Abstract
Plant species in at least 66 families produce extrafloral nectar (EFN) on their leaves or shoots and therewith attract predators and parasitoids, such as ants and wasps, which in turn defend them against herbivores. We investigated whether EFN secretion is induced by herbivory and/or artificial damage, and thus can be regarded as an induced defensive response. In addition, we studied the underlying signaling pathway. EFN secretion by field-grown Macaranga tanarius increased after herbivory, artificial leaf damage, and exogenous jasmonic acid (JA) application. Artificial damage strongly enhanced endogenous JA concentrations. The response in EFN production to artificial damage was much less pronounced in those leaves that were treated with phenidone to inhibit endogenous JA synthesis. Quantitative dose–response relations were found between the increase in nectar production and both the intensity of leaf damage and the amounts of exogenously applied JA. The amount of endogenously produced JA was positively correlated with the intensity of leaf damage. Increased numbers of defending insects and decreased numbers of herbivores were observed on leaves after inducing EFN production by exogenous JA treatment. Over 6 weeks, repeatedly applied JA or artificial damage resulted in a ten-fold reduction in herbivory. These results demonstrate that EFN production represents an alternative mechanism for induced, indirect defensive plant responses that are mediated via the octadecanoid signal transduction cascade.
Extrafloral nectaries are nectar-secreting glands that are not involved in pollination (1). They are known in at least 66 plant families (2). Many studies have shown that extrafloral nectar (EFN) can play an important role in a plant's defense against herbivores (for reviews, see ref. 3 and 4). Given a choice, ants forage preferentially on plants with extrafloral nectaries (5–7), and several ant and wasp species directly defend the nectary-bearing plant parts against other insects (8). Both insect groups reduce numbers of herbivorous insects on plants possessing extrafloral nectaries and thus lessen damage by herbivores (5, 6, 8–12).
Several studies have already indicated that EFN secretion or amino acid concentrations in EFN may increase in response to herbivory (10, 13–15) and that this reaction does not require herbivore-specific elicitors (16, 17). However, most of these studies have suffered from methodological problems (discussed in ref. 17). Because of the lack of specific elicitors, it has been discussed whether EFN can actually be considered as an induced defense (18). Moreover, nothing is known about the underlying signaling pathway, and no study has focused on the effects of induced EFN production on nectary-visiting insects and herbivores.
In this study, we used saplings of the southeast Asian pioneer tree Macaranga tanarius (L.) Muell. Arg. (Euphorbiaceae) to address the questions of (i) whether EFN production can be induced by natural or artificial damage; (ii) whether the plant hormone jasmonic acid (JA) is involved in the signal transduction; and (iii) whether induced EFN flow attracts more predators, resulting in reduced herbivory.
In addition to EFN, the myrmecophilic (“ant-loving”) species M. tanarius produces cellular food bodies. It is defended by ants and other food-body-collecting and nectary-visiting insects (19–21). All experiments, except measurements of endogenous JA, were conducted under natural field conditions near Tampin (peninsular Malaysia) in August and September 1999 and August 2000.
In the present study we provide the first evidence that the herbivore-induced EFN flow meets the criteria required for a typical “induced defense.” The initial damage of herbivorous insects up-regulates the octadecanoid signaling pathway controlling the enhanced EFN flow. The nutritious liquid attracts defending organisms (e.g., ants) that strongly reduce herbivory.
Materials and Methods
Design of Field Experiments and Measurements of EFN Secretion.
The extent of EFN secretion of M. tanarius strongly depends on plant size (17, 22). The heights and total leaf areas of all plants were therefore measured in advance of the experiments. To quantify each plant's leaf area, the length and width of each single leaf were measured to calculate their area based on a regression equation (data from 100 leaves with known area, r2 > 0.99; see ref. 22). Preparation of plants and quantification of nectar followed Heil et al. (17). All vegetation that had contact with the experimental plants was pruned and a ring of sticky resin (Tangletrap; Tanglefoot, Grand Rapids, MI) was applied around each plant's stem to exclude foraging ants. All leaves were washed with pure water to remove accumulated EFN. The leaves were then put into gauze bags (mesh size 0.5 mm) and the “upper,” nectary-bearing parts were recurved to protect the nectaries against rain and flying nectar consumers.
EFN was removed and quantified with graduated 5-μl micropipettes. When quantified apart from each other, nectar volume and nectar concentration are strongly biased by abiotic factors such as air temperature and relative humidity. They must therefore be measured together to obtain reliable data on the secreted amounts of soluble solids (i.e., sugars and amino acids; see ref. 17). Nectar concentration was measured immediately on its removal as a concentration of soluble solids with a portable, temperature-compensated refractometer (ATAGO hand refractometer, L. Kübler, Karlsruhe, Germany). To remove EFN quantitatively, 5 μl of pure water was then applied to all nectaries. The resulting solution was removed and measured as described above, and the entire procedure was repeated up to five times until the resulting solution had concentrations <1%. Values from all collections conducted for the nectaries on one leaf were summed to quantify a leaf's overall EFN production as amounts of soluble solids (in μg of sucrose equivalents produced per day and per cm2 leaf area). As a reference value, the nectar production of each experimental leaf was measured before the experiments. Twenty-four hours after treatment, nectar production of the same leaves was measured again, and the difference from the reference value was calculated.
Induction of EFN Secretion.
In September 1999, an initial experiment was conducted to compare EFN flow after (i) artificial damage, (ii) herbivory, and (iii) exogenous application of JA, to untreated controls. For this experiment, 40 plants (1.0–2.0 m high) were used, with 10 plants subjected to each treatment. The four youngest fully expanded leaves of each plant were included, and all investigated leaves of a plant received the same treatment. To ensure that different plant sizes were equally represented in the different treatments, plants were assigned to ten groups according to their total leaf areas, with plants within each group differing by less then 10% of their total leaf areas. The plants within each group were assigned randomly to the different treatments, and all plants of one group were studied on the same days to the exclude effects of changing weather conditions. Four treatments were conducted as follows. “Control” leaves were put into gauze bags without additional treatment. Four insects—two beetles (of an as yet undetermined species of the Scarabeidae) and two grasshoppers (Xenocatantops humilis, Serville 1839, Acrididae)—were placed on each of the “herbivory” leaves. This grasshopper was the most abundant herbivore of M. tanarius at the study site (M.H., unpublished data). This treatment resulted in a mean damage level of 2.6% leaf area (mean of 40 leaves, SD = 2.1) consumed in 24 h. “Artificially damaged” leaves were punctured 100 times with a needle (diameter 1 mm—this damage corresponded to about 1.2% missing leaf area spread regularly over the leaf blade) to simulate the most typical form of herbivory (perforation of the leaves by large numbers of tiny holes; M.H., unpublished data). A 2.5-ml aliquot of an aqueous 1-mM solution of JA was sprayed on the “JA” leaves. From each plant, we used four identically treated leaves that followed each other sequentially on the stem, thus representing age classes that are comparable between different plants. Because EFN production by M. tanarius depends strongly on leaf age (17), different-aged leaves are not true replicates and should not be averaged for data analysis. Consequently, a repeated measures ANOVA was chosen for evaluation of this experiment (with “leaf” as the within-subject variable and “treatment” as the between-subject variable).
A similar experiment was conducted in August 2000 at the same site with a slightly changed design to control for the JA spraying treatment and to ensure that the observed effects can be repeated under field conditions. This time, five treatments were performed as follows (ten plants per treatment, four identically treated leaves from each plant). The control treatment and the JA treatment were repeated as described above. Several other plants were subjected to a “spray” control. Individual leaves were sprayed with 2.5 ml water to mimic the spraying used to apply exogenous JA. Solutions of JA and the spray control were made with the same water. Three grasshoppers (X. humilis) were placed on each of the herbivory leaves, leading to a mean herbivory of 1.6% leaf area (mean of 40 leaves, SD = 1.4) consumed in 24 h. Artificially damaged leaves were punctured 200 times with a needle (resulting in about 2% missing leaf area). Data analysis was conducted as described above.
Response of Endogenous JA Levels to Leaf Damage.
Young saplings cultivated in a greenhouse (12 h night/day rhythm, 24°C, 90% RH/28°C, 60% RH; plants received natural light during daytime hours, which was supplied with an additional 600 μE for 4 h per day) were used to check whether M. tanarius synthesizes endogenous JA in response to leaf damage. Leaves were punctured 300 times with a needle (damage spread regularly over the entire leaf surface) once at time 0 or they remained untreated. Leaves were harvested after 10, 30, 45, 60, 120, 180, 360, and 600 min (three leaves from each different plant per harvest). Extraction and quantification of endogenous JA followed the protocol of Koch et al. (23). Briefly, 1.0 g of leaf tissue was frozen and [9,10-2H2]dihydro-JA was added as an internal standard (24). JA was extracted and purified by using NH2-propyl-solid phase extraction-cartridges (Varian). Detection and quantification of JA was done by GC-single ion monitoring-MS without further purification.
Induction of EFN and Inhibition of Endogenous JA.
Leaf damage, as well as exogenous application of JA, led to an increase in EFN flow (see Results). To further investigate the role of JA in the underlying signal transduction cascade, the four youngest totally unfolded leaves of 40 field-grown M. tanarius plants (0.5–1.6 m high) were subjected to different treatments (all leaves of an individual plant were treated identically; this field experiment was conducted in August 2000). To obtain a reference value, EFN production of all plants was measured once on net-bagged leaves over 24 h in advance of the experiment. The nets were then removed and, on the same day, leaves of plants in the “phenidone” treatment were sprayed two times (11:00 a.m. and 3:00 p.m.) with a 2 mM aqueous solution of phenidone (3.5 ml per leaf applied on both surfaces). On the next day, leaves were wounded artificially (800 needle punches per leaf) and then immediately sprayed once more with the same amount of phenidone solution. This treatment has already been shown to reduce the increase in endogenous JA levels, which is seen normally after artificial damage (see Results), by about two-thirds (T. Koch, unpublished results; see ref. 23 and literature cited therein for the general effect of phenidone on JA synthesis). Leaves were then bagged in nets again, and EFN production was measured 24 h later. The same procedure was conducted for the “phenidone + JA” plants; however, these plants were sprayed with 2.5 ml of an aqueous, 1-mM JA solution 1 h after the final phenidone application. EFN production of untreated control plants was measured on the same 2 days as the other plants, with the control plants remaining unbagged during the second day. EFN production of plants in the “damage control” treatment was subjected to the same nectar collection schedule as that of the “phenidone-treated” plants. The plants were wounded by 800 needle punches, but lacked a phenidone or JA treatment. The relative increase in EFN production as compared with the reference value was calculated. Data evaluation was done with a repeated measures ANOVA with leaf as within-subject variable and treatment as between-subject variable.
Dose–Response Experiments.
In September 1999, 55 field-grown plants (1.0–1.5 m high) were used to study dose–response relations between artificial damage, or exogenous JA application, and the response in EFN production. From 0–11 ml of an aqueous JA solution were applied per leaf on four identically treated leaves on each of 29 plants, while a further set of 26 plants was treated by puncturing four leaves per plant 0–800 times with a needle (the maximum corresponding to approximately 7 punctures per cm2 spread regularly over the leaf blade; needle diameter 1 mm; all leaves treated identically). The response in EFN production of these plants 24 h after treatment was measured and calculated in relation to the pretreatment production of the same plants as described above. Correspondingly, 22 leaves of greenhouse-grown plants were treated with 0–5.1 punctures per cm2, and their levels of endogenous JA were measured 30 min after treatment, as described above.
Effect of JA Treatment on Visiting Insects.
The activity of naturally occurring insects on five young, totally unfolded leaves of each of 20 different plants induced with JA was compared with the activity on the same leaves in the noninduced control stage (field experiment, September 1999). Plants were censused every 2 h from noon to 10:00 a.m. the next day, and insects appearing on these plants were counted separately according to different behavioral groups. All species that successfully defended nectaries against other arthropods were called “defenders.” The “visitors” group was comprised of all individual animals that were observed at least once to feed on an extrafloral nectary within the observational time span. Thus, this group includes most of the defenders, too. “Herbivores” were all insect species that were observed to feed on M. tanarius leaves. None of the herbivores showed a “defending” behavior. All remaining species were called “others.”
On the first day, twenty similarly sized plants were divided randomly into two groups of ten plants each. One group served as untreated controls, while EFN production was induced on the other plants by spraying five leaves two times (at 9:00 a.m. and 5:00 p.m.) with 2.5 ml of an aqueous 1-mM JA solution per leaf. To exclude any spatial or individual effects, the experiment was repeated 9 days later—the former controls were induced with JA and vice versa. The effect of JA on EFN production is rather short-term, that is, detectable primarily on the first and—occasionally—the second day after JA treatment (M.H. and A.H., unpublished data). At each census, all individual leaves were surveyed for 1 min each, during which all insects present on the upper surface were counted.
To establish that the observed effects resulted from induced EFN flow and not from leaf blade volatiles (25–27), the same experiment was repeated once more by using the same plants from which all nectaries had been removed by scissors.
Effect of Induced EFN Secretion on Herbivory.
In August 1999, 45 plants were divided randomly into three treatment groups (15 plants per treatment): untreated controls, artificially damaged plants, and JA-induced plants. On each plant, the three youngest totally unfolded leaves were marked. These were either treated with artificial damage (100 punches with a needle) or sprayed with 2.5 ml 1-mM JA solution, or they remained untreated (controls). All plants were revisited every 4 days, and treatments were repeated for the marked leaves and the subsequent three youngest leaves as soon as these were fully expanded. Six weeks after the first treatment, all treated leaves and the corresponding control leaves were collected and their individual leaf damage measured. Percentage of consumed leaf area was measured with the computer program dias (Delta-T Devices, Cambridge, U.K.). Because leaves do not shrink by more than 3% during drying, data from dried leaves give a reliable estimate of missing leaf area (unpublished data). Missing leaf area represents damage adequately, because leaf chewers cause more than 95% of natural leaf damage of M. tanarius at the study site (M.H., unpublished data).
Biotests were conducted to test for direct effects (or changes in leaf biochemistry) that might have severely influenced rates of herbivory on JA-treated leaves. Feeding rates of herbivores on JA-induced leaves and on noninduced control leaves were compared under defender-free conditions. The biotests were conducted at the same site and during the same time as the long-term study. On three leaves from each of ten plants per treatment, two beetles (of an as yet undetermined species of the Scarabeidae) and two grasshoppers (X. humilis) per leaf were kept in nets for 48 h. Leaves were then removed and dried to measure the consumed leaf area, as described above.
Results
Induction of EFN Secretion.
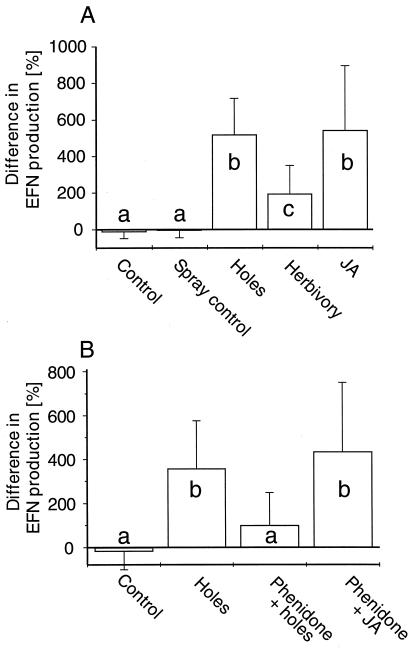
Though conducted during two different years, both experiments revealed very similar results. In 1999, all three treatments significantly increased the nectar production (repeated measures ANOVA for the effect of treatments with leaf number as within-subject variable: F(3,156) = 19.657, P < 0.001), raising it on average 200–500%, while the controls, on average, had slightly lower nectar production rates on the second day. This decrease in nectar production is a reaction of M. tanarius extrafloral nectaries to an accumulation of EFN resulting from the experimental exclusion of nectar-consuming insects (17). In 2000, both damage treatments and the application of JA again significantly increased the nectar production (Table 1), raising it on average by 200% (herbivory), 470% (artificial damage), and 550% (jasmonic acid). Both control treatments resulted in slightly lower nectar production rates on the second day (Fig. 1A).
Table 1.
Experiment on induction of EFN production: Results of repeated measures ANOVA on effects of different treatments on relative change in extrafloral nectar secretion
| Source | SS | df | F | P | |
|---|---|---|---|---|---|
| Within-subject effects | Leaf | 11564165 | 3 | 3.677 | 0.014 |
| Leaf × treatment | 26608945 | 12 | 2.115 | 0.020 | |
| Error (leaf) | 141525456 | 465 | |||
| Between-subject effects | Treatment | 71577036 | 4 | 9.849 | <0.001 |
| Error (treatment) | 81756934 | 45 |
SS, sum of squares.
Figure 1.
Changes in daily EFN production (relative difference day 1/day 0) are given separately for different treatments (mean + SD). Different letters indicate significant differences between single treatments (for all significant differences: P < 0.05, post hoc test following repeated measures ANOVA with Fisher's least significant difference (LSD); see Tables 1 and 2 for ANOVA results; n = 10 plants for each treatment with four leaves per plant perceiving identical treatment). (A) Experiment on induction of EFN production. (B) Experiment on effects of phenidone treatment.
Response of Endogenous JA Levels to Leaf Damage.
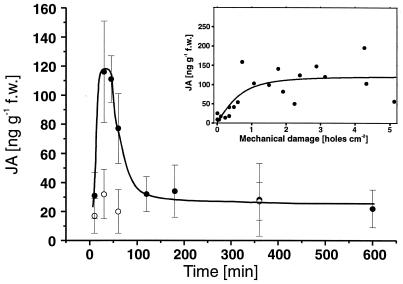
Young saplings cultivated in a greenhouse were tested for endogenous JA production after artificial leaf damage. As a response to damage, the level of endogenous JA rose within 30 min from 20–30 ng JA per g fresh mass to about 120 ng/g (Fig. 2). Within 2 h this transient accumulation leveled off to the initial concentration (Fig. 2).
Figure 2.
Induction of endogenous JA after artificial leaf damage. Changes in JA levels (mean ± SD) in leaves that were either wounded artificially by 300 punctures with a needle (diameter 1 mm—this damage corresponded to about 4% missing leaf area and was spread regularly over the entire leaf surface) once at time 0 (filled circles) or remained untreated (open circles). Leaves were harvested after 10, 30, 45, 60, 120, 180, 360, and 600 min (three leaves of different plants per harvest). (Inset) Dependence of JA production on damage intensity measured 30 min after artificial damage. The regression line indicates a saturated dose–response relation (r2 = 0.64).
Induction of EFN and Inhibition of Endogenous JA.
Artificial damage increased the nectar production on average by 360%. In contrast, the EFN production increased much less (by ca. 100%) on those artificially damaged leaves that had been treated with phenidone, while EFN production of those leaves that had received JA in addition to phenidone increased on average by 440%. Treatment had a highly significant effect on the relative change in EFN production (Table 2), with the damaged and the phenidone + JA-treated plants differing significantly from both the phenidone-treated plants and the controls (Fig. 1B). No significant difference could be detected between phenidone-treated plants and controls (Fig. 1B).
Table 2.
Experiment on effect of phenidone treatment: Results of repeated measures ANOVA on effects of different treatments on relative change in extrafloral nectar secretion
| Source | SS | df | F | P | |
|---|---|---|---|---|---|
| Within-subject effects | Leaf | 6302359 | 3 | 1.171 | n.s. |
| Leaf × treatment | 14631743 | 9 | 0.906 | n.s. | |
| Error (leaf) | 193725212 | 108 | |||
| Between-subject effects | Treatment | 75567879 | 3 | 15.911 | <0.001 |
| Error (treatment) | 56992401 | 36 |
SS, sum of squares; n.s., not significant.
Dose–Response Experiments.
Levels of endogenous JA in greenhouse-cultivated plants, determined 30 min after different intensities of artificial damage, were positively correlated with the severity of leaf damage (Spearman rank correlation tests: P < 0.001, n = 21). The quantitative relation between severity of damage and the endogenous JA level could be described by a saturation curve (Fig. 2). Correspondingly, the increase in the nectar production rate of field-grown plants was positively correlated with both the severity of artificial damage and the amounts of exogenously applied JA (Spearman rank test: P < 0.001 in both cases; n = 26 and 29 plants, respectively).
Effect of JA Treatment on Visiting Insects.
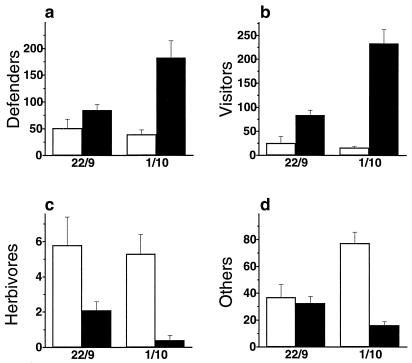
JA treatment had significant effects on all four insect groups (Fig. 3, Wilcoxon tests for matched pairs with insect numbers counted on the same plant in the induced and in the control stage serving as a “matched pair”: P < 0.001 for all four insect groups, n = 20 plants). On all plants, more “visitors” and “defenders,” and fewer “herbivores” were counted when the plant was in the induced stage as compared with the control day, regardless of the day for which a plant served as a control or as an induced plant. Significantly higher numbers of visitors and defenders were observed on the leaves just 3 h after JA treatment (Mann–Whitney U tests on the effects of EFN induction on the number of defenders and visitors counted on leaves of treated and control plants 3 h after first application of JA: P = 0.014 for defenders and P = 0.001 for visitors, at the first census, and P < 0.001 for both groups at the second census). None of the insect groups showed significant differences between JA-treated and control plants when extrafloral nectaries were removed with scissors (U tests for comparison of ten JA-treated and ten control plants: P = 0.395 for defenders, P = 0.253 for herbivores, P = 0.388 for “others”). The number of defenders was significantly lower in this part of the experiment, as compared with the activity data on the same plants when they still possessed their nectaries. On average, 52.3 defenders occurred per day on noninduced control plants with nectaries, and 15.5 defenders occurred on the same plants without nectaries (Wilcoxon test for matched pairs with insect numbers counted on the same plant with and without nectaries serving as matched pairs: P = 0.001, n = 20 plants). No comparable effect appeared in the numbers of herbivores and others (same test, P > 0.20 in both cases).
Figure 3.
Numbers of insects (mean of 10 plants + SD) counted per day on two census dates (22 September and 1 October 1999: untreated controls, open bars; JA-induced, dark bars). Numbers of defenders (a), visitors (b), herbivores (c), and others (d) were summed for the twelve single counts conducted on each census date for each plant. See Materials and Methods (Effect of JA Treatment on Visiting Insects) for definition of functional insect groups and a description of JA treatment.
Effect of Induced EFN Secretion on Herbivory.
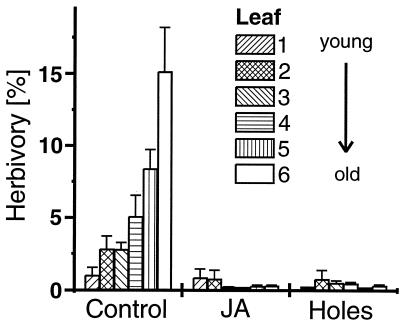
After 6 weeks, control plants suffered significantly more from herbivory than did those treated with JA or damaged artificially [repeated measures ANOVA with leaf number as within-subject variable on treatment effects (as between-subject variable) on missing leaf area: F(2, 267) = 36.053, P < 0.001]. On average, leaves of control plants had lost 5.9% of their leaf area, whereas those of treated plants had lost only 0.4% for both JA treatment and artificial damage [post hoc tests (least significant difference): P < 0.001 for significant difference between controls and each type of induction treatment, P = 0.815 for a difference between JA-induced and artificially damaged plants]. An increase of accumulated herbivory with increasing leaf age occurred in the control leaves, but not in the induced leaves (Fig. 4). In the biotests, JA treatment had no significant effect on the leaf area consumed by the most abundant herbivores (repeated measures ANOVA with leaf number as within-subject factor: F(1,58) = 0.410, P = 0.529).
Figure 4.
Herbivory on leaves of control plants, plants treated with JA, and artificially damaged plants 6 weeks after the beginning of treatment (mean + SD). The percentage of leaf area consumed by chewing herbivores was measured on the youngest six leaves (number 1 representing the youngest leaf) of 15 plants per treatment. Treatments were conducted every 4 days by puncturing leaves (Holes) 100 times with a needle (diameter 1 mm; punctures spread regularly over the entire leaf surface) and spraying 2.5 ml of an aqueous 1-mM solution of JA on the JA leaves. Control plants remained untreated during the entire study period.
Discussion
This study was designed (i) to test whether EFN production is induced as a response to herbivory, (ii) to obtain information on the signaling pathway, and (iii) to check whether, under field conditions, an induced nectar flow has beneficial effects for the EFN-secreting plant. The results show that EFN flow of the Asian myrmecophilic plant, M. tanarius, can indeed be induced under field conditions in response to both artificial damage and damage resulting from herbivore feeding. Twenty-four hours after damage, EFN flow was 200–500% higher than that of untreated controls (Fig. 1A). Similar effects were found in two field experiments that were conducted in different years. These results are in line with earlier reports indicating that EFN flow might be induced by herbivory (10, 13–15), and that this reaction does not require herbivore-specific elicitors (16, 17). Exogenous application of the plant stress hormone JA led to a comparable increase in EFN flow (Fig. 1A), and artificial damage led to a strong increase in endogenous JA levels (Fig. 2). A control spray of the solvent used (water) had no detectable effect on EFN flow, indicating that JA had elicited the response. Quantitative relationships could be established independently between (i) the intensity of damage and the resulting increase in the level of endogenous JA, (ii) the amount of exogenously applied JA and the intensity of the final response (increased EFN flow), and (iii) the intensity of damage and the intensity of the final response. Finally, inhibiting endogenous JA synthesis by application of phenidone dramatically reduced the wound response in EFN flow, whereas the response occurred when the effect of phenidone was “bypassed” by applying JA exogenously under otherwise identical experimental conditions (Fig. 1B). Although the involvement of a further signaling pathway cannot be excluded completely, these data strongly suggest that EFN production by M. tanarius is an induced response that is elicited via the octadecanoid signal cascade (27–30).
Further field experiments were conducted to investigate whether EFN-visiting insects respond to increased nectar flow, and whether this finally reduces herbivory at the plant's natural growing sites. Many more of those insects that visited extrafloral nectaries, and that defended these and the surrounding leaf tissue against other insects, occurred on leaves on which EFN production had been induced by exogenous JA application (Fig. 3). This reduced the numbers of herbivores and other insects occurring on these leaves (Fig. 3). Site effects and effects of weather conditions—which might have biased these results—were excluded by rotating plants among treatments and among days on which the individual plant served as treated plant or as control. The effect of JA treatment was missing in the same plants when nectaries had been removed with scissors. We therefore conclude that induced plant volatiles had no marked effect on insect behavior in this experimental system, which is all the more surprising because this treatment represents an artificial wounding that so far has been reported to induce, rather than suppress, production of volatile compounds (25–27). Plants on which EFN flow had been induced regularly over 6 weeks by either artificially damaging leaves or by exogenous JA application suffered much less from herbivory than did uninfluenced control plants (Fig. 4). Biotests demonstrated that strong effects of induced changes in leaf biochemistry on the feeding behavior of the most important herbivores in this system are not likely. Although the involvement of induced plant volatiles or biochemical changes in the leaves cannot be ruled out completely, our data suggest that an induction of EFN flow does lead to higher numbers of defending (and thus mutualistic) insects and to lower numbers of herbivores present on the induced plants.
In general, only a few studies have shown that induced responses of plants offer benefits under field conditions (31–33). Even fewer data have been published on indirect defenses of plants, which act via higher trophic level interactions—i.e., by emphasizing the suppressive effects of carnivores or parasitoids that attack herbivorous arthropods (34–37). In recent studies, Thaler has demonstrated that JA-induced plant volatiles can cause increased parasitism among herbivores in an agricultural field system (38), and that JA-induced plants received less leaf damage (39). However, comparable studies on EFN were missing. To our knowledge, this is the first case where a reduction in herbivory has been demonstrated as a consequence of EFN flow induced by JA or artificial damage. According to Karban and Baldwin (40), the term “induced defenses” is coined to denote those responses that have positive consequences for the fitness of the responding plants. Data on plant fitness are hard to obtain in the case of a long-lived tree. Therefore, induced EFN flow of M. tanarius should until now have been termed “resistance” in the sense of Karban & Baldwin. However, despite the absence of data on the fitness of M. tanarius, our results demonstrate that important traits that are indirectly linked to the fitness of a plant are clearly affected. Analogous to the well established indirect defense via the herbivore-induced biosynthesis of volatiles that attract defending insects (35, 36, 41), the damage-induced EFN flow also attracts defending insects that strongly reduce herbivory. Therefore, we propose to consider the induction of the EFN flow also as an induced defensive response. Being a liquid plant secretion mediating indirect defense by nourishing mutualistic insects, EFN represents an additional mechanism in the context of wound- and elicitor-induced responses that depend on the octadecanoid signal transduction cascade. In the future, more studies with other plants and other insect species are required to test whether EFN represents, in general, a mechanism by which plants respond to herbivory via the octadecanoid pathway.
Acknowledgments
We thank Ian T. Baldwin, Bert Hölldobler, Martin Heisenberg, Jürgen Tautz, Anurag Agrawal, Neil Oldham, Erhard Strohm, and Jörn Hopke for critical reading of the manuscript; an anonymous referee for linguistic improvement; R. Krüger for field assistance; K. Heil for further practical help; and Sigfried Ingrisch for identification of the grasshoppers. Mr. Lee Kung Wu and Mr. Loo Wong Poo allowed us to conduct field work on their property, and the Economic Planning Unit (EPU) gave permits to conduct field studies in Malaysia. We are grateful for financial support from the Deutsche Forschungsgemeinschaft (TP C8, SFB 251, and Grants He3169/1–1 and He3169/1–2) and the Max-Planck-Gesellschaft, and for much logistic assistance provided by the Malaysian Airline System (MAS).
Abbreviations
- EFN
extrafloral nectar
- JA
jasmonic acid
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031563398.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031563398
References
- 1.Bentley B L. Ann Rev Ecol Syst. 1977;8:407–427. [Google Scholar]
- 2.Elias T S. In: The Biology of Nectaries. Bentley B, Elias T S, editors. New York: Columbia Univ. Press; 1983. pp. 174–203. [Google Scholar]
- 3.Buckley R C. In: Ant-Plant Interactions in Australia. Buckley R C, editor. The Hague, The Netherlands: W. Junk; 1982. pp. 111–162. [Google Scholar]
- 4.Koptur S. In: Insect-Plant Interactions. Bernays E A, editor. Vol. 4. Boca Raton, FL: CRC Press; 1992. pp. 81–129. [Google Scholar]
- 5.Barton A M. Ecology. 1986;67:495–504. [Google Scholar]
- 6.Koptur S, Rico-Gray V, Palacios-Rios M. Am J Bot. 1998;85:736–739. [PubMed] [Google Scholar]
- 7.Oliveira P S, Rico-Gray V, Diaz-Castelazo C, Castillo-Guevara C. Funct Ecol. 1999;13:623–631. [Google Scholar]
- 8.O'Dowd D J. Oecologia. 1979;43:233–248. doi: 10.1007/BF00344773. [DOI] [PubMed] [Google Scholar]
- 9.Pickett C H, Clark D W. Am J Bot. 1979;66:618–625. [Google Scholar]
- 10.Stephenson A G. Ecology. 1982;63:663–669. [Google Scholar]
- 11.Koptur S. Ecology. 1984;65:1787–1793. [Google Scholar]
- 12.Oliveira P S, da Silva A F, Martins A B. Oecologia. 1987;74:228–230. doi: 10.1007/BF00379363. [DOI] [PubMed] [Google Scholar]
- 13.Koptur S. In: The Evolutionary Ecology of Plants. Bock J H, Linhart Y B, editors. Boulder, Colorado: Westview Press; 1989. pp. 323–339. [Google Scholar]
- 14.Smith L L, Lanza J, Smith G S. Ecology. 1990;71:107–115. [Google Scholar]
- 15.Swift S, Lanza J. Bull Ecol Soc Am. 1993;74:451. [Google Scholar]
- 16.Wäckers F L, Wunderlin R. Entomol Exp Appl. 1999;91:149–154. [Google Scholar]
- 17.Heil M, Fiala B, Baumann B, Linsenmair K E. Funct Ecol. 2000;14:749–757. [Google Scholar]
- 18.Agrawal A A, Rutter M T. Oikos. 1998;83:227–236. [Google Scholar]
- 19.Fiala B, Maschwitz U. Biol J Linn Soc. 1991;44:287–305. [Google Scholar]
- 20.Fiala B, Grunsky H, Maschwitz U, Linsenmair K E. Oecologia. 1994;97:186–192. doi: 10.1007/BF00323148. [DOI] [PubMed] [Google Scholar]
- 21.Heil, M., Fiala, B., Maschwitz, U. & Linsenmair, K. E. (2001) Oecologia, in press. [DOI] [PubMed]
- 22.Heil M. Quantitative Kosten-Nutzen-Analyse Verschiedener Ameisen-Pflanzen-Assoziationen Innerhalb der Gattung Macaranga. Berlin: Wissenschaft & Technik Verlag; 1998. [Google Scholar]
- 23.Koch T, Krumm T, Jung V, Engelberth J, Boland W. Plant Phys. 1999;121:153–162. doi: 10.1104/pp.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldwin I T, Zhang Z-P, Diab N, Ohnmeiss T E, McCloud E S, Lynds G Y, Schmelz E A. Planta. 1997;201:397–404. [Google Scholar]
- 25.Boland W, Koch T, Krumm T, Piel J, Jux A. In: Insect-Plant Interactions and Induced Plant Defence. Chadwick D J, Goode J A, editors. NY: Wiley; 1999. pp. 110–131. [Google Scholar]
- 26.Tumlinson J H, Paré P W, Turlings W J. In: Insect-Plant Interactions and Induced Plant Defence. Chadwick D J, Goode J A, editors. NY: Wiley; 1999. pp. 95–109. [Google Scholar]
- 27.Wasternack C, Parthier B. Trends Plant Sci. 1997;2:302–307. [Google Scholar]
- 28.Weiler E W. Naturwissenschaften. 1997;84:340–349. [Google Scholar]
- 29.Blechert S, Brodschelm W, Hölder S, Kammerer L, Kutchan T M, Müller M J, Xia Z Q, Zenk M H. Proc Natl Acad Sci USA. 1995;92:4099–4105. doi: 10.1073/pnas.92.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creelman R A, Mullet J E. Ann Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- 31.Baldwin I T. Proc Natl Acad Sci USA. 1998;95:8113–8118. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agrawal A A. Science. 1998;279:1201–1202. doi: 10.1126/science.279.5354.1201. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal A A. Ecology. 1999;80:1713–1723. [Google Scholar]
- 34.Price P W, Bouton C E, Gross P, McPheron B A, Thompson J N, Weis A E. Annu Rev Ecol Syst. 1980;11:41–65. [Google Scholar]
- 35.Takabayashi J, Dicke M. Trends Plant Sci. 1996;1:109–113. [Google Scholar]
- 36.Baldwin I T, Preston C A. Planta. 1999;208:137–145. [Google Scholar]
- 37.Dicke M. In: The Ecology and Evolution of Inducible Defenses. Tollrian R, Harvell C D, editors. Princeton: Princeton Univ. Press; 1999. pp. 62–88. [Google Scholar]
- 38.Thaler J S. Nature (London) 1999;399:686–688. [Google Scholar]
- 39.Thaler J S. Environ Entomol. 1999;28:30–37. [Google Scholar]
- 40.Karban R, Baldwin I T. Induced Responses to Herbivory. Chicago: Univ. Chicago Press; 1997. [Google Scholar]
- 41.Dicke M. J Plant Phys. 1994;143:465–472. [Google Scholar]