Chronic thromboembolic pulmonary hypertension (CTEPH) is the end result of persistent obstruction of the pulmonary arteries by acute or recurrent pulmonary emboli. In the era of successful surgical therapy, CTEPH, although historically considered a rare entity, is being diagnosed more frequently. In a recent prospective study, 3.8% of 314 consecutive patients who presented with acute pulmonary emboli developed symptomatic pulmonary hypertension within 2 years.1
Pulmonary hypertension likely stems from a redistribution of blood flow through a pulmonary arterial bed with a small cross-sectional area producing a vasculopathy of the small pulmonary vessels. CTEPH is typically not related to an underlying thrombophilic disorder, and many patients develop pulmonary hypertension in the face of adequate anticoagulation. Once pulmonary hypertension occurs, the prognosis is poor, with a median survival of 12–24 months and a 5-year survival of only 10%.2
As with idiopathic pulmonary arterial hypertension, the clinical presentation is often nonspecific. Patients with CTEPH typically present with a history of progressive dyspnea that has worsened over a period of months to years. Occasionally, syncopal episodes, chest pain or hemoptysis are present. A history of acute pulmonary emboli or deep venous thrombosis is reported in about 50% of patients.
Two investigations, Doppler echocardiography and a ventilation–perfusion scan, must be performed to establish the diagnosis of CTEPH. Although the results of a lung perfusion scan do not enable differentiation between acute pulmonary emboli and CTEPH, the scan is superior to CT angiography in excluding the diagnosis of CTEPH.3 Transthoracic Doppler echocardiography allows for an estimate of the severity of the pulmonary hypertension and the degree of right ventricular dysfunction and distension, as well as the right ventricular size and its impact on left ventricular filling (ventricular interdependence). Echocardiography can also exclude other cardiac anomalies and be used to demonstrate the presence of a right-to-left shunt through a reopening of the foramen ovale.
The definitive diagnosis of pulmonary hypertension is based on measurements made during a right-heart catheterization. If a pulmonary embolus is suspected on the ventilation–perfusion scan and signs of pulmonary hypertension are seen on the echocardiogram, a right-heart catheterization must always be performed to confirm and determine the severity of the pulmonary hypertension. It is occasionally difficult to differentiate between acute pulmonary emboli and CTEPH, and it is only by measuring the right-heart pressures and pulmonary vascular resistance during a cardiac catheterization that the distinction between the 2 clinical entities can be made. Because the normal right ventricle cannot tolerate even moderate elevations in pulmonary vascular resistance, the finding of a mean pulmonary artery pressure greater than 40 mm Hg in the presence of demonstrable pulmonary emboli supports a diagnosis of CTEPH.4
Pulmonary endarterectomy is the treatment of choice for patients with CTEPH. Candidates for surgery are determined on the basis of pulmonary angiography results and helical CT of the chest (Fig. 1). Pulmonary angiography confirms the diagnosis of CTEPH and helps in determining whether endarterectomy is feasible by demonstrating the location of the obstruction. Obstructions starting at the level of the lobar and segmental branches of the pulmonary artery are usually accessible to pulmonary endarterectomy. New-generation helical CT can show partial obstruction of the arterial lumen and abnormal thickening of the arterial wall down to the segmental branches, and it is therefore a good complement to pulmonary angiography for assessing patients for surgery. However, a normal CT scan result does not exclude the diagnosis of CTEPH, nor does it preclude the possibility of endarterectomy. All patients presenting with pulmonary arterial hypertension and segmental perfusion defect on the ventilation–perfusion scan must have a pulmonary angiogram to determine the feasibility of endarterectomy.
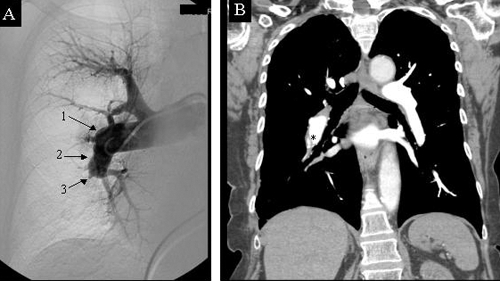
Fig. 1: (A) Preoperative angiogram of the right pulmonary artery showing typical signs of chronic thromboembolic pulmonary hypertension with: (1) abrupt change in the calibre of the artery, (2) irregularities of the arterial wall and (3) sacciform stop from obstruction of the pulmonary artery. (B) Preoperative CT scan of the same patient showing intraluminal webs that are secondary to partial resolution of the pulmonary emboli (*).
CT scanning also aids in excluding rare conditions that can mimic CTEPH.4 These conditions include fibrous mediastinitis, sarcoma of the pulmonary artery, tumor emboli into the pulmonary artery, hydatic emboli, or pulmonary arteritis such as Behcet's arteritis or Takayashu's arteritis. A CT scan can also delineate arthromatous calcifications of the pulmonary artery that could increase the technical difficulty of the endarterectomy in long-standing disease, and demonstrate the presence of a mosaic perfusion and the development of the bronchial circulation that are typical signs of CTEPH.
Pulmonary endarterectomy can restore near-normal cardiopulmonary function after surgery. The surgery is performed on cardiopulmonary bypass with a period of circulatory arrest to remove the obstructive material from each lobar and segmental branches of the pulmonary artery. The intraluminal material is composed of fibrous tissue that is inseparable from the intima, and it is thus inaccessible to thrombectomy or thrombolysis. Hence, a true endarterectomy is required, starting at the level of the right and left pulmonary arteries inside the pericardium and extending distally into each branch of the pulmonary arterial tree. In our experience, the video-assisted technique has been helpful in performing the endarterectomy in the segmental and subsegmental vessels by providing more light and by magnifying the plane of dissection. It has allowed us to expand the indication of pulmonary endarterectomy to patients with previously inaccessible distal disease in the segmental branches of the pulmonary artery.4
Histologic examination of the endarterectomy specimen reveals a true cast of the pulmonary vascular tree covered with elastic fibrin (Fig. 2). In situ thrombosis can occasionally occur as a result of obstruction of segmental and subsegmental branches associated with localized poor blood flow. However, in situ thrombus is the consequence of the disease and not its cause. Hence, thrombectomy (without a true endarterectomy) does not result in reduction of the pulmonary vascular resistance, and a true endarterectomy is required to release the segmental and subsegmental obstruction of the pulmonary arterial tree (Fig. 3).
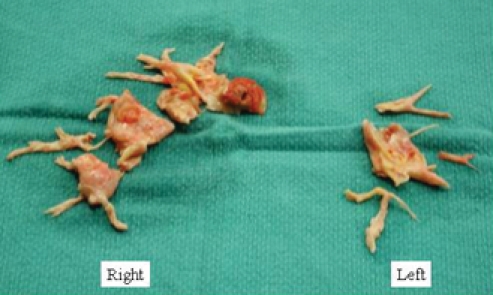
Fig. 2: Material removed by endarterectomy from the right and left pulmonary arteries. Histologic examination of the endarterectomy specimen reveals a true cast of the pulmonary vascular tree covered with elastic fibrin.
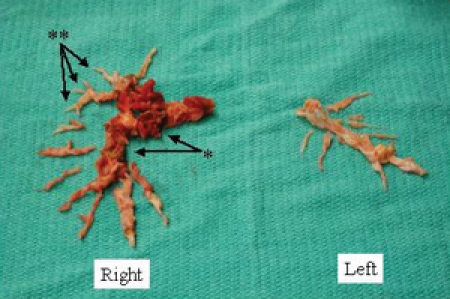
Fig. 3: In situ thrombosis can occasionally occur as a result of obstruction of segmental and subsegmental branches (*). However, thrombectomy does not result in reduction of the pulmonary vascular resistance, and a true endarterectomy is required in order to release the segmental and subsegmental obstruction (**).
Results of pulmonary endarterectomy are good to excellent in experienced centres. The overall operative rate of death ranges between 5% and 10%, but it is closely related to the preoperative hemodynamic compromise. If the pulmonary vascular resistance is below 900 dynes.s.cm-5, the rate of death is around 4%. It increases to 10% in patients with resistance between 900 and 1200 dynes.s.cm-5, and to 20% for resistance higher than 1200 dynes.s.cm-5.4 Hence, it is advantageous to perform pulmonary endarterectomy early in the course of the disease to limit the operative risk.
Diminution of the pulmonary vascular resistance by at least 50% is usually observed after successful surgery and translates into significant improvement in the functional status of the patient. In the long term, most patients return to New York Heart Association class I or II dyspnea and resume a normal life. For most patients, the only long-term treatment required is adequate anticoagulation. Survival at 10 and 15 years after the surgery is about 75%.4
In conclusion, pulmonary endarterectomy is the treatment of choice for CTEPH. It can restore normal cardiopulmonary function in the long term and should be performed early in the course of the disease to minimize the operative risk. However, it is a complex procedure requiring considerable expertise and should be performed only in highly specialized centres.
Marc de Perrot John Granton Pulmonary Hypertension Program Division of Thoracic Surgery Toronto General Hospital University of Toronto Toronto, Ont. Elie Fadel Department of Thoracic and Vascular Surgery Hospital Marie-Lannelongue University Paris-Sud Paris, France
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
REFERENCES
- 1.Pengo V, Lensing AW, Prins MH, et al. Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl JMed 2004;350:2257-64. [DOI] [PubMed]
- 2.Riedel M, Stanek V, Widimsky J, et al. Long-term follow-up of patients with pulmonary thromboembolism: late prognosis and evolution of hemodynamic and respiratory data. Chest 1982;81:151-8. [DOI] [PubMed]
- 3.Hoeper MM, Mayer E, Simonneau G, et al. Chronic thromboembolic pulmonary hypertension. Circulation 2006;113(16):2011-20. [DOI] [PubMed]
- 4.Dartevelle P, Fadel E, Mussot S, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2004;4:637-48. [DOI] [PubMed]


