Abstract
Burns are one of the most common and devastating forms of trauma. Patients with serious thermal injury require immediate specialized care in order to minimize morbidity and mortality. Significant thermal injuries induce a state of immunosuppression that predisposes burn patients to infectious complications. A current summary of the classifications of burn wound infections, including their diagnosis, treatment, and prevention, is given. Early excision of the eschar has substantially decreased the incidence of invasive burn wound infection and secondary sepsis, but most deaths in severely burn-injured patients are still due to burn wound sepsis or complications due to inhalation injury. Burn patients are also at risk for developing sepsis secondary to pneumonia, catheter-related infections, and suppurative thrombophlebitis. The introduction of silver-impregnated devices (e.g., central lines and Foley urinary catheters) may reduce the incidence of nosocomial infections due to prolonged placement of these devices. Improved outcomes for severely burned patients have been attributed to medical advances in fluid resuscitation, nutritional support, pulmonary and burn wound care, and infection control practices.
INTRODUCTION
Burns are one of the most common and devastating forms of trauma. Patients with serious thermal injury require immediate specialized care in order to minimize morbidity and mortality. Data from the National Center for Injury Prevention and Control in the United States show that approximately 2 million fires are reported each year which result in 1.2 million people with burn injuries (7, 318, 319, 369). Moderate to severe burn injuries requiring hospitalization account for approximately 100,000 of these cases, and about 5,000 patients die each year from burn-related complications (7, 8, 215, 318, 319, 369). In Canada, the estimated numbers of burn victims and deaths in serious cases are proportionally smaller on a per capita basis (265, 349, 403).
The survival rates for burn patients have improved substantially in the past few decades due to advances in modern medical care in specialized burn centers. Improved outcomes for severely burned patients have been attributed to medical advances in fluid resuscitation, nutritional support, pulmonary care, burn wound care, and infection control practices. As a result, burn-related deaths, depending on the extent of injury, have been halved within the past 40 years (7, 252, 320, 369, 373, 439). In patients with severe burns over more than 40% of the total body surface area (TBSA), 75% of all deaths are currently related to sepsis from burn wound infection or other infection complications and/or inhalation injury (15, 20, 24, 32, 140).
This review focuses on modern aspects of the epidemiology, diagnosis, management, and prevention of burn wound infections and sepsis. Recent factors contributing to the development of burn wound infection are also discussed, including the nature and extent of the burn injury itself and the secondary immunosuppression resulting from thermal injury. The prevention of burn wound infection is reviewed in the context of newer therapeutic strategies employed by specialized burn care facilities.
HUMAN SKIN—A MAJOR HOST DEFENSE
An intact human skin surface is vital to the preservation of body fluid homeostasis, thermoregulation, and the host's protection against infection. The skin also has immunological, neurosensory, and metabolic functions such as vitamin D metabolism. Thermal injury creates a breach in the surface of the skin. A basic knowledge of skin anatomy and physiology is required to understand emergency burn assessment and approaches to burn care (96, 114, 469).
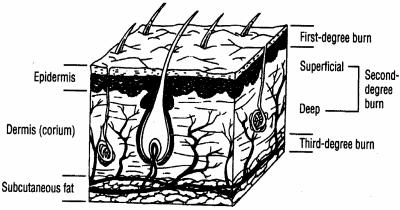
Figure 1 provides a schematic representation of the skin layers in relation to the depth of burn injury (96, 113, 369). The skin is derived from ectoderm and mesoderm and has two anatomic layers: the epidermis or outermost nonvascular layer consists of several layers of epidermal cells that vary in thickness over various body surfaces, and the dermis or corium is largely made of collagen and contains the microcirculation, a complex vascular plexus of arterioles, venules, and capillaries. The two skin layers are bound together by a complex mechanism that is essential for normal function. Epidermal appendages are distributed throughout the dermis layer, including the sweat glands, sebaceous glands, and hair follicles. The dermal layer is capable of producing new epithelial cells to replace those lost from the epidermis by burning or other injury to the skin because the shafts of these appendages are lined with epithelial cells. Nerve endings occur throughout both skin layers, and the connective tissue of the dermis also provides a firm structural base for the skin. Burn injury is a very painful form of trauma because of the multitude of pain receptors and nerves that traverse the skin layers. Beneath the skin lie the subcutaneous tissues, muscle, and bone.
FIG. 1.
Basic skin anatomy, showing the depth of injury for first-, second-, and third-degree burns. (Adapted from reference 369 with permission of the publisher.)
The skin is one of the largest organs in the human body, in terms of both its overall size and weight. In an adult male the skin weighs between 6 and 10 kg (∼13 and 22 lbs). The average adult skin surface area is 1.5 to 2.0 square meters, in contrast to that of a newborn, whose skin surface area is only 0.2 to 0.3 square meter. The two skin layers together are up to several millimeters thick, but both epidermal and dermal thickness varies depending on the body site. The epidermis is the thinnest (0.05 mm) over the eyelid but thicker (up to 1 mm) over the soles of the feet (114, 369). The dermis is thickest on the back. Males generally have thicker skin than females. General skin thickness peaks in midlife and gradually thins as part of the aging process (113, 114, 216, 217, 369). Infants, young children, and elderly adults have a much thinner dermal layer to their skin, resulting in an increased propensity for deeper burn injury. Epidermal cells are constantly being shed and replaced every month through a process that continually pushes new cells to the surface. This natural process is designed to continually replenish and heal breaches in the outermost protective skin barrier, be it from the microtraumas sustained as part of daily living or from overt injury. The epidermis therefore heals itself after superficial injury.
Several important physiological functions of the skin are altered by thermal injury. Survival of the severely burned patient requires immediate access to a specialized burn care unit. Modern emergency burn resuscitation and ongoing treatment are designed to alleviate the systemic changes that result from acute disruption of a large part of the skin barrier. Meticulous attention is given to the replacement and prevention of fluid loss, the maintenance of body temperature homeostasis within a constant normal range, the easing of severe pain, and the prevention of infection.
BURN INJURY IN CIVILIANS
Magnitude and Risk Factors of Civilian Burn Injury
In North America, burn injury is one of the main causes of injury deaths, particularly in children under the age of 14 years (7, 68-70, 318, 320, 349). Although the age-adjusted death rate from burn injury in the United States has decreased substantially since 1985, the United States still has one of the highest per capita burn death rates of any industrialized country (318, 320). Between 1993 and 1995, there were 18.7 burn-related deaths per million population in the United States, compared with 15 for Canada and 5.5 for Switzerland (318, 344, 349). The highest fatality rates occur among children 4 years of age or younger and adults over the age of 55 years (252, 318, 320, 457). Burn-related deaths in these two age groups account for more than two-thirds of all fire deaths. Males are twice as likely to die of burn-related injury as females in all age groups.
Adult burn injury may also result from an industrial or work-related accident or occur as a result of suicide attempts, assault, and unintentional injury due to alcohol and/or drug use (32, 211, 265, 332, 368). A significant proportion of adult burn patients also suffer from a high degree of mental illness (344). Since legal action is taken in many of these cases, it is important to document the etiology and extent of the burn injury.
Burn injuries incur a significant cost to the health care system in North America and worldwide. In the United States and Canada there are currently 167 centers specializing in burn care, with over 2,000 beds (369). Although the overall hospitalization rates from less-serious burn injuries have declined by 50% since 1971, the proportion of patients admitted to burn centers has increased (7, 369). Recent estimates in the United States show that 45,000 patients are admitted to acute-care hospitals annually with burn injuries, and in approximately 50% of these cases the extent of thermal injury is severe enough to warrant admission to a specialized burn center (7, 68-70, 369). Burn care centers in North America currently admit an average of more than 200 patients per year, whereas other hospital units admit an average of fewer than five burn patients per year (7, 369).
Initial hospitalization costs and physicians' fees for specialized care of a patient with a major burn injury are currently estimated to be US$200,000 (292, 318, 369). Overall, costs escalate for major burn cases because of repeated admissions for reconstruction and rehabilitation therapy. In the United States, current annual estimates show that more than US$18 billion is spent on specialized care of patients with major burn injuries (292, 318, 369).
Pathogenesis and Etiology of Burns
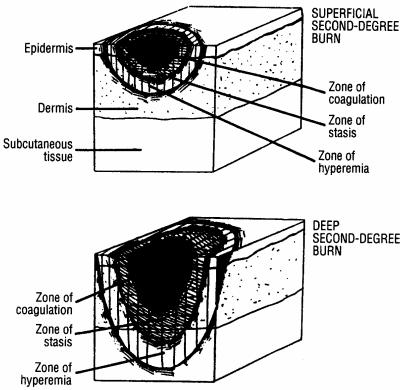
The breached skin barrier is the hallmark of thermal injury. The body tries to maintain homeostasis by initiating a process of contraction, retraction, and coagulation of blood vessels immediately after a burn injury. Three distinct zones have been defined within the burn wound: (i) the zone of coagulation, which comprises the dead tissues that form the burn eschar that is located at the center of the wound nearest to the heat source; (ii) the zone of stasis, which comprises tissues adjacent to the area of burn necrosis that is still viable but at risk for ongoing ischemic damage due to decreased perfusion; and (iii) the zone of hyperemia, which comprises normal skin with minimal cellular injury that has predominant vasodilation and increased blood flow as a response to injury (Fig. 2) (163, 195, 369). Serious thermal injury causes total loss of the skin surface over large areas of the body. Because of the importance of the skin as a barrier to microbial host invasion, it is not surprising that the risk of subsequent burn wound infection and systemic infection correlates with the size of the burn injury (377, 387).
FIG. 2.
Zones of injury for superficial and deep second-degree burns. (Adapted from reference 369 with permission of the publisher.)
Thermal injury.
Direct contact with flame, a hot surface or hot liquid (scald), or a source of heat conduction, convection, or radiation causes a degree of cellular damage to the skin that varies with the temperature and duration of exposure (21, 179, 222, 232, 299, 352, 355, 369). As the temperature rises, increasing molecular collisions occur, resulting in altered molecular conformation and the disruption of intermolecular bonds. This process leads to cell membrane dysfunction as ion channels are disrupted, resulting in sodium and water intake. As the temperature rises further, protein denaturation occurs, oxygen radicals are liberated, and eventually cells die with the formation of the burn eschar (299).
Chemical injury.
Chemical interaction may also damage protein structures. A classification system that was described in 1974 and remains in use groups chemicals according to their mode of action (Table 1) (9, 56, 57, 74, 223, 305).
TABLE 1.
Classification of chemicals that cause burn injurya
| Class | Example(s) | Mode of action |
|---|---|---|
| Reducing agents | Hydrochloric acid | Bind free electrons in tissue proteins |
| Oxidizing agents | Sodium hypochlorite | Oxidized on contacting proteins producing toxic by-products |
| Corrosive agents | Phenol | Denatures tissue proteins |
| Protoplasmic poisons | Hydrofluoric acid | Bind calcium or other ions essential to cell function |
| Acetic acid | ||
| Vesicants | Dimethyl sulforide | Ischemia with anoxic necrosis |
| Cantharides | ||
| Mustard gas | ||
| Desiccants | Sulfuric acid | Dehydration |
| Muriatic acid | Exothermic reaction |
Extent and Location of Burn Injury
A burn patient is a trauma patient. The initial assessment and resuscitation are therefore focused on the patient's airway, breathing, and circulation and an examination for other major injuries besides the burn itself. Assessment of the burn injury should include a determination of the etiology of the burn as well as the extent of the burn injury. Although the assessment of the extent and depth of all types of burn injury is clinically difficult, chemical injuries are particularly challenging. The severity of injury is not only related to the areas and sites of skin injury, but also depends on the chemical agent and the duration of exposure. In these cases, morbidity may be high even with small areas of injury, such as alkali injury to the eye, with inhalation of vapors such as anhydrous ammonia due to the systemic effects from absorption (9, 56).
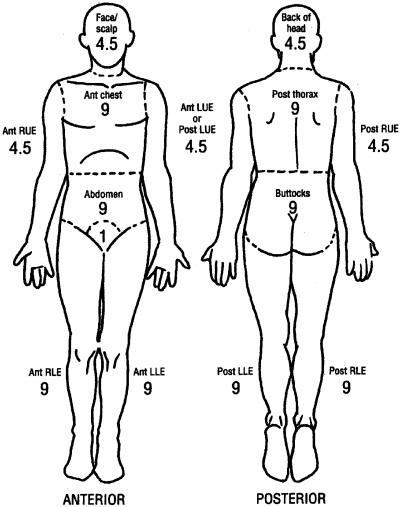
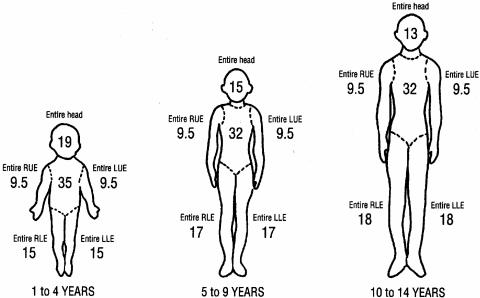
Subjective clinical methods have historically been used to determine the depth of burn injury. Body diagrams provide an estimate of the percentage of total body surface area (%TBSA) of the burn exposure and injury and document a patient's initial and clinical ongoing assessment in this regard (202, 369). Areas of partial and full-thickness burn injury are described, noting areas of circumferential involvement and burn injury across joints. Figures 3 and 4 outline a schematic assessment of %TBSA for adult and pediatric burn patients, respectively, using the rule of nines. A Berkow's percentage chart can also be used to obtain a more accurate estimate of %TBSA (40).
FIG. 3.
Body diagram for estimation of total burned surface area (%TBSA) in adults, using the rule of nines (numbers are for anterior only and posterior only). (Adapted from reference 369 with permission of the publisher.)
FIG. 4.
Body diagram for estimation of total burned surface area (%TBSA) in children, using the rule of nines (numbers include anterior and posterior). (Adapted from reference 369 with permission of the publisher.)
However, the clinical methods outlined above may not provide sufficient accuracy of evaluation of burn depth to support crucial treatment decisions such as the extent of excision and grafting required. Laser Doppler imaging (LDI) has recently been shown to provide a more objective measurement on which to base the decision to operate (25, 199, 224). A recent prospective blinded trial compared the clinical outcome of using LDI versus clinical judgment to assess injury depth. A total of 23 burn patients and 41 wounds were analyzed by both methods. LDI and the surgeon agreed on determination of wound depth only 56% of the time (P = 0.031) (224). LDI agreed with wound biopsy confirmation when the scan indicated a need for excision. LDI also enabled excision to proceed earlier even when the surgeon's clinical assessment agreed with the LDI scan results. LDI may therefore be used as an effective aid to clinical judgment in modern burn centers for deciding to excise burn wounds of indeterminate depth.
Inhalation Injury
Inhalation injury occurs in anywhere from 3 to 21% of burn patients and is a major cause of mortality; 80% of fire-related deaths occur from hypoxia due to oxygen deprivation or from inhalation of the toxins found in smoke (15, 32, 43, 342, 372, 425, 438). Inhalation injury of the lung in adults is usually proportional to the depth and extent of body surface area burned (252, 279, 342, 440). Children have a lower rate of inhalation injury because of the prominence of scald injury in this group (200, 320). Hypoxia occurs when the carbon monoxide generated by combustion is inhaled and binds to hemoglobin. Even a relatively low concentration of carbon monoxide in inhaled air can be significant because its affinity for hemoglobin is much greater than that of oxygen (e.g., 200-fold). Hydrogen cyanide and other agents generated in smoke are potent toxins and exacerbate the acidosis that occurs as a result of burn injury (43, 275).
The pathogenesis of pulmonary injury from smoke inhalation has been well described (99-102, 201, 203, 433). Direct heat injury is restricted to the upper airway above the glottis and is manifested by rapid swelling with the threat of obstruction. Steam inhalation is the only type of heat that damages the lower respiratory tract. However, inhalation of smoke and products of combustion and destruction of the tracheobronchial respiratory epithelium cause chemical injury. Inhalation injury progresses during the first few days following a burn and results in edema and sloughing of the respiratory tract mucosa and impairment of the normal mucociliary clearance mechanism. Damage of the mucociliary lining of the respiratory tract decreases the clearance of invading microorganisms. Pulmonary edema results from direct microvascular injury and the release of oxygen free radicals and inflammatory mediators. Cast formation due to aggregates of mucus and cellular debris causes obstruction of moderate-size airways when the mucosa sloughs. Disruption of endothelial and epithelial integrity results in exudation of protein-rich plasma into terminal airways, which, in combination with atelectasis, leads to bacterial growth and the subsequent development of pneumonia. Smoke inhalation also destroys type II pneumocytes, which results in impaired surfactant production (15, 251, 411).
Advances in respiratory resuscitation support in trauma intensive care units have improved the prognosis for burn patients with inhalation injury (15, 104, 139, 275). Inhalation injury should be suspected if the patient was burned in an enclosed space, has facial burns, and/or develops progressive hoarseness or stridor or a cough productive of carbonaceous sputum. The clinical effects of thermal inhalation injury typically become manifest within a few hours after injury, whereas chemical injury of the lower respiratory tract progresses more slowly (e.g., 1 to 2 days) (101, 322). Stridor that develops immediately after heat injury associated with an increased respiratory rate, worsening hypoxemia, and trouble expectorating secretions are signs of worsening edema of the upper airway (e.g., glottis), and immediate airway intubation is required to maintain patency (15, 95, 275). Similar signs of impending respiratory failure also develop in burn patients with a smoke inhalation injury and require immediate respiratory resuscitation. Intubation and mechanical ventilation as well as intensive tracheobronchial care (e.g., regular airway suctioning and therapeutic bronchoscopy) are required to assist clearance of bronchial mucus and debris (275, 312). High-frequency ventilation may also be beneficial in the clearance of secretions and also stabilizes collapsed and diseased lung segments (104, 275).
Patients with inhalation injury have greater fluid requirements than those who have only sustained a cutaneous injury. More fluid must be given in the immediate period following thermal injury in patients with inhalation injury (208, 369). Various agents have been administered, including inhaled heparin along with bronchodilators or free-radical scavenging agents such as dimethyl sulfoxide or N-acetylcysteine, in the treatment of inhalation injury in order to decrease cast formation and small-airway obstruction (15, 61, 229, 275). Nitric oxide is a potent vasodilator that has recently been administered as inhalation therapy to burn patients with acute respiratory distress syndrome due to lung injury in order to reduce ventilation-perfusion mismatch by dilating blood vessels perfusing lung alveoli (15, 122).
Early Excision and Burn Wound Closure
Prior to the widespread use of early surgical excision of burn wounds, conservative management was practiced. Colonization of the burn wound was permitted to break down the burn eschar so that it separated spontaneously. Daily cleansing and immersion hydrotherapy were used to debride necrotic surface eschar (49, 73, 113, 216, 385). Skin grafting occurred only after the development of granulation tissue on the burn wound's surface.
Although early surgical excision and grafting have been repeatedly attempted in the 20th century, the outcomes were initially poor (218, 219, 295, 296). However, an improved understanding of the pathophysiology of burns allowed the advancement of multiple intra- and postoperative medical and surgical techniques that has resulted in gradual decreases in morbidity and mortality (66, 87, 127, 181, 196, 200, 470). Medical support to maintain hemodynamic and respiratory function within the trauma intensive care unit and operating theater, the provision of early adequate nutrition, and the use of surgical techniques that minimize blood and heat loss allowed this approach to become the standard of care for large thermal injuries in modern burn centers.
Early burn wound excision now occurs within the first few days after burn injury and has resulted in improved survival (30, 127, 170, 185, 196, 200, 253, 334, 390, 429). Full-thickness and deep partial-thickness wounds are excised as soon after injury as possible once the patient has been hemodynamically stabilized. An appropriate burn care plan that includes a surgical timeline for wound closure must be developed based on the age of the patients and their clinical condition and extent of burn injury. A more conservative surgical approach may be required for patients with severe inhalation lung injury on ventilator support, the elderly, and those with underlying medical conditions that increase the risk of operation (202, 217, 457).
The primary aims of early excision are removal of the dead tissue that stimulates an overwhelming systemic inflammatory response syndrome and prevention of infection by temporary or permanent closure of the burn wound. Furthermore, shortening the period of wound inflammation, which in turn reduces the development of hypertrophic scarring, may optimize the outcome in terms of function and appearance (12, 97, 398). This is achieved by early removal of necrotic tissue (e.g., eschar) and wound closure with autograft, allograft, or skin substitutes in selected patients (15, 66, 196, 286, 390, 470).
Surgical excision of the burn wound may be carried out in a variety of ways, but the two most common methods are excision to fascia and tangential excision, whereby the eschar is removed in layers until viable tissue is reached (195, 286, 295, 296). The extent of excision at any one operation is limited by factors such as blood loss and temperature control. Usually no more than 20% of the burned area is excised during any single procedure (66, 195, 286, 297, 351, 369, 430). The open wound is usually covered with autograft, fresh allograft, or frozen allograft, in descending order of preference (297, 369). In otherwise healthy adults with burns, this process is repeated during several successive operative procedures until the entire burn wound has undergone debridement and secondary covering with new skin grafts. However, skin substitutes may be used for resurfacing in burn patients who have limited skin graft donor sites because of the extent of the injury (52, 53, 198, 225, 230, 293, 460).
Biobrane, a bilaminar temporary skin substitute, has been used in burn treatment centers since the early 1980s (28, 98, 231). Biobrane has recently been shown to be as effective as 1% silver sulfadiazine topical antibiotic therapy in the treatment of pediatric partial-thickness burns. Application of Biobrane in the immediate (e.g., 24 h after injury) postburn period decreased the children's pain, pain medication requirements, wound healing time, and length of hospital stay. However, older wounds and those with large areas of full-thickness injury may not be suitable for Biobrane treatment.
IMMUNOLOGICAL RESPONSE TO BURN INJURY
Significant thermal injuries induce a state of immunosuppression that predisposes burn patients to infectious complications. Early observations of the immunodeficiency that follows thermal injury were linked to works on “burn toxins” published by Wertheim, Avdakoff, and Sevitt (17, 384, 455). More recently, these observations have been supported by the findings of prolonged allograft survival, anergy, and increased susceptibility to infection in burn patients (75, 227, 324, 402, 405, 462). Despite improvements in the early care of burn patients, systemic inflammatory response syndrome, severe sepsis, and multiple-organ dysfunction syndrome remain major causes of morbidity and mortality (47, 194, 382). As a result, further efforts in the development of immune modulators may hold some promise for the future pending ongoing research.
Host defense against infection can be divided into innate and adaptive immune responses. The innate immune response acts immediately after the integument system is breached and relies on a phylogenetically ancient system for microbial recognition in which germ line-encoded receptors (pattern recognition receptors) recognize structural components of microorganisms and viruses (pathogen-associated molecular patterns) (412). The adaptive immune response often takes longer, especially if it involves exposure to new antigens. However, the adaptive immune response is a more efficient system for dealing with recurrent infections, relying on immune cell memory, antigen recognition, and clonal proliferation. The immunosuppression associated with burn injuries has effects on both of these systems.
Many in vitro and in vivo studies have been conducted to characterize the immune responses and the relationships between various cell types and inflammatory mediators. Several reviews have been written on the topic, discussing the findings of original works in more detail (82, 173, 194, 244, 412). This review is a synthesis of summarized data and original research that have contributed to our current understanding of the immune response following burn injury.
Systemic Response to Burn Injury
Local inflammation following injury is essential for wound healing and host defense against infection. However, trauma or burns of sufficient magnitude can incite a systemic inflammatory response, along a continuum from systemic inflammatory response syndrome through septic shock, which has the ability to cause significant cellular and end-organ damage (46, 47). Initially, the immunologic response to severe burn injury is proinflammatory but later becomes predominately anti-inflammatory in an effort to maintain homeostasis and restore normal physiology. Cytokines and cellular responses mediate both of these phases.
Inflammatory response to burn injury.
Increased serum levels of proinflammatory cytokines characterize the systemic response to burns. Interleukin-1β (IL-1β) and tumor necrosis factor alpha are produced by a wide variety of cells in response to injury, of which leukocytes are key players. Both of these cytokines contribute to the production of fever, acute-phase proteins, and an overall state of catabolism. They also up-regulate the production of prostaglandin E2 (PGE2), IL-6, and platelet-activating factor by endothelial cells and macrophages (80, 454). Levels of IL-6 are increased after injury through its production by a number of different cells (1, 42). Like IL-1β and tumor necrosis factor alpha, IL-6 induces fever and the production of acute-phase reactants that contribute to T-cell activation (471). Levels of IL-6 peak approximately 1 week after injury (178), and high levels have been associated with increased rates of morbidity and mortality, for which it is likely a marker of disease severity rather than an etiologic factor. Gamma interferon (IFN-γ) is another proinflammatory cytokine, produced by NK cells and Th-1 cells in response to injury. It has an important role in macrophage activation and the differentiation of CD4+ T cells into Th-1 cells while inhibiting their differentiation into Th-2 cells (167). Cell types that are important in facilitating a proinflammatory response to injury are proinflammatory macrophages and CD4+ T helper cells.
Anti-inflammatory response to burn injury.
The anti-inflammatory response and the subsequent immunosuppression following burn injury are characterized by a set of opposing cell types and cytokines. The production and release of monocytes/macrophages are decreased following burn injury and sepsis (151). Under these circumstances, macrophages produce increased amounts of PGE2 and decreased amounts of IL-12, which have a cooperative effect on T-cell differentiation (82, 166). T helper cells begin to preferentially differentiate into Th-2 cells, which produce the anti-inflammatory cytokines IL-4 and IL-10 (107, 167).
The exact sequence of events that result in immunosuppression after burn injury remains unknown; however, biochemical changes that may affect the immune system include those to the endocrine system, the arachidonic acid cascade, and the cytokine network. Following severe burn injury, there is an increase in the levels of vasopressin, aldosterone, growth hormone, cortisol, glucagon, and catecholamines (362, 454). Elevated levels of glucocorticoids inhibit the production of IFN-γ and IL-2, but not IL-4 and IL-10 (132, 353, 454). Similarly, norepinephrine released early after injury inhibits Th-1 cell function, but not that of Th-2 cells (376). Increased production of PGE2 by inhibitory macrophages has been observed after severe injury (454). PGE2 may have an important role in secondary immunosuppression, as it has been shown to decrease lymphocyte proliferation, to decrease the levels of the proinflammatory cytokines IL-1β and IL-2, to diminish the response to IL-2, to inhibit the activity of NK cells, and to activate suppressor T cells (4, 167). Many of the changes in cytokine levels represent alterations of the adaptive immune system following burn injury, more specifically within the T-lymphocyte population.
Innate Immune System Response to Burn Injury
Natural resistance to infection in traumatic wounds is predominantly a function of the innate immune system. Following thermal injury, the innate immune system responds immediately by stimulating localized and systemic inflammatory reactions. The innate immune response participates in activating the adaptive immune response; however, in so doing it has an adverse affect on the burn victim's ability to mount a vigorous immune response to invading microorganisms and, therefore, predisposes the burn victim to infectious complications. The innate immune system itself is composed of natural barriers to microbial invasion as well as cellular (leukocyte) and humoral (complement) elements.
Before a pathogen can establish invasive infection within the host it must break through the natural barriers of the skin or mucosa. For example, there is a loss of barrier function of the gastrointestinal epithelium in burn patients, which may be induced by up-regulation of the nitric oxide synthetase gene and the overproduction of nitric oxide (311); postoperative changes, such as decreased intestinal motility and mucus secretion; and increased exposure to endotoxin (4). The development of multiple-organ dysfunction syndrome in critically ill patients has also been associated with a derangement in intestinal permeability (109). As a result, higher rates of bacterial translocation and endotoxin absorption through the gastrointestinal mucosa may contribute to the inflammatory response seen in burn patients.
The cellular elements of the innate immune system have important roles in antimicrobial killing and in coordinating the immune response. Decreased macrophage and natural killer cell activation results in reduced levels of IFN-γ following burn injury (88, 187). The function of NK cells is diminished following significant injury (362). Neutrophil dysfunction after significant thermal injuries has also been reported (44, 137, 175, 242). Endothelial adherence of neutrophils is initially decreased after injury and then increases (374); however, the site of endothelial adhesion may not be at the point of injury, and this misguided neutrophil adhesion and activation contribute to neutrophil-mediated endothelial injury, which may play a significant role in the pathogenesis of systemic inflammatory response syndrome and multiple-organ dysfunction syndrome.
Neutrophil chemotaxis and intracellular killing are impaired following major burns (1, 173, 174). Diminished cytotoxic activity follows from a surge of degranulation early after injury and a subsequent inability to replenish intralysosomal enzymes and defensins (173, 362). Macrophages also demonstrate diminished phagocytic capacity following severe injury (5, 381). Lower levels of major histocompatibility complex class II expression and antigen presentation disrupt their roles in coordination of the immune response (362, 413). They also produce larger quantities of PGE2, resulting in the suppression of B- and T-cell reactivity (281). Increased levels of IL-4 and IL-10 inhibit macrophage antigen presentation, decrease the production of proinflammatory cytokines such as IL-1β, and suppress bactericidal and fungicidal activity (110, 130, 138, 186, 329, 442, 447).
The complement cascade represents an important humoral component of the innate immune system. Following significant burn injuries, the alternative pathway of the complement cascade is primarily depressed, while there is little effect on the classical pathway (150). Complement levels fall in proportion to injury severity and then rise to supranormal levels (150). Activation of the complement cascade by thermal injury (39) increases levels of C3a and C5a, which may result in changes in blood pressure, vascular permeability, and leukocyte function (214, 473). Small amounts of C5a have been shown to stimulate leukocyte function; however, large amounts lead to suppression of activity (453). Membrane attack complexes may target normal cells near the site of injury, contributing to reactive cell lysis, which may induce end-organ damage (194). Lastly, increased levels of C3b may be directly immunosuppressive, as they have been shown to decrease phagocytosis and contribute to lymphocyte dysfunction (4).
These alterations to the innate immune system have the combined effect of increasing the burn patient's exposure to pathogens and decreasing the natural defenses that are responsible for counteracting them. Exposure to pathogens occurs via the burn wound, invasive monitoring devices, and the gastrointestinal tract, which loses some of its capacity to act as an effective barrier to bacterial translocation. The effects of an anti-inflammatory cytokine milieu on NK cells, neutrophils, and macrophages impair the eradication of these pathogens by the innate immune system. Furthermore, the activation of complement following burn injury may be directly immunosuppressive. As a result of these phenomena and subsequent alterations to the adaptive immune system, burn patients are more susceptible to wound infections, severe sepsis, and multiple organ failure.
Adaptive Immune System in Response to Burn Injury
Following significant injury, several changes in the T-lymphocyte population have been observed. Total numbers of T lymphocytes fall in proportion to injury severity during the first week after injury (194, 362) and there is a decrease in T-cell-dependent immune functions (75, 227, 402, 405, 462). Diminished T-cell proliferation in response to mitogens (210, 364, 462) is associated with, and may be the result of, decreased production of IL-2 and IFN-γ by monocytes (133, 463). The production of immunoglobulin G (IgG) in response to T-cell-dependent antigens is also impaired after serious injury; however, no impairment of antibody formation to T-cell-independent antigens has been observed (325). There is a decreased ratio of CD4+ T helper cells to CD8-positive T suppressor cells (67, 327). After an initial proinflammatory phase, injury results in a loss of Th-1 cells associated with depressed levels of IL-1β and IFN-γ. Concomitantly, Th-2 lymphocytes are present in increased numbers along with higher levels of the anti-inflammatory cytokines IL-4 and IL-10, which may inhibit Th-1 cell activation by suppressing antigen presentation (167).
A correlation between increased levels of IL-10 and septic events has been reported (256, 395). It remains uncertain whether the relative predominance of Th-2 cells over Th-1 cells represents a phenotypic change or an increase in the rate of apoptosis of Th-1 cells (244). Alterations in the balance between T suppressor lymphocytes and T helper lymphocytes and the ratio of Th-1 to Th-2 cells appear to be important etiologic factors in the suppression of the adaptive immune response.
Altering the Immunologic Response to Burn Injury
Despite our increasingly detailed understanding of the immunological suppression that follows thermal injuries, no attempts at directly modulating the immune response at a specific site have been shown to be clinically effective. It is becoming increasingly clear that any therapies directed at addressing this immunodeficiency in burn patients will likely have to target multiple points in the inflammatory response and the neuroendocrine axis.
Immune function in burn patients can only be restored through intensive resuscitation and support. Early excision of burn eschar and prompt wound coverage remove a significant inflammatory stimulus and restore the barrier function of the skin. Providing adequate analgesia and maintaining adequate tissue perfusion, ambient temperature, and blood volume help optimize the oxidative killing capacity of neutrophils (235). Early and adequate nutritional support is also important in restoring protein synthesis and normal immune function. Research efforts have focused on the topic of immune-modifying diets, such as glutamine-enriched diets, and their clinical benefits (155). However, there is insufficient evidence to support the use of such diets in burn patients at this time.
EPIDEMIOLOGY OF BURN WOUND INFECTIONS
Burn wound infections are one of the most important and potentially serious complications that occur in the acute period following injury (10, 11, 38, 108, 189, 243). The most important patient characteristics that influence morbidity and mortality from burn wound infection and sepsis are outlined below. In addition, the impact of early excision on reducing burn wound infections is discussed. Other factors that have played a significant role in decreasing the overall fatality rates from burn wound infection and sepsis include the use of topical and prophylactic antibiotics and advances in infection control measures in modern burn units (see Prevention of Burn Wound Infections, below).
Impact of Patient Demographics and Burn Severity
Very young children and the elderly have an increased risk of being burned and worse clinical outcomes than patients in other age groups (68-70, 72, 217, 320, 344). Individuals with deliberate self-inflected burn injuries and the disabled have been shown to have more severe injuries and longer hospital stays than those with accidental injuries (18, 211, 332). Obese adults and those who have an underlying medical condition such as diabetes have also been shown to have higher morbidity and mortality (169, 276, 290). AIDS patients appear to have more complications due to infection, delayed wound healing, and increased mortality, although reported outcome data for human immunodeficiency virus-infected and AIDS patients are limited (115, 289, 310, 400). It is expected that burn patients with other types of severe immunosuppression would have similar problems, particularly increased problems with wound infection and sepsis and a higher mortality, although this group has not been studied.
Burns in the elderly constitute more severe injuries than in the general population and result in a higher number of fatalities. A recent review of adult patients admitted to a burn center over a 7-year period showed that 221 of 1,557 (11%) were >59 years of age and a higher proportion were women (279). Most elderly burn patients had one or more existing medical conditions and impaired judgment and/or mobility. Approximately one-third of the elderly patients in this study also sustained smoke inhalation injury. Substance abuse was a factor in some elderly patients, because toxicology screening showed that 10% had used alcohol and almost one-third tested positive for other drugs. Mortality was highest in elderly patients who had more severe burns and/or smoke inhalation injury that had existing underlying disease.
A recent study also assessed the factors affecting burn mortality in the elderly and analyzed changes that occurred over the past three decades (252). The study included 201 patients 75 years of age of older that had been admitted to a university-based burn center between 1972 and 2000. Almost half of these patients died (95, or 47.3%), and the severity of the burn injury as measured by TBSA and the abbreviated burn severity index were both strongly correlated with mortality. Due to improved burn care, however, the elderly are much less likely to die from burns now than in the 1970s unless they have an inhalation injury. Mortality increased significantly with inhalation injury despite advances in intensive respiratory support.
Children have a much higher risk of being burned than adults (344). In the United States in 2001 to 2002, an estimated 92,500 children aged 14 years and under required emergency care for burn-related injuries, and approximately 500 of these children died (320). Approximately two-thirds of these children sustained thermal injuries, while children <4 years of age are particularly prone to scald injury (320). Male children have a higher risk of burn injury and burn-related death than females, and obese boys represented a disproportionate number of the patients admitted to a pediatric burn center from 1991 to 1997 (26). Children who show failure to thrive (e.g., height and/or weight <5% of that expected by age) also have a higher risk of burn injury, perhaps due to the combined effects of malnutrition and neglect or abuse (26, 344).
Impact of Changes in Burn Wound Care
Much of the steady decline in burn wound infections, subsequent tissue invasion and sepsis, and associated mortality that has been realized in the past 50 years has been attributed to the substantial advances that have occurred in burn wound care, particularly early excision (15, 79, 171, 189, 196, 202, 209, 291, 369). There was a substantial reduction in one burn center in 1978 in the incidence of both burn wound infection and sepsis after the advent of early excision therapy (253). During the study period, the incidence of burn wound sepsis fell from 6% to 1% and the mortality rate for burn-related complications decreased from 40% to 18%. However, there are only two randomized, controlled trials of early excision versus conservative exposure therapy, and neither of these studies demonstrated a significant reduction in burn wound infections in patients with a major thermal injury (e.g., >15% TBSA) (127, 170). Limited data have been published that provide a clear picture of the epidemiology of different types of burn wound infections according to the recently published classification system (see Classification of Burn Wound Infections, below).
Most of our understanding of the epidemiology of burn wound infections has been gleaned from studies carried out in the 1950s through 1990 during the preexcision era of burn care (273). It is not surprising that the overall morbidity and mortality of burn wound infections, tissue invasion, and secondary sepsis were extremely high during this time period because the growth of bacteria on the burn wound surface was controlled but not eradicated. Case fatality rates were 40% or higher depending on the extent of the burn injury (272, 285, 340, 341). Immediate colonization by the patient's normal skin flora (i.e., Staphylococcus aureus and Streptococcus pyogenes) occurred following injury (23, 164, 249, 259, 333). Subsequent colonization by the patient's own gut flora added to the complex microbial ecology on the burn wound surface shortly thereafter (106, 248, 266, 267, 269, 371).
Nosocomial transmission of microorganisms to the burn wound also occurred by transfer from the hands of health care personnel and through immersion hydrotherapy treatment (73, 273, 450, 468). Burn unit outbreaks of infection were attributed mainly to contaminated Hubbard hydrotherapy tanks or water but in other cases to contaminated surfaces such as the patient's mattress (126, 274, 280, 397, 436). Despite the recognized infection risk of immersion hydrotherapy treatment in burn units, this was standard practice in many specialized burn centers until the 1990s. In a survey of burn centers in North America in 1990, 81.4% still used immersion hydrotherapy regardless of the size of the burn wound, and most centers also continued this therapy throughout hospitalization on all patients (385). Aside from microbial contamination of the tank water, aerators and agitators in hydrotherapy tubs were difficult to clean (280, 436) Hydrotherapy water continued to be cross-contaminated between patients despite the removal of these devices from the tanks (436). Sodium hypochlorite and chloramine-T disinfectants added to the hydrotherapy tank water decreased the microbial load on the burn wound surface and health care workers' hands (73, 414). However, the hydrotherapy water irritated the mucosal surfaces (e.g., conjunctiva and nares) of the patient and health care personnel, although this practice was effective in eliminating gram-negative microorganisms from burn wounds after several days of treatment (73).
Showering with a hand-held sprayer has gradually replaced hydrotherapy for cleansing and debridement of the burn wound. This practice decreases the transfer of bacteria on surfaces to the patient's burn wound. However, outbreaks related to shower hydrotherapy have also recently been reported. Pseudomonas organisms were recovered from the hydrotherapy tank used to initially remove the patient's adherent dressings in one outbreak (436), and another outbreak was caused by contamination of the shower hand grip and showering stretcher by methicillin-resistant Staphylococcus aureus (MRSA) (126). Performing local wound care in the patient's room has controlled burn unit outbreaks due to immersion hydrotherapy.
PATHOGENESIS OF BURN WOUND INFECTIONS
Pathogenesis
Thermal destruction of the skin barrier and concomitant depression of local and systemic host cellular and humoral immune responses are pivotal factors contributing to infectious complications in patients with severe burns (4, 173, 182, 194, 244). The burn wound surface (in deep partial-thickness and in all full-thickness burns) is a protein-rich environment consisting of avascular necrotic tissue (eschar) that provides a favorable niche for microbial colonization and proliferation (29, 129, 267, 268, 315). The avascularity of the eschar results in impaired migration of host immune cells and restricts delivery of systemically administered antimicrobial agents to the area, while toxic substances released by eschar tissue impair local host immune responses (see Immunological Response to Burn Injury, above).
Although burn wound surfaces are sterile immediately following thermal injury, these wounds eventually become colonized with microorganisms (129, 469). The nature and extent of the thermal injury along with the types and amounts of microorganisms colonizing the burn wound appear to influence the future risk of an invasive wound infection (29, 129, 268, 315). Gram-positive bacteria that survive the thermal insult, such as staphylococci located deep within sweat glands and hair follicles, heavily colonize the wound surface within the first 48 h unless topical antimicrobial agents are used (6, 129, 164). Eventually (after an average of 5 to 7 days), these wounds are subsequently colonized with other microbes, including gram-positive bacteria, gram-negative bacteria, and yeasts derived from the host's normal gastrointestinal and upper respiratory flora and/or from the hospital environment or that are transferred via a health care worker's hands (6, 129, 267, 268, 356, 449, 450, 468).
Over the last several decades, gram-negative organisms have emerged as the most common etiologic agents of invasive infection by virtue of their large repertoire of virulence factors and antimicrobial resistance traits (84, 92, 162, 358, 360, 363, 388, 401, 404, 436). If the patient's host defenses and therapeutic measures (including excision of necrotic tissue and wound closure) are inadequate or delayed, microbial invasion of viable tissue occurs, which is the hallmark of an invasive burn wound infection (see “Histological analysis” under Analysis of Burn Wound Specimens, below).
Biofilm Formation
Biofilms are complex communities of surface-attached aggregates of microorganisms embedded in a self-secreted extracellular polysaccharide matrix, or slime (419, 421). They are found in a wide range of natural and artificial environments and provide their constituent microbial cells with a plethora of protected dynamic microenvironments (419, 421). Once mature, biofilms act as efficient barriers against antimicrobial agents and the host immune system, resulting in persistent colonization and/or infection at the site of biofilm formation (118, 326).
Although biofilms are best known for their role in foreign device-related infections, recent studies have confirmed the importance of biofilms in the pathogenesis of burn wound infections (434). In animals with experimentally inflicted partial-thickness cutaneous burns, mature biofilms develop in 48 to 72 h, while in vitro experiments with Pseudomonas aeruginosa strains recovered from human burn wounds demonstrate that mature biofilms can form in about 10 h (184). Factors delaying the formation of biofilms in vivo may be related to the need for microbial nutrient replenishment, exposure to killing by the immune system, and immediate wound cleansing (184).
Bacteria within a biofilm typically undergo a phenotypic change whereby microbial virulence factor production is altered and metabolic rate and motility are reduced (118, 419, 421). Channels formed within the protective environment of the biofilm facilitate the transport of nutrients and microbial waste products (118, 184, 419, 421). Intercellular signaling molecules produced by bacteria within the biofilm are able to traverse these channels and influence the overall growth pattern and behavior of the biofilm in response to various host and environmental factors (258, 350, 419, 421). Persister cells within the biofilm are the cells that have remained within the biofilm after treatment with antimicrobial agents and antiseptics (434). These persister cells temporarily disable their inherent mechanisms of programmed cell death in the presence of harsh environmental conditions and help in repopulating the biofilm, often leading to failure in biofilm eradication (419, 421).
Microbial Etiology
Bacteria rapidly colonize open skin wounds after burn injury. Microorganisms colonizing the burn wound originate from the patient's endogenous skin and gastrointestinal and respiratory flora (29, 129, 267, 268, 356). Microorganisms may also be transferred to a patient's skin surface via contact with contaminated external environmental surfaces, water, fomites, air, and the soiled hands of health care workers (450, 468). Immediately following injury, gram-positive bacteria from the patient's endogenous skin flora or the external environment predominantly colonize the burn wound (29, 164, 469). Endogenous gram-negative bacteria from the patient's gastrointestinal flora also rapidly colonize the burn wound surface in the first few days after injury (266, 267, 269, 357). Wound colonization by yeasts and fungi usually occurs later due to the use of broad-spectrum antibiotic therapy (65, 105, 123). Microorganisms transmitted from the hospital environment tend to be more resistant to antimicrobial agents than those originating from the patient's normal flora (84, 146, 148, 190, 358, 363). Table 2 lists the most common microorganisms colonizing and infecting burn wounds.
TABLE 2.
Microorganisms causing invasive burn wound infectiona
| Group | Species |
|---|---|
| Gram-positive organisms | Staphylococcus aureus |
| Methicillin-resistant S. aureus | |
| Coagulase-negative staphylococci | |
| Enterococcus spp. | |
| Vancomycin-resistant enterococci | |
| Gram-negative organisms | Pseudomonas aeruginosa |
| Escherichia coli | |
| Klebsiella pneumoniae | |
| Serratia marcescens | |
| Enterobacter spp. | |
| Proteus spp. | |
| Acinetobacter spp. | |
| Bacteroides spp. | |
| Fungi | Candida spp. |
| Aspergillus spp. | |
| Fusarium spp. | |
| Alternaria spp. | |
| Rhizopus spp. | |
| Mucor spp. | |
| Viruses | Herpes simplex virus |
| Cytomegalovirus | |
| Varicella-zoster virus |
Prior to the antibiotic era, Streptococcus pyogenes (group A beta-hemolytic streptococci) was the predominant pathogen implicated in burn wound infections and was a major cause of death in severely burned patients (23, 246, 249). Staphylococcus aureus became the principal etiological agent of burn wound infections (250, 333) shortly after the introduction of penicillin G in the early 1950s, which resulted in the virtual elimination of Streptococcus pyogenes as a cause of infection in thermally injured patients. Although Staphylococcus aureus remains a common cause of early burn wound infection, Pseudomonas aeruginosa from the patient's endogenous gastrointestinal flora and/or an environmental source is the most common cause of burn wound infections in many centers (6). The incidence of infections due to less commonly encountered microbes, including other gram-positive and gram-negative bacteria, fungi, and viruses, has also increased steadily in subsequent decades (3, 23, 37, 65, 92, 105, 143, 149, 360, 389) (Table 2). While less common, infections due to anaerobic bacteria typically occur secondary to electrical burns or when open wound dressings are used in place of occlusive dressings (308).
The emergence worldwide of antimicrobial resistance among a wide variety of human bacterial and fungal burn wound pathogens, particularly nosocomial isolates, limits the available therapeutic options for effective treatment of burn wound infections (6, 84, 120, 148, 190, 358). MRSA, methicillin-resistant coagulase-negative staphylococci, vancomycin-resistant enterococci, and multiply resistant gram-negative bacteria that possess several types of beta-lactamases, including extended-spectrum beta-lactamases, ampC beta-lactamases, and metallo-beta-lactamases, have been emerging as serious pathogens in hospitalized patients (84, 92, 126, 152, 190, 241, 358). Fungal pathogens, particularly Candida spp., have increasingly become important opportunistic pathogens due to the use of broad-spectrum topical and systemic agents when infection occurs in the burned patient and have demonstrated increasing degrees of antifungal drug resistance (10, 19, 233, 302).
Virulence Factors and Tissue Invasion
The risk of invasive burn wound infection is influenced by the extent and depth of the burn injury, various host factors, and the quantity and virulence of the microbial flora colonizing the wound. Common burn wound pathogens such as Pseudomonas aeruginosa and Staphylococcus aureus produce a number of virulence factors that are important in the pathogenesis of invasive infection. Pseudomonas aeruginosa produces a number of cell-associated (adhesins, alginate, pili, flagella, and lipopolysaccharide) and extracellular (elastase, exoenzyme S, exotoxin A, hemolysins, iron-binding proteins, leukocidins, and proteases) virulence factors that mediate a number of processes, including adhesion, nutrient acquisition, immune system evasion, leukocyte killing, tissue destruction, and bloodstream invasion (437, 441). Pseudomonas aeruginosa also carries many intrinsic and acquired antimicrobial resistance traits that make infected burn wounds difficult to treat (212, 241).
Staphylococcus aureus also has a diverse array of virulence factors that facilitate adherence to host tissues, immune system evasion, and destruction of host cells and tissues, including coagulase, protein A, leukocidins, hemolysins, and superantigens (142). Resistance to methicillin in Staphylococcus aureus, and more recently emergence of resistance to glycopeptides and oxazolidinones, also complicate the treatment of burn wound infections and sepsis caused by this highly virulent organism (183, 288, 309, 391).
CLASSIFICATION OF BURN WOUND INFECTIONS
Burn wound infection is a serious problem because it causes a delay in epidermal maturation and leads to additional scar tissue formation (118, 398). Invasion of microorganisms into the tissue layers below the dermis may also result in bacteremia, sepsis, and multiple-organ dysfunction syndrome (20, 272, 346, 365). Clinical diagnosis of burn wound infection relies on regular monitoring of vital signs and inspection of the entire burn wound surface, preferably during each dressing change. Local signs of burn wound infection with invasion include conversion of a partial-thickness injury to a full-thickness wound, rapidly extending cellulitis of healthy tissue surrounding the injury, rapid eschar separation, and tissue necrosis.
Burn wound infections were previously classified based on changes in the burn wound and/or eschar appearance, time of occurrence, and associated mortality into distinct conditions, including impetigo, cellulitis, and invasive infection. Due to the advent of early excision therapy, new classifications for burn wound infections related to surgical wound infection at the excision site(s) have been developed by a subcommittee of the Committee on the Organization and Delivery of Burn Care of the American Burn Association (273, 331, 369). Each of these distinct clinical conditions that make up the spectrum of burn wound infections is described briefly below. Burn wound impetigo may or may not be associated with systemic signs of infection, but fever (temperature of >38.4°C) or leukocytosis (white blood cell count of >10,000 cells/mm3) and/or thrombocytopenia is present in all of the other types of burn wound infections outlined. The development of burn wound cellulitis or invasive burn wound infection may also be heralded by bacteremia or septicemia.
In addition to burn wound surface and/or tissue cultures, patients with signs of systemic infections should have a complete septic workup that includes blood and urine cultures as well as burn wound sample cultures. Effective treatment of burn wound infections combines an increased frequency of burn wound dressing changes with optimization of the patient's antimicrobial therapy regimen according to microbiology culture and susceptibility results from burn wound cultures.
Types of Burn Wound Infection
Burn wound impetigo.
Impetigo involves the loss of epithelium from a previously reepithelialized surface, such as grafted burns, partial-thickness burns allowed to close by secondary intention, or healed donor sites. Burn wound impetigo is not related to inadequate excision of the burn, mechanical disruption of the graft, or hematoma formation.
Burn-related surgical wound infection.
Surgical wound infections in burn patients include both excised burn and donor sites that have not yet epithelialized. The wound has purulent exudate that is culture positive. Surgical wound infections in open areas of the burn show loss of synthetic or biological covering of the wound, changes in wound appearance (such as hyperemia), and erythema in the uninjured skin surrounding the wound.
Burn wound cellulitis.
Burn wound cellulitis results from an extension of infection into the healthy, uninjured skin and soft tissues surrounding the burn wound or donor site. This condition is recognized by extension of erythema in the uninjured skin surrounding the burn beyond what is expected from the injury itself. Burn wound cellulitis is not associated with other signs of wound infection, but at least one of the following manifestations is present: localized pain or tenderness, swelling or heat at the affected site, progression of erythema and swelling, and signs of lymphangitis and/or lymphadenitis extending from the affected skin area along routes of lymphatic drainage to the area.
Invasive infection in unexcised burn wounds.
Patients with areas of unexcised deep partial-thickness or full-thickness burn wound have an increased risk of developing an invasive infection (10, 11, 27, 29, 253). This complication may be heralded by a rapid associated change in burn wound appearance or character such as separation of the eschar or dark brown, black, or violaceous discoloration of the eschar. Manifestations of invasive infection of unexcised burn wounds include inflammation of the surrounding uninjured skin, such as edema, erythema, warmth or tenderness, evidence of microbial invasion into adjacent viable tissue on histological examination, and positive blood cultures with isolation of a pathogen in the absence of another identifiable source of infection and systemic signs of sepsis, i.e., tachypnea, hypotension, oliguria, unexplained hyperglycemia (e.g., increased serum glucose level that develops at a previously tolerated level of dietary carbohydrate), and/or mental confusion. Effective treatment requires surgical excision of the burn in addition to the medical measures outlined previously.
MICROBIOLOGICAL ANALYSIS OF BURN WOUND INFECTIONS
Diagnosis of burn wound infection based on clinical signs and symptoms alone is difficult. Regular sampling of the burn wound either by surface swab or tissue biopsy for culture is also done to monitor for the presence of infection. Quantitative culture of tissue biopsy samples and histological verification of microbial invasion into viable unburned tissue have been the “gold standard” for confirming the presence of invasive burn wound infection, particularly in unexcised areas of eschar. More recently, however, the value of laborious and costly quantitative burn wound tissue biopsy cultures has been questioned (145, 282, 458). Many burn centers have correspondingly shifted to the more convenient practice of procuring burn wound surface swabs for qualitative or semiquantitative culture for infection surveillance since the advent of early excision. This section discusses the various diagnostic microbiological approaches to diagnosis of burn wound infection and current recommendations for a best approach to burn wound infection surveillance.
Best Approach for Burn Wound Infection Surveillance
Review of the studies that have compared burn wound infection surveillance by surface swabs and burn wound biopsy provides conflicting results about the best approach. Conflicting results have been obtained by different studies for the following reasons: burn patients do not have homogenous injuries (e.g., the severity and extent of burn injury vary greatly from patient to patient), various sampling techniques and laboratory methods have been used, and most comparative studies were done before the advent of early excision therapy (41, 48, 247, 254, 255, 282, 399, 407, 408, 423). Steer and colleagues (407, 408) have reported the largest recent studies that compared the results of surface swab versus biopsy cultures. In their initial study (408), a comparison was made of the qualitative results and quantitative bacterial counts of 141 surface swabs and 141 wound biopsy samples taken from 74 burn patients. Although there was significant correlation between the bacterial counts obtained by biopsy and swab, the counts obtained by one method were poorly predictive of the counts obtained by the other. In addition, parallel cultures taken on multiple occasions showed a significant correlation between bacterial counts obtained from two biopsies or two swabs taken simultaneously, but there was wide variation in bacterial densities from the same burn wound at the same time. These investigators concluded that the use of quantitative microbiology in burns is limited by the unreliability of a single surface swab or biopsy sample to represent the whole burn wound.
Steer and coworkers (407) subsequently performed a clinical-outcome study to determine the relationship between bacterial counts obtained by burn wound biopsy culture and surface swabs. These investigators collected 69 paired biopsy-surface swab specimens from 47 patients (mean 16% TBSA burned) on 64 separate occasions, either immediately prior to excision and grafting or during routine dressing changes. There was a significant positive correlation between total bacterial count by biopsy and total white cell count and a significant negative correlation between total bacterial count by swab and percent TBSA burned. No relationship was observed between clinical outcome and bacterial counts obtained with either method. Hence, this study demonstrated that quantitative bacteriology by burn wound biopsy or surface swab sample does not aid the prediction of sepsis or graft loss.
Loebl and colleagues (254, 255) originally demonstrated that the recovery of bacterial flora from the unexcised burn wound surface showed poor correlation with that from tissue biopsy samples taken from deep sites beneath the eschar. Freshwater and Su (145) also found that the results of quantitative burn wound cultures needed to be interpreted in conjunction with clinical observations of burn wound infection in order to be a useful guide to the management of burn patients with large TBSA burns. Tahlan and colleagues (423), in a study comparing surface swabs and burn wound biopsy cultures in 17 patients with second- and third-degree burns, found no difference in the types of microorganisms cultured from swabs versus those cultured from biopsies. Levine and colleagues (247) additionally noted a linear numerical relationship between quantitative surface swab and biopsy sample counts of viable bacteria from burn wounds, whereby counts of 105 bacteria per gram of biopsy sample were equated with counts of 106 bacteria obtained from surface swab samples.
McManus and colleagues found that quantitative cultures of tissue biopsy samples provided a better determination of the predominant bacterial types present in the burn wound (282). Herruzo-Cabrera and colleagues (205) showed that a semiquantitative surface swab method distinguished between wound contamination and infection, using 105 organisms/g as a threshold for the definition of infection by biopsy. Sjoberg and colleagues (399) recently reported that quantitative tissue biopsies gave a better prediction of sepsis than surface swabs but concluded that the amount of labor involved in collection and analysis of multiple biopsy samples limited the clinical relevance of this approach. Bharadwaj and colleagues (41) also assessed the value of blood cultures in the diagnosis of burn wound sepsis compared to burn wound cultures by either swab or tissue biopsy. Fifty patients with burns ranging from 30 to 50% TBSA were monitored for clinical signs of sepsis, and only 62.5% had positive burn wound cultures according to surface swabs, compared to 87.5% who had a significant bacterial count on biopsy sample culture. Blood cultures were found to be of only prognostic value in this study (41). Blood cultures have also been shown to be a late sign of invasive burn wound infection even when they are positive (270).
The best approach for routine infection surveillance of burn wounds is to use the most appropriate sampling technique for the type of burn wound area being cultured, since no single method provides a clinically relevant, reliable result for unexcised wounds (e.g., eschar) versus those that have been excised. Superficial swabs provide an adequate sampling of the microbial flora present on the wound surface and are the most convenient and least invasive approach currently available for sampling excised burn areas. Surface swabs are also the only type of sample that may be taken from areas where the skin is too thin to do a biopsy, such as over the ears, eyes, and digits. However, quantitative cultures of burn wound tissue biopsy samples along with concomitant histological analysis are the preferred infection surveillance approach for burn areas that have not been or cannot be excised. Tissue biopsy samples should also be sent for quantitative culture from infected burn wound areas in patients with sepsis.
Simultaneous culture of quantitative tissue biopsy, blood, and urine samples provides the best approach for recovery and identification of the causative organisms and their antimicrobial susceptibilities in the septic burn patient. This method also provides an accurate assessment of the depth and extent of burn infection in areas of indeterminate injury. Tissue biopsy analyses are also necessary in order to diagnose unusual types of burn wound infection due to fungi and viruses (see Other Types of Infection in Burn Patients, below).
Burn Wound Sampling Techniques
A variety of different approaches have been described for assessing the nature and extent of microbial involvement in burn wounds, although the optimal sampling technique continues to be debated. Infection surveillance of the burn wound requires taking samples on a regular basis, by either biopsying tissue or collecting surface swabs (55, 189, 202, 340, 341, 369, 458, 459). Multiple samples from several areas of the burn wound should be collected in order to obtain the most accurate assessment of the types and amounts of microorganisms present regardless of the sampling technique. Samples should be collected frequently in the first few days to weeks following injury (e.g., daily or every 48 h during dressing changes) when the microbial flora is evolving. Sampling frequency may be decreased to weekly once the burn wound has been excised, provided clinical signs of infection are not present.
Superficial wound samples.
Clinical microbiology laboratories routinely provide semiquantitative or qualitative results from cultures of superficial wound samples. A number of techniques for the collection of burn wound surface cultures have been described over the last several decades, including the collection of swabs or contact plates and capillarity gauze sampling (48, 160, 247, 399, 407, 408, 423, 459). Although each of these methods is described for historical completeness, modern burn units universally rely on the collection of surface swabs. Specimens collected by superficial sampling of the burn wound surface must be done after the removal of dressings and topical antibacterial agents and cleansing of the wound surface with 70% alcohol (41, 247, 407, 408).
Burn wound surface swabs are a convenient and effective method for routinely collecting multiple superficial wound samples (247, 408). Although there have been no studies that have compared different commercially available swabs for their ability to recover pathogens from the burn wound surface, a recent general comparison of three swab transport systems (Starplex StarSwab II, Copan VI-Pak Amies agar gel and transport swabs, and BBL Port-A-Cul) showed that the Copan VI-Pak system outperformed the other two by maintaining viability of both anaerobic and fastidious aerobic bacteria for 24 h for the organisms evaluated (206). In order to obtain enough cellular material for culture, the end of a sterile swab is moved over a minimum 1-centimeter area of the open wound. Sufficient pressure should be applied to the tip of the swab to cause minimal bleeding in the underlying tissue. Evaluations of the recovery of organisms using both dry and moistened swabs have shown that the moist-swab technique provides better reproducibility (48).
Capillarity gauze sample collections are done by applying gauze squares moistened in nonbacteriostatic saline to the open burn wound surface for several minutes, followed by use of the contaminated surface of the gauze to inoculate agar culture plates (160, 459). Although this method is relatively time-consuming and expensive, it may be superior to swab cultures. Since the capillarity gauze surface permits a more inclusive harvest of the resident bacteria, the quantitative culture result is more reproducible (459).
Agar contact plates may also be applied directly to the open wound surface, but this method has not been adopted into clinical practice because it is the least reproducible sampling technique, and culture medium sterility is not easily maintained outside of the microbiology laboratory (85, 160, 161).
Tissue biopsy.
Serial harvesting of multiple samples from beneath the eschar for quantitative culture has historically been the primary method used for accurate infection surveillance on the unexcised burn wound (254, 255, 282, 464, 465). The quantitative burn biopsy culture method was widely adopted into practice following the studies by Loebl and colleagues (254, 255). After the burn wound surface is cleansed with isopropyl alcohol, two parallel incisions are made in the skin approximately 1 to 2 cm in length and 1.5 cm apart. Sterile tissue forceps are then used to elevate and biopsy a sample with a sterile scalpel from the subcutaneous tissue at sufficient depth to obtain a small portion of the healthy underlying fat. Biopsy samples may also be collected by 3-mm punch biopsy. Tissue biopsy samples obtained by this method typically weigh between 0.02 and 0.05 g. Biopsy specimens are then placed on a nonbacteriostatic moistened sterile gauze pad within a sterile container in order to prevent tissue sample desiccation during transport.
Other investigators have also shown that quantitative burn wound biopsy cultures are more accurate than superficial surface cultures for diagnosing invasive infection in unexcised burn wounds (41). However, tissue biopsy samples must be taken from deep sites beneath the eschar (145). Woolfrey and colleagues (465) found poor reproducibility of quantitative bacterial counts between different eschar biopsy samples and showed that even high bacterial tissue levels did not correlate with the development of burn wound sepsis. Clinical microbiology laboratories may routinely perform burn wound surveillance cultures using quantitative methods on tissue biopsy samples and correlate the results with the histological analysis performed on a portion of the same biopsy sample.
Sampling techniques for other microbial pathogens.
Limited data are available regarding the optimal burn wound sampling technique to reliably detect other microbial pathogens that may cause burn wound infection, including various anaerobic bacteria, fungi, and viruses. Anaerobic swab systems and prereduced anaerobic media that provide an optimal environment for the transport of inoculated surface swabs for culture are commercially available (83). The Copan VI-Pak agar gel collection system has recently been shown to maintain the viability of anaerobic bacteria for 24 h in transport (206). However, tissue biopsy samples placed in nonbacteriostatic saline-moistened gauze in a sterile container may be more reliable for recovery of all anaerobic species from burn wounds. For viruses and fungi, tissue biopsy for culture, immunofluorescence testing (for viruses such as herpes simplex virus), and histology appear to be the most reliable diagnostic methods (see Histological analysis, below).
Specimen Transport
Although there are no published standards for transport of burn wound specimens, both superficial swabs and tissue samples should be received by the laboratory as soon after collection as possible to ensure optimal recovery of all types of microorganisms. The recovery of fastidious aerobes and anaerobes may be impaired if transport to the laboratory is delayed even though commercial swabs are directly inoculated into buffered and prereduced media (e.g., most commonly Amies, Stuart's, and PRAS). Tissue samples should be placed onto sterile nonbacteriostatic saline-moistened gauze in a leak-proof sterile container for immediate transport so that the laboratory receives and inoculates that sample onto culture media within 1 to 2 h after collection (464). Liaison between clinicians and microbiologists is essential to establish and monitor expected transportation time thresholds for delivery of burn wound biopsy specimens to the clinical microbiology laboratory.
Analysis of Burn Wound Specimens
The clinical microbiology laboratory, in order to recover and identify all potential pathogens and to perform antibiotic susceptibility testing, analyzes both superficial swab and tissue samples. The primary analytical procedures used to culture both swab and tissue samples are outlined herein.
Gram stain.
The utility of Gram staining for routine microbiological analysis of burn wound surfaces was recently evaluated in 375 serially collected specimens from 50 burn patients at our center (125). Overall, the degree of correlation between surface swab Gram stain and culture in that study was found to be fair. While Gram staining may provide an index of the degree of microbial colonization of the burn wound (125, 422), it is not suitable for diagnosing burn wound infection and does not provide information on the antimicrobial susceptibility profiles of microbes colonizing or infecting the burn wound.
Surface swab culture.
Cultures of burn wound surface swabs are routinely performed to provide a qualitative or semiquantitative result. However, methods have also been published for reporting a quantitative result based on the area of the surface of the burn wound sampled by the swabbing procedure (247, 407).
Swabs are used to inoculate blood and MacConkey agar plates using a sterile loop and inoculating the surface using the four-quadrant method. Plates are inspected for growth after 24 h of aerobic incubation at 37°C. A qualitative microbiology report provides the identification of all potential pathogens regardless of amount and the results of antibiotic susceptibility testing by isolate. A semiquantitative microbiology report includes an estimation of the relative predominance of all potential pathogens according to growth in each of the four plated quadrants (e.g., 1+, 2+, 3+, and 4+) as well as identification of each pathogen to the genus and/or species level and their antibiotic susceptibility test results.
Quantitative counts may be reported from surface swab cultures provided a standard area was swabbed (e.g., 4 cm2 of the burn wound surface) (247, 407). A bacterial suspension is first made by vortexing the swab in 1 ml of Tween 80 for a minute. The bacterial suspension is then plated onto blood and MacConkey agar in 0.1- and 0.01-ml quantities by spreading the sample evenly over the agar surface using a sterile spreading rod. The plates are then incubated aerobically for 24 h at 37°C. Colony counts are done to obtain the counts per cm2 of the surface of the burn for all potential pathogens isolated. A quantitative microbiology culture report provides the exact amount per cm2 of all potential pathogens, including the identification of each pathogen to the genus and/or species level and their antibiotic susceptibility test results.
Quantitative tissue culture.
Over three decades ago, Loebl and colleagues (254, 255) developed and evaluated a method for quantitative bacterial cultures of burn wound samples that have been widely adopted into practice. Their original method was based on the collection of burn wound biopsy specimens placed in sterile nonbacteriostatic saline, followed by maceration and preparation of doubling 10-fold dilutions. A 0.1-ml aliquot of each dilution was plated onto the surface of nonselective agar medium, followed by overnight incubation at appropriate temperature and atmospheric conditions to support microbial growth. Burn wound infection was present by quantitative culture that demonstrated a bacterial potential pathogen load in the tissue of ≥104 or ≥105 CFU/g of tissue. Subsequent clinical outcome studies performed by Loebl and colleagues and others (254, 255, 282, 294, 343) demonstrated that quantitative burn wound tissue biopsy culture results correlate well with histological evidence of bacterial invasion of tissues beneath the eschar and established that a tissue density of >105 CFU/g tissue for potential pathogens established a diagnosis of burn wound infection and predicted the development of sepsis. Pruitt and Foley (343) also demonstrated that quantitative cultures of 105 or more bacteria per gram of tissue correlated with a high (75%) mortality rate.
Clinical microbiology laboratories still use the original method developed by Loebl (255) to perform quantitative tissue biopsy cultures. The burn wound tissue biopsy sample is first weighed and homogenized in 1 ml of 1% Tween 80 using a disposable tissue grinder until only minimal tissue fibers remain. Bacterial suspensions in 0.1-ml and 0.01-ml quantities from the undiluted homogenized biopsy sample are then spread evenly over the surface of blood and MacConkey agar plates using a sterile spreading rod. If high counts are suspected, then the original homogenized tissue is diluted 1:10 to 1:10,000 and 0.1-ml and 0.01-ml quantities are also plated using the same technique. The plates are incubated aerobically for 24 h at 37°C. Colony counts are performed the next day in order to obtain the bacterial count per gram of tissue. All potential pathogens present in significant amounts (e.g., >105 CFU/ml) are identified to the genus and/or species level and have antibiotic susceptibility testing performed.
Buchanan and colleagues (64) have also reported a semiquantitative modification of this method, which provided a predictive index of burn wound sepsis similar to that of quantitative biopsy culture. Real-time PCR has recently been used experimentally to quantitate Pseudomonas aeruginosa in wound biopsy samples (336). Although molecular methods allow rapid quantitation of individual organisms, bacterial tissue biopsy culture is still necessary in order to identify and perform antibiotic susceptibility testing on the different types of bacteria present in a given sample.
Histological analysis.
Histological diagnosis of burn wound infection is based on the observation of microorganisms invading viable tissue beneath the eschar surface. Mitchell and colleagues (294) performed one of the few studies that have compared the results of histological analysis and those of quantitative tissue biopsy culture. Tissue blocks were immediately immersed in Bouin's fixative solution for 2 h and then dehydrated and embedded in paraffin using a modification of a previously published rapid manual technique that allowed biopsy sections to be evaluated within 4 h. Microtome-cut tissue sections were stained with methylene blue, hematoxylin and eosin, and periodic acid-Schiff stains. Observation of 86 burn biopsy specimens permitted the development of a grading system for microbial involvement based on the degree and depth of microbial penetration (Table 3). No growth of microorganisms on quantitative biopsy culture correlated with grades of 0 and 1a on histological analysis, while grade Ib, II, and III biopsy specimens gave positive quantitative cultures in the range of 103 to 106 organisms/gram or more, and all grade IV biopsy specimens displayed counts of bacteria greater than 104 organisms/gram of tissue. Correlation with quantitative tissue cultures shows that grades 0 and 1a document colonization but not infection of the burn wound, grades 1b and II document increasing colonization and early invasion of microorganisms into the superficial dermis, and grades III and IV document burn wound infection and the need for more aggressive therapy.
TABLE 3.
Tissue biopsy histological grading for burn wound infectiona
| Grade | Histological description |
|---|---|
| 0 | No microorganisms observed throughout the section |
| I | Microorganisms limited to burn wound surface |
| Ia | Contamination by a few bacteria |
| Ib | Colonization by numerous organisms |
| II | Microorganisms penetrated superficial dermis |
| III | Bacterial colonization observed throughout dermis |
| IV | Important microbial invasion occurred in burn wound eschar of subjacent viable tissue and hypodermis |
Data are from reference 294.
Quantitative microbiology is not, however, a diagnostic substitute for histological examination, since high tissue counts may be found during colonization that do not correlate with microscopic tissue invasion (282, 294). Burn depth can also be accurately assessed using histological measurement. A new technique that measures burn depth according to dermal microvascular occlusion has recently been described (448). Five separate sections are read in each burn biopsy sample, and the burn depth is expressed as a percentage of total dermal thickness. This histological assessment was found to correlate well with clinical estimation of burn depth as well as laser Doppler measurement. Thus, the primary advantages of histological analysis are the provision of an accurate picture of the degree and depth of colonization of microorganisms in the burn eschar and adjacent viable tissue that cannot be obtained by microbiological testing, and an accurate measurement of burn depth.
Distinguishing Burn Wound Colonization from Infection
Acute infection is clinically suspected when there is excessive inflammatory response surrounding the wound, which manifests clinically as redness, pain, and edema surrounding the wound in association with purulence of the surface in the presence of systemic signs of infection (i.e., fever and leukocytosis) (see Classification of Burn Wound Infections, above). Gram staining of a wound sample taken during acute infection will also show evidence of purulence, defined by the presence of a moderate to high number of polymorphonuclear leukocytes (125). Because burn wound swabs may show purulence due to the inflammatory response from the injury, the presence of polymorphonuclear leukocytes must be correlated with the amounts and types of bacteria present (125, 159). Burn wound infection will demonstrate purulence and the presence of a moderate to heavy amount of one or more pathogenic bacterial morphotypes that should be recovered in culture in predominant amounts (see Microbial Etiology, above). However, histological analysis of tissue biopsy samples may be required to definitively diagnose invasive burn wound infection (282, 294). Histological analysis shows invasion of bacteria into the dermis beneath the eschar and surrounding healthy tissues (294).
Clinical symptoms and signs of infection will be absent when a burn wound is colonized. Colonization is present when bacteria are cultured from the burn wound surface in the absence of clinical or microscopic evidence of infection. Gram staining of a sample taken from a colonized wound normally shows little or no purulence (e.g., no or few polymorphonuclear leukocytes) (125). However, polymorphonuclear leukocytes may be present in burn wound samples because of the secondary inflammatory response due to the injury (159). Gram stains typically show a mixture of normal skin flora and potential pathogens, with a lack of predominance of any potential pathogen. Several species of bacteria are found on normal human skin, including Staphylococcus spp., especially coagulase-negative staphylococci, Micrococcus spp., Corynebacterium spp., Propionibacterium acnes, Streptococcus viridans group, Neisseria spp., Brevibacterium spp., and Peptococcus spp. (469). Bacteria will also grow from colonized burn wounds, but typically in smaller amounts than are present during infection, and potential pathogens are not predominant. The semiquantitative or quantitative wound bacteriologic cultures outlined above therefore assist in differentiation between colonized and infected wounds (247). Histological analysis shows superficial colonization of the burn wound surface by bacteria but no invasion into the deeper tissues (294).
Antimicrobial Susceptibility Testing
Although clinical microbiology laboratories perform conventional antibiotic susceptibility testing on bacterial pathogens isolated from burn swab and tissue samples, the information provided by these methods must be interpreted within the context of the burn wound milieu. Microorganisms entrenched within a complex burn wound biofilm are in a physiologically and metabolically different state from their in vitro planktonic forms (419, 421). There are currently no published standards for the routine antibiotic susceptibility testing of bacteria that form part of a biofilm, although recent studies suggest that a modified broth microdilution plate method may provide an accurate in vitro result (77, 326). Only conventional broth microdilution methods provide antibiotic susceptibility MIC information about planktonic forms (383), but most laboratories routinely use an automated system (i.e., VITEK from Bio-Mérieux, Durham, NC; Phoenix from Becton Dickinson, Franklin Lakes, NJ; or Microscan from Dade-Behring, Deerfield, IL) to perform conventional antibiotic susceptibility testing in combination with disk diffusion, E-test, and agar dilution for confirmation of some automated system results. Most antimicrobial agents have not been subjected to controlled clinical efficacy trials in the treatment of burn wound infections, and there are limited data about the ability of systemically administered antibiotics to penetrate the devascularized burn eschar (307, 339). Hence, burn patients infected with bacterial strains that appear to be susceptible to a particular antimicrobial agent in vitro may fail to respond clinically.
Most antimicrobial therapy prescribed for burn patients is administered topically (see Topical Antimicrobial Therapy, below). Standard methods have not yet been published to allow clinical microbiology laboratories to routinely perform antibiotic susceptibility testing of burn wound isolates against commonly used topical antimicrobial agents. Although studies by Nathan and colleagues (317) as well as others (193, 207, 367) have described an agar diffusion method for this purpose, its use remains experimental and it has not been widely adopted due to lack of reproducibility and standardization. Pathogenic burn wound isolates are spread over the surface of an agar plate with 6-mm wells cut into the agar that contain various concentrations of the topical antibiotic being tested (317). The size of the zone of inhibition is predictive of the agent's specific antibacterial activity. Although procedures have been studied for assessing the antimicrobial susceptibilities of bacteria growing in a biofilm (77, 326), this method has not yet been widely adopted into practice and may be too laborious for routine use in the clinical microbiology laboratory. Standardized methods need to be developed for routine antibiotic susceptibility testing of burn wound isolates against the topical antimicrobial agents currently used by burn units.
Antimicrobial resistance and burn units.
Emerging antimicrobial resistance trends in burn wound bacterial pathogens represent a serious therapeutic challenge for clinicians caring for burn patients (6, 124, 129, 307). Antibiotic-resistant organisms such as MRSA, vancomycin-resistant enterococci, and multiply-resistant gram-negative rods, including Pseudomonas aeruginosa, Acinetobacter spp., and various members of the family Enterobacteriaceae, have been associated with infections of the burn wound and other anatomic sites in patients with major thermal injury, occasionally in the form of nosocomial outbreaks (51, 126, 136, 146, 212, 271, 274, 287, 337, 360, 361, 363, 386, 397, 436, 437). Risk factors for acquisition of an antibiotic-resistant organism include receipt of antibiotics prior to the development of infection, extended duration of hospitalization, previous hospitalization, invasive procedures, comatose state, and advancing age.
Strict infection control practices (i.e., physical isolation in a private room, use of gowns and gloves during patient contact, and handwashing before and after each patient visit) and appropriate empirical antimicrobial therapy are essential to help reduce the incidence of infections due to these antibiotic-resistant organisms (124, 129, 172). An official institutional policy guiding appropriate selection and use of antimicrobials for the treatment of infections in burn patients will further support efforts to reduce the burden of illness due to antibiotic-resistant organisms while potentially reducing hospital costs, length of hospital stay, and adverse effects due to these agents.
Burn unit antibiogram.
Burn centers should routinely determine and track the specific pattern of burn wound microbial colonization, time-related changes in the predominant microbial flora of the burn wound in individual patients, the antimicrobial susceptibility profiles of microorganisms implicated in burn wound infections in a given time period, and trends in the nosocomial spread of these pathogens (3, 6). Some burn centers rely on a laboratory microbiologic surveillance system involving periodic sampling of burn wounds and other anatomic sites to provide this information (117, 131, 202). This would facilitate the selection and administration of appropriate empirical systemic antimicrobial agents prior to the availability of microbiological culture and susceptibility test results. It is recognized that the antimicrobial susceptibility profiles of the burn unit microbial flora may not necessarily correlate with identical pathogens recovered from other units in the same hospital, and hence, the general hospital antibiogram cannot be relied upon for guiding empirical antimicrobial therapeutic decisions in burn unit patients (124, 313). Liaison between plastic surgeons, infectious disease physicians, and clinical microbiologists is essential to facilitate the development of burn unit-specific empirical treatment algorithms based on an updated yearly antibiogram data and outcome analyses.
Other Types of Infection
Nonbacterial causes of invasive burn wound infection have increasingly been recognized over the last two decades as important determinants of morbidity and mortality in severely burned patients.
Fungal infections.
Invasive burn wound infections due to Candida spp., Aspergillus spp., and other opportunistic fungi (including Alternaria spp., Fusarium spp., Rhizopus spp., and Mucor spp.) are important emerging causes of late-onset morbidity and mortality in patients with major burns and severely perturbed immune systems (37, 63, 141, 300-303, 314, 345, 406, 416-418, 456). Thermally injured children are at particular risk for fungal infections involving the burn wound (262). Such infections usually occur after the second week of thermal injury, usually following a period of burn wound colonization with fungi found in the surrounding environment and/or, in the case of Candida spp., from the patient's own gastrointestinal or upper respiratory tract flora (37, 63, 301, 303).
Infections are most commonly due to Candida spp. and Aspergillus spp. and generally appear as a darkening of the burn wound area, with an appearance similar to that of ecthyma gangrenosum (37, 105, 302, 418). Burn wound infections due to zygomycetes such as Rhizopus spp. and Mucor spp., although very rare, are important because of their remarkable ability to spread rapidly across fascial tissue planes and to invade the vasculature and are associated with a very high mortality rate (65, 424). Diagnosis of fungal infections of burn wounds is most reliably made by histological examination of burn wound tissue samples along with culture of the biopsy sample. Aggressive wide surgical excision of the infected areas of the burn wound before the development of deep invasion, combined with systemic antifungal agents, may lead to an improved chance of survival (65, 105, 141, 424). The use of a laminar-airflow isolation room, in conjunction with optimal local wound care, appears to decrease the incidence of both disseminated and local fungal infection (103, 303). Topical prophylaxis with an antifungal cream such as clotrimazole or nystatin may lead to a reduced incidence of burn wound infections and septicemia (31, 189, 192).
Viral infections.
Viruses of the herpesvirus group, particularly herpes simplex virus and varicella-zoster virus but less commonly cytomegalovirus, are rarely reported but increasingly recognized causes of wound infections in thermally injured patients (50, 143, 149, 226, 278, 345, 346, 389, 392, 404, 432). Such infections may be due to primary viral infection, reactivation of latent virus, or exogenous reinfection in previously infected patients. Burn wound infections due to herpes simplex virus occur most commonly in healing or recently healed partial-thickness burns (50, 54, 149, 226, 278, 389, 392), particularly those in the nasolabial area, or occasionally from skin graft donor sites, while those due to cytomegalovirus typically involve full-thickness burns (22, 226). In the case of herpes simplex virus, clusters of small vesicles or vesiculopustules may be found within or around the margins of the burn wound, usually occurring anywhere from 2 to 6 weeks following thermal injury. Herpetic viral infections of the burn wound are usually self-limited, although systemic spread may occur (226, 389). Diagnosis is most reliably made by histological examination or culture of burn wound biopsy specimens.
OTHER TYPES OF INFECTION IN BURN PATIENTS
Sepsis and Toxic Shock Syndrome
Most burn-related deaths (54%) in modern burn units occur because of septic shock and organ dysfunction rather than osmotic shock and hypovolemia (47, 78, 140, 272, 365). Bloodstream infection and the subsequent development of sepsis are among the most common infection complications occurring in burn patients in the intensive care unit (378). Sepsis syndrome is clinically heralded by the onset of hypothermia or hyperthermia, hypotension, decreased urinary output, hyperglycemia, neutropenia or neutrophilia, and thrombocytopenia (20, 47, 86, 140, 177). Burn wound sepsis was predominantly due to invasive wound infection prior to the advent of early burn wound excision (30, 127, 170, 185, 253, 296, 334, 429).
Teplitz and colleagues (426, 427) studied an experimental model of Pseudomonas burn wound sepsis and showed that bacteremia originated from the junction of the burn wound and the unburned hypodermis. Burn patients with sepsis from invasive burn wound infection have transient or intermittent bacteremia or fungemia from seeding of microorganisms into the bloodstream, but positive blood cultures are a late sign of infection (41, 109, 123, 152, 270, 444). Bacteremia also occurs from endogenous intestinal flora because of the decreased blood flow and gut perfusion that occur following thermal injury (248, 266, 371). Peak endotoxin concentrations have been documented to occur within 12 h after injury (283). Following burn injury in an animal model, potent gut vasoconstrictors, including thromboxane and systemic vasopressin, are released, and their levels increase in parallel in the gut (257, 266, 380). However, preventing gut mucosal atrophy and improving gut mucosal blood flow through early enteral feeding of burn patients have reduced subsequent translocation and endogenous bacteremia (185).
Mason and colleagues (272) performed the largest study of bacteremia and mortality in 5,882 burn patients consecutively admitted to a major burn center over a 25-year period from 1959 to 1983. They developed a predictor of mortality index based on the 75% of patients who did not have bacteremia. Comparison between the observed and predicted levels of mortality in subsets of patients with bacteremia showed that those with gram-negative bacteremia had a significantly increased mortality, whereas those with gram-positive bacteremia had no increase in their attributed mortality. Overall, sepsis resulted in a 21% increase in total mortality in patients with bacteremia due to either gram-negative organisms or yeasts. Most of these deaths (75%) also occurred in patients whose predicted mortality from burn injury in the absence of bacteremia was low.
Although the incidence of invasive wound infection as the primary source for burn wound sepsis has decreased substantially since the advent of early excision therapy, septic shock and multiple-organ dysfunction syndrome remain important causes of death after burn trauma despite immediate admission to an intensive care unit (46, 47, 452). In the last two decades, sepsis in burn patients was more often secondary to catheter-related infection or pneumonia rather than a result of the burn wound itself (94, 165, 347, 359, 393). Fitzwater and colleagues (140) recently studied the risk factors and temporal relationship between the development of multiple-organ dysfunction syndrome and sepsis; 175 adult patients with a >20% TBSA burn injury were studied, and 27% developed severe multiple-organ dysfunction syndrome, while 17% developed complicated sepsis, and almost a quarter of the patients died. Full-thickness burn size, age, and inhalation injury were associated with the development of multiple-organ dysfunction syndrome, sepsis, and death. Infection preceded multiple-organ dysfunction syndrome in 83% of the patients.
Burn patients may also manifest signs of toxic shock syndrome if they become colonized or infected with a strain of Staphylococcus aureus that produces TSST-1 toxin (59, 119, 121, 461). However, differentiating this severe form of toxicosis from systemic inflammatory response syndrome or even the metabolic response to the burn injury itself may be difficult, particularly in the immediate postinjury period. Toxic shock syndrome may be more prevalent in children with burn injury, and the associated mortality can be high, especially if there is a delay in recognition and appropriate management (59). Few toxic shock syndrome cases have been reported from adult burn centers (461). The Centers for Disease Control and Prevention criteria should be applied to define cases of toxic shock syndrome (76). Confirmation of the production of TSST-1 toxin can be obtained by sending a patient's Staphylococcus aureus isolate(s) to a reference laboratory for testing to detect toxin production. Antistaphylococcal antibody titers are not useful in the diagnosis of toxic shock syndrome, since many patients who do not have acute disease will have positive titers (121).
Burn patients with sepsis should be examined immediately to determine the site and source of infection, including inspection of the entire burn wound surface. Diagnostic tests should be done to identify the site and source of infection, including blood, urine, and sputum cultures. Empirical broad-spectrum antibiotic therapy directed at the most recent bacteria isolated from burn wound cultures and other sources should be instituted promptly (86, 108, 307). Patients in the intensive care unit with bloodstream infections may develop severe sepsis if initial empirical antimicrobial treatment provides inadequate activity against the organism causing infection (474). Additional drugs that block a part of the septic cascade may also be be administered to burn patients with severe sepsis. Activated protein C appears to hold the most promise for improving outcomes in intensive care unit patients with severe sepsis, but sepsis drug intervention trials have shown divergent results for other agents, including cytokine inhibitors, antiendotoxin, and other naturally occurring anticoagulants (338).
Pneumonia
Pulmonary complications are common in burn patients with inhalation lung injury. Burn patients with severe inhalation injury requiring prolonged intubation are also at risk for developing ventilator-associated pneumonia (VAP) (35, 86, 306, 378, 431, 445, 446). VAP is defined as pneumonia that develops more than 48 h after intubation (e.g., late onset) in a mechanically ventilated patient who had no signs of this complication at the time the endotracheal tube was inserted. The estimated prevalence of nosocomial pneumonia in the intensive care unit setting ranges from 10% to 65%, and mortality rates are >25% in most reported studies (60, 89, 228, 466).
The nosocomial bacteria that usually cause VAP tend to be more antibiotic resistant (306). Ramzy and colleagues (356) demonstrated that although the organisms colonizing the burn wound were similar to those recovered from quantitative culture of bronchoalveolar lavage fluid samples in pediatric burn patients with VAP, quantitative wound culture was not predictive of cross-infection in the lung. Bronchoalveolar lavage with collection of samples from the deep lung should therefore be performed in order to rapidly establish the microbial etiology of the pneumonia (34, 356, 445, 446). Quantitative culture of bronchoalveolar lavage fluid samples has shown that the presence of a bacterial pathogen(s) at ≥104 CFU/ml of bronchoalveolar lavage fluid is diagnostic of VAP in intensive care unit patients (34, 91, 396).
Invasive pulmonary diagnostic procedures such as bronchoalveolar lavage allow more appropriate use of antibiotic therapy but have not been shown to change the overall mortality from VAP (396). The results of preliminary bronchoalveolar lavage fluid sample tests such as the Gram stain are unreliable in directing prescription of initial empirical antibiotic therapy (93, 111). Laupland and colleagues (239) recently evaluated the utility of using a rapid bacterial ATP assay to screen bronchoalveolar lavage fluid samples from 477 intensive care unit patients with suspected VAP. This rapid screen demonstrated excellent performance compared to quantitative cultures in accurately detecting bronchoalveolar lavage fluid samples with significant bacterial counts. This diagnostic strategy may allow physicians to selectively prescribe antibiotics to those with a positive screening assay. Antibiotic therapy for VAP in the burn patient should be tailored to the reported antibiotic susceptibility profile of the primary bacterial pathogen(s) causing the pneumonia (323).
Burn patients may also have pulmonary complications even when the lungs have not sustained direct thermal damage (101, 201). Atelectasis and hypostatic pneumonia are common due to the hyperventilation and decreased lung expansion that occur in patients with large burns, >30% TBSA, due in part to chest wall restriction from circumferential eschar formation (101). Burn patients also have a high risk of repeated aspiration episodes (99, 101), and respiratory therapy with regular suctioning of upper airway secretions and expectoration of sputum in addition to escharotomy are critical to maintaining pulmonary function (261, 275).
Endotracheal or sputum samples should be sent for culture to determine the microbial etiology of bronchopneumonia. A clinical history of burn injury should be provided to the clinical microbiology laboratory on the requisition. Endotracheal and sputum samples are initially microscopically screened for contamination by saliva (e.g., presence of >25 epithelial cells per high-power field) to determine if the specimen has been adequately collected (33). All potential pathogens isolated from an adequately collected sputum sample from a burn patient should be identified and tested for antibiotic susceptibility (307). The culture results from sputum samples should be correlated to those from the burn wound since there is often cross-colonization between the burn wound and the tracheobronchial tree (356).
Although hematogenous pneumonia is a much less common complication in burn patients today than in prior decades, it is nevertheless a serious complication that is largely preventable. Blood cultures should be drawn prior to the start of antibiotic therapy in burn patients with fever and suspected pneumonia in order to document bacteremia (86). The occurrence of transient bacteremia associated with manipulation of the burn wound has been well documented (304, 379). The bacteremia occurrence rate ranges from 1.6 to 60% of severe burn cases, with the risk being proportional to the extent of the burn and the duration and intensity of manipulation (304). However, the incidence of bacteremia after burn wound manipulation has been substantially reduced by early excision therapy (30, 170, 185, 253).
The source of infection in bacteremic burn patients must be rapidly identified and eradicated to prevent secondary seeding of the lungs and other deep tissues through sustained high-grade circulation of microorganisms in the blood (86). Appropriate empirical systemic antibiotic therapy should be directed against the organisms recovered from recent cultures of the burn wound surface and other sources such as sputum, urine, and blood samples. Antibiotic regimens should be altered as necessary based on the results of the antibiotic susceptibility profile(s) of the isolate(s) recovered from the patient's blood cultures (307). Sustained high-grade bacteremia without an obvious focus of infection should be attributed to endocarditic or endovascular infection and treated as such.
Urinary Tract Infections
Burn patients may develop a urinary tract infection in association with prolonged bladder catheterization. Patients will develop significant bacteriuria after 72 h of urinary catheter insertion, so these devices should be removed after the initial period of fluid resuscitation and output monitoring (156, 172, 450, 468). Recent use of silver-impregnated Foley catheters in burn patients showed promise in reducing the incidence of urinary tract infections compared to that in patients with standard Foley catheters for a similar period of time (321).
In order to minimize contamination, urine samples for culture should not be collected from the drainage bag, but should be aspirated through the rubber catheter using a large-bore needle. Immediate removal of the urine catheter and institution of an appropriate antibiotic(s) based upon the latest urine culture report should be used to treat urinary tract infections. Candiduria may represent contamination of the urine from the periurethral area or vaginal area in women. However, repeated isolation of Candida spp. from urine samples in the burn patient should not be ignored because it may signal that the patient has candidemia as a sign of disseminated candidiasis (213, 316). Antifungal therapy should be instituted immediately in burn patients with an active or disseminated infection due to Candida spp. While Candida albicans and most non-C. albicans Candida spp. currently remain susceptible to fluconazole, one of the newer antifungal agents, either casopofungin or voriconazole, may be required to treat serious infections due to Candida glabrata and Candida krusei because they are typically resistant to fluconazole (19, 234, 240, 277). Most Candida spp. currently remain susceptible to amphotericin B.
Catheter Infections and Suppurative Thrombophlebitis
Burn patients are particularly susceptible to the complications associated with insertion of intravenous and intra-arterial catheters and lines, particularly infection. Catheter-associated infections have been reported to affect from 8 to 57% of burn patients (144, 245). Infected peripheral and central catheters are a significant source of sepsis in the burn patient (86, 165, 263, 347, 359, 393, 450). Suppurative thrombophlebitis may also be diagnosed in 5 to 10% of hospitalized burn patients with severe burn injury, >20% TBSA, and the mortality reaches 60% even in those who receive prompt treatment (58, 165, 328, 348, 359, 410, 443).
Suppurative thrombophlebitis should be suspected in burn patients who have high-grade bacteremia without an obvious focus of infection, including endocarditis. Suppurative thrombophlebitis serves as a source of bacteremia, sepsis syndrome, and the seeding of other deep organ tissues (i.e., endocarditis and brain abscesses) (359). Since only a third of cases have local signs of infection over the affected vein, a diagnosis of suppurative thrombophlebitis is difficult to make (165, 328, 348, 410). An infected vein may only be identified through sequential venotomy and examination of veins for intraluminal pus by vein wall biopsy for histological examination for bacterial colonization of the intimal surface. Careful nursing records must therefore be kept about sequential peripheral and central intravenous catheter locations.
Burn patients have a high frequency of peripheral intravenous and central line-related infections for a number of reasons, including a prolonged need for intravenous infusion, frequent hyperalimentation fluids, frequent episodes of bacteremia secondary to wound manipulation, a high density of surface microorganisms, and the impairment of host defenses due to the burn injury (144, 245). A recent study of burn patients demonstrated a high correlation between the microorganisms cultured from the catheter tip and hubs within 48 h of insertion, and the incidence of catheter-associated infection was inversely proportional to the distance of the line insertion site from the burn wound (354). Catheter infections in burn patients likely arise from the adherence and migration of burn wound flora microorganisms along the catheter surface to the tip, with the creation of a thick biofilm (326, 378, 419, 421). Although peripheral and central catheters should be inserted through an area of normal skin in order to minimize the development of infection, this may not be possible in patients with severe burns. It may also be necessary to cut down into a usable vein or artery through the burn wound itself in burn patients with extensive injuries to a large area of the cutaneous surface.
Rates of catheter-associated infection can nevertheless be decreased with the use of rigorous aseptic technique during the insertion procedure, the use of Teflon catheters, change of the peripheral intravenous equipment, and rotation of the peripheral catheter site every 72 h (263, 264). In addition, catheters impregnated with either antibiotics or antiseptic (chlorohexidine and silver sulfadiazine) may decrease the incidence of central catheter infection in burn patients, although comparative studies are needed (370). New catheters impregnated with nanocrystalline silver show decreased microbial colonization, but there have been no studies of the use of these devices in burn patients (375).
Treatment of suppurative thrombophlebitis requires prompt institution of empirical systemic antibiotic therapy directed against the burn wound flora and operative excision of the infected vein or vein segment. However, these measures are adjunctive to prompt removal of the infected line and relocation of a clean device at another site in a healthy noninfected vein (328, 348, 410).
Myonecrosis
Burn patients may sustain deep tissue injuries beneath the cutaneous layers that provide a focus for infection to develop. Muscle necrosis and pyomyositis occur in thermally injured extremities with circumferential full-thickness injury due to vascular compromise. These serious infections may be difficult to diagnose in limbs that are already edematous and painful due to the thermal injury. In particular, direct electrical contact results in deep muscular necrosis and delayed infection (180, 232, 352). Effective treatment of these serious infections requires prompt surgical excision of the affected deep tissues involved along with institution of broad-spectrum antibiotics against aerobic and anaerobic microorganisms (300, 308).
PREVENTION OF BURN WOUND INFECTIONS
Several studies have demonstrated the role of topical antimicrobials in decreasing morbidity and mortality in patients with major burn injuries (partial- or full-thickness skin involvement), particularly before early excision (189, 298, 307). A recent study conducted by the U.S. Army Burn Center compared the levels of mortality of adult patients according to age and burn size before (1950 to 1963) and after (1964 to 1968) the introduction of mafenide acetate topical antibiotic therapy (62). Use of mafenide acetate was associated with a greater than 10% reduction in mortality for those with burns of 40 to 79% TBSA, but its use had only a minimal effect on mortality in patients with smaller or much larger burn injuries.
The efficacy of various topical antimicrobials in common use in modern burn centers is dynamic due to the ability of microorganisms to develop resistance rapidly (6). The sustained potency of individual agents depends on the extent of use and the resident nosocomial flora within any specialized burn center. In order to accurately detect and track emerging trends in topical antimicrobial resistance in modern burn units, it is essential that standard reproducible methods be published for clinical implementation (see Antibiotic Susceptibility Testing, above).
Topical Antimicrobial Therapy
Widespread application of an effective topical antimicrobial agent substantially reduces the microbial load on the open burn wound surface and reduces the risk of infection (189, 298, 307). By controlling infection, effective topical antimicrobial therapy decreases the conversion of partial-thickness to full-thickness wounds, but its use is adjunctive to early excision therapy. Selection of topical antimicrobial therapy should be based on the agent's ability to inhibit the microorganisms recovered from burn wound surveillance cultures and monitoring of the nosocomial infections acquired in the burn unit. Prescription is also based on the individual preparation of the topical agent (e.g., ointment or cream versus solution or dressing) and its pharmacokinetic properties. Burn units may rotate the use of various topical antimicrobial preparations on a regular basis to decrease the potential for development of antibiotic resistance (6, 124, 313). Topical antibiotic agents should first be applied directly to the patient's dressings before application to the burn wound to prevent contamination of the agent's container by burn wound flora.
Table 4 outlines the most widely used topical antimicrobial agents and new silver nanocrystalline dressings that are based on the bactericidal properties of the silver ion (134, 189, 298). The inhibitory action of silver is due to its strong interaction with thiol groups present in the respiratory enzymes in the bacterial cell (237, 238). Silver has also been shown to interact with structural proteins and preferentially bind with DNA nucleic acid bases to inhibit replication (236, 237). For this reason, silver has recently been shown to be highly toxic to keratinocytes and fibroblasts and may delay burn wound healing if applied indiscriminately to debrided healing tissue areas (53, 90, 236). Moist exposure therapy using a moisture-retentive ointment (MEBO-Julphar; Gulf Pharmaceutical Industries, United Arab Emirates) has recently been shown to act as an effective antibacterial agent while promoting rapid autolysis debridement and optimal moist wound healing in partial-thickness injury (13, 16). Moisture-retentive ointment also resulted in earlier recovery of keratinocytes with improved wound healing and decreased scar formation (14). The topical antimicrobial agents reviewed are primarily used in burn center patients with full-thickness or deep partial-thickness burn wounds.
TABLE 4.
Profile of commonly used topical antimicrobial agentsa
| Topical agent | Preparation | Eschar penetration | Antibacterial activityb | Major toxicity |
|---|---|---|---|---|
| Silver nitrate (AgNO3) | 0.5% solution | None | Bacteriostatic against aerobic gram-negative bacilli, P. aeruginosa, limited antifungal | Electrolyte imbalance |
| Silver sulfadiazine (Silvodene, Flamazine, Thermazine, Burnazine) | 1% water-soluble cream (oil-in-water emulsion) | None | Bactericidal against aerobic gram-negative bacilli, P. aeruginosa, some C. albicans | Leukopenia |
| Mafenide acetate (Sulfamylon) | 10% water-soluble cream (oil-in-water emulsion), 5% solution | Limited | Broad spectrum against aerobic gram-negative bacilli, P. aeruginosa, anaerobes | Metabolic acidosis |
| Nanocrystalline silver dressings (Acticoat A.B. dressing, Silverlon) | Dressing consisting of two sheets of high-density polyethylene mesh coated with nanocrystalline silver | Moderate | Potent activity against aerobic gram-negative bacilli, P. aeruginosa, aerobic gram-positive bacilli, MRSA, VRE, multidrug-resistant Enterobacteriaceae | Limited toxicity |
Silver nitrate.
Silver nitrate is rarely used nowadays in modern burn units because the deposition of silver discolors the wound bed and other topical agents are available that are easier to use and have less potential toxicity. Silver nitrate is most effective before the burn wound becomes colonized. The burn wound needs to be cleansed of emollients and other debris before a multilayered dressing is applied to the burn wound and subsequently saturated with silver nitrate solution. Effective use of this preparation therefore requires continuous application with secondary occlusive dressings, making examination of the wound difficult. The silver ion in AgNO3 also quickly binds to elemental chlorine ions, so that repeated or large-surface application of this solution may lead to electrolyte imbalance (e.g., hyponatremia and hypochloremia) (189, 298). Silver nitrate antibacterial activity is limited to the burn wound surface (204, 409). This agent demonstrates bacteriostatic activity against gram-negative aerobic bacteria such as Pseudomonas aeruginosa and Escherichia coli, but it is not active against other genera, including Klebsiella, Providencia, and Enterobacter (189, 260). Silver nitrate also has limited antifungal activity, so that nystatin should be used concomitantly (192, 467).
Silver sulfadiazine.
Silver sulfadiazine is the most commonly used topical antibiotic agent for both ambulatory and hospitalized burn patients. This agent is a combination of sodium sulfadiazine and silver nitrate. The silver ion binds to the microorganism's nucleic acid, releasing the sulfadiazine, which then interferes with the metabolism of the microbe (237). It is easy to use and painless when applied and can be used with or without a dressing. Limited systemic toxicity with repeated daily or twice-daily application has occurred aside from the development of leukopenia (81, 147). Silver sulfadiazine has excellent broad-spectrum antibacterial coverage against Pseudomonas aeruginosa and other gram-negative enteric bacteria, although some resistance has recently been reported (189, 335). This agent also has some activity against Candida albicans, but enhanced antifungal activity can be achieved by using nystatin in combination with silver sulfadiazine (192, 467).
Although silver sulfadiazine dissociates more slowly than silver nitrate, there is still poor penetration into the wound (204, 409). Silver sulfadiazine is only absorbed within the surface epidermal layer, which limits its effectiveness in some patients with severe injuries. In Europe, a combination of cerium nitrate and silver sulfadiazine (Flammacerium; Solvay Duphar, The Netherlands) has been used to combat this problem (153, 154). Flammacerium has been shown to reduce the inflammatory response to burn injury, decrease bacterial colonization, and provide a firm eschar for easier wound management (154).
Mafenide acetate.
Topical mafenide acetate cream allows open burn wound therapy and regular examination of the burn wound surface because it is used without dressings. The burn wound surface is cleansed of debris prior to application of the cream, and the treated burn wound surface is left exposed after the cream is applied for maximal antimicrobial effect. Mafenide acetate is applied a minimum of twice daily and has been shown to penetrate the burn eschar (409). The 5% solution must be applied to saturate gauze dressings, and these need to be changed every 8 hours for maximal effect. Mafenide acetate solution appears to be as effective as the cream preparation when used in this way (135, 189).
Mafenide acetate (Sulfamylon) cream has a broad spectrum of activity against gram-negative bacteria, particularly Pseudomonas aeruginosa, but has little activity against gram-positive aerobic bacteria such as Staphylococcus aureus (189). This agent also inhibits anaerobes such as Clostridium spp. Because protracted use of mafenide acetate favors the overgrowth of Candida albicans and other fungi, this agent should be used in combination with nystatin to prevent this complication due to its limited antifungal activity (192, 467).
Despite its antibacterial potency, mafenide acetate is not as widely used as other agents because of its toxicity profile. This compound is converted to p-sulfamylvanzoic acid by monoamide oxidase, a carbonic anhydrase inhibitor, causing metabolic acidosis in the burn patient (189, 298). In burn patients with inhalation injury and a concomitant respiratory acidosis, the use of mafenide acetate over a large burn surface area or the repeated application of this compound can be fatal. Mafenide acetate also decreases the breaking strength of healed wounds and delays healing (53).
Acticoat A.B. dressing/Silverlon.
This product is a specialized dressing that consists of two sheets of high-density polyethylene mesh coated with nanocrystalline silver (e.g., ionic silver with a rayon-polyester core) (112, 435, 472). The more controlled and prolonged release of nanocrystalline silver to the burn wound area allows less-frequent dressing changes, reducing the risk of tissue damage, nosocomial infection, patient discomfort, and the overall cost of topical therapy (112, 188). Nanocrystalline dressings may also provide better penetration of unexcised burn wounds because of their prolonged mechanism of action. Acticoat A.B. dressing with SILCRYST+ (Smith & Nephew Wound Management, Largo, FL), Silverlon (Argentum Medical, L.L.C., Lakemont, GA), and Silvasorb (Medline Industries Inc., Mundelein, IL) provides the most comprehensive broad-spectrum bactericidal coverage against important burn wound pathogens of any topical antimicrobial preparation currently marketed (112, 188). These dressings have potent antibacterial activity against most aerobic gram-negatives, including Pseudomonas aeruginosa and antibiotic-resistant members of the family Enterobacteriaceae as well as aerobic gram-positive bacteria, including MRSA and vancomycin-resistant enterococci (112, 188, 472). If the burn wound surface has minimal exudates, these specialized dressings can remain in place for several days and retain antibacterial activity (188). This approach is replacing the use of other silver-based topical antibiotics in many burn centers.
Mupirocin (Bactroban).
Mupirocin (pseudomonic acid A) is a fermentation product of Pseudomonas fluorescens (189, 330). This antibiotic has potent inhibitory activity against gram-positive skin flora such as coagulase-negative staphylococci and Staphylococcus aureus, including MRSA (287, 366, 367, 420). Although primarily marketed for nasal decontamination, mupirocin has increasingly been used as a burn wound topical agent in burn units in North America, where MRSA has become a problem (126, 287). Mupirocin is currently not licensed in Europe for use as a topical burn wound agent. Various topical antibiotic preparations, including 1% silver sulfadiazine, 2% mupirocin, and 2% fusidic acid, were recently compared for their antibacterial effect in an MRSA-infected full-skin-thickness rat burn model (2). All of these agents were found to be equally effective against MRSA in reducing local burn wound bacterial counts and preventing systemic infection. Burn centers where MRSA is a problem may therefore rotate the use of topical mupirocin in combination with these other agents in order to decrease the development of resistance.
Nystatin.
Nystatin (Mycostatin or Nilstat) is produced by Streptomyces noursei and has potent antifungal effect by binding to the sterols in the fungal cell membrane (189, 330). A lower concentration (3 μg/ml) of this agent inhibits Candida albicans, but a higher concentration (6.25 μg/ml) is needed to inhibit other Candida spp. and fungi (19, 302). A recent study of nystatin powder at a concentration of 6 million units/g showed that this approach was effective in treating four burn patients with severe angioinvasive fungal infections due to either Aspergillus or Fusarium spp. (31). Both superficial and deep-tissue burn wound infections were eradicated using nystatin powder without any other interventions or adverse effects on wound healing (31, 302). However, since nystatin has no activity against bacteria, it should be used in combination with a topical agent that has activity against the broad spectrum of pathogenic bacteria that cause burn wound colonization and infection (189).
Other topical antimicrobials.
Several other topical antimicrobials have also been used for topical burn therapy, including gentamicin sulfate (0.1% water-soluble cream), betadine (10% povidone-iodine ointment), bacitracin-polymyxin ointment, and nitrofurantoin (189, 330). However, these compounds are no longer used extensively because significant resistance has developed and/or they have been shown to be toxic or ineffective at controlling localized burn wound infections. Topical bacitracin-polymyxin is primarily used as a nonadherent, nontoxic petroleum-based ointment for skin graft dressings and for dressing partial-thickness burn wounds, particularly in children (330).
Prophylactic Systemic Antibiotics
Studies of the clinical benefit of prophylactic courses of systemic antibiotics in burn patients in decreasing the occurrence of burn wound infections have not demonstrated improved outcome compared to the use of topical therapy along with surgical excision. In a recent study of pediatric burn patients, the efficacy of antibiotic prophylaxis was studied in 77 children; 47 children received prophylactic antibiotics, while the rest received no prophylaxis (128). Children in both groups with wound colonization and infection had a larger burn injury, but the administration of antibiotics did not prevent the development of burn wound infection. The group of children that received prophylactic antibiotics had a higher burn wound infection rate (21.3% versus 16.7%, P < 0.05) (128). Most of the children who developed sepsis were also on prophylactic antibiotics. Use of prophylactic antibiotics also promoted the development of other secondary infections (i.e., upper and lower respiratory tract and urinary tract infections and otitis media). Overall length of hospital stay was also prolonged in the children receiving prophylactic antibiotics.
Administration of systemic antibiotic therapy may also cause antibiotic-associated diarrhea due to the overgrowth of toxigenic strains of Clostridium difficile (176, 404, 415). Exposure to prophylactic antibiotic therapy may also increase the resistance of endogenous and pathogenic bacteria to a wide variety of antibiotics, making the subsequent treatment of clinically overt infections in the burn patient more difficult (6, 307).
Systemic antibiotic administration in burn patients should therefore only be used selectively and for a short period of time. Because of the secondary bacteremia associated with prolonged burn wound manipulation and/or excision, prophylactic systemic antibiotic therapy may be given immediately before, during, and for one or two doses after the procedure, particularly in burn patients with extensive injury (e.g., 40% TBSA) (304). Culture-specific laboratory information obtained from bacterial culture and susceptibility results for the burn wound and other sources (i.e., blood, urine, and respiratory cultures) should be used to guide the selection of effective antimicrobial agents for use as preoperative prophylaxis as well as treatment of overt clinical infections (307).
Selective Bowel Decontamination
The relationship between the translocation of bowel microflora due to increased intestinal permeability and the subsequent colonization of the burn wound by enteric gram-negative microorganisms has been well described (168, 257). Several studies compared the ability of oral prophylactic antibiotic regimens to selectively decontaminate the normal bowel flora in burn patients, thereby reducing burn wound colonization and infection (106, 220, 221, 267, 269). Jarrett and colleagues (220, 221) showed that burn wound colonization was delayed and the rate of infection was reduced in patients who received an oral bowel flora-suppressive antibiotic regimen (neomycin, erythromycin, and nystatin). However, this broad-spectrum decontamination regimen not only suppressed aerobic gram-negative bacteria but also destroyed the anaerobic flora that is important in host “colonization resistance.” Manson and colleagues (267, 269) later showed that selective bowel decontamination using oral polymyxin either alone or in combination with oral trimethoprim-sulfamethoxazole and/or amphotericin B prevented burn wound colonization. Selective oral bowel decontamination therapy, however, was never widely adopted as routine therapy and became unnecessary with the advent of early-excision burn wound therapy.
Recent studies have shown that early enteral feeding in combination with excision of the burn wound in severely burn-injured children improved their clinical recovery and outcome. Hart and colleagues (185) found that early enteral feeding diminished the incidence of wound colonization and infection by bowel flora and sepsis but did not accelerate the hypermetabolic state induced by the burn injury. Early enteral feeding is likely effective because it increases circulation to the bowel, thereby decreasing ischemia postinjury and the translocation of bowel flora.
Prevention of Tetanus
Since thermal injury creates an open dirty wound, burn patients are prone to develop tetanus (45, 116, 394). Burn centers routinely administer human tetanus immunoglobulin (250 to 500 IU) to provide immediate passive immunization regardless of the patient's active immunization status. Active immunization with tetanus toxoid is also given (0.5 ml intramuscularly) to burn patients who have not received a complete primary immunizing series or who have not received a tetanus toxoid booster within the past 10 years (45, 116). For children <7 years of age, a trivalent diphtheria-pertussis-tetanus vaccine is administered, while adults are given tetanus-diphtheria vaccine. Patients who have never been immunized against tetanus or who have been partially immunized should also receive subsequent doses of tetanus vaccine to ensure antibody levels are protective (45, 116).
Infection Control in the Burn Unit
Modern burn centers have a contained perimeter that is designed to minimize the unnecessary traffic of health care workers and visitors alike through the unit (36, 191, 202, 449). Cross-contamination is further diminished within the unit by housing burn patients in individual nursing units composed of individual isolation rooms, each with its own laminar airflow (103, 449). Nosocomial outbreaks due to antibiotic-resistant organisms have been described in modern burn units because critically ill burn patients and equipment had to be moved between the burn unit and the trauma intensive care unit (36). Modern burn unit designs should allow all intensive and burn care procedures, including ventilation and operative procedures, to be done within the burn center itself, or, as a minimum, the facility design should minimize the need to transfer patients out of the burn unit for different aspects of their care. A recent study showed that the rate of cross-colonization with resistant organisms in 66 critically ill children with severe burns and inhalation injury on ventilator support during a 5-year period was extremely low (3.2 cases per 1,000 patient-days) in such a facility (451).
Modern infection control practice has been effective in reducing or eliminating endemic pathogenic and/or antibiotic-resistant organisms, preventing the establishment of newly introduced pathogenic and/or antibiotic-resistant organisms as the predominant nosocomial flora of the burn unit, and preventing reseeding of such strains back into the burn unit from patients housed in the adjacent convalescent ward (284, 449). The infection control program for burn centers requires strict compliance with a number of environmental control measures that include strictly enforced hand washing and the universal use of personal protective equipment (i.e., gowns, gloves, and masks) (284, 449). Health care personnel must be gowned (including use of disposable or reusable gowns and disposable plastic aprons to prevent soiling of health care workers' clothing during wound care procedures) and gloved at each entry to the burn patient's isolation room. Monitoring and diagnostic equipment is housed in each burn patient's room to prevent cross-contamination between patients. All equipment in the isolation room must be regularly cleaned with appropriate disinfectants. Procedures that may predispose burn patients to cross-contamination, such as exposure hydrotherapy, are kept to a minimum (126, 436, 449). Most burn wound care in units practicing early excision therapy is now performed at the patient's bedside.
Cohort nursing care is another important component of environmental control that is utilized in the burn unit (71, 428). Nurses and other health care personnel are assigned to care for a specific patient or group of patients as a team, and the movements of assigned personnel between patients are strictly limited. Convalescent burn patients are also separated from those with an acute injury because they represent a reservoir for more antibiotic-resistant organisms that may have been acquired during a prolonged hospital stay. Admission surveillance cultures are also done to screen burn patients for colonization by antibiotic-resistant organisms (e.g., MRSA and vancomycin-resistant enterococci). Patients who are colonized on admission or who acquire an antibiotic-resistant organism during their burn unit stay are physically isolated from other burn unit patients.
Infection control practitioners also play an integral part in any burn center's prevention program. Burn wound infections should be rigorously monitored according to the standard definitions previously provided (see Classification of Burn Wound Infections, above) in order to generate accurate epidemiological data about infection rates. Routine surveillance should also be carried out for other types of nosocomial infections commonly diagnosed in burn patients, including catheter-related infections, pneumonia, and urinary tract infections. In all cases, published standard definitions should be used in identifying these types of infection complications (157, 158). Laboratory surveillance cultures (e.g., culture of nasal, rectal, or groin swabs for MRSA and culture of rectal swabs for vancomycin-resistant enterococci) as well as routine microbial surveillance cultures of the burn wound and other sources (i.e., blood, respiratory, and urine samples) should be monitored to rapidly identify epidemic pathogens and/or antibiotic-resistant strains so that control measures can be immediately implemented (6, 124). Antibiotic utilization should be rotated or changed based on monitoring of antibiotic resistance trends (e.g., antibiograms) within individual burn centers (3). Finally, adverse outcomes, including morbidity and mortality due to burn wound infection, sepsis, or another nosocomial infection complication, should be monitored in burn patients according to the extent of burn injury in order to assess the effectiveness of existing infection control practices within the institute's modern burn therapy program.
FUTURE DIRECTIONS IN MICROBIAL BURN WOUND SURVEILLANCE
Infection control programs need to document and report burn wound infections according to the recently established definitions of the classification system. Future studies of burn wound infections should use this standardized burn wound classification system so that clinical outcomes can be compared for burn patients with a specific condition (e.g., burn wound cellulitis) (273, 331). More research is required to determine the best methods for sampling excised and unexcised burn wound areas over the course of a severe deep partial-thickness and/or full-thickness injury. Reproducible standardized methods should be developed so that clinical microbiology laboratories can routinely test burn wound bacterial isolates for susceptibility to the topical antimicrobial agents on formulary at a particular burn center. A rotation program for topical antimicrobial use may also retard the development of resistance. Laboratory surveillance should include the reporting of burn unit-specific antibiograms for topical antimicrobial agents once standardized methods are available for performing susceptibility testing.
REFERENCES
- 1.Abraham, E., and A. A. Freitas. 1989. Hemorrhage produces abnormalities in lymphocyte function and lymphokine generation. J. Immunol. 142:899-906. [PubMed] [Google Scholar]
- 2.Acikel, C., O. Oncul, E. Ulkur, I. Bayram, B. Celikoz, and S. Cavuslu. 2003. Comparison of silver sulfadiazine 1%, mupirocin 2%, and fusidic acid 2% for topical antibacterial effect in methicillin-resistant staphylococci-infected, full-skin thickness rat burn wounds. J. Burn Care Rehabil. 24:37-41. [DOI] [PubMed] [Google Scholar]
- 3.Agnihotri, N., V. Gupta, and R. M. Joshi. 2004. Aerobic bacterial isolates from burn wound infections and their antibiograms—a five-year study. Burns 30:241-243. [DOI] [PubMed] [Google Scholar]
- 4.Alexander, J. W. 1990. Mechanism of immunologic suppression in burn injury. J. Trauma 30:S70-S75. [DOI] [PubMed] [Google Scholar]
- 5.Altman, L. C., C. T. Furukawa, and S. J. Klebanoff. 1977. Depressed mononuclear leukocyte chemotaxis in thermally injured patients. J. Immunol. 119:199-205. [PubMed] [Google Scholar]
- 6.Altoparlak, U., S. Erol, M. N. Akcay, F. Celebi, and A. Kadanali. 2004. The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns 30:660-664. [DOI] [PubMed] [Google Scholar]
- 7.American Burn Association. 2000. Burn incidence and treatment in the US: 2000 fact sheet. http://www.ameriburn.org. [Online.]
- 8.American Burn Association. 2000. Burn incidence and treatment in the U.S. National health interview survey (1991-1993 data). American Burn Association, Philadelphia, Pa.
- 9.Amshel, C. E., M. H. Fealk, B. J. Phillips, and D. M. Caruso. 2000. Anhydrous ammonia burns: case report and review of the literature. Burns 26:493-497. [DOI] [PubMed] [Google Scholar]
- 10.Appelgren, P., V. Bjornhagen, K. Bragderyd, C. E. Jonsson, and U. Ransjo. 2002. A prospective study of infections in burn patients. Burns 28:39-46. [DOI] [PubMed] [Google Scholar]
- 11.Arons, M. S. 1965. Burn wound infection—a review. Conn. Med. 29:718-722. [PubMed] [Google Scholar]
- 12.Atiyeh, B. S., C. A. Amm, and K. A. El Musa. 2003. Improved scar quality following primary and secondary healing of cutaneous wounds. Aesthetic Plast. Surg. 27:411-417. [DOI] [PubMed] [Google Scholar]
- 13.Atiyeh, B. S., R. Dham, M. Kadry, A. F. Abdallah, M. Al-Oteify, O. Fathi, and A. Samir. 2002. Benefit-cost analysis of moist exposed burn ointment. Burns 28:659-663. [DOI] [PubMed] [Google Scholar]
- 14.Atiyeh, B. S., K. A. El-Musa, and R. Dham. 2003. Scar quality and physiologic barrier function restoration after moist and moist-exposed dressings of partial-thickness wounds. Dermatol. Surg. 29:14-20. [DOI] [PubMed] [Google Scholar]
- 15.Atiyeh, B. S., S. W. Gunn, and S. N. Hayek. 2005. State of the art in burn treatment. World J. Surg. 29:131-148. [DOI] [PubMed] [Google Scholar]
- 16.Atiyeh, B. S., J. Ioannovich, G. Magliacani, M. Masellis, M. Costagliola, R. Dham, and M. Al-Farhan. 2002. Efficacy of moist exposed burn ointment in the management of cutaneous wounds and ulcers: a multicenter pilot study. Ann. Plast. Surg. 48:226-227. [DOI] [PubMed] [Google Scholar]
- 17.Avdakoff, V. 1876. Modifications pathologiques des tissus apres brulures. Vestrik 16:4. [Google Scholar]
- 18.Backstein, R., W. Peters, and P. Neligan. 1993. Burns in the disabled. Burns 19:192-197. [DOI] [PubMed] [Google Scholar]
- 19.Baddley, J. W., and S. A. Moser. 2004. Emerging fungal resistance. Clin. Lab. Med. 24:721-735, vii. [DOI] [PubMed] [Google Scholar]
- 20.Baker, C. C., C. L. Miller, and D. D. Trunkey. 1979. Predicting fatal sepsis in burn patients. J. Trauma 19:641-648. [DOI] [PubMed] [Google Scholar]
- 21.Baker, S. P., B. O'Neill, N. J. Ginsberg, and G. Li. 1992. Fire burns and lightening, p. 161-173. In Injury fact book. Oxford University Press, New York, N.Y.
- 22.Bale, J. F., Jr., G. P. Kealey, C. L. Ebelhack, C. E. Platz, and J. A. Goeken. 1992. Cytomegalovirus infection in a cyclosporine-treated burn patient: case report. J. Trauma 32:263-267. [DOI] [PubMed] [Google Scholar]
- 23.Bang, R. L., R. K. Gang, S. C. Sanyal, E. M. Mokaddas, and A. R. Lari. 1999. Beta-haemolytic Streptococcus infection in burns. Burns 25:242-246. [DOI] [PubMed] [Google Scholar]
- 24.Bang, R. L., P. N. Sharma, S. C. Sanyal, and I. Al Najjadah. 2002. Septicaemia after burn injury: a comparative study. Burns 28:746-751. [DOI] [PubMed] [Google Scholar]
- 25.Banwell, P. E., M. P. Tyler, A. M. Watts, A. H. Roberts, and D. A. McGrouther. 1999. Burn depth estimation: use of laser Doppler flowmetry. Plast. Reconstr. Surg. 103:334-335. [DOI] [PubMed] [Google Scholar]
- 26.Barillo, D. J., T. S. Burge, D. T. Harrington, E. C. Coffey, K. Z. Shirani, and C. W. Goodwin. 1998. Body habitus as a predictor of burn risk in children: do fat boys still get burned? Burns 24:725-727. [DOI] [PubMed] [Google Scholar]
- 27.Barillo, D. J., A. T. McManus, L. C. Cancio, A. Sofer, and C. W. Goodwin. 2003. Burn center management of necrotizing fasciitis. J. Burn Care Rehabil. 24:127-132. [DOI] [PubMed] [Google Scholar]
- 28.Barret, J. P., P. Dziewulski, P. I. Ramzy, S. E. Wolf, M. H. Desai, and D. N. Herndon. 2000. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast. Reconstr. Surg. 105:62-65. [DOI] [PubMed] [Google Scholar]
- 29.Barret, J. P., and D. N. Herndon. 2003. Effects of burn wound excision on bacterial colonization and invasion. Plast. Reconstr. Surg. 111:744-750. [DOI] [PubMed] [Google Scholar]
- 30.Barret, J. P., and D. N. Herndon. 2003. Modulation of inflammatory and catabolic responses in severely burned children by early burn wound excision in the first 24 hours. Arch. Surg. 138:127-132. [DOI] [PubMed] [Google Scholar]
- 31.Barret, J. P., P. I. Ramzy, J. P. Heggers, C. Villareal, D. N. Herndon, and M. H. Desai. 1999. Topical nystatin powder in severe burns: a new treatment for angioinvasive fungal infections refractory to other topical and systemic agents. Burns 25:505-508. [DOI] [PubMed] [Google Scholar]
- 32.Barrow, R. E., M. Spies, L. N. Barrow, and D. N. Herndon. 2004. Influence of demographics and inhalation injury on burn mortality in children. Burns 30:72-77. [DOI] [PubMed] [Google Scholar]
- 33.Bartlett, J. G. 1987. Diagnosis of bacterial infections of the lung. Clin. Chest Med. 8:119-134. [PubMed] [Google Scholar]
- 34.Baselski, V. S., and R. G. Wunderink. 1994. Bronchoscopic diagnosis of pneumonia. Clin. Microbiol. Rev. 7:533-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baughman, R. P. 2005. Diagnosis of ventilator-associated pneumonia. Microbes Infect. 7:262-267. [DOI] [PubMed]
- 36.Bayat, A., H. Shaaban, A. Dodgson, and K. W. Dunn. 2003. Implications for Burns Unit design following outbreak of multi-resistant Acinetobacter infection in ICU and Burns Unit. Burns 29:303-306. [DOI] [PubMed] [Google Scholar]
- 37.Becker, W. K., W. G. Cioffi, Jr., A. T. McManus, S. H. Kim, W. F. McManus, A. D. Mason, and B. A. Pruitt, Jr. 1991. Fungal burn wound infection. A 10-year experience. Arch. Surg. 126:44-48. [DOI] [PubMed] [Google Scholar]
- 38.Beetstra, J. E. 1986. Recognition and treatment of burn wound infection. Can. Oper. Room Nurs. J. 4:28-32. [PubMed] [Google Scholar]
- 39.Bengtson, A., and M. Heideman. 1987. Anaphylatoxin formation in plasma and burn bullae fluid in the thermally injured patient. Burns Incl. Therm. Inj. 13:185-189. [DOI] [PubMed] [Google Scholar]
- 40.Berkow, S. G. 1924. A method for estimating the extensiveness of lesions (burns and scalds) based on surface area proportions. Arch. Surg. 8:138-148. [PubMed] [Google Scholar]
- 41.Bharadwaj, R., B. N. Joshi, and S. A. Phadke. 1983. Assessment of burn wound sepsis by swab, full thickness biopsy culture and blood culture—a comparative study. Burns Incl. Therm. Inj. 10:124-126. [DOI] [PubMed] [Google Scholar]
- 42.Biffl, W. L., E. E. Moore, F. A. Moore, and V. M. Peterson. 1996. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann. Surg. 224:647-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birky, M. M., and F. B. Clarke. 1981. Inhalation of toxic products from fires. Bull. N. Y. Acad. Med. 57:997-1013. [PMC free article] [PubMed] [Google Scholar]
- 44.Bjornson, A. B., H. S. Bjornson, and W. A. Altemeier. 1981. Serum-mediated inhibition of polymorphonuclear leukocyte function following burn injury. Ann. Surg. 194:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bleck, T. P. 2005. Clostridium tetani (tetanus), p. 2817-2822. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Elsevier Churchill Livingstone, Philadelphia, Pa.
- 46.Bone, R. C. 1996. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann. Intern. Med. 125:680-687. [DOI] [PubMed] [Google Scholar]
- 47.Bone, R. C., W. J. Sibbald, and C. L. Sprung. 1992. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 101:1481-1483. [DOI] [PubMed] [Google Scholar]
- 48.Bornside, G. H., and B. B. Bornside. 1979. Comparison between moist swab and tissue biopsy methods for quantitation of bacteria in experimental incisional wounds. J. Trauma 19:103-105. [DOI] [PubMed] [Google Scholar]
- 49.Boswick, J. A., Jr. 1967. Long-term management of the burn patient. Mod. Treat. 4:1282-1290. [PubMed] [Google Scholar]
- 50.Bourdarias, B., G. Perro, M. Cutillas, J. C. Castede, M. E. Lafon, and R. Sanchez. 1996. Herpes simplex virus infection in burned patients: epidemiology of 11 cases. Burns 22:287-290. [DOI] [PubMed] [Google Scholar]
- 51.Boyce, J. M., R. L. White, W. A. Causey, and W. R. Lockwood. 1983. Burn units as a source of methicillin-resistant Staphylococcus aureus infections. JAMA 249:2803-2807. [PubMed] [Google Scholar]
- 52.Boyce, S. T., R. J. Kagan, K. P. Yakuboff, N. A. Meyer, M. T. Rieman, D. G. Greenhalgh, and G. D. Warden. 2002. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann. Surg. 235:269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyce, S. T., A. P. Supp, V. B. Swope, and G. D. Warden. 1999. Topical sulfamylon reduces engraftment of cultured skin substitutes on athymic mice. J. Burn Care Rehabil. 20:33-36. [DOI] [PubMed] [Google Scholar]
- 54.Brandt, S. J., C. G. Tribble, A. D. Lakeman, and F. G. Hayden. 1985. Herpes simplex burn wound infections: epidemiology of a case cluster and responses to acyclovir therapy. Surgery 98:338-343. [PubMed] [Google Scholar]
- 55.Bretano, L., and A. C. Gravens. 1967. A method for quantitation of bacteria in burn wounds. Appl. Microbiol. 15:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brodovsky, S. C., C. A. McCarty, G. Snibson, M. Loughnan, L. Sullivan, M. Daniell, and H. R. Taylor. 2000. Management of alkali burns: an 11-year retrospective review. Ophthalmology 107:1829-1835. [DOI] [PubMed] [Google Scholar]
- 57.Brodzka, W., H. L. Thornhill, and S. Howard. 1985. Burns: causes and risk factors. Arch. Phys. Med. Rehabil. 66:746-752. [PubMed] [Google Scholar]
- 58.Brook, I., and E. H. Frazier. 1996. Aerobic and anaerobic microbiology of superficial suppurative thrombophlebitis. Arch. Surg. 131:95-97. [DOI] [PubMed] [Google Scholar]
- 59.Brown, A. P., K. Khan, and S. Sinclair. 2003. Bacterial toxicosis/toxic shock syndrome as a contributor to morbidity in children with burn injuries. Burns 29:733-738. [DOI] [PubMed] [Google Scholar]
- 60.Brown, D. L., E. S. Hungness, R. S. Campbell, and F. A. Luchette. 2001. Ventilator-associated pneumonia in the surgical intensive care unit. J. Trauma 51:1207-1216. [DOI] [PubMed] [Google Scholar]
- 61.Brown, M., M. Desai, L. D. Traber, D. N. Herndon, and D. L. Traber. 1988. Dimethylsulfoxide with heparin in the treatment of smoke inhalation injury. J. Burn Care Rehabil. 9:22-25. [DOI] [PubMed] [Google Scholar]
- 62.Brown, T. P., L. C. Cancio, A. T. McManus, and A. D. Mason, Jr. 2004. Survival benefit conferred by topical antimicrobial preparations in burn patients: a historical perspective. J. Trauma 56:863-866. [DOI] [PubMed] [Google Scholar]
- 63.Bruck, H. M., G. Nash, J. M. Stein, and R. B. Lindberg. 1972. Studies on the occurrence and significance of yeasts and fungi in the burn wound. Ann. Surg. 176:108-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buchanan, K., D. M. Heimbach, B. H. Minshew, and M. B. Coyle. 1986. Comparison of quantitative and semiquantitative culture techniques for burn biopsy. J. Clin. Microbiol. 23:258-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burdge, J. J., F. Rea, and L. Ayers. 1988. Noncandidal, fungal infections of the burn wound. J. Burn Care Rehabil. 9:599-601. [DOI] [PubMed] [Google Scholar]
- 66.Burke, J. F., C. C. Bondoc, and W. C. Quinby. 1974. Primary burn excision and immediate grafting: a method shortening illness. J. Trauma 14:389-395. [DOI] [PubMed] [Google Scholar]
- 67.Burleson, D. G., A. D. Mason, Jr., and B. A. Pruitt, Jr. 1988. Lymphoid subpopulation changes after thermal injury and thermal injury with infection in an experimental model. Ann. Surg. 207:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burn Foundation. 1999. Burn incidence and treatment in the United States:1999 fact sheet. http://www.burnfoundation.org. [Online.]
- 69.Burn Foundation. 1999. Adult burn fact sheet. http://www.burnfoundation.org. [Online.]
- 70.Burn Foundation. 1999. Pediatric burn fact sheet. http://www.burnfoundation.org. [Online.]
- 71.Busby, H. C. 1979. Nursing management of the acute burn patient and nursing management of optimal burn recovery. J. Contin. Educ. Nurs. 10:16-30. [DOI] [PubMed] [Google Scholar]
- 72.Cadier, M. A., and P. G. Shakespeare. 1995. Burns in octogenarians. Burns 21:200-204. [DOI] [PubMed] [Google Scholar]
- 73.Cardany, C. R., G. T. Rodeheaver, J. H. Horowitz, J. G. Kenney, and R. F. Edlich. 1985. Influence of hydrotherapy and antiseptic agents on burn wound bacterial contamination. J. Burn Care Rehabil. 6:230-232. [DOI] [PubMed] [Google Scholar]
- 74.Cartotto, R. C., W. J. Peters, P. C. Neligan, L. G. Douglas, and J. Beeston. 1996. Chemical burns. Can. J. Surg. 39:205-211. [PMC free article] [PubMed] [Google Scholar]
- 75.Casson, P., A. C. Solowey, J. M. Converse, and F. T. Rapaport. 1966. Delayed hypersensitivity status of burned patients. Surg. Forum 17:268-270. [PubMed] [Google Scholar]
- 76.Centers for Disease Control. 1980. Follow-up on toxic shock syndrome. Morb. Mortal. Wkly. Rep. 29:442. [Google Scholar]
- 77.Ceri, H., M. Olson, D. Morck, D. Storey, R. Read, A. Buret, and B. Olson. 2001. The MBEC assay system: multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzymol. 337:377-385. [DOI] [PubMed] [Google Scholar]
- 78.Chai, J., Z. Sheng, L. Diao, H. Yang, J. Gao, and M. Xu. 2000. Effect of extensive excision of burn wound with invasive infection on hypermetabolism in burn patients with sepsis. Zhonghua Wai Ke Za Zhi 38:405-408. [PubMed] [Google Scholar]
- 79.Chai, J., Z. Sheng, H. Yang, L. Diao, and L. Li. 2000. Successful treatment of invasive burn wound infection with sepsis in patients with major burns. Chin. Med. J. (Engl. Ed.) 113:1142-1146. [PubMed] [Google Scholar]
- 80.Chaudry, I. H., and A. Ayala. 1993. Mechanism of increased susceptibility to infection following hemorrhage. Am. J. Surg. 165:59S-67S. [DOI] [PubMed] [Google Scholar]
- 81.Choban, P. S., and W. J. Marshall. 1987. Leukopenia secondary to silver sulfadiazine: frequency, characteristics and clinical consequences. Am. Surg. 53:515-517. [PubMed] [Google Scholar]
- 82.Cioffi, W. G. 2001. What's new in burns and metabolism. J. Am. Coll. Surg. 192:241-254. [DOI] [PubMed] [Google Scholar]
- 83.Citron, D. M. 1984. Specimen collection and transport, anaerobic culture techniques, and identification of anaerobes. Rev. Infect. Dis. 6(Suppl. 1):S51-S58. [DOI] [PubMed] [Google Scholar]
- 84.Clark, N. M., J. Patterson, and J. P. Lynch III. 2003. Antimicrobial resistance among gram-negative organisms in the intensive care unit. Curr. Opin. Crit. Care 9:413-423. [DOI] [PubMed] [Google Scholar]
- 85.Clarkson, J. G., C. G. Ward, and H. C. Polk. 1967. Quantitative bacteriologic study of the burn wound surface. Surg. Forum 18:506-507. [Google Scholar]
- 86.Cohen, J., C. Brun-Buisson, A. Torres, and J. Jorgensen. 2004. Diagnosis of infection in sepsis: an evidence-based review. Crit. Care Med. 32:S466-S494. [DOI] [PubMed] [Google Scholar]
- 87.Cole, J. K., L. H. Engrav, D. M. Heimbach, N. S. Gibran, B. A. Costa, D. Y. Nakamura, M. L. Moore, C. B. Blayney, and C. L. Hoover. 2002. Early excision and grafting of face and neck burns in patients over 20 years. Plast. Reconstr. Surg. 109:1266-1273. [DOI] [PubMed] [Google Scholar]
- 88.Collart, M. A., D. Belin, J. D. Vassalli, S. de Kossodo, and P. Vassalli. 1986. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J. Exp. Med. 164:2113-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cook, D. J., S. D. Walter, R. J. Cook, L. E. Griffith, G. H. Guyatt, D. Leasa, R. Z. Jaeschke, and C. Brun-Buisson. 1998. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann. Intern. Med. 129:433-440. [DOI] [PubMed] [Google Scholar]
- 90.Cooper, M. L., S. T. Boyce, J. F. Hansbrough, T. J. Foreman, and D. H. Frank. 1990. Cytotoxicity to cultured human keratinocytes of topical antimicrobial agents. J. Surg. Res. 48:190-195. [DOI] [PubMed] [Google Scholar]
- 91.Croce, M. A., T. C. Fabian, E. W. Mueller, G. O. Maish III, J. C. Cox, T. K. Bee, B. A. Boucher, and G. C. Wood. 2004. The appropriate diagnostic threshold for ventilator-associated pneumonia using quantitative cultures. J. Trauma 56:931-934. [DOI] [PubMed] [Google Scholar]
- 92.Dalamaga, M., K. Karmaniolas, C. Chavelas, S. Liatis, H. Matekovits, and I. Migdalis. 2003. Stenotrophomonas maltophilia: a serious and rare complication in patients suffering from burns. Burns 29:711-713. [DOI] [PubMed] [Google Scholar]
- 93.Davis, K. A., M. J. Eckert, R. L. Reed, T. J. Esposito, J. M. Santaniello, S. Poulakidas, and F. A. Luchette. 2005. Ventilator-associated pneumonia in injured patients: do you trust your Gram's stain? J. Trauma 58:462-466. [DOI] [PubMed] [Google Scholar]
- 94.Davis, K. A., J. M. Santaniello, L. K. He, K. Muthu, S. Sen, S. B. Jones, R. L. Gamelli, and R. Shankar. 2004. Burn injury and pulmonary sepsis: development of a clinically relevant model. J. Trauma 56:272-278. [DOI] [PubMed] [Google Scholar]
- 95.DeBoer, S., C. Felty, and M. Seaver. 2004. Burn care in EMS. Emerg. Med. Serv. 33:69-76. [PubMed] [Google Scholar]
- 96.DeBoer, S., and A. O'Connor. 2004. Prehospital and emergency department burn care. Crit. Care Nurs. Clin. N. Am. 16:61-73. [DOI] [PubMed] [Google Scholar]
- 97.Deitch, E. A., T. M. Wheelahan, M. P. Rose, J. Clothier, and J. Cotter. 1983. Hypertrophic burn scars: analysis of variables. J. Trauma 23:895-898. [PubMed] [Google Scholar]
- 98.Demling, R. H. 1995. Use of Biobrane in management of scalds. J. Burn Care Rehabil. 16:329-330. [DOI] [PubMed] [Google Scholar]
- 99.Demling, R. H., L. DeSanti, and D. Orgill. 2005. Tracheobronchitis from inhational injury. http://www.burnsurgery.org. [Online.]
- 100.Demling, R. H., L. DeSanti, and D. Orgill. 2005. Pulmonary problems in the inflammation-infection phase. http://www.burnsurgery.org. [Online.]
- 101.Demling, R. H., L. DeSanti, and D. P. Orgill. 2005. Pulmonary problems in the burn patient. http://www.burnsurgery.org. [Online.]
- 102.Demling, R. H., C. LaLonde, Y. P. Liu, and D. G. Zhu. 1989. The lung inflammatory response to thermal injury: relationship between physiologic and histologic changes. Surgery 106:52-59. [PubMed] [Google Scholar]
- 103.Demling, R. H., A. Perea, J. Maly, J. A. Moylan, F. Jarrett, and E. Balish. 1978. The use of a laminar airflow isolation system for the treatment of major burns. Am. J. Surg. 136:375-378. [DOI] [PubMed] [Google Scholar]
- 104.Derdak, S. 2005. High-frequency oscillatory ventilation for adult acute respiratory distress syndrome: a decade of progress. Crit. Care Med. 33:S113-S114. [DOI] [PubMed] [Google Scholar]
- 105.Desai, M. H., and D. N. Herndon. 1988. Eradication of Candida burn wound septicemia in massively burned patients. J. Trauma 28:140-145. [DOI] [PubMed] [Google Scholar]
- 106.Deutsch, D. H., S. F. Miller, and R. K. Finley, Jr. 1990. The use of intestinal antibiotics to delay or prevent infections in patients with burns. J. Burn Care Rehabil. 11:436-442. [DOI] [PubMed] [Google Scholar]
- 107.DiPiro, J. T., T. R. Howdieshell, J. K. Goddard, D. B. Callaway, R. G. Hamilton, and A. R. Mansberger, Jr. 1995. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch. Surg. 130:1159-1162. [DOI] [PubMed] [Google Scholar]
- 108.Dodd, D., and H. R. Stutman. 1991. Current issues in burn wound infections. Adv. Pediatr. Infect. Dis. 6:137-162. [PubMed] [Google Scholar]
- 109.Doig, C. J., L. R. Sutherland, J. D. Sandham, G. H. Fick, M. Verhoef, and J. B. Meddings. 1998. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am. J. Respir. Crit. Care Med. 158:444-451. [DOI] [PubMed] [Google Scholar]
- 110.Donnelly, R. P., M. J. Fenton, J. D. Kaufman, and T. L. Gerrard. 1991. IL-1 expression in human monocytes is transcriptionally and posttranscriptionally regulated by IL-4. J. Immunol. 146:3431-3436. [PubMed] [Google Scholar]
- 111.Duflo, F., B. Allaouchiche, R. Debon, F. Bordet, and D. Chassard. 2001. An evaluation of the Gram stain in protected bronchoalveolar lavage fluid for the early diagnosis of ventilator-associated pneumonia. Anesth. Analg. 92:442-447. [DOI] [PubMed] [Google Scholar]
- 112.Dunn, K., and V. Edwards-Jones. 2004. The role of Acticoat with nanocrystalline silver in the management of burns. Burns 30(Suppl. 1):S1-S9. [DOI] [PubMed] [Google Scholar]
- 113.Dyer, C. 1988. Burn wound management: an update. Plast. Surg. Nurs. 8:6-12. [DOI] [PubMed] [Google Scholar]
- 114.Dyer, C., and D. Roberts. 1990. Thermal trauma. Nurs. Clin. N. Am. 25:85-117. [PubMed] [Google Scholar]
- 115.Edge, J. M., A. E. Van der Merwe, C. H. Pieper, and P. Bouic. 2001. Clinical outcome of HIV positive patients with moderate to severe burns. Burns 27:111-114. [DOI] [PubMed] [Google Scholar]
- 116.Edlich, R. F., L. G. Hill, C. A. Mahler, M. J. Cox, D. G. Becker, J. H. Horowitz, L. S. Nichter, M. L. Martin, and W. C. Lineweaver. 2003. Management and prevention of tetanus. J. Long-Term Eff. Med. Implants 13:139-154. [PubMed] [Google Scholar]
- 117.Edlich, R. F., G. T. Rodeheaver, M. Spengler, J. Herbert, and M. T. Edgerton. 1977. Practical bacteriologic monitoring of the burn victim. Clin. Plast. Surg. 4:561-569. [PubMed] [Google Scholar]
- 118.Edwards, R., and K. G. Harding. 2004. Bacteria and wound healing. Curr. Opin. Infect. Dis. 17:91-96. [DOI] [PubMed] [Google Scholar]
- 119.Edwards-Jones, V., M. M. Dawson, and C. Childs. 2000. A survey into toxic shock syndrome (TSS) in UK burns units. Burns 26:323-333. [DOI] [PubMed] [Google Scholar]
- 120.Edwards-Jones, V., and J. E. Greenwood. 2003. What's new in burn microbiology? James Laing Memorial Prize essay 2000. Burns 29:15-24. [DOI] [PubMed] [Google Scholar]
- 121.Edwards-Jones, V., and S. G. Shawcross. 1997. Toxic shock syndrome in the burned patient. Br. J. Biomed. Sci. 54:110-117. [PubMed] [Google Scholar]
- 122.Eichacker, P. Q. 1997. Inhaled nitric oxide in adult respiratory distress syndrome: do we know the risks versus benefits? Crit. Care Med. 25:563-565. [DOI] [PubMed] [Google Scholar]
- 123.Ekenna, O., R. J. Sherertz, and H. Bingham. 1993. Natural history of bloodstream infections in a burn patient population: the importance of candidemia. Am. J. Infect. Control 21:189-195. [DOI] [PubMed] [Google Scholar]
- 124.Elliott, T. S., and P. A. Lambert. 1999. Antibacterial resistance in the intensive care unit: mechanisms and management. Br. Med. Bull. 55:259-276. [DOI] [PubMed] [Google Scholar]
- 125.Elsayed, S., D. B. Gregson, T. Lloyd, M. Crichton, and D. L. Church. 2003. Utility of Gram stain for the microbiological analysis of burn wound surfaces. Arch. Pathol. Lab. Med. 127:1485-1488. [DOI] [PubMed] [Google Scholar]
- 126.Embil, J. M., J. A. McLeod, A. M. Al-Barrak, G. M. Thompson, F. Y. Aoki, E. J. Witwicki, M. F. Stranc, A. M. Kabani, D. R. Nicoll, and L. E. Nicolle. 2001. An outbreak of methicillin resistant Staphylococcus aureus on a burn unit: potential role of contaminated hydrotherapy equipment. Burns 27:681-688. [DOI] [PubMed] [Google Scholar]
- 127.Engrav, L. H., D. M. Heimbach, J. L. Reus, T. J. Harnar, and J. A. Marvin. 1983. Early excision and grafting vs. nonoperative treatment of burns of indeterminant depth: a randomized prospective study. J. Trauma 23:1001-1004. [DOI] [PubMed] [Google Scholar]
- 128.Ergun, O., A. Celik, G. Ergun, and G. Ozok. 2004. Prophylactic antibiotic use in pediatric burn units. Eur. J. Pediatr. Surg. 14:422-426. [DOI] [PubMed] [Google Scholar]
- 129.Erol, S., U. Altoparlak, M. N. Akcay, F. Celebi, and M. Parlak. 2004. Changes of microbial flora and wound colonization in burned patients. Burns 30:357-361. [DOI] [PubMed] [Google Scholar]
- 130.Essner, R., K. Rhoades, W. H. McBride, D. L. Morton, and J. S. Economou. 1989. IL-4 down-regulates IL-1 and TNF gene expression in human monocytes. J. Immunol. 142:3857-3861. [PubMed] [Google Scholar]
- 131.Fabri, P. J. 1986. Monitoring of the burn patient. Clin. Plast. Surg. 13:21-27. [PubMed] [Google Scholar]
- 132.Faist, E., T. S. Kupper, C. C. Baker, I. H. Chaudry, J. Dwyer, and A. E. Baue. 1986. Depression of cellular immunity after major injury. Its association with posttraumatic complications and its reversal with immunomodulation. Arch. Surg. 121:1000-1005. [DOI] [PubMed] [Google Scholar]
- 133.Faist, E., A. Mewes, T. Strasser, A. Walz, S. Alkan, C. Baker, W. Ertel, and G. Heberer. 1988. Alteration of monocyte function following major injury. Arch. Surg. 123:287-292. [DOI] [PubMed] [Google Scholar]
- 134.Falcone, A. E., and J. A. Spadaro. 1986. Inhibitory effects of electrically activated silver material on cutaneous wound bacteria. Plast. Reconstr. Surg. 77:455-459. [DOI] [PubMed] [Google Scholar]
- 135.Falcone, P. A., H. N. Harrison, G. O. Sowemimo, and G. P. Reading. 1980. Mafenide acetate concentrations and bacteriostasis in experimental burn wounds treated with a three-layered laminated mafenide-saline dressing. Ann. Plast. Surg. 5:266-269. [DOI] [PubMed] [Google Scholar]
- 136.Falk, P. S., J. Winnike, C. Woodmansee, M. Desai, and C. G. Mayhall. 2000. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect. Control Hosp. Epidemiol. 21:575-582. [DOI] [PubMed] [Google Scholar]
- 137.Fikrig, S. M., S. C. Karl, and K. Suntharalingam. 1977. Neutrophil chemotaxis in patients with burns. Ann. Surg. 186:746-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147:3815-3822. [PubMed] [Google Scholar]
- 139.Fitzpatrick, J. C. and W. G. Cioffi, Jr. 1997. Ventilatory support following burns and smoke-inhalation injury. Respir. Care Clin. N. Am. 3:21-49. [PubMed] [Google Scholar]
- 140.Fitzwater, J., G. F. Purdue, J. L. Hunt, and G. E. O'Keefe. 2003. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J. Trauma 54:959-966. [DOI] [PubMed] [Google Scholar]
- 141.Foley, F. D., and J. M. Shuck. 1968. Burn-wound infection with phycomycetes requiring amputation of hand. JAMA 203:596. [PubMed] [Google Scholar]
- 142.Foster, T. J. 2004. The Staphylococcus aureus “superbug.” J. Clin. Investig. 114:1693-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frame, J. D., L. Kangesu, and W. M. Malik. 1992. Changing flora in burn and trauma units: experience in the United Kingdom. J. Burn Care Rehabil. 13:281-286. [DOI] [PubMed] [Google Scholar]
- 144.Franceschi, D., R. L. Gerding, G. Phillips, and R. B. Fratianne. 1989. Risk factors associated with intravascular catheter infections in burned patients: a prospective, randomized study. J. Trauma 29:811-816. [DOI] [PubMed] [Google Scholar]
- 145.Freshwater, M. F., and C. T. Su. 1980. Potential pitfalls of quantitative burn wound biopsy cultures. Ann. Plast. Surg. 4:216-218. [PubMed] [Google Scholar]
- 146.Fuchs, P. C., J. Kopp, H. Hafner, U. Kleiner, and N. Pallua. 2002. MRSA-retrospective analysis of an outbreak in the burn centre Aachen. Burns 28:575-578. [DOI] [PubMed] [Google Scholar]
- 147.Fuller, F. W., and P. E. Engler. 1988. Leukopenia in non-septic burn patients receiving topical 1% silver sulfadiazine cream therapy: a survey. J. Burn Care Rehabil. 9:606-609. [DOI] [PubMed] [Google Scholar]
- 148.Gales, A. C., R. N. Jones, J. Turnidge, R. Rennie, and R. Ramphal. 2001. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S146-S155. [DOI] [PubMed] [Google Scholar]
- 149.Gallagher, W. F. 1970. Burn-wound infection with viruses. N. Engl. J. Med. 282:1272. [PubMed] [Google Scholar]
- 150.Gallinaro, R., W. G. Cheadle, K. Applegate, and H. C. Polk, Jr. 1992. The role of the complement system in trauma and infection. Surg. Gynecol. Obstet. 174:435-440. [PubMed] [Google Scholar]
- 151.Gamelli, R. L., L. K. He, and H. Liu. 1994. Marrow granulocyte-macrophage progenitor cell response to burn injury as modified by endotoxin and indomethacin. J. Trauma 37:339-346. [DOI] [PubMed] [Google Scholar]
- 152.Gang, R. K., S. C. Sanyal, R. L. Bang, E. Mokaddas, and A. R. Lari. 2000. Staphylococcal septicaemia in burns. Burns 26:359-366. [DOI] [PubMed] [Google Scholar]
- 153.Garner, J. P., and P. S. Heppell. 2005. The use of Flammacerium in British burns units. Burns 31:379-382. [DOI] [PubMed] [Google Scholar]
- 154.Garner, J. P., and P. S. Heppell. 2005. Cerium nitrate in the management of burns. Burns 31:539-547. [DOI] [PubMed] [Google Scholar]
- 155.Garrel, D., J. Patenaude, B. Nedelec, L. Samson, J. Dorais, J. Champoux, M. D'Elia, and J. Bernier. 2003. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: a prospective, controlled, randomized clinical trial. Crit. Care Med. 31:2444-2449. [DOI] [PubMed] [Google Scholar]
- 156.Gastmeier, P., O. Weigt, D. Sohr, and H. Ruden. 2002. Comparison of hospital-acquired infection rates in paediatric burn patients. J. Hosp. Infect. 52:161-165. [DOI] [PubMed] [Google Scholar]
- 157.Gaynes, R. P., D. H. Culver, T. G. Emori, T. C. Horan, S. N. Banerjee, J. R. Edwards, W. R. Jarvis, J. S. Tolson, T. S. Henderson, and J. M. Hughes. 1991. The National Nosocomial Infections Surveillance System: plans for the 1990s and beyond. Am. J. Med. 91:116S-120S. [DOI] [PubMed] [Google Scholar]
- 158.Gaynes, R. P., and S. Solomon. 1996. Improving hospital-acquired infection rates: the CDC experience. Joint Comm. J. Qual. Improv. 22:457-467. [DOI] [PubMed] [Google Scholar]
- 159.Gelfand, J. A. 1984. Infections in burn patients: a paradigm for cutaneous infection in the patient at risk. Am. J. Med. 76:158-165. [DOI] [PubMed] [Google Scholar]
- 160.Georgiade, N. G., M. C. Lucas, W. M. O'Fallon, and S. Osterhout. 1970. A comparison of methods for the quantitation of bacteria in burn wounds. I. Experimental evaluation. Am. J. Clin. Pathol. 53:35-39. [DOI] [PubMed] [Google Scholar]
- 161.Georgiade, N. G., M. C. Lucas, and S. Osterhout. 1970. A comparison of methods for the quantitation of bacteria in burn wounds. II. Clinical evaluation. Am. J. Clin. Pathol. 53:40-42. [DOI] [PubMed] [Google Scholar]
- 162.Geyik, M. F., M. Aldemir, S. Hosoglu, and H. I. Tacyildiz. 2003. Epidemiology of burn unit infections in children. Am. J. Infect. Control 31:342-346. [DOI] [PubMed] [Google Scholar]
- 163.Gibran, N. S., and D. M. Heimbach. 2000. Current status of burn wound pathophysiology. Clin. Plast. Surg. 27:11-22. [PubMed] [Google Scholar]
- 164.Gibson, C. D., Jr., and W. C. Thompson, Jr. 1955. The response of burn wound staphylococci to alternating programs of antibiotic therapy. Antibiot. Annu. 3:32-34. [PubMed] [Google Scholar]
- 165.Gillespie, P., H. Siddiqui, and J. Clarke. 2000. Cannula related suppurative thrombophlebitis in the burned patient. Burns 26:200-204. [DOI] [PubMed] [Google Scholar]
- 166.Goebel, A., E. Kavanagh, A. Lyons, I. B. Saporoschetz, C. Soberg, J. A. Lederer, J. A. Mannick, and M. L. Rodrick. 2000. Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann. Surg. 231:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gosain, A., and R. L. Gamelli. 2005. A primer in cytokines. J. Burn Care Rehabil. 26:7-12. [DOI] [PubMed] [Google Scholar]
- 168.Gosain, A., and R. L. Gamelli. 2005. Role of the gastrointestinal tract in burn sepsis. J. Burn Care Rehabil. 26:85-91. [DOI] [PubMed] [Google Scholar]
- 169.Gottschlich, M. M., T. Mayes, J. C. Khoury, and G. D. Warden. 1993. Significance of obesity on nutritional, immunologic, hormonal, and clinical outcome parameters in burns. J. Am. Diet. Assoc. 93:1261-1268. [DOI] [PubMed] [Google Scholar]
- 170.Gray, D. T., R. W. Pine, T. J. Harnar, J. A. Marvin, L. H. Engrav, and D. M. Heimbach. 1982. Early surgical excision versus conventional therapy in patients with 20 to 40 percent burns. A comparative study. Am. J. Surg. 144:76-80. [DOI] [PubMed] [Google Scholar]
- 171.Greenfield, E., and B. Jordan. 1996. Advances in burn wound care. Crit. Care Nurs. Clin. N. Am. 8:203-215. [PubMed] [Google Scholar]
- 172.Greenfield, E., and A. T. McManus. 1997. Infectious complications: prevention and strategies for their control. Nurs. Clin. N. Am. 32:297-309. [PubMed] [Google Scholar]
- 173.Griswold, J. A. 1993. White blood cell response to burn injury. Semin. Nephrol. 13:409-415. [PubMed] [Google Scholar]
- 174.Grogan, J. B. 1976. Altered neutrophil phagocytic function in burn patients. J. Trauma 16:734-738. [DOI] [PubMed] [Google Scholar]
- 175.Grogan, J. B., and R. C. Miller. 1973. Impaired function of polymorphonuclear leukocytes in patients with burns and other trauma. Surg. Gynecol. Obstet. 137:784-788. [PubMed] [Google Scholar]
- 176.Grube, B. J., D. M. Heimbach, and J. A. Marvin. 1987. Clostridium difficile diarrhea in critically ill burned patients. Arch. Surg. 122:655-661. [DOI] [PubMed] [Google Scholar]
- 177.Gullo, A. 1999. Sepsis and organ dysfunction/failure. An overview. Minerva Anestesiol. 65:529-540. [PubMed] [Google Scholar]
- 178.Guo, Y., C. Dickerson, F. J. Chrest, W. H. Adler, A. M. Munster, and R. A. Winchurch. 1990. Increased levels of circulating interleukin 6 in burn patients. Clin. Immunol. Immunopathol. 54:361-371. [DOI] [PubMed] [Google Scholar]
- 179.Haberal, M., Z. Oner, H. Gulay, U. Bayraktar, and N. Bilgin. 1989. Severe electrical injury. Burns Incl. Therm. Inj. 15:60-63. [DOI] [PubMed] [Google Scholar]
- 180.Haberal, M. A. 1995. An eleven-year survey of electrical burn injuries. J. Burn Care Rehabil. 16:43-48. [DOI] [PubMed] [Google Scholar]
- 181.Hansbrough, J. F. 1994. The promises of excisional therapy of burn wounds: have they been achieved? J. Intensive Care Med. 9:1-3. [DOI] [PubMed] [Google Scholar]
- 182.Hansbrough, J. F., T. O. Field, Jr., M. A. Gadd, and C. Soderberg. 1987. Immune response modulation after burn injury: T cells and antibodies. J. Burn Care Rehabil. 8:509-512. [PubMed] [Google Scholar]
- 183.Haraga, I., S. Nomura, S. Fukamachi, H. Ohjimi, H. Hanaki, K. Hiramatsu, and A. Nagayama. 2002. Emergence of vancomycin resistance during therapy against methicillin-resistant Staphylococcus aureus in a burn patient—importance of low-level resistance to vancomycin. Int. J. Infect. Dis. 6:302-308. [DOI] [PubMed] [Google Scholar]
- 184.Harrison-Balestra, C., A. L. Cazzaniga, S. C. Davis, and P. M. Mertz. 2003. A wound-isolated Pseudomonas aeruginosa grows a biofilm in vitro within 10 hours and is visualized by light microscopy. Dermatol. Surg. 29:631-635. [DOI] [PubMed] [Google Scholar]
- 185.Hart, D. W., S. E. Wolf, D. L. Chinkes, R. B. Beauford, R. P. Mlcak, J. P. Heggers, R. R. Wolfe, and D. N. Herndon. 2003. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J. Trauma 54:755-761. [DOI] [PubMed] [Google Scholar]
- 186.Hart, P. H., G. F. Vitti, D. R. Burgess, G. A. Whitty, D. S. Piccoli, and J. A. Hamilton. 1989. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc. Natl. Acad. Sci. USA 86:3803-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hayes, M. P., S. L. Freeman, and R. P. Donnelly. 1995. IFN-gamma priming of monocytes enhances LPS-induced TNF production by augmenting both transcription and MRNA stability. Cytokine 7:427-435. [DOI] [PubMed] [Google Scholar]
- 188.Heggers, J., R. E. Goodheart, J. Washington, L. McCoy, E. Carino, T. Dang, P. Edgar, C. Maness, and D. Chinkes. 2005. Therapeutic efficacy of three silver dressings in an infected animal model. J. Burn Care Rehabil. 26:53-56. [DOI] [PubMed] [Google Scholar]
- 189.Heggers, J. P., H. Hawkins, P. Edgar, C. Villarreal, and D. N. Herndon. 2002. Treatment of infections in burns, p. 120-169. In D. N. Herndon (ed.), Total burn care. Saunders, London, England.
- 190.Heggers, J. P., L. McCoy, B. Reisner, M. Smith, P. Edgar, and R. J. Ramirez. 1998. Alternate antimicrobial therapy for vancomycin-resistant enterococci burn wound infections. J. Burn Care Rehabil. 19:399-403. [DOI] [PubMed] [Google Scholar]
- 191.Heggers, J. P., and M. C. Robson. 1986. Infection control in burn patients. Clin. Plast. Surg. 13:39-47. [PubMed] [Google Scholar]
- 192.Heggers, J. P., M. C. Robson, D. N. Herndon, and M. H. Desai. 1989. The efficacy of nystatin combined with topical microbial agents in the treatment of burn wound sepsis. J. Burn Care Rehabil. 10:508-511. [DOI] [PubMed] [Google Scholar]
- 193.Heggers, J. P., V. Velanovich, M. C. Robson, S. M. Zoellner, R. Schileru, J. Boertman, and X. T. Niu. 1987. Control of burn wound sepsis: a comparison of in vitro topical antimicrobial assays. J. Trauma 27:176-179. [DOI] [PubMed] [Google Scholar]
- 194.Heideman, M., and A. Bengtsson. 1992. The immunologic response to thermal injury. World J. Surg. 16:53-56. [DOI] [PubMed] [Google Scholar]
- 195.Heimbach, D., R. Mann, and L. Engrav. 2002. Evaluation of the burn wound management decisions, p. 101-108. In D. Herndon (ed.), Total burn care. Saunders, London, England.
- 196.Heimbach, D. M. 1987. Early burn excision and grafting. Surg. Clin. N. Am. 67:93-107. [DOI] [PubMed] [Google Scholar]
- 197.Heimbach, D. M., T. D. Kirksey, J. M. Shuck, and B. A. Pruitt, Jr. 1969. Gastrointestinal complications of burns. GP. 40:110-115. [PubMed] [Google Scholar]
- 198.Heitland, A., A. Piatkowski, E. M. Noah, and N. Pallua. 2004. Update on the use of collagen/glycosaminoglycate skin substitute-six years of experiences with artificial skin in 15 German burn centers. Burns 30:471-475. [DOI] [PubMed] [Google Scholar]
- 199.Hemington-Gorse, S. J. 2005. A comparison of laser Doppler imaging with other measurement techniques to assess burn depth. J. Wound. Care 14:151-153. [DOI] [PubMed] [Google Scholar]
- 200.Herndon, D. N., D. Gore, M. Cole, M. H. Desai, H. Linares, S. Abston, T. Rutan, T. Van Osten, and R. E. Barrow. 1987. Determinants of mortality in pediatric patients with greater than 70% full-thickness total body surface area thermal injury treated by early total excision and grafting. J. Trauma 27:208-212. [DOI] [PubMed] [Google Scholar]
- 201.Herndon, D. N., F. Langner, P. Thompson, H. A. Linares, M. Stein, and D. L. Traber. 1987. Pulmonary injury in burned patients. Surg. Clin. N. Am. 67:31-46. [DOI] [PubMed] [Google Scholar]
- 202.Herndon, D. N., and M. Spies. 2001. Modern burn care. Semin. Pediatr. Surg. 10:28-31. [DOI] [PubMed] [Google Scholar]
- 203.Herndon, D. N., D. L. Traber, G. D. Niehaus, H. A. Linares, and L. D. Traber. 1984. The pathophysiology of smoke inhalation injury in a sheep model. J. Trauma 24:1044-1051. [DOI] [PubMed] [Google Scholar]
- 204.Herruzo-Cabrera, R., V. Garcia-Torres, J. Rey-Calero, and M. J. Vizcaino-Alcaide. 1992. Evaluation of the penetration strength, bactericidal efficacy and spectrum of action of several antimicrobial creams against isolated microorganisms in a burn centre. Burns 18:39-44. [DOI] [PubMed] [Google Scholar]
- 205.Herruzo-Cabrera, R., M. J. Vizcaino-Alcaide, C. Pinedo-Castillo, and J. Rey-Calero. 1992. Diagnosis of local infection of a burn by semiquantitative culture of the eschar surface. J. Burn Care Rehabil. 13:639-641. [DOI] [PubMed] [Google Scholar]
- 206.Hindiyeh, M., V. Acevedo, and K. C. Carroll. 2001. Comparison of three transport systems (Starplex StarSwab II, the new Copan Vi-Pak Amies Agar Gel collection and transport swabs, and BBL Port-A-Cul) for maintenance of anaerobic and fastidious aerobic organisms. J. Clin. Microbiol. 39:377-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Holder, I. A., M. Schwab, and L. Jackson. 1979. Eighteen months of routine topical antimicrobial susceptibility testing of isolates from burn patients: results and conclusions. J. Antimicrob. Chemother. 5:455-463. [DOI] [PubMed] [Google Scholar]
- 208.Holm, C., J. Tegeler, M. Mayr, U. Pfeiffer, G. Henckel von Donnersmarck, and W. Muhlbauer. 2002. Effect of crystalloid resuscitation and inhalation injury on extravascular lung water: clinical implications. Chest 121:1956-1962. [DOI] [PubMed] [Google Scholar]
- 209.Honari, S. 2004. Topical therapies and antimicrobials in the management of burn wounds. Crit. Care Nurs. Clin. N. Am. 16:1-11. [DOI] [PubMed] [Google Scholar]
- 210.Horgan, A. F., M. V. Mendez, D. S. O'Riordain, R. G. Holzheimer, J. A. Mannick, and M. L. Rodrick. 1994. Altered gene transcription after burn injury results in depressed T-lymphocyte activation. Ann. Surg. 220:342-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Horner, B. M., H. Ahmadi, R. Mulholland, S. R. Myers, and J. Catalan. 2005. Case-controlled study of patients with self-inflicted burns. Burns 31:471-475. [DOI] [PubMed] [Google Scholar]
- 212.Hsueh, P. R., L. J. Teng, P. C. Yang, Y. C. Chen, S. W. Ho, and K. T. Luh. 1998. Persistence of a multidrug-resistant Pseudomonas aeruginosa clone in an intensive care burn unit. J. Clin. Microbiol. 36:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Huang, C. T., and H. S. Leu. 1995. Candiduria as an early marker of disseminated infection in critically ill surgical patients. J. Trauma 39:616. [DOI] [PubMed] [Google Scholar]
- 214.Hugli, T. E. 1984. Complement and cellular triggering reactions. Introductory remarks. Fed. Proc. 43:2540-2542. [PubMed] [Google Scholar]
- 215.Hunt, J. L. 2000. The 2000 presidential address. Back to the future: the ABA and burn prevention. J. Burn Care Rehabil. 21:474-483. [PubMed] [Google Scholar]
- 216.Hunt, J. L., B. G. McGranahan, and B. A. Pruitt, Jr. 1973. Burn-wound management. Heart Lung 2:690-695. [PubMed] [Google Scholar]
- 217.Hunt, J. L., and G. F. Purdue. 1992. The elderly burn patient. Am. J. Surg. 164:472-476. [DOI] [PubMed] [Google Scholar]
- 218.Janzekovic, J. 1970. A new concept in the early excision and immediate grafting of burns. J. Trauma 10:1103-1108. [PubMed] [Google Scholar]
- 219.Janzekovic, Z. 1975. The burn wound from the surgical point of view. J. Trauma 15:42-62. [PubMed] [Google Scholar]
- 220.Jarrett, F., E. Balish, J. A. Moylan, and S. Ellerbe. 1978. Clinical experience with prophylactic antibiotic bowel suppression in burn patients. Surgery 83:523-527. [PubMed] [Google Scholar]
- 221.Jarrett, F., C. K. Chan, E. Balish, and J. Moylan. 1976. Antibiotic bowel preparation and burn wound colonization. Surg. Forum 27:67-68. [PubMed] [Google Scholar]
- 222.Jay, K. M., R. H. Bartlett, R. Danet, and P. A. Allyn. 1977. Burn epidemiology: a basis for burn prevention. J. Trauma 17:943-947. [PubMed] [Google Scholar]
- 223.Jelenko, C., III. 1974. Chemicals that “burn.” J. Trauma 14:65-72. [PubMed] [Google Scholar]
- 224.Jeng, J. C., A. Bridgeman, L. Shivnan, P. M. Thornton, H. Alam, T. J. Clarke, K. A. Jablonski, and M. H. Jordan. 2003. Laser Doppler imaging determines need for excision and grafting in advance of clinical judgment: a prospective blinded trial. Burns 29:665-670. [DOI] [PubMed] [Google Scholar]
- 225.Jones, I., L. Currie, and R. Martin. 2002. A guide to biological skin substitutes. Br. J. Plast. Surg. 55:185-193. [DOI] [PubMed] [Google Scholar]
- 226.Kagan, R. J., S. Naraqi, T. Matsuda, and O. M. Jonasson. 1985. Herpes simplex virus and cytomegalovirus infections in burned patients. J. Trauma 25:40-45. [DOI] [PubMed] [Google Scholar]
- 227.Kay, G. D. 1957. Prolonged survival of a skin homograft in a patient with very extensive burns. Ann. N. Y. Acad. Sci. 64:767-774. [DOI] [PubMed] [Google Scholar]
- 228.Keith, D. D., K. M. Garrett, G. Hickox, B. Echols, and E. Comeau. 2004. Ventilator-associated pneumonia: improved clinical outcomes. J. Nurs. Care Qual. 19:328-333. [DOI] [PubMed] [Google Scholar]
- 229.Kimura, R., L. D. Traber, D. N. Herndon, G. D. Neuhaus, and D. L. Traber. 1988. Treatment of smoke-induced pulmonary injury with nebulized dimethylsulfoxide. Circ. Shock 25:333-341. [PubMed] [Google Scholar]
- 230.Klein, M. B., L. H. Engrav, J. H. Holmes, J. B. Friedrich, B. A. Costa, S. Honari, and N. S. Gibran. 2005. Management of facial burns with a collagen/glycosaminoglycan skin substitute-prospective experience with 12 consecutive patients with large, deep facial burns. Burns 31:257-261. [DOI] [PubMed] [Google Scholar]
- 231.Klein, R. L., B. F. Rothmann, and R. Marshall. 1984. Biobrane—a useful adjunct in the therapy of outpatient burns. J. Pediatr. Surg. 19:846-847. [DOI] [PubMed] [Google Scholar]
- 232.Koumbourlis, A. C. 2002. Electrical injuries. Crit. Care Med. 30:S424-S430. [DOI] [PubMed] [Google Scholar]
- 233.Kuhn, D. M., and M. A. Ghannoum. 2004. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr. Opin. Investig. Drugs 5:186-197. [PubMed] [Google Scholar]
- 234.Kullberg, B. J., J. D. Sobel, M. Ruhnke, P. G. Pappas, C. Viscoli, J. H. Rex, J. D. Cleary, E. Rubinstein, L. W. Church, J. M. Brown, H. T. Schlamm, I. T. Oborska, F. Hilton, and M. R. Hodges. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 366:1435-1442. [DOI] [PubMed] [Google Scholar]
- 235.Kuroda, T., T. Harada, H. Tsutsumi, and M. Kobayashi. 1997. Hypernatremic suppression of neutrophils. Burns 23:338-340. [DOI] [PubMed] [Google Scholar]
- 236.Lansdown, A. B. 2002. Silver. 2. Toxicity in mammals and how its products aid wound repair. J. Wound Care 11:173-177. [DOI] [PubMed] [Google Scholar]
- 237.Lansdown, A. B. 2002. Silver. 1. Its antibacterial properties and mechanism of action. J. Wound Care 11:125-130. [DOI] [PubMed] [Google Scholar]
- 238.Lansdown, A. B., A. Williams, S. Chandler, and S. Benfield. 2005. Silver absorption and antibacterial efficacy of silver dressings. J. Wound Care 14:155-160. [DOI] [PubMed] [Google Scholar]
- 239.Laupland, K. B., D. L. Church, and D. B. Gregson. 2005. Validation of a rapid diagnostic strategy for determination of significant bacterial counts in bronchoalveolar lavage samples. Arch. Pathol. Lab. Med. 129:78-81. [DOI] [PubMed] [Google Scholar]
- 240.Laupland, K. B., D. B. Gregson, D. L. Church, T. Ross, and S. Elsayed. 2005. Invasive Candida species infections: a 5 year population-based assessment. J. Antimicrob. Chemother. 56:532-537. [DOI] [PubMed] [Google Scholar]
- 241.Laupland, K. B., M. D. Parkins, D. L. Church, D. B. Gregson, T. J. Louie, J. M. Conly, S. Elsayed, and J. D. Pitout. 2005. Population-based epidemiological study of infections caused by carbapenem-resistant Pseudomonas aeruginosa in the Calgary Health Region: importance of metallo-beta-lactamase (MBL)-producing strains. J. Infect. Dis. 192:1606-1612. [DOI] [PubMed] [Google Scholar]
- 242.Lavaud, P., J. Mathieu, P. Bienvenu, M. Braquet, P. Gerasimo, J. F. Kergonou, and R. Ducousso. 1988. Modulation of leucocyte activation in the early phase of the rabbit burn injury. Burns Incl. Therm. Inj. 14:15-20. [DOI] [PubMed] [Google Scholar]
- 243.Lawrence, J. C., and J. Soothill. 1989. Burn wound infection. Burns 15:342. [DOI] [PubMed] [Google Scholar]
- 244.Lederer, J. A., M. L. Rodrick, and J. A. Mannick. 1999. The effects of injury on the adaptive immune response. Shock 11:153-159. [DOI] [PubMed] [Google Scholar]
- 245.Lesseva, M. 1998. Central venous catheter-related bacteraemia in burn patients. Scand. J. Infect. Dis. 30:585-589. [DOI] [PubMed] [Google Scholar]
- 246.Lesseva, M., B. P. Girgitzova, and C. Bojadjiev. 1994. Beta-haemolytic streptococcal infections in burned patients. Burns 20:422-425. [DOI] [PubMed] [Google Scholar]
- 247.Levine, N. S., R. B. Lindberg, A. D. Mason, Jr., and B. A. Pruitt, Jr. 1976. The quantitative swab culture and smear: a quick, simple method for determining the number of viable aerobic bacteria on open wounds. J. Trauma 16:89-94. [PubMed] [Google Scholar]
- 248.LeVoyer, T., W. G. Cioffi, Jr., L. Pratt, R. Shippee, W. F. McManus, A. D. Mason, Jr., and B. A. Pruitt, Jr. 1992. Alterations in intestinal permeability after thermal injury. Arch. Surg. 127:26-29. [DOI] [PubMed] [Google Scholar]
- 249.Liedberg, N. C., L. R. Kuhn, B. A. Barnes, E. Reiss, and W. H. Mspacher. 1954. Infection in burns. II. The pathogenicity of streptococci. Surg. Gynecol. Obstet. 98:693-699. [PubMed] [Google Scholar]
- 250.Lilly, H. A., E. J. Lowbury, M. D. Wilkins, and J. S. Cason. 1979. Staphylococcal sepsis in a burns unit. J. Hyg. (London) 83:429-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Linares, H. A., D. N. Herndon, and D. L. Traber. 1989. Sequence of morphologic events in experimental smoke inhalation. J. Burn Care Rehabil. 10:27-37. [DOI] [PubMed] [Google Scholar]
- 252.Lionelli, G. T., E. J. Pickus, O. K. Beckum, R. L. Decoursey, and R. A. Korentager. 2005. A three decade analysis of factors affecting burn mortality in the elderly. Burns 31:958-963. [DOI] [PubMed]
- 253.Lloyd, J. R., and D. W. Hight. 1978. Early laminar excision: improved control of burn wound sepsis by partial dermatome debridement. J. Pediatr. Surg. 13:698-706. [DOI] [PubMed] [Google Scholar]
- 254.Loebl, E. C., J. A. Marvin, E. L. Heck, P. W. Curreri, and C. R. Baxter. 1974. The use of quantitative biopsy cultures in bacteriologic monitoring of burn patients. J. Surg. Res. 16:1-5. [DOI] [PubMed] [Google Scholar]
- 255.Loebl, E. C., J. A. Marvin, E. L. Heck, P. W. Curreri, and C. R. Baxter. 1974. The method of quantitative burn-wound biopsy cultures and its routine use in the care of the burned patient. Am. J. Clin. Pathol. 61:20-24. [DOI] [PubMed] [Google Scholar]
- 256.Lyons, A., J. L. Kelly, M. L. Rodrick, J. A. Mannick, and J. A. Lederer. 1997. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann. Surg. 226:450-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Macintire, D. K., and T. L. Bellhorn. 2002. Bacterial translocation: clinical implications and prevention. Vet. Clin. N. Am. Small Anim. Pract. 32:1165-1178. [DOI] [PubMed] [Google Scholar]
- 258.Mack, D., P. Becker, I. Chatterjee, S. Dobinsky, J. K. Knobloch, G. Peters, H. Rohde, and M. Herrmann. 2004. Mechanisms of biofilm formation in Staphylococcus epidermidis and Staphylococcus aureus: functional molecules, regulatory circuits, and adaptive responses. Int. J. Med. Microbiol. 294:203-212. [DOI] [PubMed] [Google Scholar]
- 259.MacMillan, B. G. 1980. Infections following burn injury. Surg. Clin. N. Am. 60:185-196. [DOI] [PubMed] [Google Scholar]
- 260.MacMillan, B. G., E. O. Hill, and W. A. Altemeier. 1967. Use of topical silver nitrate, mafenide, and gentamicin in the burn patient. A comparative study. Arch. Surg. 95:472-481. [DOI] [PubMed] [Google Scholar]
- 261.Madden, M. R., J. L. Finkelstein, and C. W. Goodwin. 1986. Respiratory care of the burn patient. Clin. Plast. Surg. 13:29-38. [PubMed] [Google Scholar]
- 262.Majeski, J. A., and B. G. MacMillan. 1977. Fatal systemic mycotic infections in the burned child. J. Trauma 17:320-322. [DOI] [PubMed] [Google Scholar]
- 263.Maki, D. G. 1976. Preventing infection in intravenous therapy. Hosp. Pract. 11:95-104. [DOI] [PubMed] [Google Scholar]
- 264.Maki, D. G., J. T. Botticelli, M. L. LeRoy, and T. S. Thielke. 1987. Prospective study of replacing administration sets for intravenous therapy at 48- vs 72-hour intervals. 72 hours is safe and cost-effective. JAMA 258:1777-1781. [PubMed] [Google Scholar]
- 265.Mandelcorn, E., M. Gomez, and R. C. Cartotto. 2003. Work-related burn injuries in Ontario, Canada: has anything changed in the last 10 years? Burns 29:469-472. [DOI] [PubMed] [Google Scholar]
- 266.Manson, W. L., J. M. Coenen, H. J. Klasen, and E. H. Horwitz. 1992. Intestinal bacterial translocation in experimentally burned mice with wounds colonized by Pseudomonas aeruginosa. J. Trauma 33:654-658. [DOI] [PubMed] [Google Scholar]
- 267.Manson, W. L., H. J. Klasen, E. W. Sauer, and A. Olieman. 1992. Selective intestinal decontamination for prevention of wound colonization in severely burned patients: a retrospective analysis. Burns 18:98-102. [DOI] [PubMed] [Google Scholar]
- 268.Manson, W. L., P. C. Pernot, V. Fidler, E. W. Sauer, and H. J. Klasen. 1992. Colonization of burns and the duration of hospital stay of severely burned patients. J. Hosp. Infect. 22:55-63. [DOI] [PubMed] [Google Scholar]
- 269.Manson, W. L., A. W. Westerveld, H. J. Klasen, and E. W. Sauer. 1987. Selective intestinal decontamination of the digestive tract for infection prophylaxis in severely burned patients. Scand. J. Plast. Reconstr. Surg. Hand Surg. 21:269-272. [DOI] [PubMed] [Google Scholar]
- 270.Marvin, J. A., E. L. Heck, E. C. Loebl, P. W. Curreri, and C. R. Baxter. 1975. Usefulness of blood cultures in confirming septic complications in burn patients: evaluation of a new culture method. J. Trauma 15:657-662. [DOI] [PubMed] [Google Scholar]
- 271.Maslow, J. N., T. Glaze, P. Adams, and M. Lataillade. 2005. Concurrent outbreak of multidrug-resistant and susceptible subclones of Acinetobacter baumannii affecting different wards of a single hospital. Infect. Control Hosp. Epidemiol. 26:69-75. [DOI] [PubMed] [Google Scholar]
- 272.Mason, A. D., Jr., A. T. McManus, and B. A. Pruitt, Jr. 1986. Association of burn mortality and bacteremia. A 25-year review. Arch. Surg. 121:1027-1031. [DOI] [PubMed] [Google Scholar]
- 273.Mayhall, C. G. 2003. The epidemiology of burn wound infections: then and now. Clin. Infect. Dis. 37:543-550. [DOI] [PubMed] [Google Scholar]
- 274.Mayhall, C. G., V. A. Lamb, W. E. Gayle, Jr., and B. W. Haynes, Jr. 1979. Enterobacter cloacae septicemia in a burn center: epidemiology and control of an outbreak. J. Infect. Dis. 139:166-171. [DOI] [PubMed] [Google Scholar]
- 275.McCall, J. E., and T. J. Cahill. 2005. Respiratory care of the burn patient. J. Burn Care Rehabil. 26:200-206. [PubMed] [Google Scholar]
- 276.McCampbell, B., N. Wasif, A. Rabbitts, L. Staiano-Coico, R. W. Yurt, and S. Schwartz. 2002. Diabetes and burns: retrospective cohort study. J. Burn Care Rehabil. 23:157-166. [DOI] [PubMed] [Google Scholar]
- 277.McCormack, P. L., and C. M. Perry. 2005. Caspofungin: a review of its use in the treatment of fungal infections. Drugs 65:2049-2068. [DOI] [PubMed] [Google Scholar]
- 278.McGill, S. N., and R. C. Cartotto. 2000. Herpes simplex virus infection in a paediatric burn patient: case report and review. Burns 26:194-199. [DOI] [PubMed] [Google Scholar]
- 279.McGill, V., A. Kowal-Vern, and R. L. Gamelli. 2000. Outcome for older burn patients. Arch. Surg. 135:320-325. [DOI] [PubMed] [Google Scholar]
- 280.McGuckin, M. B., R. J. Thorpe, and E. Abrutyn. 1981. Hydrotherapy: an outbreak of Pseudomonas aeruginosa wound infections related to Hubbard tank treatments. Arch. Phys. Med. Rehabil. 62:283-285. [PubMed] [Google Scholar]
- 281.McLoughlin, G. A., A. V. Wu, I. Saporoschetz, R. Nimberg, and J. A. Mannick. 1979. Correlation between anergy and a circulating immunosuppressive factor following major surgical trauma. Ann. Surg. 190:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.McManus, A. T., S. H. Kim, W. F. McManus, A. D. Mason, Jr., and B. A. Pruitt, Jr. 1987. Comparison of quantitative microbiology and histopathology in divided burn-wound biopsy specimens. Arch. Surg. 122:74-76. [DOI] [PubMed] [Google Scholar]
- 283.McManus, A. T., R. B. Lindberg, B. A. Pruitt, Jr., and A. D. Mason, Jr. 1979. Association of endotoxemia and bacteremia in burn patients: a prospective study. Prog. Clin. Biol. Res. 29:275-278. [PubMed] [Google Scholar]
- 284.McManus, A. T., A. D. Mason, Jr., W. F. McManus, and B. A. Pruitt, Jr. 1994. A decade of reduced gram-negative infections and mortality associated with improved isolation of burned patients. Arch. Surg. 129:1306-1309. [DOI] [PubMed] [Google Scholar]
- 285.McManus, W. F., C. W. Goodwin, A. D. Mason, Jr., and B. A. Pruitt, Jr. 1981. Burn wound infection. J. Trauma 21:753-756. [DOI] [PubMed] [Google Scholar]
- 286.McManus, W. F., A. D. Mason, Jr., and B. A. Pruitt, Jr. 1989. Excision of the burn wound in patients with large burns. Arch. Surg. 124:718-720. [DOI] [PubMed] [Google Scholar]
- 287.Meier, P. A., C. D. Carter, S. E. Wallace, R. J. Hollis, M. A. Pfaller, and L. A. Herwaldt. 1996. A prolonged outbreak of methicillin-resistant Staphylococcus aureus in the burn unit of a tertiary medical center. Infect. Control Hosp. Epidemiol. 17:798-802. [DOI] [PubMed] [Google Scholar]
- 288.Meka, V. G., S. K. Pillai, G. Sakoulas, C. Wennersten, L. Venkataraman, P. C. DeGirolami, G. M. Eliopoulos, R. C. Moellering, Jr., and H. S. Gold. 2004. Linezolid resistance in sequential Staphylococcus aureus isolates associated with a T2500A mutation in the 23S rRNA gene and loss of a single copy of rRNA. J. Infect. Dis. 190:311-317. [DOI] [PubMed] [Google Scholar]
- 289.Mele, J. A., III, S. A. Linder, R. Calabria, and C. J. Ikeda. 1998. HIV seropositivity in a burn center's population. J. Burn Care Rehabil. 19:138-141. [DOI] [PubMed] [Google Scholar]
- 290.Memmel, H., A. Kowal-Vern, and B. A. Latenser. 2004. Infections in diabetic burn patients. Diabetes Care 27:229-233. [DOI] [PubMed] [Google Scholar]
- 291.Merz, J., C. Schrand, D. Mertens, C. Foote, K. Porter, and L. Regnold. 2003. Wound care of the pediatric burn patient. AACN Clin. Issues 14:429-441. [DOI] [PubMed] [Google Scholar]
- 292.Miller, T. R., N. M. Pindus, J. B. Douglas, and B. Rossman. 1995. Databook on nonfatal injury: incidence, costs and consequences. The Urban Institute Press, Washington, D.C.
- 293.Mis, B., E. Rolland, and V. Ronfard. 2004. Combined use of a collagen-based dermal substitute and a fibrin-based cultured epithelium: a step toward a total skin replacement for acute wounds. Burns 30:713-719. [DOI] [PubMed] [Google Scholar]
- 294.Mitchell, V., J. P. Galizia, and L. Fournier. 1989. Precise diagnosis of infection in burn wound biopsy specimens. Combination of histologic technique, acridine orange staining, and culture. J. Burn Care Rehabil. 10:195-202. [DOI] [PubMed] [Google Scholar]
- 295.Monafo, W. W. 1974. Tangential excision. Clin. Plast. Surg. 1:591-601. [PubMed] [Google Scholar]
- 296.Monafo, W. W., C. E. Aulenbacher, and C. Pappalardo. 1972. Early tangential excision of the eschars of major burns. Arch. Surg. 104:503-508. [DOI] [PubMed] [Google Scholar]
- 297.Monafo, W. W., and P. Q. Bessey. 2002. Wound care, p. 109-119. In D. Herndon (ed.), Total burn care. Saunders, London, England.
- 298.Monafo, W. W., and M. A. West. 1990. Current treatment recommendations for topical burn therapy. Drugs 40:364-373. [DOI] [PubMed] [Google Scholar]
- 299.Moritz, A. R., and F. C. Heuriquez. 1947. Studies of thermal injury. II. The relative importance of time and surface temperature in the causation of cutaneous burns. Am. J. Pathol. 23:695-720. [PMC free article] [PubMed] [Google Scholar]
- 300.Mousa, H. A. 1997. Aerobic, anaerobic and fungal burn wound infections. J. Hosp. Infect. 37:317-323. [DOI] [PubMed] [Google Scholar]
- 301.Mousa, H. A. 1999. Fungal infection of burn wounds in patients with open and occlusive treatment methods. East. Mediterr. Health J. 5:333-336. [PubMed] [Google Scholar]
- 302.Mousa, H. A., and S. M. al-Bader. 2001. Yeast infection of burns. Mycoses 44:147-149. [DOI] [PubMed] [Google Scholar]
- 303.Mousa, H. A., S. M. al-Bader, and D. A. Hassan. 1999. Correlation between fungi isolated from burn wounds and burn care units. Burns 25:145-147. [DOI] [PubMed] [Google Scholar]
- 304.Mozingo, D. W., A. T. McManus, S. H. Kim, and B. A. Pruitt, Jr. 1997. Incidence of bacteremia after burn wound manipulation in the early postburn period. J. Trauma 42:1006-1010. [DOI] [PubMed] [Google Scholar]
- 305.Mozingo, D. W., A. A. Smith, W. F. McManus, B. A. Pruitt, Jr., and A. D. Mason, Jr. 1988. Chemical burns. J. Trauma 28:642-647. [DOI] [PubMed] [Google Scholar]
- 306.Mueller, E. W., S. D. Hanes, M. A. Croce, G. C. Wood, B. A. Boucher, and T. C. Fabian. 2005. Effect from multiple episodes of inadequate empiric antibiotic therapy for ventilator-associated pneumonia on morbidity and mortality among critically ill trauma patients. J. Trauma 58:94-101. [DOI] [PubMed] [Google Scholar]
- 307.Murphy, K. D., J. O. Lee, and D. N. Herndon. 2003. Current pharmacotherapy for the treatment of severe burns. Expert Opin. Pharmacother. 4:369-384. [DOI] [PubMed] [Google Scholar]
- 308.Murray, P. M., and S. M. Finegold. 1984. Anaerobes in burn-wound infections. Rev. Infect. Dis. 6(Suppl. 1):S184-S186. [DOI] [PubMed] [Google Scholar]
- 309.Mutnick, A. H., V. Enne, and R. N. Jones. 2003. Linezolid resistance since 2001: SENTRY Antimicrobial Surveillance Program. Ann. Pharmacother. 37:769-774. [DOI] [PubMed] [Google Scholar]
- 310.Mzezewa, S., K. Jonsson, E. Sibanda, M. Aberg, and L. Salemark. 2003. HIV infection reduces skin graft survival in burn injuries: a prospective study. Br. J. Plast. Surg. 56:740-745. [DOI] [PubMed] [Google Scholar]
- 311.Nadler, E. P., J. S. Upperman, E. C. Dickinson, and H. R. Ford. 1999. Nitric oxide and intestinal barrier failure. Semin. Pediatr. Surg. 8:148-154. [DOI] [PubMed] [Google Scholar]
- 312.Nakae, H., H. Tanaka, and H. Inaba. 2001. Failure to clear casts and secretions following inhalation injury can be dangerous: report of a case. Burns 27:189-191. [DOI] [PubMed] [Google Scholar]
- 313.Namias, N., L. Samiian, D. Nino, E. Shirazi, K. O'Neill, D. H. Kett, E. Ginzburg, M. G. McKenney, D. Sleeman, and S. M. Cohn. 2000. Incidence and susceptibility of pathogenic bacteria vary between intensive care units within a single hospital: implications for empiric antibiotic strategies. J. Trauma 49:638-645. [DOI] [PubMed] [Google Scholar]
- 314.Nash, G., F. D. Foley, M. N. Goodwin, Jr., H. M. Bruck, K. A. Greenwald, and B. A. Pruitt, Jr. 1971. Fungal burn wound infection. JAMA 215:1664-1666. [PubMed] [Google Scholar]
- 315.Nasser, S., A. Mabrouk, and A. Maher. 2003. Colonization of burn wounds in Ain Shams University Burn Unit. Burns 29:229-233. [DOI] [PubMed] [Google Scholar]
- 316.Nassoura, Z., R. R. Ivatury, R. J. Simon, N. Jabbour, and W. M. Stahl. 1993. Candiduria as an early marker of disseminated infection in critically ill surgical patients: the role of fluconazole therapy. J. Trauma 35:290-294. [DOI] [PubMed] [Google Scholar]
- 317.Nathan, P., E. J. Law, D. F. Murphy, and B. G. McMillan. 1979. A laboratory method for selection of topical antimicrobial agents to treat infected burn wounds. Burns 4:177-187. [Google Scholar]
- 318.National Center for Injury Prevention and Control. 2002. United States fire/burn deaths and rates per 100,000. Office of Statistics and Programming, Centers for Disease Control and Prevention, Atlanta, Ga.
- 319.National Fire Data Center. 1993. Fire in the United States, 1983 to 1990. National Fire Data Center, Emmitsburg, Md.
- 320.National Safe Kids Campaign. 2002. Burn injury fact sheet. http://www.safekids.ca. [Online.]
- 321.Newton, T., J. M. Still, and E. Law. 2002. A comparison of the effect of early insertion of standard latex and silver-impregnated latex foley catheters on urinary tract infections in burn patients. Infect. Control Hosp. Epidemiol. 23:217-218. [DOI] [PubMed] [Google Scholar]
- 322.Nguyen, T. T., D. A. Gilpin, N. A. Meyer, and D. N. Herndon. 1996. Current treatment of severely burned patients. Ann. Surg. 223:14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Niederman, M. S. 2004. Therapy of ventilator-associated pneumonia: what more can we do to use less antibiotics? Crit. Care Med. 32:2344-2345. [DOI] [PubMed] [Google Scholar]
- 324.Ninnemann, J. L., J. C. Fisher, and H. A. Frank. 1978. Prolonged survival of human skin allografts following thermal injury. Transplantation 25:69-72. [DOI] [PubMed] [Google Scholar]
- 325.Nohr, C. W., N. V. Christou, H. Rode, J. Gordon, and J. L. Meakins. 1984. In vivo and in vitro humoral immunity in surgical patients. Ann. Surg. 200:373-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Olson, M. E., H. Ceri, D. W. Morck, A. G. Buret, and R. R. Read. 2002. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can. J. Vet. Res. 66:86-92. [PMC free article] [PubMed] [Google Scholar]
- 327.O'Mahony, J. B., J. J. Wood, M. L. Rodrick, and J. A. Mannick. 1985. Changes in T lymphocyte subsets following injury. Assessment by flow cytometry and relationship to sepsis. Ann. Surg. 202:580-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.O'Neill, J. A., Jr., B. A. Pruitt, Jr., F. D. Foley, and J. A. Moncrief. 1968. Suppurative thrombophlebitis—a lethal complication of intravenous therapy. J. Trauma 8:256-267. [DOI] [PubMed] [Google Scholar]
- 329.Oswald, I. P., T. A. Wynn, A. Sher, and S. L. James. 1992. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor alpha required as a costimulatory factor for interferon gamma-induced activation. Proc. Natl. Acad. Sci. USA 89:8676-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Palmieri, T. L., and D. G. Greenhalgh. 2002. Topical treatment of pediatric patients with burns: a practical guide. Am. J. Clin. Dermatol. 3:529-534. [DOI] [PubMed] [Google Scholar]
- 331.Peck, M. D., J. Weber, A. McManus, R. Sheridan, and D. Heimbach. 1998. Surveillance of burn wound infections: a proposal for definitions. J. Burn Care Rehabil. 19:386-389. [DOI] [PubMed] [Google Scholar]
- 332.Pham, T. N., J. R. King, T. L. Palmieri, and D. G. Greenhalgh. 2003. Predisposing factors for self-inflicted burns. J. Burn Care Rehabil. 24:223-227. [DOI] [PubMed] [Google Scholar]
- 333.Phillips, L. G., J. P. Heggers, M. C. Robson, J. A. Boertman, T. Meltzer, and D. J. Smith, Jr. 1989. The effect of endogenous skin bacteria on burn wound infection. Ann. Plast. Surg. 23:35-38. [DOI] [PubMed] [Google Scholar]
- 334.Pietsch, J. B., D. T. Netscher, H. S. Nagaraj, and D. B. Groff. 1985. Early excision of major burns in children: effect on morbidity and mortality. J. Pediatr. Surg. 20:754-757. [DOI] [PubMed] [Google Scholar]
- 335.Pirnay, J. P., D. De Vos, C. Cochez, F. Bilocq, J. Pirson, M. Struelens, L. Duinslaeger, P. Cornelis, M. Zizi, and A. Vanderkelen. 2003. Molecular epidemiology of Pseudomonas aeruginosa colonization in a burn unit: persistence of a multidrug-resistant clone and a silver sulfadiazine-resistant clone. J. Clin. Microbiol. 41:1192-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Pirnay, J. P., V. D. De, L. Duinslaeger, P. Reper, C. Vandenvelde, P. Cornelis, and A. Vanderkelen. 2000. Quantitation of Pseudomonas aeruginosa in wound biopsy samples: from bacterial culture to rapid ‘real-time’ polymerase chain reaction. Crit. Care 4:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Podnos, Y. D., M. E. Cinat, S. E. Wilson, J. Cooke, W. Gornick, and L. D. Thrupp. 2001. Eradication of multi-drug resistant Acinetobacter from an intensive care unit. Surg. Infect. (Larchmont) 2:297-301. [DOI] [PubMed] [Google Scholar]
- 338.Polderman, K. H., and A. R. Girbes. 2004. Drug intervention trials in sepsis: divergent results. Lancet 363:1721-1723. [DOI] [PubMed] [Google Scholar]
- 339.Polk, R. E., C. G. Mayhall, J. Smith, G. Hall, B. J. Kline, E. Swensson, and B. W. Haynes. 1983. Gentamicin and tobramycin penetration into burn eschar. Pharmacokinetics and microbiological effects. Arch. Surg. 118:295-302. [DOI] [PubMed] [Google Scholar]
- 340.Pruitt, B. A., Jr. 1990. Infection and the burn patient. Br. J. Surg. 77:1081-1082. [DOI] [PubMed] [Google Scholar]
- 341.Pruitt, B. A., Jr. 1984. The diagnosis and treatment of infection in the burn patient. Burns Incl. Therm. Inj. 11:79-91. [DOI] [PubMed] [Google Scholar]
- 342.Pruitt, B. A., Jr., W. G. Cioffi, T. Shimazu, H. Ikeuchi, and A. D. Mason, Jr. 1990. Evaluation and management of patients with inhalation injury. J. Trauma 30:S63-S68. [DOI] [PubMed] [Google Scholar]
- 343.Pruitt, B. A., Jr., and F. D. Foley. 1973. The use of biopsies in burn patient care. Surgery 73:887-897. [PubMed] [Google Scholar]
- 344.Pruitt, B. A., C. W. Goodwin, and D. Mason Jr. 2002. Epidemiological, demographic, and outcome characteristics of burn injury, p. 16-30. In D. Herndon (ed.), Total burn care. Saunders, London, England.
- 345.Pruitt, B. A., Jr., and A. T. McManus. 1992. The changing epidemiology of infection in burn patients. World J. Surg. 16:57-67. [DOI] [PubMed] [Google Scholar]
- 346.Pruitt, B. A., Jr., A. T. McManus, S. H. Kim, and C. W. Goodwin. 1998. Burn wound infections: current status. World J. Surg. 22:135-145. [DOI] [PubMed] [Google Scholar]
- 347.Pruitt, B. A., Jr., W. F. McManus, S. H. Kim, and R. C. Treat. 1980. Diagnosis and treatment of cannula-related intravenous sepsis in burn patients. Ann. Surg. 191:546-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pruitt, B. A., Jr., J. M. Stein, F. D. Foley, J. A. Moncrief, and J. A. O'Neill, Jr. 1970. Intravenous therapy in burn patients. Suppurative thrombophlebitis and other life-threatening complications. Arch. Surg. 100:399-404. [DOI] [PubMed] [Google Scholar]
- 349.Public Health Agency of Canada. 1999. Mechanism of injury, mortality by province/territory: deaths per 100,000 crude rates. Health Surveillance and Epidemiology Division, CHHD. http://dsol-smed.phac-aspc.gc.ca. [Online.]
- 350.Purevdorj, B., J. W. Costerton, and P. Stoodley. 2002. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 68:4457-4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Quinby, W. C., Jr., J. F. Burke, and C. C. Bondoc. 1981. Primary excision and immediate wound closure. Intensive Care Med. 7:71-76. [DOI] [PubMed] [Google Scholar]
- 352.Rai, J., M. G. Jeschke, R. E. Barrow, and D. N. Herndon. 1999. Electrical injuries: a 30-year review. J. Trauma 46:933-936. [DOI] [PubMed] [Google Scholar]
- 353.Ramirez, F., D. J. Fowell, M. Puklavec, S. Simmonds, and D. Mason. 1996. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J. Immunol. 156:2406-2412. [PubMed] [Google Scholar]
- 354.Ramos, G. E., A. N. Bolgiani, O. Patino, G. E. Prezzavento, P. Guastavino, R. Durlach, L. B. Fernandez Canigia, and F. Benaim. 2002. Catheter infection risk related to the distance between insertion site and burned area. J. Burn Care Rehabil. 23:266-271. [DOI] [PubMed] [Google Scholar]
- 355.Ramzy, P. I., J. P. Barret, and D. N. Herndon. 1999. Thermal injury. Crit. Care Clin. 15:333-352, ix. [DOI] [PubMed] [Google Scholar]
- 356.Ramzy, P. I., D. N. Herndon, S. E. Wolf, O. Irtun, J. P. Barret, R. J. Ramirez, and J. P. Heggers. 1998. Comparison of wound culture and bronchial lavage in the severely burned child: implications for antimicrobial therapy. Arch. Surg. 133:1275-1280. [DOI] [PubMed] [Google Scholar]
- 357.Ramzy, P. I., S. E. Wolf, O. Irtun, D. W. Hart, J. C. Thompson, and D. N. Herndon. 2000. Gut epithelial apoptosis after severe burn: effects of gut hypoperfusion. J. Am. Coll. Surg. 190:281-287. [DOI] [PubMed] [Google Scholar]
- 358.Rennie, R. P., R. N. Jones, and A. H. Mutnick. 2003. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn. Microbiol. Infect. Dis. 45:287-293. [DOI] [PubMed] [Google Scholar]
- 359.Reper, P., P. Van Der Rest, A. Creemers, D. Vandenen, and A. Vanderkelen. 2001. Medical treatment of a central vein suppurative thrombosis with cerebral metastatic abscesses in a burned child. Burns 27:662-663. [DOI] [PubMed] [Google Scholar]
- 360.Revathi, G., J. Puri, and B. K. Jain. 1998. Bacteriology of burns. Burns 24:347-349. [DOI] [PubMed] [Google Scholar]
- 361.Revathi, G., K. P. Shannon, P. D. Stapleton, B. K. Jain, and G. L. French. 1998. An outbreak of extended-spectrum, beta-lactamase-producing Salmonella senftenberg in a burns ward. J. Hosp. Infect. 40:295-302. [DOI] [PubMed] [Google Scholar]
- 362.Rich, R. R. 2001. Clinical immunology principles and practices, p. 44.3. Mosby, Baltimore, Md.
- 363.Richard, P., R. Le Floch, C. Chamoux, M. Pannier, E. Espaze, and H. Richet. 1994. Pseudomonas aeruginosa outbreak in a burn unit: role of antimicrobials in the emergence of multiply resistant strains. J. Infect. Dis. 170:377-383. [DOI] [PubMed] [Google Scholar]
- 364.Riddle, P. R., and M. C. Berenbaum. 1967. Postoperative depression of the lymphocyte response to phytohaemagglutinin. Lancet i:746-748. [DOI] [PubMed] [Google Scholar]
- 365.Robson, M. C. 1988. Burn sepsis. Crit. Care Clin. 4:281-298. [PubMed] [Google Scholar]
- 366.Rode, H., D. Hanslo, P. M. De Wet, A. J. Millar, and S. Cywes. 1989. Efficacy of mupirocin in methicillin-resistant Staphylococcus aureus burn wound infection. Antimicrob. Agents Chemother. 33:1358-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Rodgers, G. L., J. E. Mortensen, M. C. Fisher, and S. S. Long. 1997. In vitro susceptibility testing of topical antimicrobial agents used in pediatric burn patients: comparison of two methods. J. Burn Care Rehabil. 18:406-410. [DOI] [PubMed] [Google Scholar]
- 368.Rossignol, A. M., J. A. Locke, C. M. Boyle, and J. F. Burke. 1986. Epidemiology of work-related burn injuries in Massachusetts requiring hospitalization. J. Trauma 26:1097-1101. [DOI] [PubMed] [Google Scholar]
- 369.Roth, J. J., and W. B. Hughes. 2004. The essential burn unit handbook. Quality Medical Publishing, St. Louis, Mo.
- 370.Rupp, M. E., S. J. Lisco, P. A. Lipsett, T. M. Perl, K. Keating, J. M. Civetta, L. A. Mermel, D. Lee, E. P. Dellinger, M. Donahoe, D. Giles, M. A. Pfaller, D. G. Maki, and R. Sherertz. 2005. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann. Intern. Med. 143:570-580. [DOI] [PubMed] [Google Scholar]
- 371.Ryan, C. M., S. H. Bailey, E. A. Carter, D. A. Schoenfeld, and R. G. Tompkins. 1994. Additive effects of thermal injury and infection on gut permeability. Arch. Surg. 129:325-328. [DOI] [PubMed] [Google Scholar]
- 372.Ryan, C. M., D. A. Schoenfeld, W. P. Thorpe, R. L. Sheridan, E. H. Cassem, and R. G. Tompkins. 1998. Objective estimates of the probability of death from burn injuries. N. Engl. J. Med. 338:362-366. [DOI] [PubMed] [Google Scholar]
- 373.Saffle, J. R., B. Davis, and P. Williams. 1995. Recent outcomes in the treatment of burn injury in the United States: a report from the American Burn Association Patient Registry. J. Burn Care Rehabil. 16:219-232. [DOI] [PubMed] [Google Scholar]
- 374.Salo, M. 1992. Effects of anaesthesia and surgery on the immune response. Acta Anaesthesiol. Scand. 36:201-220. [DOI] [PubMed] [Google Scholar]
- 375.Samuel, U., and J. P. Guggenbichler. 2004. Prevention of catheter-related infections: the potential of a new nano-silver impregnated catheter. Int. J. Antimicrob. Agents 23(Suppl. 1):S75-S78. [DOI] [PubMed] [Google Scholar]
- 376.Sanders, V. M., R. A. Baker, D. S. Ramer-Quinn, D. J. Kasprowicz, B. A. Fuchs, and N. E. Street. 1997. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J. Immunol. 158:4200-4210. [PubMed] [Google Scholar]
- 377.Santaniello, J. M., F. A. Luchette, T. J. Esposito, H. Gunawan, R. L. Reed, K. A. Davis, and R. L. Gamelli. 2004. Ten year experience of burn, trauma, and combined burn/trauma injuries comparing outcomes. J. Trauma 57:696-700. [DOI] [PubMed] [Google Scholar]
- 378.Santucci, S. G., S. Gobara, C. R. Santos, C. Fontana, and A. S. Levin. 2003. Infections in a burn intensive care unit: experience of seven years. J. Hosp. Infect. 53:6-13. [DOI] [PubMed] [Google Scholar]
- 379.Sasaki, T. M., G. W. Welch, D. N. Herndon, J. Z. Kaplan, R. B. Lindberg, and B. A. Pruitt, Jr. 1979. Burn wound manipulation-induced bacteremia. J. Trauma 19:46-48. [DOI] [PubMed] [Google Scholar]
- 380.Saydjari, R., G. I. Beerthuizen, C. M. Townsend, Jr., D. N. Herndon, and J. C. Thompson. 1991. Bacterial translocation and its relationship to visceral blood flow, gut mucosal ornithine decarboxylase activity, and DNA in pigs. J. Trauma 31:639-643. [DOI] [PubMed] [Google Scholar]
- 381.Schildt, B. E. 1970. Function of the RES after thermal and mechanical trauma in mice. Acta Chir. Scand. 136:359-364. [PubMed] [Google Scholar]
- 382.Schwacha, M. G., L. T. Holland, I. H. Chaudry, and J. L. Messina. 2005. Genetic variability in the immune-inflammatory response after major burn injury. Shock 23:123-128. [DOI] [PubMed] [Google Scholar]
- 383.Sepandj, F., H. Ceri, A. Gibb, R. Read, and M. Olson. 2004. Minimum inhibitory concentration (MIC) versus minimum biofilm eliminating concentration (MBEC) in evaluation of antibiotic sensitivity of gram-negative bacilli causing peritonitis. Perit. Dial. Int. 24:65-67. [PubMed] [Google Scholar]
- 384.Sevitt, S. 1957. Burns: pathology and therapeutic applications, p. 171-176. Buttersworth, London, England.
- 385.Shankowsky, H. A., L. S. Callioux, and E. E. Tredget. 1994. North American survey of hydrotherapy in modern burn care. J. Burn Care Rehabil. 15:143-146. [DOI] [PubMed] [Google Scholar]
- 386.Sherertz, R. J., and M. L. Sullivan. 1985. An outbreak of infections with Acinetobacter calcoaceticus in burn patients: contamination of patients' mattresses. J. Infect. Dis. 151:252-258. [DOI] [PubMed] [Google Scholar]
- 387.Sheridan, R. L. 2000. Evaluating and managing burn wounds. Dermatol. Nurs. 12:17-18. [PubMed] [Google Scholar]
- 388.Sheridan, R. L., C. M. Ryan, M. S. Pasternack, J. M. Weber, and R. G. Tompkins. 1993. Flavobacterial sepsis in massively burned pediatric patients. Clin. Infect. Dis. 17:185-187. [PubMed] [Google Scholar]
- 389.Sheridan, R. L., J. T. Schulz, J. M. Weber, C. M. Ryan, M. S. Pasternack, and R. G. Tompkins. 2000. Cutaneous herpetic infections complicating burns. Burns 26:621-624. [DOI] [PubMed] [Google Scholar]
- 390.Sheridan, R. L., R. G. Tompkins, and J. F. Burke. 1994. Management of burn wounds with prompt excision and immediate closure. J. Intensive Care Med. 9:6-17. [DOI] [PubMed] [Google Scholar]
- 391.Sheridan, R. L., J. Weber, J. Benjamin, M. S. Pasternack, and R. G. Tompkins. 1994. Control of methicillin-resistant Staphylococcus aureus in a pediatric burn unit. Am. J. Infect. Control 22:340-345. [DOI] [PubMed] [Google Scholar]
- 392.Sheridan, R. L., J. M. Weber, M. M. Pasternak, J. M. Mulligan, and R. G. Tompkins. 1999. A 15-year experience with varicella infections in a pediatric burn unit. Burns 25:353-356. [DOI] [PubMed] [Google Scholar]
- 393.Sheridan, R. L., J. M. Weber, H. F. Peterson, and R. G. Tompkins. 1995. Central venous catheter sepsis with weekly catheter change in paediatric burn patients: an analysis of 221 catheters. Burns 21:127-129. [DOI] [PubMed] [Google Scholar]
- 394.Sherman, R. T. 1970. The prevention and treatment of tetanus in the burn patient. Surg. Clin. N. Am. 50:1277-1281. [DOI] [PubMed] [Google Scholar]
- 395.Sherry, R. M., J. I. Cue, J. K. Goddard, J. B. Parramore, and J. T. DiPiro. 1996. Interleukin-10 is associated with the development of sepsis in trauma patients. J. Trauma 40:613-616. [DOI] [PubMed] [Google Scholar]
- 396.Shorr, A. F., J. H. Sherner, W. L. Jackson, and M. H. Kollef. 2005. Invasive approaches to the diagnosis of ventilator-associated pneumonia: a meta-analysis. Crit. Care Med. 33:46-53. [DOI] [PubMed] [Google Scholar]
- 397.Simor, A. E., M. Lee, M. Vearncombe, L. Jones-Paul, C. Barry, M. Gomez, J. S. Fish, R. C. Cartotto, R. Palmer, and M. Louie. 2002. An outbreak due to multiresistant Acinetobacter baumannii in a burn unit: risk factors for acquisition and management. Infect. Control Hosp. Epidemiol. 23:261-267. [DOI] [PubMed] [Google Scholar]
- 398.Singer, A. J., and S. A. McClain. 2002. Persistent wound infection delays epidermal maturation and increases scarring in thermal burns. Wound Repair Regen. 10:372-377. [DOI] [PubMed] [Google Scholar]
- 399.Sjoberg, T., S. Mzezewa, K. Jonsson, V. Robertson, and L. Salemark. 2003. Comparison of surface swab cultures and quantitative tissue biopsy cultures to predict sepsis in burn patients: a prospective study. J. Burn Care Rehabil. 24:365-370. [DOI] [PubMed] [Google Scholar]
- 400.Sjoberg, T., S. Mzezewa, K. Jonsson, and L. Salemark. 2004. Immune response in burn patients in relation to HIV infection and sepsis. Burns 30:670-674. [DOI] [PubMed] [Google Scholar]
- 401.Skoll, P. J., D. A. Hudson, and J. A. Simpson. 1998. Aeromonas hydrophila in burn patients. Burns 24:350-353. [DOI] [PubMed] [Google Scholar]
- 402.Slade, M. S., R. L. Simmons, E. Yunis, and L. J. Greenberg. 1975. Immunodepression after major surgery in normal patients. Surgery 78:363-372. [PubMed] [Google Scholar]
- 403.Smartrisk. 1998. The economic burden of unintentional injury in Canada. http://www.smartrisk.ca. [Online.]
- 404.Smith, D. J., Jr., and P. D. Thomson. 1992. Changing flora in burn and trauma units: historical perspective—experience in the United States. J. Burn Care Rehabil. 13:276-280. [DOI] [PubMed] [Google Scholar]
- 405.Solowey, A. C., and F. T. Rapaport. 1966. The immunologic response to repeated individual-specific skin allografts. Transplantation 4:178-181. [DOI] [PubMed] [Google Scholar]
- 406.Spebar, M. J., and B. A. Pruitt, Jr. 1981. Candidiasis in the burned patient. J. Trauma 21:237-239. [DOI] [PubMed] [Google Scholar]
- 407.Steer, J. A., R. P. Papini, A. P. Wilson, D. A. McGrouther, and N. Parkhouse. 1996. Quantitative microbiology in the management of burn patients. II. Relationship between bacterial counts obtained by burn wound biopsy culture and surface alginate swab culture, with clinical outcome following burn surgery and change of dressings. Burns 22:177-181. [DOI] [PubMed] [Google Scholar]
- 408.Steer, J. A., R. P. Papini, A. P. Wilson, D. A. McGrouther, and N. Parkhouse. 1996. Quantitative microbiology in the management of burn patients. I. Correlation between quantitative and qualitative burn wound biopsy culture and surface alginate swab culture. Burns 22:173-176. [DOI] [PubMed] [Google Scholar]
- 409.Stefanides, M. M., Sr., C. E. Copeland, S. D. Kominos, and R. B. Yee. 1976. In vitro penetration of topical antiseptics through eschar of burn patients. Ann. Surg. 183:358-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Stein, J. M., and B. A. Pruitt, Jr. 1970. Suppurative thrombophlebitis. A lethal iatrogenic disease. N. Engl. J. Med. 282:1452-1455. [DOI] [PubMed] [Google Scholar]
- 411.Stein, M. D., D. N. Herndon, J. M. Stevens, L. D. Traber, and D. L. Traber. 1986. Production of chemotactic factors and lung cell changes following smoke inhalation in a sheep model. J. Burn Care Rehabil. 7:117-121. [DOI] [PubMed] [Google Scholar]
- 412.Steinstraesser, L., Y. Oezdogan, S. C. Wang, and H. U. Steinau. 2004. Host defense peptides in burns. Burns 30:619-627. [DOI] [PubMed] [Google Scholar]
- 413.Stephan, R. N., A. Ayala, J. M. Harkema, R. E. Dean, J. R. Border, and I. H. Chaudry. 1989. Mechanism of immunosuppression following hemorrhage: defective antigen presentation by macrophages. J. Surg. Res. 46:553-556. [DOI] [PubMed] [Google Scholar]
- 414.Steve, L., P. Goodhart, and J. Alexander. 1979. Hydrotherapy burn treatment: use of chloramine-T against resistant microorganisms. Arch. Phys. Med. Rehabil. 60:301-303. [PubMed] [Google Scholar]
- 415.Still, J., E. Law, B. Friedman, T. Newton, and J. Wilson. 2002. Clostridium difficile diarrhea on a burn unit. Burns 28:398-399. [DOI] [PubMed] [Google Scholar]
- 416.Still, J. M., Jr., E. J. Law, G. I. Pereira, and E. Singletary. 1993. Invasive burn wound infection due to Curvularia species. Burns 19:77-79. [DOI] [PubMed] [Google Scholar]
- 417.Still, J. M., K. Orlet, and E. J. Law. 1994. Trichosporon beigelii septicaemia in a burn patient. Burns 20:467-468. [DOI] [PubMed] [Google Scholar]
- 418.Stone, H. H., J. Z. Cuzzell, L. D. Kolb, M. S. Moskowitz, and J. E. McGowan, Jr. 1979. Aspergillus infection of the burn wound. J. Trauma 19:765-767. [DOI] [PubMed] [Google Scholar]
- 419.Stoodley, P., K. Sauer, D. G. Davies, and J. W. Costerton. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187-209. [DOI] [PubMed] [Google Scholar]
- 420.Strock, L. L., M. M. Lee, R. L. Rutan, M. H. Desai, M. C. Robson, D. N. Herndon, and J. P. Heggers. 1990. Topical Bactroban (mupirocin): efficacy in treating burn wounds infected with methicillin-resistant staphylococci. J. Burn Care Rehabil. 11:454-459. [PubMed] [Google Scholar]
- 421.Sutherland, I. W. 2001. The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol. 9:222-227. [DOI] [PubMed] [Google Scholar]
- 422.Taddonio, T. E., P. D. Thomson, M. J. Tait, J. K. Prasad, and I. Feller. 1988. Rapid quantification of bacterial and fungal growth in burn wounds: biopsy homogenate Gram stain versus microbial culture results. Burns Incl. Therm. Inj. 14:180-184. [DOI] [PubMed] [Google Scholar]
- 423.Tahlan, R. N., R. K. Keswani, S. Saini, and O. P. Miglani. 1984. Correlation of quantitative burn wound biopsy culture and surface swab culture to burn wound sepsis. Burns Incl. Therm. Inj. 10:217-224. [DOI] [PubMed] [Google Scholar]
- 424.Tang, D., and W. Wang. 1998. Successful cure of an extensive burn injury complicated with mucor wound sepsis. Burns 24:72-73. [DOI] [PubMed] [Google Scholar]
- 425.Tasaki, O., C. W. Goodwin, D. Saitoh, D. W. Mozingo, S. Ishihara, W. W. Brinkley, W. G. Cioffi, Jr., and B. A. Pruitt, Jr. 1997. Effects of burns on inhalation injury. J. Trauma 43:603-607. [DOI] [PubMed] [Google Scholar]
- 426.Teplitz, C., D. Davis, A. D. Mason, Jr., and J. A. Moncrief. 1964. Pseudomonas burn wound sepsis. I. Pathogenesis of experimental Pseudomonas burn wound sepsis. J. Surg. Res. 54:200-216. [DOI] [PubMed] [Google Scholar]
- 427.Teplitz, C., D. Davis, H. L. Walker, G. L. Raulston, A. D. Mason, Jr., and J. A. Moncrief. 1964. Pseudomonas burn wound sepsis. II. Hematogenous infection at the junction of the burn wound and the unburned hypodermis. J. Surg. Res. 54:217-222. [DOI] [PubMed] [Google Scholar]
- 428.Thompson, J. T., J. W. Meredith, and J. A. Molnar. 2002. The effect of burn nursing units on burn wound infections. J. Burn Care Rehabil. 23:281-286. [DOI] [PubMed] [Google Scholar]
- 429.Thompson, P., D. N. Herndon, S. Abston, and T. Rutan. 1987. Effect of early excision on patients with major thermal injury. J. Trauma 27:205-207. [DOI] [PubMed] [Google Scholar]
- 430.Tompkins, R. G., J. P. Remensnyder, J. F. Burke, D. M. Tompkins, J. F. Hilton, D. A. Schoenfeld, G. E. Behringer, C. C. Bondoc, S. E. Briggs, and W. C. Quinby, Jr. 1988. Significant reductions in mortality for children with burn injuries through the use of prompt eschar excision. Ann. Surg. 208:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Torres, A., and S. Ewig. 2004. Diagnosing ventilator-associated pneumonia. N. Engl. J. Med. 350:433-435. [DOI] [PubMed] [Google Scholar]
- 432.Torstenson, O. L., W. J. Meyer, and P. G. Quie. 1970. Burn-wound infection with viruses. N. Engl. J. Med. 282:1272-1273. [PubMed] [Google Scholar]
- 433.Traber, D. L., D. N. Herndon, and K. Soejima. 2005. The pathopysiology of inhalation injury, p. 221-231. In D. N. Herndon (ed.), Total burn care. Saunders, London, England.
- 434.Trafny, E. A. 1998. Susceptibility of adherent organisms from Pseudomonas aeruginosa and Staphylococcus aureus strains isolated from burn wounds to antimicrobial agents. Int J. Antimicrob. Agents 10:223-228. [DOI] [PubMed] [Google Scholar]
- 435.Tredget, E. E., H. A. Shankowsky, A. Groeneveld, and R. Burrell. 1998. A matched-pair, randomized study evaluating the efficacy and safety of Acticoat silver-coated dressing for the treatment of burn wounds. J. Burn Care Rehabil. 19:531-537. [DOI] [PubMed] [Google Scholar]
- 436.Tredget, E. E., H. A. Shankowsky, A. M. Joffe, T. I. Inkson, K. Volpel, W. Paranchych, P. C. Kibsey, J. D. Alton, and J. F. Burke. 1992. Epidemiology of infections with Pseudomonas aeruginosa in burn patients: the role of hydrotherapy. Clin. Infect. Dis. 15:941-949. [DOI] [PubMed] [Google Scholar]
- 437.Tredget, E. E., H. A. Shankowsky, R. Rennie, R. E. Burrell, and S. Logsetty. 2004. Pseudomonas infections in the thermally injured patient. Burns 30:3-26. [DOI] [PubMed] [Google Scholar]
- 438.Tredget, E. E., H. A. Shankowsky, T. V. Taerum, G. L. Moysa, and J. D. Alton. 1990. The role of inhalation injury in burn trauma. A Canadian experience. Ann. Surg. 212:720-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.U.S. Department of Health and Human Services. 1993. Injury mortality: national summary of injury mortality data, 1984-1990. U.S. Department of Health and Human Services, Washington, D.C.
- 440.Valova, M., R. Konigova, L. Broz, R. Zajicek, and P. Toupalik. 2002. Early and late fatal complications of inhalation injury. Acta Chir. Plast. 44:51-54. [PubMed] [Google Scholar]
- 441.Van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Vannier, E., L. C. Miller, and C. A. Dinarello. 1992. Coordinated antiinflammatory effects of interleukin 4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc. Natl. Acad. Sci. USA 89:4076-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Villani, C., D. H. Johnson, and B. A. Cunha. 1995. Bilateral suppurative thrombophlebitis due to Staphylococcus aureus. Heart Lung 24:342-344. [DOI] [PubMed] [Google Scholar]
- 444.Vindenes, H., and R. Bjerknes. 1993. The frequency of bacteremia and fungemia following wound cleaning and excision in patients with large burns. J. Trauma 35:742-749. [DOI] [PubMed] [Google Scholar]
- 445.Wahl, W. L., K. S. Ahrns, M. M. Brandt, S. A. Rowe, M. R. Hemmila, and S. Arbabi. 2005. Bronchoalveolar lavage in diagnosis of ventilator-associated pneumonia in patients with burns. J. Burn Care Rehabil. 26:57-61. [DOI] [PubMed] [Google Scholar]
- 446.Wahl, W. L., G. A. Franklin, M. M. Brandt, L. Sturm, K. S. Ahrns, M. R. Hemmila, and S. Arbabi. 2003. Does bronchoalveolar lavage enhance our ability to treat ventilator-associated pneumonia in a trauma-burn intensive care unit? J. Trauma 54:633-638. [DOI] [PubMed] [Google Scholar]
- 447.Wang, P., P. Wu, M. I. Siegel, R. W. Egan, and M. M. Billah. 1994. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J. Immunol. 153:811-816. [PubMed] [Google Scholar]
- 448.Watts, A. M., M. P. Tyler, M. E. Perry, A. H. Roberts, and D. A. McGrouther. 2001. Burn depth and its histological measurement. Burns 27:154-160. [DOI] [PubMed] [Google Scholar]
- 449.Weber, J., and A. McManus. 2004. Infection control in burn patients. Burns 30:A16-A24. [DOI] [PubMed] [Google Scholar]
- 450.Weber, J. M., R. L. Sheridan, M. S. Pasternack, and R. G. Tompkins. 1997. Nosocomial infections in pediatric patients with burns. Am. J. Infect. Control 25:195-201. [DOI] [PubMed] [Google Scholar]
- 451.Weber, J. M., R. L. Sheridan, J. T. Schulz, R. G. Tompkins, and C. M. Ryan. 2002. Effectiveness of bacteria-controlled nursing units in preventing cross-colonization with resistant bacteria in severely burned children. Infect. Control Hosp. Epidemiol. 23:549-551. [DOI] [PubMed] [Google Scholar]
- 452.Weber, J. M., and D. M. Tompkins. 1993. Improving survival: infection control and burns. AACN Clin. Issues Crit. Care Nurs. 4:414-423. [PubMed] [Google Scholar]
- 453.Webster, R. O., S. R. Hong, R. B. Johnston, Jr., and P. M. Henson. 1980. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology 2:201-219. [DOI] [PubMed] [Google Scholar]
- 454.Weissman, C. 1990. The metabolic response to stress: an overview and update. Anesthesiology 73:308-327. [DOI] [PubMed] [Google Scholar]
- 455.Wertheim, G. 1868. Uber die Vesandesungen bei vesbrennungen, p. 309. Weiner Medical Press, Vienna, Austria.
- 456.Wheeler, M. S., M. R. McGinnis, W. A. Schell, and D. H. Walker. 1981. Fusarium infection in burned patients. Am. J. Clin. Pathol. 75:304-311. [DOI] [PubMed] [Google Scholar]
- 457.Wibbenmeyer, L. A., M. J. Amelon, L. J. Morgan, B. K. Robinson, P. X. Chang, R. Lewis, and G. P. Kealey. 2001. Predicting survival in an elderly burn patient population. Burns 27:583-590. [DOI] [PubMed] [Google Scholar]
- 458.Williams, H. B., W. C. Breidenbach, W. B. Callaghan, G. K. Richards, and J. J. Prentis. 1984. Are burn wound biopsies obsolete? A comparative study of bacterial quantitation in burn patients using the absorbent disc and biopsy techniques. Ann. Plast. Surg. 13:388-395. [DOI] [PubMed] [Google Scholar]
- 459.Winkler, M., G. Erbs, W. Konig, and F. E. Muller. 1987. Comparison of 4 methods of bacterial count determination in burn wounds. Zentbl. Bakteriol. Mikrobiol. Hyg. A 265:82-98. (In German.) [PubMed] [Google Scholar]
- 460.Wisser, D., and J. Steffes. 2003. Skin replacement with a collagen based dermal substitute, autologous keratinocytes and fibroblasts in burn trauma. Burns 29:375-380. [DOI] [PubMed] [Google Scholar]
- 461.Withey, S. J., N. Carver, J. D. Frame, and C. C. Walker. 1999. Toxic shock syndrome in adult burns. Burns 25:659-662. [DOI] [PubMed] [Google Scholar]
- 462.Wolfe, J. H., A. V. Wu, N. E. O'Connor, I. Saporoschetz, and J. A. Mannick. 1982. Anergy, immunosuppressive serum, and impaired lymphocyte blastogenesis in burn patients. Arch. Surg. 117:1266-1271. [DOI] [PubMed] [Google Scholar]
- 463.Wood, J. J., M. L. Rodrick, J. B. O'Mahony, S. B. Palder, I. Saporoschetz, P. D'Eon, and J. A. Mannick. 1984. Inadequate interleukin 2 production. A fundamental immunological deficiency in patients with major burns. Ann. Surg. 200:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Woolfrey, B. F. 1986. Burn wound biopsy techniques. J. Clin. Microbiol. 24:503. (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Woolfrey, B. F., J. M. Fox, and C. O. Quall. 1981. An evaluation of burn wound quantitative microbiology. I. Quantitative eschar cultures. Am. J. Clin. Pathol. 75:532-537. [DOI] [PubMed] [Google Scholar]
- 466.Woske, H. J., T. Roding, I. Schulz, and H. Lode. 2001. Ventilator-associated pneumonia in a surgical intensive care unit: epidemiology, etiology and comparison of three bronchoscopic methods for microbiological specimen sampling. Crit. Care 5:167-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Wright, J. B., K. Lam, D. Hansen, and R. E. Burrell. 1999. Efficacy of topical silver against fungal burn wound pathogens. Am. J. Infect Control 27:344-350. [DOI] [PubMed] [Google Scholar]
- 468.Wurtz, R., M. Karajovic, E. Dacumos, B. Jovanovic, and M. Hanumadass. 1995. Nosocomial infections in a burn intensive care unit. Burns 21:181-184. [DOI] [PubMed] [Google Scholar]
- 469.Wysocki, A. B. 2002. Evaluating and managing open skin wounds: colonization versus infection. AACN Clin. Issues 13:382-397. [DOI] [PubMed] [Google Scholar]
- 470.Xiao-Wu, W., D. N. Herndon, M. Spies, A. P. Sanford, and S. E. Wolf. 2002. Effects of delayed wound excision and grafting in severely burned children. Arch. Surg. 137:1049-1054. [DOI] [PubMed] [Google Scholar]
- 471.Xing, Z., J. Gauldie, G. Cox, H. Baumann, M. Jordana, X. F. Lei, and M. K. Achong. 1998. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 101:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Yin, H. Q., R. Langford, and R. E. Burrell. 1999. Comparative evaluation of the antimicrobial activity of ACTICOAT antimicrobial barrier dressing. J. Burn Care Rehabil. 20:195-200. [DOI] [PubMed] [Google Scholar]
- 473.Yurt, R. W., and B. A. Pruitt, Jr. 1986. Base-line and postthermal injury plasma histamine in rats. J. Appl. Physiol. 60:1782-1788. [DOI] [PubMed] [Google Scholar]
- 474.Zaragoza, R., A. Artero, J. J. Camarena, S. Sancho, R. Gonzalez, and J. M. Nogueira. 2003. The influence of inadequate empirical antimicrobial treatment on patients with bloodstream infections in an intensive care unit. Clin. Microbiol. Infect. 9:412-418. [DOI] [PubMed] [Google Scholar]