mTg islets. (E–H) Sections of pdx-1(−/−); mTg islets. pdx-1(−/−); mTg islets are much smaller and are located in close proximity to the ducts. Immunohistochemistry (brown) shows insulin-positive (F), glucagon-positive (G), and somatostatin-positive (H) cells in pdx-1(−/−); mTg islets. Glucagon- and somatostatin-positive cells (G and H) are not localized at the islet periphery as in wild-type islets (compare with C and D).
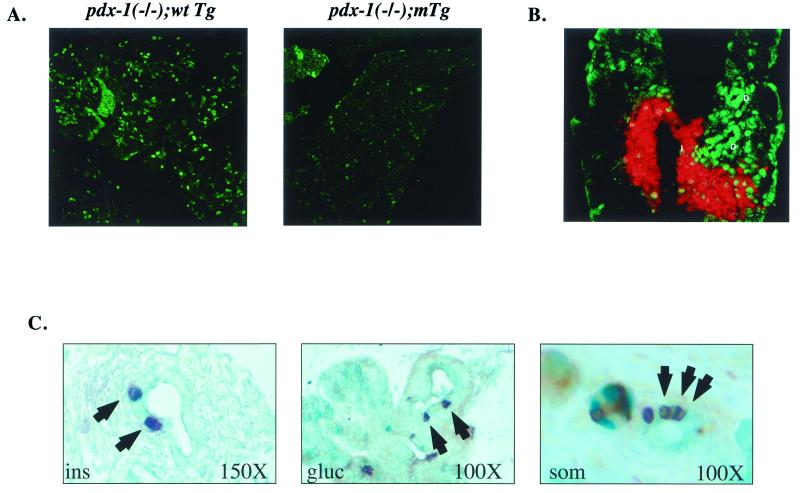
The reduced size of the pancreas in pdx-1(−/−);mTg mice prompted us to attempt to determine whether the proliferation of pancreatic cells was correspondingly lower in these animals. By using the marker Ki-67 (20) as a reliable index of ongoing cellular proliferation, we observed intense staining in about 20–30% of pancreatic cells from wild-type newborn animals (Fig. 4A, Left). Consistent with their relative abundance in the neonatal pancreas, acinar cells accounted for the highest percentage of proliferating cells, followed by ductal and islet cells. Ki-67 staining was severely reduced in pdx-1(−/−);mTg mice; both the number and intensity of immunopositive cells were markedly lower, and all cell types within the pancreas were equally affected (Fig. 4A, Right). These results suggest that PDX-1:PBX complexes promote the expansion of various lineages within the pancreas by enhancing cellular proliferation.
Figure 4.
Pancreata from pdx-1 (−/−);mTgt mice show a defect in proliferation and contain increased numbers of hormone-positive cells in the ductal epithelium. (A) Immunocytochemical staining of whole-pancreas sections from pdx-1 (−/−);wtTg and pdx-1 (−/−);mTg neonates, with Ki-67 antiserum as the marker of cell proliferation. (B) Immunocytochemistry of pancreas sections from neonatal wild-type mice, showing the highest levels of PBX-1 (green) in ductal cells (D), followed by islet cells. Acinar cells stain weakly for PBX-1. Insulin immunostaining (red) is overlaid to indicate the islet. (C) Immunohistochemical detection of insulin (Left), glucagon (Middle), and somatostatin (Right) cells (arrowheads) within the pancreatic ductal epithelium of neonatal pdx-1 (−/−);mTg mice. Mice of all other genotypes did not show hormone-positive cells within ducts in any sections examined.
To identify particular cell types where PDX-1:PBX complexes are likely to form, we conducted immunocytochemical studies with PBX-1 antiserum. PBX-1 was localized in the nucleus in all cells of the neonatal pancreas; the highest levels of PBX-1 were detected in ductal cells, with modest to low staining in islets and acinar cells, respectively (Fig. 4B). These results support the notion that PDX-1 interacts with PBX primarily in ductal epithelial cells, the site of presumed stem cells and the origin of islet endocrine cells. Remarkably, pancreatic ducts of pdx-1(−/−);mTg neonates contained numerous hormone-positive cells (Fig. 4C), whereas wild-type neonates did not (not shown). Indeed, all major hormone (insulin, glucagon, somatostatin) cell types were detected, suggesting a general defect in islet cell migration in these mice (Fig. 4C). Taken together, these results indicate that the interaction of PDX-1 with PBX is critical for early events in pancreatic development, including migration of differentiating endocrine cells or precursors out of the duct to form proper islets and proliferation of pancreatic cell types.
Discussion
PDX-1 has been shown to stimulate pancreatic gene expression in vitro, in part, as a heterodimer with PBX (14, 15), although the functional importance of this complex relative to the PDX-1 monomer has not been appreciated. Our results demonstrate a differential requirement in vivo for PDX-1:PBX heterodimers with respect to the initial specification of exocrine/endocrine cell types and the formation of the normal architecture of the mature organ.
In the absence of PDX-1, pancreatic development is arrested at the early bud stage, with complete absence of the major cell types (proper endocrine, exocrine, or ductal cells; refs. 9–11). Each of these pancreatic cell types is present, however, in pdx-1(−/−);mTg neonates, suggesting that monomeric (non-PBX-associated) PDX-1 activity is sufficient to promote initiation of a complete pancreatic genetic program. PDX-1:PBX complexes were also dispensable for glucose homeostasis, inasmuch as overexpression of the mutant PDX-1 transgene rescued glucose intolerance in pdx-1 heterozygotes. Rather, the PBX interaction motif in PDX-1, and therefore the PDX-1:PBX complex, was essential for the expansion of each cell type within the developing pancreas. Pancreata from pdx-1 (−/−);mTg mice were markedly smaller than those of control littermates, owing in large part to a reduction in cellular proliferation among all endocrine/exocrine compartments.
Our immunocytochemical analyses indicate that PDX-1:PBX complexes are likely to form most abundantly in ducts of the developing pancreas, the sites of presumed islet cell precursors. Although cellular levels of the PDX-1:PBX heterodimer may be regulated in part by nuclear translocation of PBX via Meis/Prep proteins during development (21), PBX-1 protein was exclusively nuclear, at least in cells of the neonatal pancreas. Rather, PDX-1:PBX complexes appear to be limited primarily by the level of cellular PDX-1; although highly expressed in ductal cells during development, PDX-1 protein is, except for a few cells (22), undetectable in ducts of the mature pancreas (23). Remarkably, ductal expression of PDX-1 in adult cells is induced after islet cell injury (24) or subtotal pancreatectomy (25). PDX-1:PBX heterodimers may therefore contribute importantly to ductal cell proliferation and subsequent islet regeneration.
We imagine that the PDX-1:PBX heterodimer acts chiefly to induce the expression of genes involved in islet neogenesis. Thus the highly increased number of hormone-positive cells in ducts of pdx-1 (−/−); mTg mice may reflect in part premature specification toward the endocrine cell lineage because these complexes normally maintain the ductal proliferative state. Alternatively, PDX-1:PBX may induce the expression of certain genes that are required for the subsequent migration of hormone positive cells from ducts. In either case, failure of these cells to migrate from the duct may contribute to the abnormal islet morphology in pdx-1 (−/−); mTg mice. In this regard, it will be of interest to identify PDX-1:PBX target genes that function in this developmental process.
Acknowledgments
We thank Christine Reyes for excellent technical assistance, Judy Lebet for histology, and Dr. Nitin Trevedi for help with confocal microscopy. We also thank Hiroshi Asahara for help and advice. This work was supported in part by the Foundation for Medical Research, by National Institutes of Health RO1 Grants DK49777 and DK 44523, and by the Joslin National Institutes of Health, Diabetes Endocrinology Research Care cores for Animal Facility and Advanced Microscopy.
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031561298.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031561298
References
- 1.Offield M, Jetton T, Labosky P, Ray M, Stein R, Magnuson M, Hogan B, Wright C. Development (Cambridge, UK) 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson J, Carlsson L, Edlund T, Edlund H. Nature (London) 1994;13:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 3.Stoffers D, Zinkin N, Stanojevic V, Clarke W L, Habener J F. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 4.Dutta S, Bonner-Wier S, Wright C, Montminy M. Nature (London) 1998;392:560. doi: 10.1038/33311. [DOI] [PubMed] [Google Scholar]
- 5.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoffers D, Ferrer J, Clarke W, Habener J. Nat Genet. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 7.Miller C, McGehee R, Habener J. EMBO J. 1994;13:1145–1156. doi: 10.1002/j.1460-2075.1994.tb06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonard J, Peers B, Johnson T, Ferreri K, Lee S, Montminy M. Mol Endocrinol. 1993;7:1275–1283. doi: 10.1210/mend.7.10.7505393. [DOI] [PubMed] [Google Scholar]
- 9.Ohlsson H, Karlsson K, Edlund T. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peers B, Leonard J, Sharma S, Teitelman G, Montminy M R. Mol Endocrinol. 1994;8:1798–1806. doi: 10.1210/mend.8.12.7708065. [DOI] [PubMed] [Google Scholar]
- 11.Peshavaria M, Gamer L, Henderson E, Teitelman G, Wright C, Stein R. Mol Endocrinol. 1994;8:806–816. doi: 10.1210/mend.8.6.7935494. [DOI] [PubMed] [Google Scholar]
- 12.Waeber G, Thompson N, Nicod P, Bonny C. Mol Endocrinol. 1996;10:1327–1334. doi: 10.1210/mend.10.11.8923459. [DOI] [PubMed] [Google Scholar]
- 13.Watada H, Kajimoto Y, Miyagawa J, Hanafusa T, Hamaguchi K, Matsuoka T, Yamamoto K, Matsuzawa Y, Kawamori R, Yamasaki Y. Diabetes. 1996;45:1826–1831. doi: 10.2337/diab.45.12.1826. [DOI] [PubMed] [Google Scholar]
- 14.Peers B, Sharma S, Johnson T, Kamps M, Montminy M. Mol Cell Biol. 1995;15:7091–7097. doi: 10.1128/mcb.15.12.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swift G, Liu Y, Rose S, Bischof L, Steelman S, Buchberg A, Wright C, MacDonald R. Mol Cell Biol. 1998;18:5109–5120. doi: 10.1128/mcb.18.9.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma S, Leonard J, Chapman H, Leiter E, Montminy M. J Biol Chem. 1996;271:2294–2299. doi: 10.1074/jbc.271.4.2294. [DOI] [PubMed] [Google Scholar]
- 17.Asahara H, Dutta S, Kao H, Evans R, Montminy M. Mol Cell Biol. 1999;19:8219–8225. doi: 10.1128/mcb.19.12.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvo K, Sykes D, Pasillas M, Kamps M. Mol Cell Biol. 2000;20:3274–3285. doi: 10.1128/mcb.20.9.3274-3285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu K, Gannon M, Peshavaria M, Offield M, Henderson E, Ray M, Marks A, Gamer L, Wright C, Stein R. Mol Cell Biol. 1997;17:6002–6013. doi: 10.1128/mcb.17.10.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers J, Bernard P, Boisseau M R. Exp Cell Res. 1994;210:145–153. doi: 10.1006/excr.1994.1022. [DOI] [PubMed] [Google Scholar]
- 21.Rieckhof G, Casares F, Ryoo H, Abu-Shaar M, Mann R. Cell. 1997;91:171–183. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- 22.Song S Y, Gannon M, Washington M K, Scoggins C, Meszoely I, Goldenring J R, Marino C R, Sandgren E P, Coffey R J, Jr, Wright C V, Leach S D. Gastroenterology. 1999;117:1416–1426. doi: 10.1016/s0016-5085(99)70292-1. [DOI] [PubMed] [Google Scholar]
- 23.Guz Y, Montminy M, Stein R, Leonard J, Gamer L, Wright C, Teitelman G. Development (Cambridge, UK) 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 24.Kritzik M, Jones E, Chen Z, Krakowski M, Krahl T, Good A, Wright C, Fox H, Sarvetnick N. J Endocrinol. 1999;163:523–530. doi: 10.1677/joe.0.1630523. [DOI] [PubMed] [Google Scholar]
- 25.Sharma A, Zangen D, Reitz P, Taneja M, Lissauer M, Weir G, Habener J, Bonner-Weir S. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]