Abstract
Renal cell carcinoma (RCC) is the most lethal of urologic malignancies, accounting for an estimated 36,000 new cases of carcinoma and 12,000 deaths in 2005. Nephrectomy is the usual treatment; however, after nephrectomy, RCC recurs in 20% to 40% of patients with clinically localized disease. A consensus surveillance protocol does not exist for follow-up of RCC after nephrectomy. In this article, available protocols are reviewed with a goal of developing an evidence-based system including the prognostic factors for recurrent disease, chronology and sites of recurrence, available treatment options if recurrent disease is found, and modalities of diagnostic testing available to urologists. New surveillance recommendations are presented based on prognostic factors as well as the University of California, Los Angeles Integrated Staging System for RCC.
Key words: Cancer, Kidney, Renal, Nephrectomy, Surveillance, Recurrence
In the United States, renal cell carcinoma (RCC) will account for an estimated 36,000 new cases and over 12,000 deaths in 2005.1 RCC is the most lethal of the urologic malignancies, with approximately 20% to 30% of patients with RCC presenting with metastatic disease and more than 40% of patients eventually dying from it.1,2 Surgical resection for clinically localized disease remains the mainstay for curative intervention. However, the aggressive and often insidious nature of RCC is reflected by recurrence rates of 20% to 40% after nephrectomy for clinically localized disease.2
This high rate of recurrence for clinically localized disease after nephrectomy underscores the importance of post-surgical surveillance. With the availability of treatment modalities offering improved survival in recurrent cases, the physician is challenged to identify treatable recurrences, while minimizing low yield studies and without sacrificing patient outcomes.
Urologists play a prominent role in long-term follow-up of patients with RCC. However, a consensus surveillance protocol does not exist. The rationale for developing an evidence-based system is based on the prognostic factors for recurrent disease, chronology and sites of recurrence, treatment options available if recurrent disease is found, and modalities of diagnostic testing available. This article provides a historical review of surveillance protocols and discusses new recommendations based on prognostic factors as well as the University of California, Los Angeles (UCLA) Integrated Staging System (UISS).
Prognostic Factors in RCC
Multiple prognostic factors have been studied to help predict RCC recurrence, including tumor stage, nuclear grade, overall performance status, and molecular markers.3 However, anatomic staging systems based on the tumor, nodes, metastasis (TNM) system have been the mainstays in RCC prognosis. Using the 1997 TNM classification from the International Union Against Cancer and American Joint Committee on Cancer, 5-year cancer-specific survival rates of 91%, 74%, 67%, and 32% for stages I to IV, respectively, have been reported.4 A major reduction in survival occurs with systemic metastases between stages III and IV.
Positive lymph node status is incorporated in the TNM classification and is associated with a higher incidence of metastatic disease and poorer response rates to immunotherapy.5,6 The overall incidence of lymph node metastases is approximately 20% with a 5-year survival rate ranging from 11% to 35%. Significantly, lymph node dissection in these patients improves response to immunotherapy, because lymph nodes have been observed to respond minimally to immunotherapy.7
Tumor grade is an independent prognostic indicator for RCC. The Fuhrman nuclear grading system projects 5-year survival rates of 89%, 65%, and 46% for grades 1, 2, and 3 to 4, respectively, independent of T stage.4 Alternatively, in patients with T1 disease, 5-year cancer-specific survival rates have been reported to be 91%, 83%, 60%, and 0% for grades 1, 2, 3, and 4, respectively.4
Incorporated in the TNM staging system, the modified 2002 TNM classifies tumors less than 4 cm (T1a), between 4 and 7 cm (T1b), and greater than 7 cm. In addition to its prognostic value, size is an important criterion in selection for nephron-sparing surgery, with tumors 4 cm or less most amenable for partial nephrectomy.8
Recurrence of RCC: Timing and Location
The greatest risk of recurrence for RCC occurs within the first 5 years after nephrectomy, with the majority of recurrences occurring within 3 years. Although recurrences have been reported as late as 30 years following nephrectomy, rates of 43% in the first year, 70% within the second year, 80% within 3 years, and 93% within 5 years have been reported.9,10 Tumor stage plays an important role in timing of recurrence, with T1 tumors generally recurring between 38 and 45 months, whereas T3 tumors generally recur between 17 and 28 months following initial nephrectomy.11,12 After nephrectomy, the incidence of RCC recurrence has been reported to be 7% with a median time of 38 months for T1 tumors, 26% with a median time of 32 months for T2 disease, and 39% with a median time to recurrence at 17 months for T3 tumors.11
RCC has been shown to metastasize to almost all soft tissues in the body, but most commonly to the lung, followed by bone, liver, brain, and local recurrence.12 Metastases to brain, bone, and liver often present as widely disseminated disease. Modalities of survey are chosen to reflect the most prevalent locations of RCC recurrence. In addition, stringent surveillance to detect recurrences in areas most amenable to further therapy is paramount.
RCC metastases occur most commonly in the lung, affecting 3% to 16% of patients after nephrectomy.10,11,13–15 Metastatic lung lesions are typically identified through symptoms such as cough, dyspnea, pleuritic chest pain, or hemoptysis (over 70%), although other reports find that these lesions are detected in asymptomatic patients more readily through imaging tests (over 90%).12 A history and physical examination are performed, and serial chest radiographs are obtained. We found chest computed tomography (CT) scans to be more sensitive in detecting lung metastases.
Bone metastases occur in 2% to 8% of patients following nephrectomy.10,11,14,15 The majority of patients present with symptoms of bone pain (67% to 90%) and with elevated alkaline phosphatase levels (33% to 55%).11,16,17 Furthermore, treatment for bone metastases is usually palliative to prevent pain or pathologic fractures, or to preserve function, and thus routine radiographic surveillance or nuclear scintigraphy is not advocated but used for confirmation of suspected metastases.
The incidence of liver metastases is reported to be 1% to 7%.10,11,14,15 The majority of metastases are detected as a result of symptoms (86% to 90%) or elevated liver function tests, although most are multifocal at the time of diagnosis.11,14 As resection of liver metastases improves survival, especially resection of solitary masses, history and physical examination, laboratory studies, and surveillance abdominal CT scans are the standard of care.2
Metastases to the brain occur in 2% to 4% of patients following nephrectomy. 10,11,14,15 Metastasis to the brain usually involves neurologic symptoms in up to 98% of patients.17 Treatment is usually palliative and typically consists of corticosteroid therapy or radiation therapy; therefore, active surveillance with imaging is not justified. However, at UCLA, before immunotherapy begins, brain screening with MRI is performed to evaluate for occult metastasis because the seizure threshold is decreased with interleukin-2 (IL-2) therapy.12 Brain metastases can then be treated with gamma-knife surgery to improve tolerance to immunotherapy.
Studies report the incidence of local recurrence ranging from 1.8% to 27%, with 1 study reporting a 5-year incidence of 1.8% from a population undergoing nephrectomy for localized RCC.12,18 In the same study, only 60% of recurrences were identified secondary to symptoms.18 Along with a careful history and physical examination, abdominal CT scans are critical because resection of the renal fossa bed has been shown to improve survival.
Nephron-sparing surgery or partial nephrectomy has been advocated for localized RCC lesions generally less than 4 cm diameter. Despite the fears of higher local recurrence following partial nephrectomy, local recurrence rates of 1.2% to 9% have been reported, with breakdown by T stage at 0%, 2%, 8%, and 11% for T1, T2, T3a, and T3b disease, respectively, in one study.8,15 Furthermore, overall survival rates compared with radical nephrectomy have been similar for T1 tumors.8
Management of Recurrent RCC
The challenge in the management of recurrent RCC lies in the limited efficacy of treatment modalities because RCC is typically resistant to chemotherapy and radiation therapy. Two modes of treatment are currently available for metastatic or recurrent RCC: immunotherapy and surgery. Systemic IL-2 treatment, the only FDA-approved immunotherapy, exhibits response rates of 15% to 25%.19 This therapy includes significant side effects, including pulmonary edema, hypotension, flu-like symptoms, and central nervous system toxicity, and requires adequate renal function to tolerate treatment. Improved response to immunotherapy has been shown in patients with the lowest metastatic burden and with solitary versus multiple recurrences.20
Surgical management of recurrent RCC plays a role in solitary metastasis, locally recurrent disease, residual masses after systemic therapy, and palliation for symptomatic relief. Surgical resection of solitary metastasis can result in 5-year survival rates of 24% to 60%, with solitary lung metastasis most amenable for resection.21 Resection of local recurrences has also been shown to extend survival from 21 to 136 months with reported 5-year survival for patients treated with surgical resection, medical therapy, and observation for renal fossa recurrences of 51%, 18%, and 13%, respectively.18,22,23
Based on the more favorable out-comes when metastatic burden is detected at its infancy, an appropriate but aggressive surveillance protocol is indicated. Other factors should be weighed in as well, including general health and comorbidities, site(s) of metastases, disease course to date, and morbidity of surgery.
Traditional Surveillance Protocols
The majority of recurrent disease is detected by surveillance laboratory or radiographic studies in asymptomatic patients 50% to 80% of the time, with the remainder detected by either work-up of patient symptoms, including decreased appetite, weight loss, decreased energy, fever, and night sweats, or physical findings of cachexia, abdominal mass, localized neurologic symptoms, or adenopathy.2 Surveillance tools include a careful history and physical examination; laboratory tests for serum calcium level, alkaline phosphatase level, and liver transaminases; and plain chest radiographs and CT scans.
Traditional protocols uniformly followed patients without tailored time points reflecting the likelihood of recurrence. Montie24 proposed a generic protocol for RCC surveillance following nephrectomy with a history, physical examination, and laboratory tests every 6 months for 5 years starting 1 month following surgery, a chest radiograph every 6 months starting at 6 months, and an abdominal CT scan after 12, 24, and 48 months. Although based largely on empirical data, the increased concern of local tumor recurrence following partial nephrectomy has led to more frequent abdominal CT scans at a rate of once every 6 months for 5 years.
Stage-Based Surveillance Protocols
Several protocols have been proposed based on TNM staging. They have been reviewed in greater detail elsewhere and only the range of recommendations will be summarized here. For T1 disease, risk of metastatic disease and local recurrence is low, thus recommendations range from a history and physical examination yearly for 5 years follow-up, to including a chest radiograph every 6 months for 3 years then yearly until 5 years follow-up. Most protocols do not advocate abdominal CT surveillance.10,11,14,25
Increased risk for lung and abdominal recurrence exists for T2 tumors, thus recommendations range from a history and physical examination, laboratory tests, and chest radiograph every 6 months for 3 years, then yearly until 5 years with no follow-up CT scans, to a history and physical examination, laboratory tests, and chest radiograph yearly for 5 years and abdominal CT scans at 2 and 4 years.10,11,14,25
Surveillance for T3 and T4 disease increases abdominal CT surveillance. Along with a history and physical examination, laboratory tests, and chest radiograph every 6 months for 3 years then yearly until 5 years follow-up, most reports advocated routine abdominal CT scans, with scans at years 2 and 5, or 1, 3, and 5.10,11,14,25 Some reports advocate a first visit at 3 months.
Following partial nephrectomy, increased frequency of abdominal CT scans was advocated for T3 disease with scans every 6 months for 3 years, then a scan at year 5 com-pared to CT scans at 1, 3, and 5 years by the same study.25
Integrated Staging and University of California, Los Angeles Integrated Staging System-Based Surveillance Protocols
Contemporary systems have been developed at UCLA and other institutions to improve and simplify prognostic information based on TNM stage as well as other independent pathologic and clinical variables. Notably, Kattan and colleagues26 at Memorial Sloan-Kettering Cancer Center evaluated 601 patients under-going nephrectomy for localized RCC and found symptoms, tumor histology, tumor size, and TNM stage all independent predictors of tumor recurrence. Leibovich and colleagues27 at the Mayo Clinic evaluated 1671 patients undergoing nephrectomy for localized RCC and found tumor stage, regional lymph node status, tumor size, nuclear grade, and histologic tumor necrosis to be predictive of progression to metastatic disease. In both studies, the described factors were used to construct a nomogram to stratify patients according to risk of metastasis.
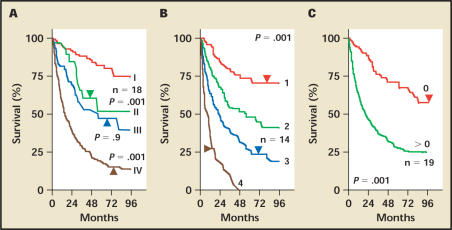
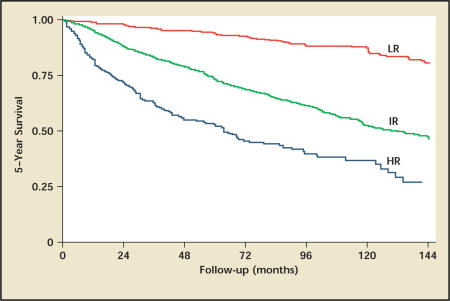
At the University of California, Los Angeles (UCLA), a novel staging system was recently developed that stratifies patients better than stage alone for survival and tumor recurrence. The development of the UCLA Integrated Staging System (UISS) incorporated the 1997 TNM classification with the Eastern Cooperative Oncology Group (ECOG) performance status and Fuhrman grade into a single prognostic system and has been validated using 4202 patients from 8 institutions28,29 (Figure 1). The UISS has undergone modification from its original form to a simplified system categorizing patients into risk groups of low, intermediate, and high risk30 (Figure 2). For localized RCC, 5-year survival rates from this study were 92%, 67%, and 44% for low-, intermediate-, and high-risk groups, respectively (Figure 3). For metastatic RCC, the UISS projected 3-year survival rates of 37%, 23%, and 12% for low-, intermediate-, and high-risk groups, respectively.
Figure 1.
Kaplan-Meier survival analysis of 661 patients based on prognostic indicators incorporated into University of California Los Angeles Integrated Staging System for renal cell carcinoma. (A) Survival curves based on 1997 tumor, nodes, metastasis (TNM) stages I–IV; (B) Survival curves based on Fuhrman grades 1–4. (C) Survival curves based on Eastern Cooperative Oncology Group performance status. Reproduced with permission from Zisman A et al.28
Figure 2.
University of California Los Angeles Integrated Staging for patients with localized renal cell carcinoma. Using the T stage, Fuhrman grade, and Eastern Cooperative Oncology Group performance status (ECOG PS), patients are stratified into low-, intermediate-, and high-risk groups. Adapted from Zisman A et al.30
Figure 3.
Kaplan-Meier survival analysis of 3119 patients based on University of California Los Angeles Integrated Staging with localized renal cell carcinoma. CT, computerized tomography; LR, low risk; IR, intermediate risk; HR, high risk. Reproduced with permission from Patard JJ et al.29
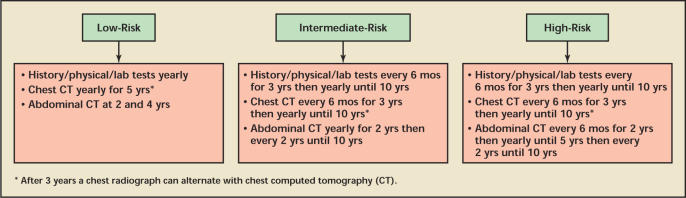
Based on the UISS stratification, the natural history of RCC, and available treatment modalities, we recommend the following guidelines (Figure 4). For low-risk patients, we recommend yearly history and physical examination, laboratory tests, and chest CT for 5 years and an abdominal CT scan at years 2 and 4, with no further surveillance beyond 5 years. For intermediate-risk patients, we recommend history and physical examination, laboratory tests, and chest CT every 6 months for the first 3 years, then yearly for 10 years of follow-up, with an abdominal CT scan at 1 year then every 2 years until 10 years follow-up. We recommend more intensive abdominal surveillance for high-risk patients, with recommendations identical to those for the intermediate-risk group except with more frequent abdominal CT scans at a rate of once every 6 months for the first 2 years, then yearly for years 2 to 5, then every 2 years until 10 years follow-up. For both medium-and high-risk groups, a chest radiograph can alternate with a chest CT scan after 3 years.12,31
Figure 4.
Surveillance protocol following nephrectomy for localized renal cell carcinoma using the University of California Los Angeles Integrated Staging System.
Compared to a non-biased protocol, this strategy has a more relaxed surveillance for the low-risk group, with increased surveillance for the high-risk population. We do not recommend any additional surveillance for patients following partial nephrectomy, with an exception perhaps in cases of familial forms of RCC such as von Hippel-Lindau disease, with which there have been documented reports of 80% recurrence in the ipsilateral kidney following partial nephrectomy within 10 years.32
Notably, all surveillance protocols must take into account patient comorbidities, patient compliance and mindset, and willingness to consider additional treatment.
Future Directions
New technologies and progressive understanding of RCC biology promise enhanced treatments as well as detection of metastases. Positron emission tomography (PET) may soon have a larger role in renal tumor imaging. Studies suggest promising results for detection of lymph node involvement and of improved differentiation of local recurrence and metastasis.33 In a study of 8 patients, PET imaging upstaged tumor burden in 3 patients and excluded recurrence in 1 patient.34
The use of molecular markers for RCC is currently in its infancy. Markers may prove beneficial as a prognostic indicator, to predict responsiveness to treatment, to monitor progression of treatment, to detect recurrences, and perhaps as a target for directed therapies or vaccines. Playing essential roles in angiogenesis, apoptosis, cell adhesion, cell cycle regulation, and proliferation, these molecules represent the key to un-locking the mysteries of RCC.19 Recent work in carbonic anhydrase (CA) IX, a member of the carbonic anhydrase family of proteins thought to regulate intracellular and extracellular pH during hypoxic periods in tumor cells, has shown that low expression of CA IX, defined as less than 85%, is an independent prognostic indicator of poor survival in patients with metastatic RCC.35,36 Furthermore, the RCC of complete responders to immunotherapy correlated with high expression of CA IX.35 An analogous correlation exists in the low expression of CA IX in papillary and chromophobe subsets of RCC, which typically respond poorly to immunotherapy. At UCLA, CA IX is now routinely screened in pathologic samples and a phase III trial of antibody against CA IX is underway.
In an era of limited medical resources, future studies will also investigate the cost-benefit analysis of aggressive routine surveillance. These data will compare the burden of early surveillance to detect early recurrences to the response rates and outcomes when recurrences are detected later.
Conclusion
Refinements in the understanding of the natural history of RCC, as well as promising new discoveries in RCC biology, result in a continuous evolution of recommendations for surveillance of patients with RCC. Currently at UCLA, a novel staging algorithm has combined prognostic factors along with the traditional TNM staging system to improve RCC staging. Together with the likely timing and location for RCC recurrences, an evidence-based protocol to survey patients following nephrectomy for clinically localized RCC has been proposed. This protocol can be used by the clinician with patient preferences and treatment options to tailor patient follow-up after nephrectomy.
Main Points.
The aggressive and often insidious nature of renal cell carcinoma (RCC) is reflected by recurrence rates of 20% to 40% after nephrectomy for clinically localized disease.
Anatomic staging systems based on the tumor, nodes, metastasis (TNM) system have been the mainstays in RCC prognosis.
Positive lymph node status is incorporated in the TNM classification and is associated with a higher incidence of metastatic disease and poorer response rates to immunotherapy.
At the University of California, Los Angeles, a novel staging algorithm has combined prognostic factors along with the traditional TNM staging system to improve RCC staging—with the likely timing and location for RCC recurrences, an evidence-based protocol to survey patients following nephrectomy for clinically localized RCC has been proposed.
Low-risk patients are observed by yearly history and physical examination, laboratory tests, and a chest CT for 5 years and an abdominal CT scan at years 2 and 4, with no further surveillance beyond 5 years.
Intermediate-risk patients are observed by history and physical examination, laboratory tests, and a chest CT every 6 months for the first 3 years, then yearly for 10 years of follow-up, with an abdominal CT scan at 1 year and then every 2 years until 10 years follow-up.
More intensive abdominal surveillance is needed for high-risk patients, with recommendations identical to those for the intermediate-risk group, except with more frequent abdominal CT scans once every 6 months for the first 2 years, then yearly for years 2 to 5, and then every 2 years until 10 years follow-up.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 3.Lam JS, Shvarts O, Leppert JT, et al. Renal cell carcinoma 2005: new frontiers in staging, prognostication and targeted molecular therapy. J Urol. 2005;173:1853–1862. doi: 10.1097/01.ju.0000165693.68449.c3. [DOI] [PubMed] [Google Scholar]
- 4.Tsui KH, Shvarts O, Smith RB, et al. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000;163:1090–1095. doi: 10.1016/s0022-5347(05)67699-9. quiz 1295. [DOI] [PubMed] [Google Scholar]
- 5.Vasselli JR, Yang JC, Linehan WM, et al. Lack of retroperitoneal lymphadenopathy predicts survival of patients with metastatic renal cell carcinoma. J Urol. 2001;166:68–72. [PubMed] [Google Scholar]
- 6.Pantuck AJ, Zisman A, Dorey F, et al. Renal cell carcinoma with retroperitoneal lymph nodes. Impact on survival and benefits of immunotherapy. Cancer. 2003;97:2995–3002. doi: 10.1002/cncr.11422. [DOI] [PubMed] [Google Scholar]
- 7.Pantuck AJ, Zisman A, Dorey F, et al. Renal cell carcinoma with retroperitoneal lymph nodes: role of lymph node dissection. J Urol. 2003;169:2076–2083. doi: 10.1097/01.ju.0000066130.27119.1c. [DOI] [PubMed] [Google Scholar]
- 8.van Ophoven A, Tsui KH, Shvarts O, et al. Current status of partial nephrectomy in the management of kidney cancer. Cancer Control. 1999;6:560–570. doi: 10.1177/107327489900600602. [DOI] [PubMed] [Google Scholar]
- 9.McNichols DW, Segura JW, DeWeerd JH. Renal cell carcinoma: long-term survival and late recurrence. J Urol. 1981;126:17–23. doi: 10.1016/s0022-5347(17)54359-1. [DOI] [PubMed] [Google Scholar]
- 10.Ljungberg B, Alamdari FI, Rasmuson T, Roos G. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int. 1999;84:405–411. doi: 10.1046/j.1464-410x.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 11.Levy DA, Slaton JW, Swanson DA, Dinney CP. Stage specific guidelines for surveillance after radical nephrectomy for local renal cell carcinoma. J Urol. 1998;159:1163–1167. [PubMed] [Google Scholar]
- 12.Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Surveillance following radical or partial nephrectomy for renal cell carcinoma. Curr Urol Rep. 2005;6:7–18. doi: 10.1007/s11934-005-0062-x. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson AJ, Chetner MP, Rourke K, et al. Guidelines for the surveillance of localized renal cell carcinoma based on the patterns of relapse after nephrectomy. J Urol. 2004;172:58–62. doi: 10.1097/01.ju.0000132126.85812.7d. [DOI] [PubMed] [Google Scholar]
- 14.Sandock DS, Seftel AD, Resnick MI. A new protocol for the followup of renal cell carcinoma based on pathological stage. J Urol. 1995;154:28–31. [PubMed] [Google Scholar]
- 15.Hafez KS, Novick AC, Campbell SC. Patterns of tumor recurrence and guidelines for followup after nephron sparing surgery for sporadic renal cell carcinoma. J Urol. 1997;157:2067–2070. [PubMed] [Google Scholar]
- 16.Shvarts O, Lam JS, Kim HL, et al. Eastern Cooperative Oncology Group performance status predicts bone metastasis in patients presenting with renal cell carcinoma: implication for preoperative bone scans. J Urol. 2004;172:867–870. doi: 10.1097/01.ju.0000135803.91207.b0. [DOI] [PubMed] [Google Scholar]
- 17.Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 18.Itano NB, Blute ML, Spotts B, Zincke H. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol. 2000;164:322–325. [PubMed] [Google Scholar]
- 19.Lam JS, Belldegrun AS, Figlin RA. Advances in immune-based therapies of renal cell carcinoma. Expert Rev Anticancer Ther. 2004;4:1081–1096. doi: 10.1586/14737140.4.6.1081. [DOI] [PubMed] [Google Scholar]
- 20.Han KR, Pantuck AJ, Bui MH, et al. Number of metastatic sites rather than location dictates overall survival of patients with node-negative metastatic renal cell carcinoma. Urology. 2003;61:314–319. doi: 10.1016/s0090-4295(02)02163-5. [DOI] [PubMed] [Google Scholar]
- 21.Piltz S, Meimarakis G, Wichmann MW, et al. Long-term results after pulmonary resection of renal cell carcinoma metastases. Ann Thorac Surg. 2002;73:1082–1087. doi: 10.1016/s0003-4975(01)03602-5. [DOI] [PubMed] [Google Scholar]
- 22.Tanguay S, Pisters LL, Lawrence DD, Dinney CP. Therapy of locally recurrent renal cell carcinoma after nephrectomy. J Urol. 1996;155:26–29. [PubMed] [Google Scholar]
- 23.Esrig D, Ahlering TE, Lieskovsky G, Skinner DG. Experience with fossa recurrence of renal cell carcinoma. J Urol. 1992;147:1491–1494. doi: 10.1016/s0022-5347(17)37605-x. [DOI] [PubMed] [Google Scholar]
- 24.Montie JE. Follow-up after partial or total nephrectomy for renal cell carcinoma. Urol Clin North Am. 1994;21:589–592. [PubMed] [Google Scholar]
- 25.Uzzo RG, Novick AC, et al. Surveillance strategies following surgery for renal cell carcinoma. In: Belldegrun AS, Ritchie AW, Figlin RA, et al., editors. Renal and Adrenal Tumors: Biology and Management. New York: Oxford University Press; 2003. pp. 324–330. [Google Scholar]
- 26.Kattan MW, Reuter V, Motzer RJ, et al., editors. A post-operative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63–67. [PubMed] [Google Scholar]
- 27.Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–1671. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 28.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–1657. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 29.Patard JJ, Kim HL, Lam JS, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol. 2004;22:3316–3322. doi: 10.1200/JCO.2004.09.104. [DOI] [PubMed] [Google Scholar]
- 30.Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–4566. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 31.Lam JS, Shvarts O, Leppert JT, et al. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol. 2005;174:466–472. doi: 10.1097/01.ju.0000165572.38887.da. [DOI] [PubMed] [Google Scholar]
- 32.Steinbach F, Novick AC, Zincke H, et al. Treatment of renal cell carcinoma in von Hippel-Lindau disease: a multicenter study. J Urol. 1995;153:1812–1816. [PubMed] [Google Scholar]
- 33.Janzen NK, Laifer-Narin S, Han KR, et al. Emerging technologies in uroradiologic imaging. Urol Oncol. 2003;21:317–326. doi: 10.1016/s1078-1439(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 34.Ramdave S, Thomas GW, Berlangieri SU, et al. Clinical role of F-18 fluorodeoxyglucose positron emission tomography for detection and management of renal cell carcinoma. J Urol. 2001;166:825–830. [PubMed] [Google Scholar]
- 35.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 36.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. [PubMed] [Google Scholar]