Abstract
Invading organisms may spread through local movements (giving rise to a diffusion-like process) and by long-distance jumps, which are often human-mediated. The local spread of invading organisms has been fit with varying success to models that couple local population growth with diffusive spread, but to date no quantitative estimates exist for the relative importance of local dispersal relative to human-mediated long-distance jumps. Using a combination of literature review, museum records, and personal surveys, we reconstruct the invasion history of the Argentine ant (Linepithema humile), a widespread invasive species, at three spatial scales. Although the inherent dispersal abilities of Argentine ants are limited, in the last century, human-mediated dispersal has resulted in the establishment of this species on six continents and on many oceanic islands. Human-mediated jump dispersal has also been the primary mode of spread at a continental scale within the United States. The spread of the Argentine ant involves two discrete modes. Maximum distances spread by colonies undergoing budding reproduction averaged 150 m/year, whereas annual jump-dispersal distances averaged three orders of magnitude higher. Invasions that involve multiple dispersal processes, such as those documented here, are undoubtedly common. Detailed data on invasion dynamics are necessary to improve the predictive power of future modeling efforts.
Keywords: stratified diffusion, invasive ants, Linepithema humile, rate of invasion
One of the triumphs of invasion biology is the well-developed mathematical theory that describes the spread of invasive species (1). Skellam (2) advanced models based on reaction-diffusion equations that have been used commonly by ecologists to predict asymptotic rate of invasion. Predicted rates of spread are often in agreement with observed rates, demonstrating the value of this approach (1, 3–6). These models are often based on two parameters: the intrinsic rate of growth and the diffusion coefficient (2, 7). An important assumption concerning the diffusion coefficient is that the distances individuals move over a given length of time are drawn from a normal distribution (8, 9).
Although theoretical models have reasonably predicted invasion rates in many cases, violations of their assumptions may limit their usefulness. In particular, the distributions of dispersal distances for many taxa are leptokurtic rather than normally distributed (7–12). Such non-normal distributions may result from a variety of processes. First, rare long-distance jump-dispersal events can skew distributions of dispersal distances. Second, deviations from normality may arise from stratified diffusion (3) where an invading species spreads by two or more modes (e.g., diffusion and jump-dispersal) simultaneously. Both long-distance dispersal and stratified diffusion can greatly increase invasion rates and result in a lack of agreement between models and empirical data (1, 3, 9, 13, 14). The frequency and distances of jump-dispersal events are thought to be stochastic, difficult to determine, and therefore have rarely been quantified (refs. 12 and 13, but see ref. 10). However, estimates of the rate and distance of long-distance dispersal events are essential for accurate model construction, a limitation that is widely recognized (1, 9, 12–14).
Despite their value in guiding modeling efforts, there are few data documenting large-scale patterns of invasion for species that spread primarily via jump-dispersal or that spread by multiple processes. To address this issue, we quantified invasion dynamics for a highly invasive species, the Argentine ant (Linepithema humile). Despite the widespread distribution of this species, no recent attempt has been made to synthesize the growing amount of information regarding its distribution and invasion history. By analyzing museum records, personal collections, and the literature, we reconstructed invasion dynamics of Argentine ants at three spatial scales. First, we determined the current worldwide distribution of the Argentine ant. Second, we constructed a chronological history of invasion for this species at a continental scale in the United States after its introduction into New Orleans around 1891. Finally, we examined patterns of spread at a local scale by providing new data and reviewing the literature. Examining invasion dynamics of the Argentine ant at these three spatial scales allowed us to gauge the relative importance of alternate modes of spread at each scale.
Methods
Biology of Linepithema humile.
Native to South America, the Argentine ant causes a variety of economic and ecological problems throughout its introduced range. Perhaps most notably, the Argentine ant competitively displaces native ant species wherever it is introduced (15–21). The loss of native ants has led to a number of indirect effects, including reduced recruitment of myrmecochorous shrubs in South Africa (16) and declines in populations of coastal horned lizards in California (22). In addition, Argentine ants have been implicated in the decline of endemic arthropods in Hawaii (23) and in the disruption of arthropod communities in California (24, 25).
Argentine ants are most successful in Mediterranean and some subtropical climates but appear unable to survive in cold-temperate, tropical, or extremely arid environments (26, 27). However, through their close association with humans, Argentine ants may persist locally in areas with unfavorable climates in the vicinity of human habitations. While Argentine ants are associated with disturbed habitats throughout their introduced range, in some locations L. humile penetrates natural areas that have experienced little anthropogenic disturbance. Examples include matorral in Chile (28), fynbos in South Africa (16), coastal sage scrub in southern California (20), riparian woodlands in California (17, 21, 29), subalpine shrubland in Hawaii (23), and oak and pine woodland in Portugal (18).
The dispersal of Argentine ants involves at least two discrete processes: diffusion and jump dispersal. Once established, Argentine ant colonies reproduce by budding; this pattern of spread resembles that of diffusion. When new colonies are formed by budding, inseminated queens leave established nests on foot along with a group of workers and form new nests nearby. This is in contrast to the prevailing mode of colony reproduction in ants where queens undergo mating flights, founding colonies independently of and often well away from their natal nest (26). Argentine ants queens are not known to undergo mating flights in their introduced range (21, 30, 31). A second form of dispersal involves human-mediated transport of colonies. Such jump dispersal is probably common for Argentine ants because they often associate closely with humans. For example, early this century it was noted that nearly every one of over 100 steamships landing between New Orleans and Baton Rouge, Louisiana, was heavily invested with Argentine ants (32, 33). Their commensal habits result in part from opportunistic nesting requirements and a general diet (32). Because Argentine ants lack mating flights, it easy to distinguish between these two distinct modes of spread. Argentine ants are also known to spread locally by rafting downstream (33). We feel that this mode of spread is of relatively minor importance compared with human-mediated jump dispersal given that patterns of expansion are predominately upstream, across watersheds, and overland (see below).
Worldwide Distribution.
We used the following methods to determine the current global distribution of the Argentine ant. First, in a thorough review of the scientific literature on ants, we examined both regional surveys as well as publications pertaining specifically to Argentine ants. Second, we contacted 140 public and personal entomological collections for information regarding the presence or absence of Argentine ants. Last, we conducted visual surveys for Argentine ants along the west coast of North America (from Guerrero Negro, Baja California, Mexico to Vancouver, Canada) and in northern Argentina (from Buenos Aires north, primarily along the Rio Parana and the Rio Uruguay).
The taxonomy of Linepithema, a genus confined to the Neotropical region, is poorly studied and unresolved. In particular, the species boundaries of L. humile in its native range are largely unknown. While introduced populations are all likely the same species, some records for Central and South America (where other native Linepithema occur) may pertain to species other than L. humile. When possible, we examined material from these regions to determine whether the specimens resembled the invasive form. In our surveys of museum records, an attempt was made to determine whether specimens were morphologically similar to those of introduced populations.
Regional Patterns of Invasion.
We used four different sources of information to construct a chronological history of invasion for the Argentine ant in the United States. These sources included published accounts, museum surveys, personal surveys, and unpublished personal communications with academics, pest company entomologists, and state agriculture extension personnel. We then constructed a GIS database (arcview for MS Windows NT) that consisted of dates Argentine ants were first detected (if ever) for counties in the United States. We used counties as our unit of measure because information in many museum records and publications was limited to the county level. This approach allowed a detailed reconstruction of invasion for the southeastern United States where counties are relatively small. In the western United States, however, where counties are much larger, county-level analysis exaggerated the area occupied. Therefore, rather than use area as our overall measure of occupied territory, we used the number of counties. To depict invasion history chronologically, we partitioned records into four periods (1891–1910, 1911–1930, 1931–1950, and 1951–1999) starting with the year of first detection (1891) in New Orleans, LA (32).
Although our sources provide information on whether Argentine ants have been recorded in a county, they do not always reveal whether they were able to persist, or if they still occur. In some locations, Argentine ants cannot survive outside of human-modified landscapes because of their inability to tolerate arid (e.g., Arizona) or cold-temperate (e.g., Minnesota and Illinois) climates. In other areas, Argentine ants have been locally eradicated through control measures (e.g., ref. 34) or displaced by the red imported fire ant, Solenopsis invicta (35). Because the purpose of our study is to examine the frequency and scale at which long-distance jump-dispersal events occur, we do not distinguish between counties that still have Argentine ants versus those that presently do not.
We used the above reconstruction of the Argentine ant's invasion of the United States along with published accounts that monitored invasion fronts for at least 1 year (see below) to assemble a distribution of rates of spread. Yearly jump-dispersal distances were estimated from the invasion history of the Argentine ant by using all new foci (newly occupied counties) through the year 1930. Early in the invasion, most jump-dispersal events likely originated from New Orleans, the site of the original introduction (32). However, later foci may have originated either from New Orleans or from a closer infested county. Because sources of introduction are not known, we estimated jump-dispersal distances in two ways. First, we determined the distribution of distances assuming New Orleans was the source for all new introductions (out of New Orleans model). This method provides maximum estimates of human-mediated jump-dispersal distances. For counties in California, only the first record was used; subsequent spread was assumed to occur from other counties in California. Second, we assumed that the source of new foci came from the nearest county that had been occupied for at least 1 year (nearest occupied county model); this method provides a distribution composed of minimum estimates.
While the contrasting models above provide a realistic range of estimates for long-distance jump-dispersal at a regional scale, two aspects of this approach may lead to overestimates of jump-dispersal distances. First, jump-dispersal events occurring within counties would not be detected by our methods. Average county diameter therefore sets a lower limit to our estimation of jump-dispersal distances. However, while important for local consolidation of occupied areas, within-county jumps contribute less to the overall pattern of colonization at the regional scale than do between-county jumps. For this reason, we feel that a county-level approach is appropriate for an analysis of the pattern of invasion at this regional scale. Second, unequal spatial sampling could bias the reconstruction of the Argentine ant's invasion history and also lead to overestimates of jump-dispersal distances. For example, the absence of Argentine ants from many intervening counties may have resulted from inadequate surveys there. This would inflate estimates of jump-dispersal events in our nearest occupied county model as the actual distances between a new infestation and its putative source would be overestimated if a closer infestation existed but remained unnoticed. There are several reasons why we believe that this error is minimal. As a result of its prominent pest status earlier this century (32), extensive surveys were conducted throughout the southeastern United States, and L. humile was not detected in many areas. Because information concerning absences is as important as presence data, collections and published surveys that did not detect Argentine ants are also included in the supplemental tables, which are published on the PNAS web site (www.pnas.org). In addition, rates of spread from budding are far too slow to account for the occupation of these distant counties (see Discussion).
Local Patterns of Invasion.
Rates of spread at invasion fronts through colony budding were collated from the literature and unpublished surveys. For each account, we noted the location, duration of study, number of fronts monitored, habitat type, and the minimum and maximum rates of spread. Subsequent analyses used the maximum rate of spread from each study.
Interannual variation in rates of spread by budding was examined by following 20 distinct invasion fronts of Argentine ants for 3–4 years (depending on the site) in riparian woodlands in northern California (see ref. 29 for complete description of study areas). Variation in invasion rate across years was examined by using repeated-measures ANOVA. Only fronts that spread at least 3 years were included in subsequent analyses (n = 10). Invasion fronts that did not advance were dropped from this analysis because the environment at these sites may not be abiotically suitable for Argentine ants (29). For example, Holway (29) found that Argentine ants spread at sites with permanent stream flow but did not at sites with intermittent stream flow. Therefore, including sites at which Argentine ants did not spread would overestimate variation in invasion rates.
Results
Worldwide Distribution.
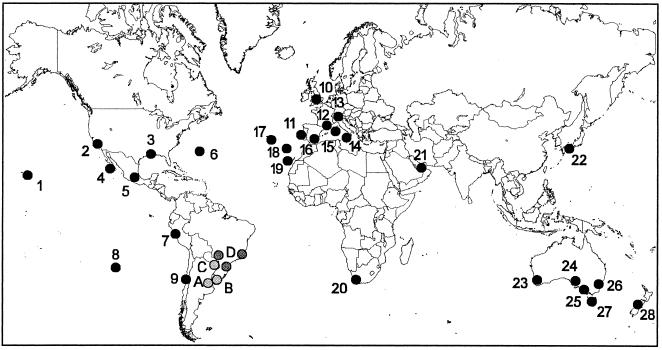
Argentine ants now occur throughout the world, with at least 28 separate introductions known from six continents and many oceanic islands (Fig. 1). The earliest reported introduction occurred in 1882 on Madeira Island. Although new records continue to accumulate, many populations were established by the 1950s (Table 1). Although Fig. 1 undoubtedly underestimates the Argentine ant's current distribution, it illustrates that this species has become a worldwide invader in areas with Mediterranean-type climates and in some temperate and subtropical areas. Given the innate limitations in the Argentine ant's natural dispersal capabilities, it is clear that the colonization ability of this species hinges on human commerce. Although the introduced range of this species is now fairly well known, its native range in South America deserves further study. Morphological and genetic similarity exists between many introduced populations and populations along the Rios Parana and Uruguay in northern Argentina (unpublished data). However, at this point it is unclear whether populations from Brazil belong to L. humile (ref. 36 and P. Ward, personal communication).
Figure 1.
Known worldwide distribution of the Argentine ant. Numbered black circles indicate introduced populations while shaded circles represent presumed native populations. See Table 1 for sources of information, estimated dates of introduction, and a brief discussion of some records for each number and letter.
Table 1.
Dates Argentine ants were first detected in countries and islands throughout the world
| No./letter | Year | Location | Collection (ref.) |
|---|---|---|---|
| Introduced populations | |||
| 1 | 1940 | United States: Hawaii | HDOA (43) |
| 2 | 1907 | United States: California | (32) |
| 3 | 1891 | United States: Louisiana | (32) |
| 4 | 1938 | Mexico: Baja California | CASC |
| 5 | 1946 | Mexico: (Mexico City) | USNH |
| 6 | 1949 | Bermuda | (44) |
| 7 | 1974 | Peru: (Lima) | (46) |
| 8 | 1987 | Easter Island | USNH (47) |
| 9 | 1910 | Chile | (32) |
| 10 | 1927 | Britain | (48) |
| 11 | 1900 | Portugal: (Lisbon) | (50) |
| 12 | 1905 | France | (51) |
| 13 | 1980 | Switzerland | (52) |
| 14 | 1926 | Italy: Sicily | (48) |
| 15 | 1970 | Corsica | (53) |
| 16 | 1923 | Spain | (54) |
| 17 | 1940 | Azores | USNH |
| 18 | 1882 | Madeira | (55) |
| 19 | 1965 | Grand Canary | FMNH |
| 20 | 1901 | South Africa: (Stellenbosch) | (56) |
| 21 | 1995 | United Arab Emirates | (57) |
| 22 | 1993 | Japan: Hiroshima | (58) |
| 23 | 1941 | Australia: Western Australia | (59) |
| 24 | ? | Australia: South Australia | P. Ward (personal communication) |
| 25 | 1939 | Australia: Victoria | USNH (59) |
| 26 | 1950 | Australia: New South Wales | FMNH |
| 27 | 1951 | Tasmania: (Hobart) | (59) |
| 28 | 1990 | New Zealand | (60) |
| Native populations | |||
| A | Argentina, Buenos Aires Prov. | Type local., Mayr 1868 | |
| Argentina, Entre Rios Prov. | MACN; this study | ||
| Argentina, Corrientes Prov. | MACN; this study | ||
| Argentina, Missiones Prov. | MACN; this study | ||
| Argentina, Chaco Prov. | MACN | ||
| Argentina, Formosa Prov. | MACN | ||
| Argentina, Tucumán Prov.* | MACN | ||
| Argentina, Catamarca Prov.* | MACN | ||
| Argentina, Jujuy Prov.* | MACN | ||
| Argentina, Cordoba Prov. | MACN | ||
| Argentina, Santa Fe Prov. | This study | ||
| B | Uruguay, (Carasco) | USNH | |
| Uruguay, Canelones Prov. | MACN | ||
| Uruguay, Colonia Prov. | MACN | ||
| C | Paraguay, (Asari) | MACN | |
| D | Brazil, Sao Paulo Prov.* | (32, 36) | |
| Brazil, Rio Grande do Sul Prov.* | (32) | ||
| Brazil, Minas Gerais Prov.* | (61) | ||
| Brazil, Mato Grosso do Sul* | (36) | ||
Numbers refer to locations in Fig. 1. HDOA, Hawaii Department of Agriculture; CASC, California Academy of Sciences; USNH, United States National Museum; FMNH, Field Museum of Natural History; MACN, Museo de Ciencas Naturales, Buenos Aires.
These records may represent a native Linepithema species other than L. humile.
Regional Patterns of Invasion.
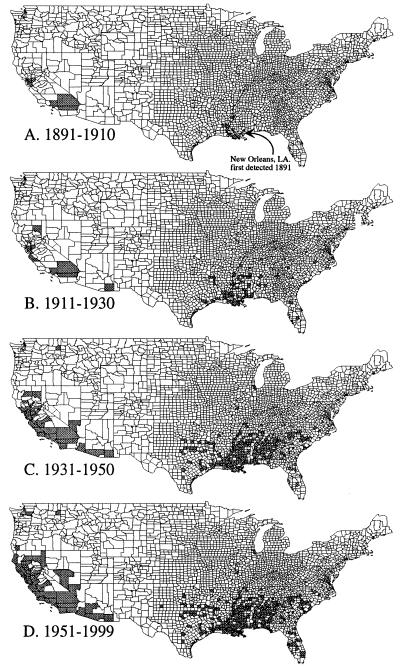
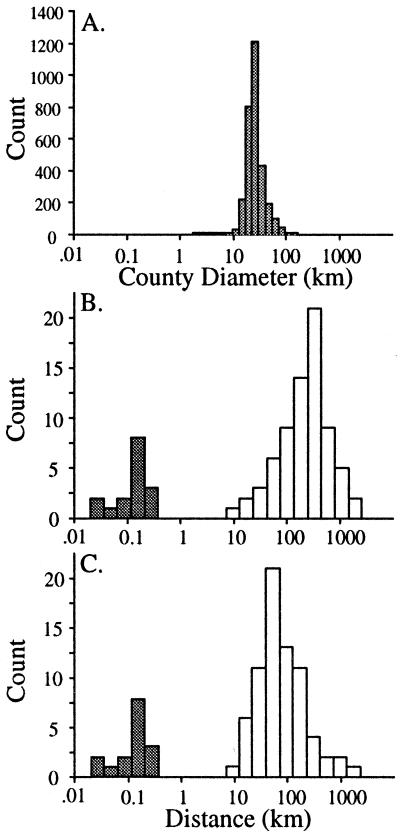
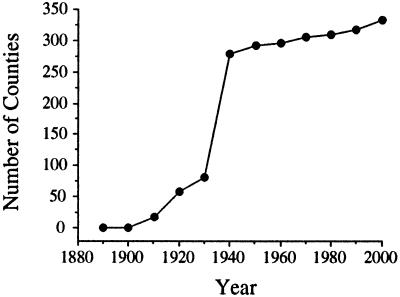
In addition to published accounts, 66 collections provided information regarding the presence or absence of Argentine ants in counties in the United States (see supplemental Tables 3 and 4). Linepithema humile has been recorded in 335 counties in 21 states (Fig. 2). Their initial spread throughout the United States was characterized by new populations established well away from the original site of introduction (Fig. 2 A and B). The two models of jump dispersal provide a range of estimates of annual jump-dispersal distances based on the pattern of spread of Argentine ants across the United States through 1930: out of New Orleans model = 361.72 ± 416.82 km (mean ± 1 SD); nearest occupied county model = 160.58 ± 323.83 km (see Fig. 4). Although these distributions are different (paired t test, P < 0.05), they are of the same order of magnitude. Moreover, these distances are an order of magnitude greater than the estimated lower limit of the distribution set by average county diameter (28.79 ± 17.69 km, n = 3,140 counties) (see Fig. 4), suggesting that, on average, jumps are occurring across more than one county. Apparent from the later stages of this invasion, Argentine ants filled in their range as new populations were discovered between originally separate colonized counties forming continuous regions of occupation (Fig. 2 C and D). When plotted against time, the pattern of occupation appears sigmoidal (Fig. 3). In the first 20 years of this invasion, few new counties were colonized. This was followed by a period of rapid expansion through the 1930s and then a period (that continues through the present) during which few new counties were colonized (Fig. 3). Although historical records for Argentine ants are fairly detailed, the current distribution of this species in the southeastern United States is unknown given the widespread presence of the red imported fire ant which may have displaced L. humile from portions of its former range (35).
Figure 2.
Reconstruction of the spread of Argentine ants throughout the United States from first detection in 1891 in New Orleans to the present.
Figure 4.
(A) The distribution of county diameters for all counties in the United States. (B and C) Distribution of yearly dispersal distances through local spread (shaded bars) and jump-dispersal events (open bars). Local rates of spread through colony budding were determined from the literature (Table 2). Yearly jump-dispersal distances were estimated through the reconstructed invasion history of the Argentine ant in the United States through 1930 (Fig. 2). Distances were estimated assuming sources for newly occupied counties originated at the site of original introduction, New Orleans (B), or from the nearest already occupied county (C).
Figure 3.
Rate of spread of the Argentine ant, measured in cumulative number of counties through time, based on the reconstruction of its invasion of the United States.
Local Patterns of Invasion.
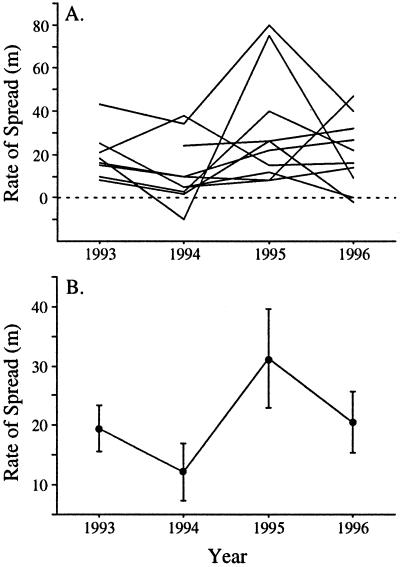
Sixteen studies followed Argentine ant invasion fronts for at least 1 year (Table 2). Although examined in different habitats throughout the world, with few exceptions, the maximum yearly rates of spread reported were largely consistent across sites (0.154 ± 0.021 km) (mean ± SE). Jump-dispersal distances, in contrast, averaged three orders of magnitude higher. When both distributions are plotted together, the disparity in dispersal distances between these two processes is clearly evident (Fig. 4). Despite the consistency among studies in their maximum rate of invasion (Table 2), within locations, rates of spread show much temporal variation. For example, at 10 sites in northern California, average rates of spread varied by a factor of 3 over a 3- to 4-year period (F = 3.14, df = 3,18, P = 0.05) (Fig. 5).
Table 2.
Reported rates of spread by budding reproduction for Argentine ants
| Ref. | Study | No. of sites* | No. of years | Rate of spread†, m/yr | Location | Habitat type |
|---|---|---|---|---|---|---|
| 62 | Woodworth (1910) | ? | 2 | 200 | Northern CA, USA | Urban? |
| 33 | Barber (1916) | ? | ? | 100–130 | Memphis, TN, USA | Urban |
| 63 | Fullaway (1944)‡ | 3 | 3 | 100–200 | Oahu, HI, USA | Urban |
| 44 | Crowell (1968)§ | 10 | 13 | 0–250 | Bermuda | Urban, woodland, mangrove |
| 59 | Pasfield (1968) | 1 | 1 | 275 | Sydney, Australia | Urban |
| 31 | Fluker and Beardsley (1970)¶ | 4 | 1.5 | 66–100 | Oahu, HI, USA | ? |
| 15 | Erickson (1972) | 1 | 6 | 50–150 | Oceanside, CA, USA | Grassland |
| 45 | Tremper (1976) | 1 | 1 | 15 | Livermore, CA, USA | Riparian woodland |
| 19 | Human and Gordon (1996) | 1 (40) | 1.4 | 0–211 | Stanford, CA, USA | Grassland |
| 50 | Way et al. (1997) | 1 | 3 | 30 | Central-south Portugal | Oak plantation |
| 29 | Holway (1998)‖ | 20 | 3–4 | 0–49 | Sacramento Valley, CA, USA | Riparian woodland |
| 49 | King (1998) | 1 | 2 | 100 | Del Mar, CA, USA | Coastal sage scrub |
| Digirolamo (unpublished work) | 1 (3) | 1 | 5–270 | Marina, CA, USA | Maritime chaparral, grassland | |
| Krushelnycky et al. (unpublished work) | 1 | 30 | 29–247 | Maui, HI, USA | Subalpine shrubland | |
| Krushelnycky et al. (unpublished work) | 1 | 15 | 24–150 | Maui, HI, USA | Subalpine shrubland | |
| A.V.S. and T.J.C. (unpublished work) | 1 | 3 | 20–200 | Chula Vista, CA, USA | Coastal sage scrub |
Figure 5.
Yearly variation in rates of spread through diffusion processes at 10 invasion fronts in riparian woodlands in northern California. (A) Rates at each site shown individually, and (B) rates averaged across sites (± SE).
Discussion
The results of this study demonstrate the importance of human-mediated jump-dispersal in determining invasion dynamics subsequent to establishment. This is evident from both the worldwide and regional reconstruction of Argentine ant invasion history. Fig. 1 illustrates that virtually no country or island is too isolated for potential establishment. The patchy and somewhat piecemeal pattern of invasion at the regional scale (Fig. 2) is in contrast to the well-defined and continuous invasion fronts that characterize some invasions (refs. 1 and 3, but see ref. 37). To account for this observed pattern through a diffusion-like processes, the rates of spread through colony budding would have to be three orders of magnitude higher (Fig. 4). Taken together, the results of this study provide an unusually thorough analysis of a worldwide invasion. In addition, unlike previous reconstructions of invasion history, this is the first comprehensive assessment for a species that spreads primarily by human-mediated jump dispersal.
Long-distance jump-dispersal events are believed to be rare and difficult to measure. Even when infrequent, long-distance dispersal events may greatly influence overall invasion rate. For example, using simulations, Higgins and Richardson (12) demonstrated that long-distance dispersal by as little as 0.001% could increase predicted rate of spread by an order of magnitude. Our study illustrates that for species that associate closely with humans these events are common and can drive the overall pattern of invasion. Specifically, by determining the distance and rate at which long-distance jump-dispersal events occur, we can estimate parameters for use in modeling the spread of invasive organisms that rely heavily on these events. The spread of the house finch (Carpodacus mexicanus) throughout the northeastern United States provides another good example of how historical data sets can enhance the predictive quality of modeling efforts. Because of long-term census data provided by Christmas Bird Counts, Veit and Lewis (10) were able to measure long-distance dispersal events and to estimate their relative contribution to overall invasion rate (9, 10).
When jump dispersal is common, simple diffusion fails to describe the pattern of spread. Such cases are better modeled by stratified diffusion, where more than one process is involved in the spread of an invading species. This is particularly clear in Argentine ant invasions given the huge disparity between rates of spread for alternate modes of dispersal (Fig. 4). Stratified diffusion “may be the rule rather than the exception” for invading organisms (3) and quantification of alternate modes of dispersal is key to the development of more realistic models to predict patterns of invasion dynamics in such cases. In particular, for species that spread through stratified diffusion, the distance and rate at which new foci are created may be more important than the rate of spread through diffusion from established foci.
The extent to which species spread by stratified diffusion may influence the implementation of control strategies. For example, the effectiveness of control measures can be greatly increased by preventing the establishment of new foci or by eliminating new foci rather than focusing efforts on established invasion fronts (37). As demonstrated in this study, the establishment of new foci through human-mediated jump dispersal is of paramount importance in the spread of Argentine ants and control efforts should focus on preventing their spread through these means. However, Argentine ants establish new populations easily. For example, laboratory experiments demonstrate that queens with as few as 10 workers exhibit high rates of colony growth, suggesting that such small propagules can easily establish beachheads (38).
Another interesting result of this study was that, at different spatial scales, rates of invasion vary through time. First, at a regional scale, there was a clear lag time in the spread of Argentine ants throughout the United States (Fig. 3). Such lag times are common features of invasions but their underlying causes often remain unclear (14, 39). Explanations include the inherent features of population growth, environmental changes that benefit invasive species such as increased urbanization, and genetic changes occurring subsequent to introduction that result in fitness increases (39). The relatively rapid spread of Argentine ants following the lag period may also reflect coincident increases in human transportation and commerce. Although unequal sampling effort could give rise to the pattern seen in Fig. 3, this is unlikely because of the intense sampling for Argentine ants early this century (32, 33). Second, at a local scale, rates of diffusion through budding reproduction varied temporally across sites (Fig. 5), possibly because of differences in abiotic conditions (e.g., precipitation) among years. A better appreciation of the causes underlying such variation will be useful in guiding theoretical work on the spread of invading organisms.
While we describe a case of stratified diffusion involving two dispersal processes, other invasions are more complex. For example, red imported fire ants (Solenopsis invicta) and zebra mussels (Dreissena polymorpha) spread via three primary modes of dispersal. In red imported fire ants, budding, mating flights and human introductions all contribute to overall spread (40). In zebra mussels, spread results from diffusive (within watershed), advective (within watershed), and jump-dispersal (across watershed) events (41, 42). Quantifying the rates and distances of these dispersal events is therefore difficult. For the spread of zebra mussels, an attempt has been made to examine the potential for over-ground dispersal between watersheds by examining the rates and distances which recreational boaters travel in Wisconsin (11). Approaches such as these offer great promise in the quantification of jump-dispersal events.
A major challenge in the study of biological invasions lies in determining factors that contribute to or limit the spread of exotic species. This can be difficult because detailed chronological histories of invasions rarely exist. In addition, despite the obvious value in making the study of biological invasions a more predictive science, estimating the rate and pattern of invasions remains a difficult task. Given the unpredictable nature of long-distance jump-dispersal events, accurately determining the range at which they occur can greatly enhance future modeling efforts. A careful reconstruction of invasion dynamics at contrasting spatial scales will also aid in the development management or eradication strategies.
Supplementary Material
Acknowledgments
This manuscript would not have been possible without the help of all of the museum curators mentioned in supplemental Table 4. In addition, we would like to especially thank A. Bachman, B. Brown, S. Cover, M. Deyrup, L. Loope, B. Norden, N. Reimer, T. Schultz, R. Snelling, D. Summers, P. Ward, and A. Wild for sharing unpublished data and for their hospitality. Work in Argentina was made possible by P. Cichero, F. Menvielle, and L. Raffo from the Administracion de Parques Nacionales Argentinas, R. (Mima) Venguet for logistical support, and I. C. Quilmes for many refreshing insights. The manuscript benefited from comments by L. Levin, T. Price, and P. Ward. This work was supported by the Canon National Parks Science Scholars Program (A.V.S.), U.S. Department of Agriculture National Research Initiative Competitive Grants Program Grants 99-35302-8675 (D.A.H.), and 00-35302-9417 (A.V.S.); and National Science Foundation Grant DEB-9610306 (T.J.C.).
References
- 1.Shigesada N, Kawasaki K. Biological Invasion: Theory and Practice. New York: Oxford Univ. Press; 1997. [Google Scholar]
- 2.Skellam J G. Biometrika. 1951;38:196–218. [PubMed] [Google Scholar]
- 3.Hengeveld R. Dynamics of Biological Invasions. London: Chapman and Hall; 1989. [Google Scholar]
- 4.Okubo A, Maini P K, Williamson M H, Murray J D. Proc R Soc London Ser B. 1989;238:113–125. doi: 10.1098/rspb.1989.0070. [DOI] [PubMed] [Google Scholar]
- 5.Andow D A, Karieva P M, Levin S A, Okubo A. Landscape Ecol. 1990;4:177–188. [Google Scholar]
- 6.Allen L J S, Allen E J, Kunst C R G. J Ecol. 1991;79:1123–1135. [Google Scholar]
- 7.Okubo A. Diffusion and Ecological Problems: Mathematical Models. New York: Springer; 1980. [Google Scholar]
- 8.Kot M, Lewis M A, van der Driessche P. Ecology. 1996;77:2027–2042. [Google Scholar]
- 9.Lewis M A. In: Spatial Ecology: The Role of Space in Population Dynamics and Interspecific Interactions. Tilman D, Kareiva P, editors. Princeton, NJ: Princeton Univ. Press; 1997. pp. 46–69. [Google Scholar]
- 10.Veit R R, Lewis M A. Am Nat. 1996;148:255–274. [Google Scholar]
- 11.Buchan L A J, Padilla D K. Ecol Appl. 1999;9:254–265. [Google Scholar]
- 12.Higgins S L, Richardson D M. Am Nat. 1999;153:464–475. doi: 10.1086/303193. [DOI] [PubMed] [Google Scholar]
- 13.Hengeveld R. Trends Ecol Evol. 1994;9:339–342. doi: 10.1016/0169-5347(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 14.Hastings A. Ecology. 1996;77:1675–1679. [Google Scholar]
- 15.Erickson J M. Psyche. 1971;78:257–266. [Google Scholar]
- 16.Bond W, Slingsby P. Ecology. 1984;65:1031–1037. [Google Scholar]
- 17.Ward P S. Hilgardia. 1987;55(2):1–16. [Google Scholar]
- 18.Cammell M E, Way M J, Paiva M R. Insectes Soc. 1996;43:37–46. [Google Scholar]
- 19.Human K G, Gordon D M. Oecologia. 1996;105:405–412. doi: 10.1007/BF00328744. [DOI] [PubMed] [Google Scholar]
- 20.Suarez A V, Bolger D T, Case T J. Ecology. 1998;79:2041–2056. [Google Scholar]
- 21.Holway D A. Oecologia. 1998;116:252–258. doi: 10.1007/s004420050586. [DOI] [PubMed] [Google Scholar]
- 22.Suarez A V, Richmond J Q, Case T J. Ecol Appl. 2000;10:711–725. [Google Scholar]
- 23.Cole F R, Medeiros A C, Loope L L, Zuehlke W W. Ecology. 1992;73:1313–1322. [Google Scholar]
- 24.Human K G, Gordon D M. Cons Biol. 1997;11:1242–1248. [Google Scholar]
- 25.Bolger D T, Suarez A V, Crooks K R, Morrison S A, Case T J. Ecol Appl. 2000;10:1230–1248. [Google Scholar]
- 26.Holldobler B, Wilson E O. The Ants. Cambridge, MA: Harvard Univ. Press; 1990. [Google Scholar]
- 27.Passera L. In: Exotic Ants: Biology, Impact, and Control of Introduced Species. Williams D F, editor. Boulder: Westview Press; 1994. pp. 23–43. [Google Scholar]
- 28.Fuentes E R. In: Biogeography of Mediterranean Invasions. Grives R H, Di Castri F, editors. Cambridge, MA: Cambridge Univ. Press; 1991. pp. 43–49. [Google Scholar]
- 29.Holway D A. Oecologia. 1998;115:206–212. doi: 10.1007/s004420050509. [DOI] [PubMed] [Google Scholar]
- 30.Markin G P. Ann Entomol Soc Am. 1970;63:1238–1242. [Google Scholar]
- 31.Fluker S S, Beardsley J W. Ann Entomol Soc Am. 1970;63:1290–1296. [Google Scholar]
- 32.Newell W, Barber T C. US Dep Agric Bur Entomol Bull. 1913;122:1–98. [Google Scholar]
- 33.Barber T C. US Dep Agric Bur Entomol Bull. 1916;377:1–23. [Google Scholar]
- 34.Harned R W, Smith M R. J Econ Entomol. 1922;15:261–264. [Google Scholar]
- 35.Wilson E O. Evolution. 1951;5:68–79. [Google Scholar]
- 36.Orr M R, Seike S H. Oecologia. 1998;117:420–425. doi: 10.1007/s004420050676. [DOI] [PubMed] [Google Scholar]
- 37.Moody M E, Mack R N. J Appl Ecol. 1988;25:1009–1021. [Google Scholar]
- 38.Hee J J, Holway D A, Suarez A V, Case T J. Cons Biol. 2000;14:559–563. [Google Scholar]
- 39.Crooks J, Soulé M E. In: in Proceedings of the Norway/UN Conference on Alien Species. Sandlund O, Schei P, Viken A, editors. Norway: Trondheim; 1996. pp. 39–46. [Google Scholar]
- 40.Porter S D, Van Eimeren B, Gilbert L E. Ann Entomol Soc Am. 1988;81:913–918. [Google Scholar]
- 41.Johnson L E, Carlton J T. Ecology. 1996;77:1686–1690. [Google Scholar]
- 42.Johnson L E, Padilla D K. Biol Cons. 1996;78:23–33. [Google Scholar]
- 43.Zimmerman E C. Proc Hawaiian Entomol Soc. 1941;11:108. [Google Scholar]
- 44.Crowell K L. Ecology. 1968;49:551–555. [Google Scholar]
- 45.Tremper B D. Ph.D. thesis. Berkeley: Univ. of California; 1976. [Google Scholar]
- 46.Dale W E. Revista Peruana Entomol. 1974;17:126–127. [Google Scholar]
- 47.Morrison L W. Acta Oecologica. 1997;18:685–695. [Google Scholar]
- 48.Donisthorpe H. Entomol Record J Var. 1930;42:13–16. [Google Scholar]
- 49.King J. M.S. thesis. San Diego: Univ. of California; 1998. [Google Scholar]
- 50.Way M J, Cammell M E, Paiva M R, Collingwood C A. Insectes Soc. 1997;44:415–433. [Google Scholar]
- 51.Chopard L. Ann Epiphyties. 1921;7:237–266. [Google Scholar]
- 52.Kutter H. Mitt Schweiz Entomol Ges. 1981;54:171–172. [Google Scholar]
- 53.Casevitz-Weulersse J. Ann Soc Entomol Fr. 1974;10:611–621. [Google Scholar]
- 54.Martinez M D, Ornosa C, Gamarra P. Boln Asoc Esp Ent. 1997;21:275–276. [Google Scholar]
- 55.Stoll O. Mittheil Schweize Entomol Gesellschaft. 1898;10:120–126. [Google Scholar]
- 56.Prins A J, Robertson H G, Prins A. In: Applied Myrmecology, A World Perspective. Vandermeer R K, Jaffe K, Cedeno A, editors. Boulder: Westview Press; 1990. pp. 25–33. [Google Scholar]
- 57.Collingwood C A, Tigas B J, Agnosti D. J Arid Environ. 1997;37:505–512. [Google Scholar]
- 58.Sugiyama T. Jpn J Appl Entomol Zool. 2000;44:125–126. [Google Scholar]
- 59.Pasfield G. Australian Nat Hist. 1968;16:12–15. [Google Scholar]
- 60.Green O R. Weta. 1990;13:14–15. [Google Scholar]
- 61.Della Lucia T M C, Loureira M C, Chandler L, Freire J A H, Galvno J D, Fernandes B. Experientiae. 1982;18:67–94. [Google Scholar]
- 62.Woodworth C W. Univ Calif Agric Exp Stn Bull. 1910;207:51–82. [Google Scholar]
- 63.Fullaway D T. Proc Hawaiian Entomol Soc. 1944;12:26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.