Abstract
Vesicles containing ternary mixtures of diphytanoylphosphatidylcholine, dipalmitoylphosphatidylcholine (DPPC), and cholesterol produce coexisting liquid phases over an unusually large range of temperature and composition. Liquid domains persist well above the DPPC chain melting temperature (41°C), resulting in a closed-loop miscibility gap bounded by two critical points at fixed temperature. Quantitative tie-lines are determined directly from 2H NMR spectra using a novel analysis, and are found to connect a liquid-disordered phase rich in diphytanoyl PC with a liquid-ordered phase rich in DPPC. The direction of the tie-lines implies that binary DPPC/cholesterol mixtures are in one uniform phase above 41°C. All 2H NMR results for tie-lines are verified by independent fluorescence microscopy results.
INTRODUCTION
Over the past five years, our group and others have established that coexisting liquid-ordered (Lo) and liquid-crystalline (Lα) phases form in vesicles composed of a wide variety of ternary lipid mixtures (1–6). These mixtures contain a lipid with high melting temperature (Tm), a lipid with low Tm, and a sterol such as cholesterol. A major question remains: why are three components, rather than only two, necessary to form micron-scale liquid domains in membranes? Furthermore, opinions vary on whether or not large-scale domains in ternary systems arise from small-scale domains in binary systems of lipid and cholesterol (7). Recent work has explored both the experimental (8–11) and theoretical aspects of these questions (12–16). We have found that the ternary system of 1,2-diphytanoyl-sn-glycero-3-phosphocholine (diPhyPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), and cholesterol (Chol) is particularly suited to addressing these fundamental questions experimentally.
The diPhyPC/DPPC/Chol mixture exhibits surprisingly high miscibility transition temperatures, and coexisting liquid phases are observed over a wider range of lipid compositions than we have reported for any other lipid mixture. As a result, a closed-loop miscibility gap is present at high temperatures in the phase diagram, isolated from interference by the lower-temperature gel phase of DPPC at 41°C (17). As an added benefit, both phospholipids are saturated, so our vesicles are less prone to photooxidation during fluorescence imaging. This stability allows for direct observation of critical fluctuations by fluorescence microscopy over the course of several minutes. Moreover, diPhyPC is interesting in itself. It has a very low melting temperature of <–120°C due to its branched acyl chains (18). Polyunsaturated lipids also have very low chain melting temperatures, and our observations may apply to membranes containing these biologically important lipids.
Here we map phase diagrams of diPhyPC/DPPC/Chol by fluorescence microscopy and independently determine the composition of coexisting liquid phases by 2H NMR. We use a novel analysis to calculate tie-lines through the composition point 35/35/30 diPhyPC/DPPC/Chol both above and below the DPPC chain melting temperature. Lastly, we present implications of our ternary tie-lines on the phase diagram of binary DPPC/Chol mixtures.
MATERIALS AND METHODS
Commercial reagents
DiPhyPC, DPPC, Chol, and 1,2-dipalmitoyl-d62-sn-glycero-3-phosphocholine (DPPCd62) were obtained from Avanti Polar Lipids (Alabaster, AL). All lipids were used without further purification and were stored in chloroform at −20°C until use. Texas Red 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (Molecular Probes, Eugene, OR) was used as a dye for contrast between phases in fluorescence experiments.
Fluorescence microscopy
Giant unilamellar vesicles (GUVs) were prepared using electroformation (19) and viewed using fluorescence microscopy, as described extensively elsewhere (3,20). Vesicles were prepared in >18 MΩ/cm water, grown for 1 h at 60 ± 3°C, and stored at the growth temperature for at most 2 h before observation. Miscibility transition temperatures were measured by monitoring the distribution of a fluorescent probe in vesicles. Specifically, GUVs were viewed after ramping a temperature-controlled stage near the transition (∼0.2°C/s) and then waiting ∼60 s for the stage and sample to equilibrate.
Although the miscibility transition temperature is well defined in a single vesicle, we find a distribution of transition temperatures among vesicles in a single GUV preparation. We attribute this to slight variations in lipid composition between vesicles, estimated at <2 mol %. Errors reported in temperatures represent the full range of measured transition temperatures in a single vesicle sample. The midpoint of this range roughly corresponds to the temperature at which half of the vesicles contain coexisting liquid phases. This reproducible midpoint is the transition temperature we report.
Deuterium NMR
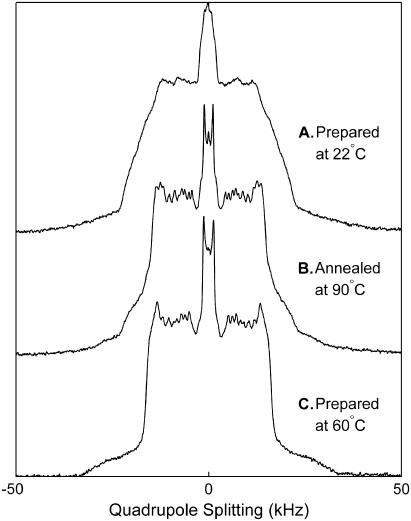
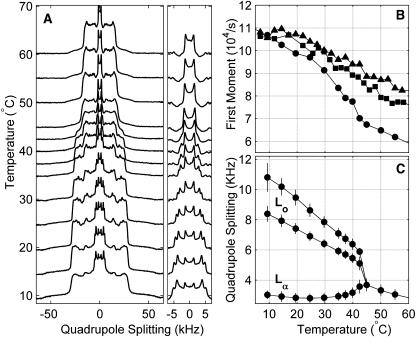
Multilamellar vesicles (MLVs) of 1:1 diPhyPC/DPPCd62 + 30% Chol, 1:2 diPhyPC/DPPCd62 + 40% Chol, and 1:4 diPhyPC/DPPCd62 + 40% Chol were prepared for 2H NMR experiments. Lipid mixtures containing 10 mg deuterated lipid (DPPCd62) were mixed in chloroform and then dried under nitrogen while rotating to form a thin film. Samples were exposed to vacuum (<10 μm Hg) overnight to remove any remaining solvent. Lipids were hydrated to 50 wt % with deuterium-depleted H2O, homogenized at 60°C, spun into ground-glass stoppered sample tubes, and sealed with parafilm. Samples were either used immediately after preparation or stored at −80°C until use. Samples were not hydrated at room temperature (∼20°C) because the resulting MLVs produced low-resolution spectra at temperatures well above the miscibility transition (Fig. 1). Resolution was restored after vesicles were annealed at high temperature (90°C) for ∼1 h. We conclude that samples that are mixed and hydrated at room temperature are inhomogeneous.
FIGURE 1.
Quadrupole spectra of vesicles of 1:1 diPhyPC/DPPCd62 + 30% cholesterol at 60°C under different sample preparations. (A) MLVs hydrated at room temperature (∼20°C) produce low resolution spectra, suggesting that lipids are not uniformly distributed between vesicles. (B) Spectral resolution improves after vesicles from a are annealed for 1 h at 80–90°C. (C) MLVs hydrated at 60°C give well resolved Lα spectra.
2H NMR powder spectra were acquired on a 500 MHz Bruker spectrometer (Billerica, MA) at a resonance frequency of 76.8 MHz using a stationary dual-resonance probe with a 4-mm solenoidal sample coil. Sample temperature was controlled to 0.1°C by a Bruker variable temperature control unit. Absolute sample temperature was calibrated to within 0.5°C. A quadrupolar echo sequence (21) was used with two 1.8-s 90° pulses and an interpulse delay of 50 μs. For all experiments, the carrier frequency of the instrument was placed at the center of the spectrum. Typically 1,024 scans were acquired with a spectral width of 200 kHz and a delay time of 0.2 s. Before Fourier transformation, the resonances, detected in quadrature detection mode, were phase-corrected to minimize intensity in the imaginary channel. The echo maximum in the real channel was determined with a resolution of 1/10 of a dwell-time unit. A time-base-corrected free induction decay was calculated by spline interpolation between data points to begin exactly at the echo maximum. An exponential line broadening of 100 Hz was applied to the free induction decay before performing the Fourier transformation.
The first moments, M1, of 2H NMR spectra were evaluated as 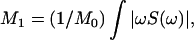 where
where 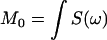 is the total area under the spectrum, and S(ω) is the intensity of the 2H NMR spectrum as a function of frequency ω. The origin of the frequency axis was defined as the center of symmetry of each spectrum. The limits of integration were symmetric around the origin and completely enclose the observed spectra. The magnitude and spectral width of the methyl splittings were determined from the dePaked spectra by fitting with a Lorentzian lineshape.
is the total area under the spectrum, and S(ω) is the intensity of the 2H NMR spectrum as a function of frequency ω. The origin of the frequency axis was defined as the center of symmetry of each spectrum. The limits of integration were symmetric around the origin and completely enclose the observed spectra. The magnitude and spectral width of the methyl splittings were determined from the dePaked spectra by fitting with a Lorentzian lineshape.
Tie-line evaluation
Tie-lines were determined through spectral subtraction as previously described in binary systems (22,23). Here we generalize the method for use in ternary systems. First, we show how to calculate the location of the tie-line endpoints when the tie-line slope is known. We then describe a novel method for evaluating tie-line slope using 2H NMR spectra from three distinct lipid compositions.
Determination of tie-line endpoints with known tie-line slope
Two normalized experimental spectra, SA and SB, have compositions that fall along the same tie-line if they can be represented as a weighted superposition of the same two endpoint spectra:
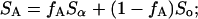 |
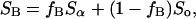 |
where Sα is the Lα-phase endpoint spectrum, So is the Lo-phase endpoint spectra, and fA (fB) represents the fraction of SA (SB) arising from lipids in the Lα phase. Experimentally, the endpoint spectra are initially unknown, and we instead perform a weighted subtraction of the two spectra until only a pure Lα or Lo endpoint spectrum is found:
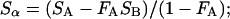 |
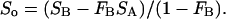 |
The terms fA, fB, FA, and FB are related by simple algebra:
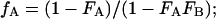 |
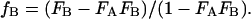 |
In a two-phase region, the fraction of lipids that are in the Lα or Lo phase is given by the lever rule:
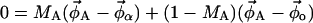 |
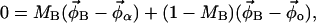 |
where MA and MB are the mole fractions of total lipid represented in the spectra SA and SB that are in the Lα phase. The vector 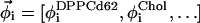 describes the lipid composition that produces spectrum Si, and the vectors
describes the lipid composition that produces spectrum Si, and the vectors 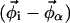 and
and 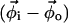 connect the composition of spectrum Si with the Lα and Lo endpoints, respectively. In our experiments, we measure the partitioning of the DPPCd62 lipid to the Lα phase (recorded in fA and fB). The measured values for fA and (1 − fA) must be normalized by the concentration of DPPC lipids in each phase (
connect the composition of spectrum Si with the Lα and Lo endpoints, respectively. In our experiments, we measure the partitioning of the DPPCd62 lipid to the Lα phase (recorded in fA and fB). The measured values for fA and (1 − fA) must be normalized by the concentration of DPPC lipids in each phase (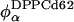 and
and 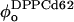 ) to give the partitioning of total lipid. The lever rule becomes:
) to give the partitioning of total lipid. The lever rule becomes:
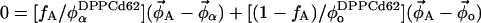 |
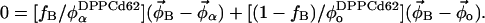 |
This system of equations is solved to determine the compositions of the two endpoint spectra (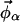 and
and 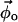 ). In a three-component system, compositions are described using two independent variables (e.g., φDPPCd62 and φChol) and thus four equations must be solved. The tie-line slope is constrained by the choice of compositions
). In a three-component system, compositions are described using two independent variables (e.g., φDPPCd62 and φChol) and thus four equations must be solved. The tie-line slope is constrained by the choice of compositions 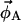 and
and 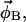 whereas the parameters fA and fB determine the location of the tie-line endpoints.
whereas the parameters fA and fB determine the location of the tie-line endpoints.
Determination of tie-line slope
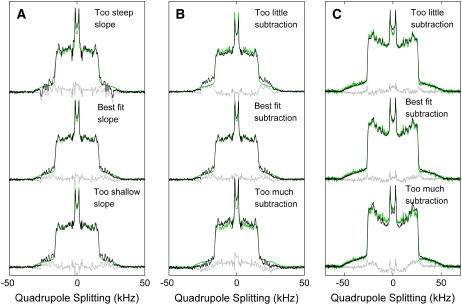
In our analysis, we do not assume a tie-line slope and instead use three separate 2H NMR spectra to determine tie-line slope and endpoints through two minimization steps. Our goal is to determine the tie-line that passes through the point 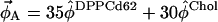 (1:1 diPhyPC/DPPC + 30% Chol). A second composition along the tie-line (φB) is determined by interpolating between any two additional spectra (here, they are 1:2 diPhyPC/DPPC + 40% Chol and 1:4 diPhyPC/DPPC + 40% Chol) so that the resulting endpoint spectrum Sα (determined by spectral subtraction) is best fit to the reference Lα spectrum (Fig. 2, a and b). This minimization step determines the tie-line slope as well as the parameter FA. Spectral subtraction is then conducted by fitting So against the reference Lo spectrum to determine the second subtraction parameter FB (Fig. 2 c).
(1:1 diPhyPC/DPPC + 30% Chol). A second composition along the tie-line (φB) is determined by interpolating between any two additional spectra (here, they are 1:2 diPhyPC/DPPC + 40% Chol and 1:4 diPhyPC/DPPC + 40% Chol) so that the resulting endpoint spectrum Sα (determined by spectral subtraction) is best fit to the reference Lα spectrum (Fig. 2, a and b). This minimization step determines the tie-line slope as well as the parameter FA. Spectral subtraction is then conducted by fitting So against the reference Lo spectrum to determine the second subtraction parameter FB (Fig. 2 c).
FIGURE 2.
Demonstration of spectral subtraction method at 35°C to determine the best fit for tie-lines passing through the point 1:1 diPhyPC/DPPC + 30% cholesterol. Measured reference spectra (green) are subtracted from calculated endpoint spectra (black), yielding residuals in gray. All reference Lα spectra in A and B are identical, and all reference Lo spectra in c are identical. (A) Calculated Lα endpoint spectrum with a tie-line slope that is too steep (top), best fit (middle), and too shallow (bottom). The goodness of fit can be assessed by eye by examining the intensity at wide splittings. (B) Calculated Lα endpoint spectrum when the subtraction is too little (top), best fit (middle), and too large (bottom). (C) Calculated Lo endpoint spectrum when the subtraction is too small (top), best fit (middle), and too large (bottom). In this example, the calculated Lo endpoint is best evaluated by examining narrow splittings and is less sensitive to changes in fitting parameters than the Lα endpoint. For clarity of demonstration, the parameter ranges shown here are larger than those described by the errors obtained directly from the fitting procedure.
Fitting procedure
Spectral subtraction is accomplished by minimizing the mean-squared difference between the calculated Sα (So) spectrum and a known reference Lα (Lo) spectrum using the fminsearch routine in Matlab6 (Fig. 2). The reference Lα spectrum is acquired from membranes of 4:1 1,2-di-oleoyl-sn-glycero-3-phosphocholine (DOPC)/DPPCd62 + 15% cholesterol, and the reference Lo spectrum is from membranes of either DPPCd62 + 40% cholesterol or DPPCd62 + 50% cholesterol. Reference spectra are scaled along the frequency axis to correct for temperature- and composition-dependent changes in order parameters. This correction assumes that order-parameter profiles of hydrocarbon chains are related by a simple scaling factor. Previous work (e.g., Barry and Gawrisch (24)) and our own analysis in Fig. 2 demonstrate that this assumption is accurate for modest variations of temperature and composition.
Errors in the fitting parameters FA, FB, and tie-line slope are determined directly from the covariance matrix and are verified through a visual inspection of the fits. A five-point smoothing filter is applied to the residual before squaring and integrating to reduce errors due to noise. Errors in the fitting parameters are propagated through the tie-line calculation, taking into consideration that fitting parameters do not vary independently. Correlation coefficients between tie-line slope and FA, which are both determined in the first fitting step, range between −0.51 and −0.59. The fitting parameter FB, which is determined in the second fitting step, and tie-line slope are likely also correlated, but it is assumed that this contributes minimally to the reported error bounds. Using this method, tie-line errors are dependent on the reference spectra used. Propagated error limits (see Fig. 7) are deemed to be sufficiently small. Errors could be further reduced by investigating samples with compositions corresponding to the tie-line endpoints.
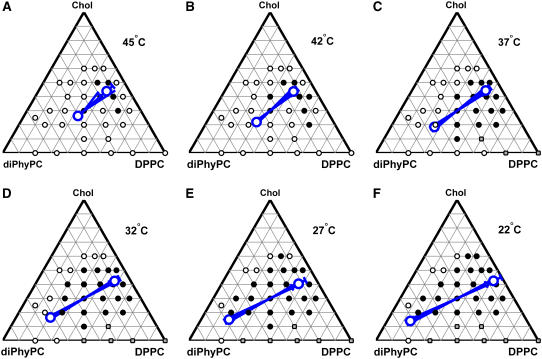
FIGURE 7.
Tie-lines calculated from 2H NMR spectra through the composition 1:1 diPhyPC/DPPC + 30% cholesterol at different temperatures are shown as thick blue lines. Calculated endpoints are represented by large open circles. Errors in the calculated endpoints are represented by the splay of the blue line, and tick marks around each endpoint. In most cases, calculated errors are on the order of the size of the large open circles. Tie-lines are superimposed on fluorescence microscopy data recording the phase behavior of GUVs of 34 different lipid compositions. Black circles, coexisting liquid phases; open circles, one uniform liquid phase; gray squares indicate coexistence of solid and liquid(s). 2H NMR tie-line temperatures are shifted +2.5°C to correct for lower transition temperatures in perdeuterated lipid samples (20).
RESULTS AND DISCUSSION
Phase boundary by fluorescence microscopy
GUVs prepared from ternary mixtures of diPhyPC, DPPC, and cholesterol produce coexisting liquid phases as temperature is lowered through the miscibility transition temperature (Fig. 3). A distinct experimental advantage of the diPhyPC system is that vesicles are generally larger and more stable than for similar domain-forming mixtures. In time, liquid phases ripen completely through a process of domain collision and coalescence. Occasionally, domains remain stable in a dispersed pattern.
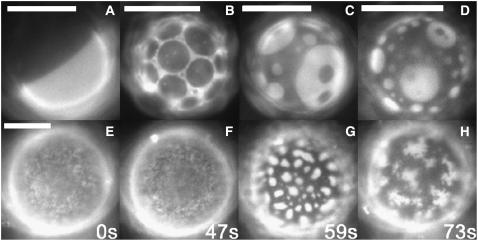
FIGURE 3.
GUVs containing diPhyPC. (A) Typically, liquid domains in giant vesicles of diPhyPC/DPPC/Chol ripen until only one bright and one dark domain remain. (B) Occasionally, domains in vesicles of diPhyPC/DPPC/Chol remain dispersed for long times. Coexisting liquid phases are also observed in GUVs of (C) diPhyPC/POPC/Chol or (D) diPhyPC/SOPC/Chol at low temperatures. (E and F) Micron-scale fluctuations persist in membranes near a critical point for extended time periods. (G) Larger, distinct domains form in the same vesicle when temperature is lowered slightly (<1°C). (H) When temperature is raised, domains break up and critical fluctuations resume. The same vesicle is shown in panels E–H and was imaged at the times shown. Membrane compositions are (A) 1:2 diPhyPC/DPPC + 40% Chol at 16°C; (B) 1:4 diPhyPC/DPPC + 20% Chol at 23°C; (C) 1:4 diPhyPC/SOPC + 40% Chol at ∼5°C; (D) 1:4 diPhyPC/POPC + 40% Chol at ∼5°C; and (E–H) 1:1 diPhyPC/DPPC + 50% Chol at ∼33°C. Scale bars, 20 μm.
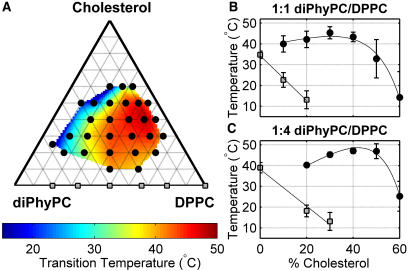
The diPhyPC/DPPC/Chol system is remarkable in the extent of its miscibility phase boundary, both in temperature and in composition (Fig. 4). Liquid domains are present in vesicles at temperatures as high as 50°C, well above the DPPC chain melting temperature of Tm = 41°C (17). As a comparison, the related system of DOPC/DPPC/Chol produces liquid domains only below 41°C, although the qualitative shape of the phase boundary is similar in both systems (3). The diPhyPC system also surpasses the DOPC system in the range of compositions that produce domains.
FIGURE 4.
(A) Phase behavior determined by fluorescence microscopy for vesicles composed of mixtures of diPhyPC, DPPC, and cholesterol. Black circles denote compositions that form coexisting liquid phases as temperature is lowered. Miscibility transition temperatures follow the color scale, where high temperatures are generally at high DPPC/Chol fractions. Gray squares denote gel-liquid coexistence, which is observed in membranes without cholesterol. (B and C) Cuts through the phase boundary at constant diPhyPC:DPPC ratio. Black circles denote the miscibility transition temperature and gray squares indicate the temperature at which a gel phase is first observed as the temperature is lowered.
By comparing systems containing diPhyPC to ones containing other lipids with low melting temperatures, it appears that lower chain melting temperatures produce higher miscibility transition temperatures. For example, liquid domains are observed at higher temperatures in vesicles of diPhyPC/DPPC/Chol (diPhyPC Tm < −120°C) than in DOPC/DPPC/Chol (DOPC Tm = −20°C) (17), just as miscibility transition temperatures are higher in DOPC/D-erythro-N-palmitoyl-sphingomyelin (PSM)/Chol than in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)/PSM/Chol (POPC Tm < −2°C) (4). In other words, we expect similar results for membranes in which the high Tm lipid component is a sphingomyelin rather than DPPC.
Observations of high miscibility transition temperatures are biologically significant because an important class of lipids, polyunsaturated lipids, also have very low chain melting temperatures (25). It has been proposed that polyunsaturated lipids can enhance interactions between saturated chains and cholesterol (26,27). Given our results for membranes containing diPhyPC, we predict that membranes with polyunsaturated lipids should sustain coexisting liquid domains at high temperatures. Indeed, there is evidence for domains rich in DPPC and cholesterol at high temperatures (50°C) in rhodopsin-containing membranes of di(22:6)PC/DPPC/Chol (28). Additionally, membranes with polyunsaturated lipids may form domains even in the absence of more traditional “raft-associated” lipids. For example, we observe liquid immiscibility in membranes in which the monounsaturated lipids POPC or 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (SOPC) function as the high Tm component (Fig. 3, C and D), a surprising result.
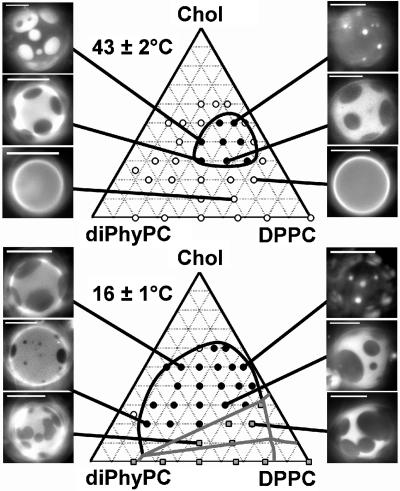
Fig. 5 shows phase diagrams of diPhyPC/DPPC/Chol vesicles at two different temperatures, 43°C and 16°C. By examining the surface fraction of bright and dark phases as a function of lipid composition at both temperatures, we conclude that the bright phase is strongly enriched in diPhyPC, whereas the dark phase is strongly enriched in DPPC and moderately enriched in cholesterol (3). Above the chain melting temperature of 41°C, we observe a closed-loop miscibility gap, such that the miscibility phase boundary does not intersect any of the three limiting binary mixtures, or any region containing a solid (gel) phase. A striking consequence of this type of miscibility gap is that the diPhyPC/DPPC/Chol system has two miscibility critical points at any fixed temperature between the DPPC chain melting temperature and the upper consolute critical point. By examining surface fractions of bright and dark phases in Fig. 5, top, we infer the presence of two critical points at high and low cholesterol compositions.
FIGURE 5.
Phase diagrams determined by fluorescence microscopy and corresponding fluorescence micrographs for GUVs of ternary mixtures of diPhyPC, DPPC, and cholesterol at constant temperature. Open circles denote one liquid phase. (Top) Above the DPPC chain melting temperature of 41°C, the miscibility region denoted by black circles lies within a closed loop and no solid phase is present. (Bottom) At a lower temperature, gray squares indicate the presence of solid phase in vesicles. The liquid-liquid coexistence region connects to the gel-liquid coexistence region through a three-phase triangle at low cholesterol. Vesicle compositions are as indicated in the Gibbs triangle. Scale bars, 20 microns.
In contrast, below the DPPC chain melting temperature, the phase diagram appears similar to those we have observed previously (4) in that the miscibility phase boundary does intersect a gel phase in a three-phase region. We have direct 2H NMR evidence for coexistence of two liquids plus one gel phase in membranes of DOPC/DPPCd62/Chol (S. L. Veatch, S. L. Keller, and K. Gawrisch, unpublished data). We can also infer the presence of a three-phase region in membranes with <30% cholesterol by fluorescence microscopy because the lipid mobility in dark regions and the ripening of dark domains is significantly slower at low temperature (Fig. 5, bottom), as we have reported for vesicles of DOPC/PSM/Chol and POPC/PSM/Chol (4).
Liquid immiscibility by 2H NMR
2H NMR is a particularly powerful tool for observing coexisting liquid phases, because spectra show a superposition of liquid endpoint spectra (Fig. 6). A significant step forward in our current work is that we have collected detailed 2H NMR spectra for three separate compositions over a wide range of temperatures of the ternary diPhyPC/DPPC/Chol vesicle system. Our choice of compositions allows us to quantitatively determine the lipid compositions of the two liquid phases along tie-lines (Fig. 7) without relying on phase boundaries determined by fluorescence as in previous work (20). As a result, our current tie-lines benefit from significantly smaller error bounds.
FIGURE 6.
2H NMR results for multilamellar vesicles containing ternary mixtures of diPhyPC, DPPCd62, and cholesterol. (A) DPPCd62 2H NMR spectra at various temperatures for 1:1 DiPhyPC/DPPCd62 + 30% cholesterol. (B) DPPCd62 first moments for 1:1 diPhyPC/DPPCd62 + 30% cholesterol (circles), 1:2 diPhyPC/DPPCd62 + 40% cholesterol (squares), and 1:4 diPhyPC/DPPCd62 + 40% cholesterol (triangles). (C) DPPCd62 methyl quadrupole splittings and peak widths (error bars) as a function of temperature for 1:1 DOPC/DPPCd62 + 30% cholesterol. Below the miscibility transition at 42.5°C, three separate methyl splittings are found, indicating coexistence of Lo and Lα phases (20). The Lo phase produces two wide (ordered) splittings, whereas the Lα phase produces a single narrow (disordered) splitting.
We find that 2H NMR tie-lines connect an Lo phase rich in both DPPCd62 and cholesterol with an Lα phase rich in diPhyPC. A series of tie-lines passing through the point 35:35:30 diPhyPC/DPPC/Chol are shown in Fig. 7. At lower temperatures, the tie-line extends to higher diPhyPC fraction (the Lα endpoint lies closer to the diPhyPC vertex in the Gibbs triangle), and the cholesterol compositions of the two liquid phases become more similar (the tie-line has a shallower slope).
Our results above are useful for critically evaluating the validity of fluorescence microscopy phase boundaries. First, we notice that the tie-lines in Fig. 7 are similar to the tie-lines we previously estimated for the ternary system of DOPC/DPPC/Chol by using a combination of 2H NMR and fluorescence microscopy results (20). Additional evidence for good correlation between 2H NMR and fluorescence is clear by directly evaluating phase boundaries both in temperature and in composition. We find good agreement between miscibility transition temperatures measured by 2H NMR and by fluorescence microscopy for the mixtures probed in Table 1. Moreover, tie-line endpoints derived from 2H NMR are close to fluorescence-microscopy phase boundaries, as can be seen by eye in Fig. 7.
TABLE 1.
Miscibility transition temperatures for mixtures of diPhyPC/DPPCd62/Chol
| Transition temperature
|
||
|---|---|---|
| Lipid mixture | Microscopy | 2H NMR |
| 1:1 DiPhyPC/DPPCd62 + 30% Chol | 42 ± 2°C | 43 ± 2°C |
| 1:2 DiPhyPC/DPPCd62 + 40% Chol | 43 ± 2°C | 42 ± 2°C |
| 1:4 DiPhyPC/DPPCd62 + 40% Chol | 43 ± 2°C | 43 ± 2°C |
Critical fluctuations
Using fluorescence microscopy, we directly observe composition fluctuations in membranes of diPhyPC/DPPC/Chol with compositions near a miscibility critical point (Fig. 3, E–H). In vesicles of diPhyPC/DPPC/Chol, we find that critical fluctuations can persist for long times (>10 min) without ripening into larger liquid domains (Fig. 3, E–F). In the same vesicle, fluctuations ripen into distinct domains when temperature is slightly lowered, although fluctuations of the domain boundaries are still visible (Fig. 3 G). When temperature is raised again, domains break apart and critical fluctuations resume, indicating that our observations are reversible (Fig. 3 H).
A distinct experimental advantage of the diPhyPC/DPPC/Chol ternary system is that it is possible to view critical fluctuations under the microscope for extended time periods. Previously, we were unable to hold vesicles containing unsaturated phospholipids near a miscibility phase boundary using fluorescence microscopy because photooxidation of unsaturated lipids shifts the phase boundary to higher temperature over time (3,7).
In this study, we did not probe lipid compositions near a critical point by 2H NMR. Critical fluctuations are observed by 2H NMR studies as broadening of spectral peaks (unpublished data for the system of DOPC/DPPCd62/Chol). We do not observe significant broadening of spectra near the transition temperature for the three mixtures probed in this study.
Implications for the DPPC/Chol binary phase diagram
The direction of our calculated tie-lines in the ternary diPhyPC/DPPC/Chol mixture has direct implications for the phase behavior of binary mixtures of DPPC and cholesterol. Numerous reports have proposed coexistence of Lo and Lα phases in DPPC (or DMPC) and cholesterol above the DPPC chain melting temperature (22,29,30). We (7) and others (12,13,31) have recently questioned this conclusion, because most evidence for this particular phase separation is indirect, and lipid organization, if present, occurs on a small lengthscale. In past work, we documented that large-scale phase separation is not directly observed in binary DPPC/Chol mixtures by fluorescence microscopy (3), yet it was unclear if limited spatial resolution or equal probe partitioning between phases obscured our ability to observe two liquid phases. Our results in the ternary diPhyPC/DPPC/Chol mixture offer much stronger evidence to suggest that binary membranes of DPPC and cholesterol are in one uniform phase above the DPPC chain melting temperature.
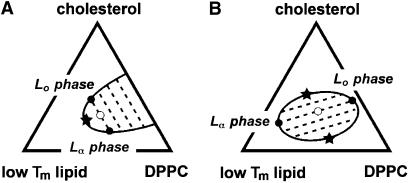
Consider the two potential phase diagrams in Fig. 8 for vesicles of diPhyPC/DPPC/Chol at temperatures above the DPPC chain melting temperature. If phase separation is present in the binary DPPC/Chol mixture (Fig. 8 A), we expect to find tie-lines roughly parallel to the binary DPPC/Chol edge, at least for mixtures with small amounts of diPhyPC. This is because the tie-line in the binary DPPC/Chol mixture is constrained to run along the binary edge, and thermodynamic rules stipulate that tie-line slopes must vary continuously. On the other hand, if phase separation is not present in the binary mixture, then there is no initial constraint on tie-line slope, and tie-lines can assume any orientation with respect to limiting binary mixtures, including an orientation perpendicular to the DPPC/Chol axis (as in Fig. 8 B).
FIGURE 8.
(A) If two liquid phases exist in vesicles containing binary mixtures of DPPC and cholesterol above the DPPC chain-melting temperature, then tie-lines through compositions close to the DPPC/cholesterol binary axis lie roughly parallel to that axis. For example, the composition at the open circle demixes along the dashed tie-line to form two liquid phases with compositions at the solid circles. Only one critical point (star) is expected. (B) In contrast, two critical points (stars) are found for a closed-loop miscibility gap, and tie-lines are allowed to run roughly perpendicular to the DPPC/cholesterol axis (as observed in the diPhyPC/DPPC/Chol system).
In our experiments, we observe that tie-lines indeed run roughly perpendicular to the binary DPPC/Chol edge, even for membranes with small amounts of diPhyPC. It is unlikely that this tie-line orientation could be achieved if phase separation were present in the binary mixture. Our results suggest that membranes of DPPC and cholesterol do not form coexisting Lo and Lα phases above the DPPC chain melting temperature for binary mixtures in the vicinity of our 2H NMR tie-lines. Instead, we conclude that these binary membranes form a single uniform liquid phase at these temperatures.
CONCLUSIONS
We observe coexisting liquid phases in ternary mixtures of diPhyPC, DPPC, and cholesterol by both fluorescence microscopy and deuterium NMR. Using both experimental methods, we find that this system is unusual because liquid domains persist well above the DPPC chain melting temperature, and coexisting phases are observed over a very wide range of lipid compositions. We directly observe critical fluctuations by fluorescence microscopy for long time periods. Ternary mixtures containing diPhyPC are striking in that monounsaturated lipids can function as the high Tm component as in diPhyPC/POPC/Chol and diPhyPC/SOPC/Chol.
We use a novel spectral subtraction method to calculate quantitative tie-lines directly from 2H NMR spectra, independent of fluorescence microscopy results. We find that one liquid phase is rich in diPhyPC and the other liquid phase is rich in DPPC. The direction of tie-lines and the location of the phase boundary are in excellent agreement with independent fluorescence microscopy results. We report that observed tie-line orientations imply an absence of liquid immiscibility in binary DPPC/Chol mixtures at temperatures above the DPPC chain melting temperature of 41°C.
TABLE 2.
Lo and Lα phase compositions for tie-lines that pass through the point 1:1 diPhyPC/DPPC + 30% cholesterol
| Lo endpoint
|
Lα endpoint
|
Tie-line slope
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| diPhyPC | DPPC | Chol | σ diPhyPC | diPhyPC | DPPC | Chol | σ diPhyPC | Slope* | σ Slope | |
| 22°C | 6 | 51 | 43 | 12 | 72 | 14 | 14 | 6 | 0.77 | 0.07 |
| 27°C | 12 | 47 | 41 | 11 | 68 | 17 | 15 | 6 | 0.84 | 0.10 |
| 32°C | 10 | 48 | 42 | 7 | 62 | 21 | 17 | 4 | 0.98 | 0.10 |
| 37°C | 10 | 45 | 45 | 8 | 55 | 27 | 18 | 5 | 1.39 | 0.26 |
| 42°C | 14 | 42 | 44 | 7 | 47 | 31 | 22 | 4 | 1.84 | 0.53 |
| 45°C | 14 | 42 | 44 | 12 | 41 | 33 | 26 | 4 | 2.00 | 1.51 |
Data in table correspond to representation in Fig. 7. Tie-lines were determined by fitting 2H NMR spectra as in Fig. 2. Tie-line errors are presented as standard deviations in both diPhyPC concentration (roughly equivalent to changes in tie-line length) and in tie-line slope, and are given by σ diPhyPC and σ slope, respectively. 2H NMR tie-line temperatures are shifted +2.5°C to correct for lower transition temperatures in perdeuterated lipid samples (20).
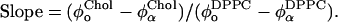
Acknowledgments
We thank Walter Teague for assistance with 2H NMR experiments and Holger Scheidt for the use of his DPPCd62/Chol 2H NMR data.
S.L.V. was supported in part through a postdoctoral fellowship from the Cancer Research Institute. K.G. was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health. S.L.K. acknowledges support from National Science Foundation Career Award Molecular and Cellular Biosciences No. 0133484 and from the Research Corporation (Research Innovation Award and Cottrell Scholar Award).
Sarah L. Veatch's present address is Dept. of Microbiology and Immunology, University of British Columbia, Vancouver, BC.
References
- 1.Dietrich, C., L. A. Bagatolli, Z. N. Volovyk, N. L. Thompson, M. Levi, K. Jacobson, and E. Gratton. 2001. Lipid rafts reconstituted in model membranes. Biophys. J. 80:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veatch, S. L., and S. L. Keller. 2002. Lateral organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 89:268101. [DOI] [PubMed] [Google Scholar]
- 3.Veatch, S. L., and S. L. Keller. 2003. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veatch, S. L., and S. L. Keller. 2005. a. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys. Rev. Lett. 94:148101. [DOI] [PubMed] [Google Scholar]
- 5.Baumgart, T., S. T. Hess, and W. W. Webb. 2003. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 425:821–824. [DOI] [PubMed] [Google Scholar]
- 6.Kahya, N., D. Scherfeld, K. Bacia, B. Poolman, and P. Schwille. 2003. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem. 278:28109–28115. [DOI] [PubMed] [Google Scholar]
- 7.Veatch, S. L., and S. L. Keller. 2005. b. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 1746:172–185. [DOI] [PubMed] [Google Scholar]
- 8.de Almeida, R. F. M., A. Fedorov, and M. Prieto. 2003. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 85:2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silvius, J. R. 2003. Fluorescence energy transfer reveals microdomain formation at physiological temperatures in lipid mixtures modeling the outer leaflet of the plasma membrane. Biophys. J. 85:1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korlach, J., T. Baumgart, W. W. Webb, and G. W. Feigenson. 2005. Detection of motional heterogeneities in lipid bilayer membranes by dual probe fluorescence correlation spectroscopy. Biochim. Biophys. Acta. 1668:158–163. [DOI] [PubMed] [Google Scholar]
- 11.Kahya, N., D. Scherfeld, and P. Schwille. 2005. Differential lipid packing abilities and dynamics in giant unilamellar vesicles composed of short-chain saturated glycerol-phospholipids, sphingomyelin and cholesterol. Chem. Phys. Lipids. 135:169–180. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishnan, A., and H. M. McConnell. 2005. Condensed complexes in vesicles containing cholesterol and phospholipids. Proc. Natl. Acad. Sci. USA. 102:12662–12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McConnell, H. 2005. Complexes in ternary cholesterol-phospholipid mixtures. Biophys. J. 88:L23–L25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komura, S., H. Shirotori, P. D. Olmsted, and D. Andelman. 2004. Lateral phase separation in mixtures of lipids and cholesterol systems. Europhys. Lett. 67:321–327. [Google Scholar]
- 15.Elliott, R., K. Katsov, M. Schick, and I. Szleifer. 2005. Phase separation of saturated and mono-unsaturated lipids as determined from a microscopic model. J. Chem. Phys. 122:44904. [DOI] [PubMed] [Google Scholar]
- 16.Ayton, G. S., J. L. McWhirter, P. McMurtry, and G. A. Voth. 2005. Coupling field theory with continuum mechanics: a simulation of domain formation in giant unilamellar vesicles. Biophys. J. 88:3855–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvius, J. R. 1982. Thermotropic phase transitions of pure lipids in model membranes and their modifications by membrane proteins. In Lipid-Protein Interactions. John Wiley and Sons, New York.
- 18.Lindsey, H., N. O. Petersen, and S. I. Chan. 1979. Physicochemical characterization of 1,2-diphytanoyl-sn-glycero-3-phosphocholine in model membrane systems. Biochim. Biophys. Acta. 555:147–167. [DOI] [PubMed] [Google Scholar]
- 19.Angelova, M. I., S. Soleau, P. Meleard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Prog. Colloid Polym. Sci. 89:127–131. [Google Scholar]
- 20.Veatch, S. L., I. V. Polozov, K. Gawrisch, and S. L. Keller. 2004. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 86:2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis, J. H., K. R. Jeffrey, M. Bloom, M. I. Valic, and T. P. Higgs. 1976. Quadrupolar echo deuteron magnetic resonance spectroscopy in ordered hydrocarbon chains. Chem. Phys. Lett. 42:390–394. [Google Scholar]
- 22.Vist, M. R., and J. H. Davis. 1990. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 29:451–464. [DOI] [PubMed] [Google Scholar]
- 23.Hsueh, Y. W., K. Gilbert, C. Trandum, M. Zuckermann, and J. Thewalt. 2005. The effect of ergosterol on dipalmitoylphosphatidylcholine bilayers: a deuterium NMR and calorimetric study. Biophys. J. 88:1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barry, J. A., and K. Gawrisch. 1995. Effects of ethanol on lipid bilayers containing cholesterol, gangliosides, and sphingomyelin. Biochemistry. 34:8852–8860. [DOI] [PubMed] [Google Scholar]
- 25.Barry, J. A., T. P. Trouard, A. Salmon, and M. F. Brown. 1991. Low-temperature 2H NMR spectroscopy of phospholipid bilayers containing docosahexaenoyl (22:6 omega 3) chains. Biochemistry. 30:8386–8394. [DOI] [PubMed] [Google Scholar]
- 26.Huster, D., K. Arnold, and K. Gawrisch. 1998. Influence of docosahexaenoic acid and cholesterol on lateral lipid organization in phospholipid mixtures. Biochemistry. 37:17299–17308. [DOI] [PubMed] [Google Scholar]
- 27.Wassall, S. R., M. R. Brzustowicz, S. R. Shaikh, V. Cherezov, M. Caffrey, and W. Stillwell. 2004. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chem. Phys. Lipids. 132:79–88. [DOI] [PubMed] [Google Scholar]
- 28.Polozova, A., and B. J. Litman. 2000. Cholesterol dependent recruitment of di22:6-PC by a G protein-coupled receptor into lateral domains. Biophys. J. 79:2632–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loura, L. M. S., A. Fedorov, and M. Prieto. 2001. Fluid-fluid membrane microheterogeneity: a fluorescence resonance energy transfer study. Biophys. J. 80:776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida, P. F., W. L. Vaz, and T. E. Thompson. 1992. Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: a free volume analysis. Biochemistry. 31:6739–6747. [DOI] [PubMed] [Google Scholar]
- 31.Huang, T. H., C. W. Lee, S. K. Das Gupta, A. Blume, and R. G. Griffin. 1993. A 13C and 2H nuclear magnetic resonance study of phosphatidylcholine/cholesterol interactions: characterization of liquid-gel phases. Biochemistry. 32:13277–13287. [DOI] [PubMed] [Google Scholar]