Abstract
Developing interpretive breakpoints for any given organism-drug combination requires integration of the MIC distribution, pharmacokinetic and pharmacodynamic parameters, and the relationship between in vitro activity and outcome from both in vivo and clinical studies. Previously, the Subcommittee for Antifungal Testing of the Clinical and Laboratory Standards Institute (CLSI [formerly National Committee for Clinical Laboratory Standards]) proposed MIC interpretive breakpoints for fluconazole and Candida spp. These breakpoints were considered to be somewhat weak, because the clinical data supporting them came largely from mucosal infections and there were very few infections involving strains with elevated fluconazole MICs. We readdress the issue of fluconazole breakpoints for Candida by using published clinical and microbiologic data to provide further validation of the breakpoints proposed by the CLSI in 1997. We also address interpretive breakpoints for agar disk diffusion testing of fluconazole. The MIC distribution for fluconazole was determined with a collection of 13,338 clinical isolates. The overall MIC at which 90% of the isolates were inhibited was 8 μg/ml: 91% were susceptible (S) at a MIC of ≤8 μg/ml and 3% were resistant (R) (MIC ≥ 64 μg/ml). Similar results were obtained for 2,190 isolates from randomized clinical trials. Analysis of available data for 1,295 patient-episode-isolate events (692 represented mucosal infections and 603 represented invasive infections) from 12 published clinical studies demonstrated an overall success rate of 77%, including 85% for those episodes in which the fluconazole MIC was ≤8 μg/ml, 67% for those episodes in which the MIC was 16 to 32 μg/ml, and 42% for those episodes with resistant (MIC ≥ 64 μg/ml) isolates. Pharmacodynamic analysis demonstrated a strong relationship between MIC, fluconazole dose, and outcome. A dose/MIC ratio of ∼25 was supportive of the following susceptibility breakpoints for fluconazole and Candida spp.: S, MIC ≤ 8 μg/ml; susceptible-dose dependent (SDD), MIC = 16 to 32 μg/ml; R, MIC ≥ 64 μg/ml. The corresponding disk test breakpoints are as follows: S, ≥19 mm; SDD, 15 to 18 mm; R, ≤14 mm.
INTRODUCTION
Fluconazole is a triazole antifungal agent that has been available for the treatment of infections due to Candida, Cryptococcus, and other opportunistic yeasts since 1990 (43, 97). The drug is available as a tablet (50, 100, or 200 mg), as an oral suspension, and as an intravenous formulation (200 or 400 mg). When used in the treatment of invasive candidiasis (e.g., bloodstream infections [BSI], deep tissue sites, other normally sterile site infections), fluconazole is administered as an initial loading dose of 800 mg (oral or intravenous) followed by a daily maintenance dose of 400 mg (oral or intravenous). Higher daily doses of 800 mg or greater may be used in selected circumstances (24, 59, 87, 105).
There is now a very broad clinical experience of using fluconazole to treat both mucosal and invasive candidiasis, to the extent that it must be considered one of the first-line agents for the treatment of all forms of candidiasis (54). Clinical resistance of Candida spp. to fluconazole has been well documented in many settings (5, 6, 14, 15, 37, 38, 78, 80-82, 88, 100, 106); however, aside from infections with Candida glabrata and C. krusei, true antimicrobial resistance is rare among species of Candida commonly associated with invasive disease (5, 16, 18, 38, 64, 68, 82, 83, 96, 97, 100, 110, 112). The application of in vitro susceptibility testing and the use of molecular methods have served to detect potentially resistant strains of Candida and to characterize completely the various mechanisms of resistance to fluconazole and other azoles in clinical isolates of Candida spp. (12, 44, 52, 64, 68, 78, 96, 110).
The Clinical and Laboratory Standards Institute (CLSI) (formerly the National Committee for Clinical Laboratory Standards [NCCLS]) Subcommittee for Antifungal Testing has developed and standardized broth microdilution (BMD), broth macrodilution, and disk diffusion methods for in vitro susceptibility testing of Candida spp. (and other yeasts) against fluconazole (50, 51). In addition to standardized testing methods, the CLSI Subcommittee for Antifungal Testing has approved quality control limits for both MIC and disk diffusion methods with fluconazole (11, 50, 51). These methods have been applied worldwide to generate a very detailed and comprehensive understanding of the in vitro susceptibility profile of Candida spp. to fluconazole (8, 9, 16-18, 20, 21, 29, 32, 33, 38, 54, 64, 68-73, 75, 83, 100, 112).
In 1997, the CLSI Subcommittee for Antifungal Testing used accumulated clinical and microbiological data to propose interpretive breakpoints for MIC testing of fluconazole against Candida spp. (84). The breakpoints, which were subsequently incorporated into NCCLS document M27-A (48), were as follows: susceptible (S), MIC ≤ 8 μg/ml; susceptible-dose dependent (SDD), MIC = 16 to 32 μg/ml; resistant (R), MIC ≥ 64 μg/ml. Despite the fact that the process and rationale for developing these breakpoints was described in great detail (84), and that the breakpoints were arrived at using the best available clinical and in vitro data (85, 86), concerns have been raised regarding the clinical utility of these breakpoints and of in vitro susceptibility testing of fluconazole in general (99). In particular, concern has been expressed that most (∼80%) of the data supporting the breakpoints were derived from studies of mucosal candidiasis and that, in addition, there was little clinical outcome data for isolates for which the MICs were elevated (85). Thus, it was unclear how useful such testing would be in predicting the outcome of more serious invasive infections treated with fluconazole and for those involving strains for which the fluconazole MICs were elevated (86).
In this review we readdress the issue of fluconazole breakpoints for Candida spp. by using the available published microbiological and clinical data to provide further validation of the breakpoints proposed by CLSI in 1997. The analytical model employed was that used previously (84) and as outlined for all types of antimicrobial testing in CLSI document M23-A2 (49). We considered the data relating the MICs to known resistance mechanisms, the MIC (and disk zone diameter) distribution profiles, pharmacokinetic (PK) and pharmacodynamic (PD) parameters, and the relationship between in vitro activity (MIC) and clinical outcome, as determined by the investigators in a total of 12 (7 mucosal and 5 invasive) published clinical efficacy studies. These analyses are summarized below.
MECHANISMS OF RESISTANCE TO FLUCONAZOLE IN CANDIDA SPP.
Fluconazole, like all azoles, acts by inhibiting the fungal cytochrome P-450-dependent enzyme lanosterol 14-α-demethylase. This enzyme functions to convert lanosterol to ergosterol, and its inhibition disrupts membrane synthesis in the fungal cell (97).
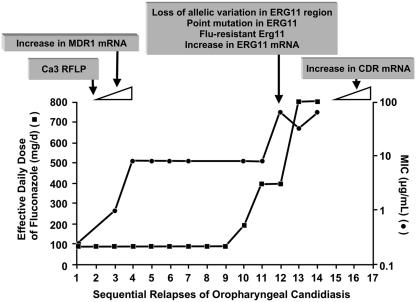
The mechanism of azole resistance in Candida has been well worked out for fluconazole and C. albicans (61, 95, 110). Resistance can arise from a modification in the quality or quantity of the target enzyme, reduced access of the drug to the target, or some combination of these mechanisms (23, 110). In the first instance, point mutations in the gene (ERG11) encoding the target enzyme, 14-α-demethylase, lead to an altered target with decreased affinity for azoles. Overexpression of ERG11 results in the production of high concentrations of the target enzyme, creating the need for higher intracellular fluconazole concentrations to inhibit all of the enzyme molecules in the cell. Loss of allelic variation in the ERG11 promoter may result in a resistant strain that is homozygous for the mutated gene (Fig. 1) (109).
FIG. 1.
Relationship between MIC, dose of fluconazole, and emergence/expression of specific resistance mechanisms in oropharyngeal candidiasis. •, MIC of fluconazole for the clinical isolate; ▪, effective daily dose of fluconazole. MICs are represented on the secondary y axis, in logarithmic scale. Boxes above the graph represent genetic changes identified at each stage. Based on data from Redding et al. (78), White (108, 109), and White et al. (110). (Reprinted from reference 23 with permission.)
The second major mechanism involves active efflux of fluconazole out of the cell through the activation of two types of multidrug efflux transporters: the major facilitators (encoded by MDR genes) and those of the ATP-binding cassette superfamily (encoded by CDR genes) (23, 61, 95, 110). Upregulation of the MDR1 gene leads to elevated fluconazole MICs (Fig. 1), whereas upregulation of CDR genes leads to resistance to multiple azoles (12, 90-93, 96, 110). Evidence that these mechanisms may act individually, sequentially, and in concert has been derived by studying serial isolates of C. albicans from AIDS patients with oropharyngeal candidiasis (39, 40, 78, 108) as well as from patients with invasive disease (44, 52). An example of the evolution of fluconazole resistance in a single patient is shown graphically in Fig. 1 (78, 108, 110). This example clearly shows the relationship between the fluconazole MIC, the dose of fluconazole, and the emergence/expression of specific resistance mechanisms. It provides excellent support for the CLSI MIC breakpoints.
It is also now well established that the mechanism of resistance to fluconazole, and other azoles, in C. glabrata involves upregulation of the CDR1 and CDR2 genes, resulting in resistance to multiple azoles (12, 94, 96). Thus, exposure of C. glabrata to subtherapeutic doses (i.e., <400 mg/day) of fluconazole may result in resistance not only to fluconazole but to other azoles (i.e., itraconazole and voriconazole) as well (12, 64, 71). Fluconazole resistance in C. krusei appears to be mediated by reduced sensitivity of the target enzyme to inhibition by the agent (53).
PK AND PD CONSIDERATIONS
Fluconazole is a bistriazole antifungal with a half-life of approximately 30 h in adults and 15 h in children. Protein binding is low (11% to 12%), and it is distributed to virtually all organs and tissues, including the central nervous system (97). Fluconazole is renally excreted, with approximately 80% recovered in the urine as active, unchanged drug. Currently used dosages span the range of 50 mg/day to as high as 2 g/day; however, the standard dose in adults is 400 to 800 mg/day and in children is 6 to 12 mg/kg (of body weight)/day (1, 26). Dosages of 100 mg/day (1.5 mg/kg/day) produce peak serum drug levels of ∼6.7 μg/ml, 400 mg/day (6 mg/kg/day) produces peak levels of 20 to 30 μg/ml, and the linear pharmacokinetics of fluconazole would predict peak serum levels of 40 to 60 μg/ml at a dosage of 800 mg/day (12 mg/kg/day) (25, 84). With daily dosing, trough levels would be approximately half the peak level. Thus, a daily dose of 400 mg/day would provide serum fluconazole levels in excess of 10 μg/ml throughout the dosing interval (2, 3, 43).
PD investigations of fluconazole and Candida have been undertaken, and both in vitro and in vivo models have demonstrated a correlation between drug dose, organism MIC, and outcome (2-4, 36, 42). Fluconazole exhibits time-dependent, concentration-independent fungistatic activity against Candida (2-4, 13, 36). This concentration-independent activity, coupled with a prolonged in vivo post-antifungal effect, increases the importance of the total amount of drug administered (2, 3). The area under the serum concentration curve (AUC) represents the total amount of drug exposure, and the AUC/MIC ratio is the predictive PD parameter (2, 3). In vivo fluconazole dose-ranging studies with C. albicans strains for which the MICs varied 64-fold found that an AUC/MIC magnitude near 25 predicted efficacy (4). Subsequent studies have included C. albicans strains for which MICs covered a range of more than 500-fold and have confirmed treatment success with a fluconazole AUC/MIC ratio near 25 (42, 89, 103).
FLUCONAZOLE MIC DISTRIBUTION PROFILE FOR CANDIDA SPECIES
The fluconazole MIC profile for each of 12 different species of Candida (13,338 isolates) is shown in Table 1. These results were all determined in a single reference laboratory (University of Iowa) by CLSI-recommended BMD methods (50). This large data set represents recent (1992 to 2004), clinically important (blood and normally sterile-site) isolates from more than 200 different medical centers throughout the world (22, 23, 64, 67-70, 73, 75). The overall MIC at which 90% of the isolates were inhibited (MIC90) for fluconazole was 8 μg/ml; 91% of the 13,338 isolates tested were encompassed by the CLSI susceptible (S) category (MIC ≤ 8 μg/ml), and 3% were resistant (R) (MIC ≥ 64 μg/ml). The MIC90 was highest for C. krusei (MIC ≥ 64 μg/ml) and C. glabrata (MIC, 32 μg/ml) and was ≤2 μg/ml for C. albicans (0.5 μg/ml), C. parapsilosis (2 μg/ml), C. tropicalis (2 μg/ml), C. lusitaniae (2 μg/ml), and C. kefyr (0.5 μg/ml).
TABLE 1.
Susceptibility of Candida BSI isolates to fluconazole by MIC: Global Antifungal Surveillance Program, 1992-2004a
| Organism | No. tested | Cumulative % at MIC (μg/ml) ofb:
|
%Rc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | |||
| C. albicans | 7,725 | 27 | 84 | 94 | 97 | 98 | 98 | 99 | 99 | >99 | 0.06 |
| C. glabrata | 1,966 | <1 | <1 | <1 | 1 | 10 | 34 | 62 | 87 | 91 | 9 |
| C. parapsilosis | 1,623 | <1 | 10 | 49 | 79 | 92 | 96 | 97 | 99 | >99 | 0.5 |
| C. tropicalis | 1,253 | 2 | 21 | 52 | 78 | 94 | 97 | 98 | 98 | 98 | 2 |
| C. krusei | 312 | <1 | 3 | 13 | 60 | 40 | |||||
| C. lusitaniae | 134 | 4 | 37 | 69 | 85 | 93 | 96 | 97 | 98 | 99 | 1 |
| C. dubliniensis | 103 | 45 | 83 | 86 | 87 | 87 | 87 | 91 | 93 | 97 | 3 |
| C. guilliermondii | 92 | 1 | 2 | 5 | 40 | 74 | 90 | 96 | 97 | 3 | |
| C. pelliculosa | 34 | 18 | 79 | 100 | 0 | ||||||
| C. kefyr | 33 | 12 | 61 | 94 | 100 | 0 | |||||
| C. famata | 19 | 5 | 21 | 37 | 42 | 63 | 74 | 100 | 0 | ||
| C. rugosa | 19 | 68 | 79 | 79 | 79 | 79 | 100 | 0 | |||
| All Candida spp. | 13,338 | 17 | 53 | 67 | 75 | 81 | 86 | 91 | 95 | 97 | 3 |
Resistance to fluconazole, designated by an MIC of ≥64 μg/ml, was ≤3% for all species of Candida with the exception of C. glabrata (9%) and C. krusei (40%). These data, including the species distribution rank order, are highly representative of that published in numerous in vitro surveys of Candida BSI isolates (Table 2) (8, 16-21, 29, 32, 33, 54, 63, 64, 66, 83, 100). It is notable that fluconazole resistance among BSI isolates of C. albicans, C. tropicalis, and C. parapsilosis, as determined by CLSI (or EUCAST [European Committee for Antimicrobial Susceptibility Testing]) BMD methods, has remained very infrequent (usually ≤3%) worldwide from 1990 to the present (Table 2). However, important variation has been seen among isolates of C. glabrata over the same period (Table 2). Although the frequency of fluconazole resistance among BSI isolates of C. glabrata was <10% in many different surveys conducted in various countries, the MICs for this species were always higher than those seen with other species (Table 1), and higher-than-standard doses of fluconazole (e.g., ≥800 mg/day) are recommended if fluconazole is used to treat infections with this species (59). Although fluconazole resistance has remained low among Candida spp. over the 14-year period in which it has been used clinically, isolates of C. glabrata collected between 2001 and 2004 appear to be progressively more resistant (Table 3) (D. J. Diekema, S. Messer, L. Boyken, S. Tendolkar, R. Hollis, and M. A. Pfaller, Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-2238, 2005). An increase in both MIC90 (16 μg/ml to 64 μg/ml) and the percent R (7% to 12%) was observed over the 4-year period despite an overall trend toward a decrease in the frequency of C. glabrata detected as a cause of BSI at the various surveillance sites (Table 3). C. glabrata remains the focus for concern regarding fluconazole resistance (71). Although fluconazole may serve as a safe, efficacious, and cost-effective treatment option for infections due to C. glabrata (59), the susceptibility of this species to fluconazole, or other azoles, is not predictable and requires confirmation by “real-time” antifungal susceptibility testing (9, 28, 31, 71, 112).
TABLE 2.
Fluconazole resistance among Candida BSI isolates as determined by different surveillance programsa
| Surveillance programb | Yr | Reference | % Resistant by species (no. tested)c
|
|||
|---|---|---|---|---|---|---|
| C. albicans | C. glabrata | C. parapsilosis | C. tropicalis | |||
| Iceland | 1980-1999 | 8 | 0 (67) | 0 (12) | 0 (11) | 0 (5) |
| CDC | 1992-1993 | 35 | 1 (183) | 14 (59) | 0 (83) | 2 (59) |
| CDC | 1998-2000 | 30 | 1 (423) | 7 (226) | 0 (123) | 6 (118) |
| Sweden | 1994-1998 | 17 | 0 (123) | 40 (52) | 15 (33) | 0 (11) |
| Quebec | 1996-1998 | 98 | 1 (240) | 9 (67) | 0 (53) | 0 (41) |
| Taiwan | 1994-1995 | 16 | 0 (59) | 0 (17) | 0 (24) | 0 (33) |
| Taiwan | 1999-2000 | 16 | 0 (62) | 0 (39) | 0 (43) | 0 (47) |
| Taiwan | 2003 | 34 | 0 (207) | 14 (59) | 0 (41) | 0 (67) |
| Argentina | 1996-1999 | 20 | 9d (94) | 50d (6) | 1d (70) | 6d (47) |
| Spain | 1996-1999 | 20 | 2d (155) | 43d (49) | 0d (201) | 0d (55) |
| Spain | 2002-2003 | 21 | 0d (178) | 19d (31) | 1d (81) | 3d (36) |
| Italy | 1997-1999 | 102 | NAe | 4 (80) | 0 (49) | 5 (44) |
| SENTRY | 1997-2000 | 66 | 1 (1,114) | 7 (334) | 0 (301) | 1 (209) |
| EIEIO | 1998-2001 | 22 | 0 (147) | 10 (51) | 0 (18) | 0 (28) |
| ARTEMIS | 2001-2002 | 69 | 1 (2,359) | 9 (607) | 1 (439) | 1 (319) |
Adapted from Pfaller (62).
All multicenter studies used CLSI or EUCAST broth microdilution methods. CDC, Centers for Disease Control and Prevention; EIEIO, Emerging Infections and the Epidemiology of Iowa Organisms.
Resistant isolates were those for which the MIC was ≥64 μg/ml.
Includes both SDD (MIC = 16 to 32 μg/ml) and R (MIC ≥ 64 μg/ml) categories.
NA, data not available.
TABLE 3.
Trends in the frequency of occurrence and resistance to fluconazole among BSI isolates of C. glabrata: ARTEMIS Global Antifungal Surveillance Program, 2001-2004a
| Yr | Total no. of Candida BSI isolates | No. (%) of C. glabrata BSI isolates | MIC (μg/ml)b
|
% Resistantc | |
|---|---|---|---|---|---|
| 50% | 90% | ||||
| 2001 | 1,932 | 302 (15.6) | 8 | 16 | 7 |
| 2002 | 2,387 | 346 (14.5) | 8 | 32 | 9 |
| 2003 | 2,495 | 325 (13.0) | 16 | 32 | 8 |
| 2004 | 1,892 | 211 (11.2) | 16 | 64 | 12 |
A total of 8,706 isolates from 91 institutions worldwide (Diekema et al., 45th ICAAC).
Broth microdilution MICs were determined in accordance with CLSI M27-A2 (50). 50% and 90%, MIC encompassing 50% and 90% of isolates tested, respectively.
Percent resistant to fluconazole at a MIC of ≥64 μg/ml.
FLUCONAZOLE MICs FOR CANDIDA SPP. ISOLATED IN RANDOMIZED TRIALS OF FLUCONAZOLE IN THE TREATMENT OF CANDIDEMIA
There have been two randomized multicenter clinical trials examining the efficacy of fluconazole in the treatment of candidemia (81, 87). The first trial compared fluconazole to amphotericin B in the treatment of candidemia in non-neutropenic patients (81), and the second compared high-dose (800 mg/day) fluconazole plus placebo with high-dose fluconazole plus amphotericin B also in the treatment of candidemia in non-neutropenic patients (87). The isolates from these two clinical trials, plus additional isolates collected during an observational study conducted concurrently with the high-dose fluconazole trial (58), were tested for susceptibility to fluconazole by CLSI broth dilution methods (54, 83) (Table 4). The five major species from these studies are identical to those shown in Table 1 and in other multicenter surveys (Table 2). Among these more common species (2,190 isolates), the relative susceptibility to fluconazole was similar to that shown in Table 1, with C. albicans representing the most susceptible species (MIC90, 1 to 2 μg/ml; <1 to 5% R) and C. krusei (MIC90, >64 μg/ml; 34% R) and C. glabrata (MIC90, 32 μg/ml; 8 to 10% R) the least susceptible species (Table 4). Interestingly, both of these studies reported high fluconazole MICs for C. tropicalis compared to those in Tables 1 and 2. C. tropicalis has been shown to exhibit a high frequency of trailing (incomplete inhibition of growth), which may result in artificially high MICs (7, 54). Indeed, in both studies the MICs for C. tropicalis were considerably lower, and in concert with those in Table 1, when the isolates were retested by CLSI BMD and the MICs read at 24 h of incubation (to minimize trailing) (54, 83). Thus, the large-scale surveys produce both MICs and species distribution profiles that are entirely representative of those seen in more formal clinical trials of fluconazole efficacy.
TABLE 4.
Species distribution and susceptibility to fluconazole among Candida isolates obtained in two randomized trials of fluconazole in the treatment of candidemia
| Species | Clinical study, time perioda
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Rex (83), 1989-1993b
|
Ostrosky-Zeichner (54), 1995-1999c
|
|||||||
| n | MIC50 | MIC90 | %R | n | MIC50 | MIC90 | %R | |
| C. albicans | 129 | 0.25 | 1 | <1 | 733 | 0.25 | 2 | 5 |
| C. glabrata | 31 | 16 | 32 | 10 | 458 | 8 | 32 | 8 |
| C. parapsilosis | 23 | 1 | 4 | 0 | 391 | 1 | 2 | 2 |
| C. tropicalis | 40 | 1d | >64d | 25d | 307 | 0.5 | 16 | 8 |
| C. krusei | 6 | 32 | ND | NA | 50 | 32 | >64 | 34 |
| C. lusitaniae | 3 | 0.5 | ND | NA | 20 | 0.5 | 2 | 0 |
MIC50 and MIC90, MIC encompassing 50% and 90% of isolates tested, respectively; %R, percent resistant at MIC of ≥64 μg/ml; ND, not done (<10 isolates); NA, not available.
Isolates tested by broth macrodilution (NCCLS, M27-A) (48).
Isolates tested by CLSI M27-A2 broth microdilution (50).
Trailing artifact. When retested using CLSI broth microdilution and reading MIC at 24 h of incubation, MIC50/90 values were 0.25 and 1 μg/ml, respectively (83).
CROSS-RESISTANCE BETWEEN FLUCONAZOLE AND OTHER AZOLES
Previous in vitro studies have suggested that cross-resistance may occur with fluconazole and other azole compounds (19, 54, 64, 66, 69, 70, 73, 74). The mechanistic basis for such cross-resistance has been clearly demonstrated in a number of elegant studies and most often involves the upregulation of genes encoding the ATP-binding cassette efflux transporters, the so-called CDR pumps (12, 90, 92, 93, 96, 110).
Isolates of Candida spp. for which fluconazole MICs are ≥64 μg/ml also tend to be less susceptible (MIC ≥ 2 μg/ml) to itraconazole (73), posaconazole (69), ravuconazole (65, 70), and voriconazole (69, 77). In general, among Candida isolates there is a strong positive correlation (R = 0.9) between fluconazole MICs and those of itraconazole, posaconazole, ravuconazole, and voriconazole, suggesting some degree of cross-resistance (54, 69, 70, 77). This is especially true for C. glabrata (69, 77).
CLINICAL CORRELATION AND SUPPORT OF CLSI MIC BREAKPOINTS FOR FLUCONAZOLE
The previously established CLSI MIC interpretive breakpoints for Candida spp. tested against fluconazole were based on an analysis of treatment outcomes in both mucosal (411 patient-episode-isolate events) and invasive (108 patient-episode-isolate events) disease (84, 85). In addition to the traditional categories of “susceptible” and “resistant,” the interpretive breakpoints included a novel category, “susceptible-dose dependent” (SDD) (84). The SDD category encompassed MICs of 16 and 32 μg/ml. Isolates for which fluconazole MICs were above this range (≥64 μg/ml) were termed resistant (R), whereas those inhibited at lower concentrations (≤8 μg/ml) were labeled susceptible (S). The purpose of the SDD category was to emphasize the importance of attaining maximal fluconazole levels in blood and tissue for isolates with higher MICs (85). The maximal dose was defined as being at least 400 mg/day in a 70-kg adult with normal renal function (59). The overall clinical response rate for these 519 patient-episode-isolate events was 87%, including 92% (370/403) for those episodes in which the fluconazole MIC for the isolate was ≤8 μg/ml (S), 82% (45/55) for those episodes in which the MIC was 16 to 32 μg/ml (SDD), and 56% (34/61) for those episodes in which the MIC was ≥64 μg/ml (R) (84). For patients with mucosal disease (411 patient-episode-isolate events), the response rates were 92%, 82%, and 41% at MICs of ≤8 μg/ml, 16 to 32 μg/ml, and ≥64 μg/ml, respectively. The corresponding success rates for patients with invasive disease (108 patient-episode-isolate events) were 71%, 91%, and 58%, respectively (84). The weaknesses of these data were noted to be that (i) the majority of the results were drawn from cases of mucosal candidiasis, (ii) the number of episodes involving isolates for which the fluconazole MICs were elevated (>8 μg/ml) was small, and (iii) the concept of dose-dependent susceptibility was most clearly proven only for mucosal disease (85).
Subsequent to this analysis, there have been several additional studies in which the efficacy of fluconazole therapy has been examined relative to the fluconazole MIC or susceptibility category (S, SDD, and R) of the infecting isolate, as determined by standardized (CLSI or EUCAST) susceptibility testing methods (Table 5). In each instance the isolate with the highest MIC from each episode of infection from each patient was defined as a separate patient-episode-isolate event (84). A total of 1,295 patient-episode-isolate events in which patients were infected with Candida spp., received fluconazole therapy, and were characterized as treatment successes or failures at the end of therapy were included in 12 published clinical databases (Table 5). These studies included seven (692 patient-episode-isolate events) in which the infection was mucosal in nature and where the daily dose of fluconazole was typically 100 mg/day (6, 14, 15, 37, 80, 84, 88) and five (603 patient-episode-isolate events) in which invasive candidiasis (bloodstream, tissue, normally sterile-site infection) was treated with higher doses (usually 400 mg/day) of fluconazole (5, 18, 38, 84, 100). Although the clinical response was not stratified by species of Candida, the species distribution in these studies was typical for the two broad infection types: mucosal (80% C. albicans, 20% non-C. albicans) and invasive (40% C. albicans, 60% non-C. albicans).
TABLE 5.
Correlations of fluconazole susceptibility testing with clinical response for mucosal and invasive Candida infections treated with fluconazolea
| Reference | Type of infection | Dose (mg/day) | MIC (μg/ml) used to determine susceptibility class
|
No. of eventsb | % Success (n/N)f by susceptibility class
|
||||
|---|---|---|---|---|---|---|---|---|---|
| S | SDD | R | S | SDD | R | ||||
| 84 | Mucosal | 100 | ≤8 | 16-32 | ≥64 | 302 | 98 (248/253) | 78 (21/27) | 73 (16/22) |
| 6 | Mucosal | 100-200 | ≤8 | 16-32 | ≥64 | 48 | 80 (28/35) | 46 (6/13) | |
| 15 | Mucosal | 100 | ≤8 | 16-32 | ≥64 | 66 | 96 (49/51) | 0 (0/7) | 0 (0/8) |
| 88 | Mucosal | 100-400 | ≤32 | ≥64 | 21 | 88 (14/16) | 0 (0/5) | ||
| 37 | Mucosal | 100 | ≤0.39 | 1.56 | ≥3.12 | 27 | 93 (14/15) | 0 (0/2) | 30 (3/10) |
| 14 | Mucosal | NAc | ≤8 | 16-32 | ≥64 | 73 | 75 (42/56) | 50 (2/4) | 23 (3/13) |
| 80 | Mucosal | 100-800 | ≤8 | 16-32 | ≥64 | 155 | 87 (93/107) | 72 (13/18) | 43 (13/30) |
| Total mucosal | 692 | 92 (488/533) | 62 (36/58) | 41 (41/101) | |||||
| 84 | Invasived | >100 | ≤8 | 16-32 | ≥64 | 217e | 81 (122/150) | 86 (24/28) | 46 (18/39) |
| 38 | Invasive | 400 | ≤8 | 16-32 | ≥64 | 32 | 79 (19/24) | 67 (4/6) | 0 (0/2) |
| 5 | Invasive | 400 | ≤8 | 16-32 | ≥64 | 80 | 92 (54/59) | 75 (6/8) | 54 (7/13) |
| 18 | Invasive | 50-400 | ≤8 | 16-32 | ≥64 | 32 | 67 (14/21) | 20 (1/5) | 0 (0/6) |
| 100 | Invasive | 200 | ≤8 | 16-32 | ≥64 | 242 | 70 (144/206) | 64 (16/25) | 55 (6/11) |
| Total invasive | 603 | 77 (353/460) | 71 (51/72) | 44 (31/71) | |||||
| Total invasive plus mucosal | 1,295 | 85 (841/993) | 67 (87/130) | 42 (72/172) | |||||
All studies were performed by CLSI or EUCAST broth dilution methods. Percent clinical success was determined by investigators at the end of treatment.
Number of individual patient-episode-isolate events.
NA, data not available.
Invasive infections including bloodstream, deep tissue, and other normally sterile sites.
Ninety-nine patient-episode-isolate events represented invasive infection, with response rates of 71% (S), 91% (SDD), and 58% (R) (84).
Abbreviations: n, number of successful events; N, number of total patient-episode-isolate events.
Among the isolates causing mucosal infection, 77% (533/692) were susceptible (MIC ≤ 8 μg/ml), 8% (58/692) were SDD (MIC, 16 to 32 μg/ml), and 15% (101/692) were R to fluconazole (Table 5). Similarly, 76% (460/603) of the isolates from invasive infections were classified as S, 12% (72/603) were SDD, and 12% (71/603) were R. By comparison to the MIC distribution profiles shown in Tables 1 and 4, these isolates tended to be less susceptible to fluconazole. These differences may be explained by the fact that the MIC data shown in Tables 1 and 4 represent that of the incident isolate (first isolate) from each episode of infection, whereas those shown in Table 5 generally represent the highest MIC determined for each patient-episode. Thus, the patient-episode-isolate events summarized in Table 5 represent a rigorous challenge to the therapeutic efficacy of fluconazole and provide significant numbers of isolates for which fluconazole MICs are elevated. Additionally, the studies shown in Table 5 serve to expand the number of patient-episode-isolate events representing more serious invasive disease and thus strengthen the potential applicability of the MIC interpretive criteria to infections other than mucosal.
The overall success rate for the 1,295 patient-episode-isolate events shown in Table 5 was 77% (1,000/1,295), including 85% (841/993) for those episodes in which the fluconazole MIC was ≤8 μg/ml, 67% (87/130) for those episodes in which the MIC was 16 to 32 μg/ml, and 42% (72/172) for those episodes with resistant (MIC ≥ 64 μg/ml) isolates. These aggregate data clearly support the interpretive breakpoints proposed earlier by the CLSI Subcommittee for Antifungal Testing and are consistent with the “90-60” rule described previously for both antibacterial and antifungal susceptibility testing (86).
Although a successful outcome was more strongly predicted by a result of S (MIC ≤ 8 μg/ml) in the case of mucosal candidiasis compared to invasive disease, the complexities surrounding those patients in which candidemia or other invasive disease develops are well known and clearly contribute to a poorer outcome irrespective of the potency of the antifungal agent administered (31, 86, 100). Perhaps more important than the ability of an antifungal test to predict clinical success when the MIC is low (i.e., susceptible) is the ability to predict failure when the result is high (i.e., resistant). In this regard, fluconazole susceptibility testing functions quite well (Table 5). Only 42% of the patient-episode-isolate events were successfully treated with fluconazole when the MIC for the infecting isolate was ≥64 μg/ml. This was true irrespective of whether the infection was mucosal or invasive. Thus, the evidence most strongly supports the usefulness of the R category. Consideration of the overall MIC distribution (Table 1), the cross-resistance and resistance mechanism data, the PK of the drug, and the relationship between MIC and clinical outcome (Table 5) are very compelling and supportive of this concept. It is clear that Candida isolates for which fluconazole MICs are ≥64 μg/ml (i) are predominantly C. glabrata and C. krusei (Table 1), (ii) represent a concentration of fluconazole that cannot be maintained over the dosing interval with currently recommended doses, (iii) exhibit several different resistance mechanisms (Fig. 1), and (iv) are significantly less likely to respond clinically to fluconazole therapy (Table 5).
The fact that most of the fluconazole-resistant isolates are C. glabrata and C. krusei raises some issues that may confound the relationship between MIC and outcome. Specifically, patients at risk for infections with C. glabrata and C. krusei may have other factors, such as advanced age, relapsed leukemia, fluconazole prophylaxis, and corticosteroid therapy, that can also be associated with poor outcome irrespective of the antifungal agent used to treat the infections (58, 100, 104, 112). Evidence to support the conclusion that decreased susceptibility to fluconazole does in fact contribute to a poor outcome may be found in several salvage therapy studies in which patients failing fluconazole therapy (and infected with isolates of Candida spp. [including C. glabrata and C. krusei] with decreased susceptibility to fluconazole) were treated successfully with voriconazole (55), micafungin (56), caspofungin (35), or amphotericin B (47, 112).
The data also support the SDD category as one in which the clinical response may approach that of S isolates as long as higher doses of fluconazole are used. This was seen most prominently in invasive disease, where the higher doses administered in such infections might be expected to be more effective in treating infections with organisms in the SDD category (2, 3, 85, 105). It is also apparent that the rate of successful therapy was higher with SDD isolates than that seen with isolates that were R. It should be noted that the concept of dose-dependent susceptibility pertains only to species such as C. glabrata, where resistance is inducible or acquired, and not to intrinsically resistant species such as C. krusei.
These data provide strong validation of the CLSI MIC interpretive breakpoints for fluconazole and Candida spp. and document their applicability in both mucosal and invasive disease. In performing this analysis we have addressed the aforementioned weaknesses of fluconazole MIC testing by expanding the number of invasive cases as well as the number of episodes of infection involving isolates for which fluconazole MICs were elevated. Furthermore, we provide additional data to support the concept of dose-dependent susceptibility for infections more serious than mucosal disease. Such a concept is even more strongly supported by PD considerations (2, 3, 18).
PD SUPPORT FOR FLUCONAZOLE BREAKPOINTS AND DOSE-DEPENDENT SUSCEPTIBILITY OF CANDIDA SPP.
Correlation of human PK and clinical trial outcome with several anti-infective agents has suggested that the PD parameter magnitude that produces efficacy in animal models also predicts efficacy in humans (2, 3). In vivo PD studies of fluconazole and invasive candidiasis have identified the AUC/MIC ratio as the key PD parameter and a magnitude of ∼25 as predictive of efficacy (2-4, 42). It is known that the AUC for healthy adults given fluconazole is almost exactly equal to the daily dose, in milligrams (85). Thus, a 70-kg adult given 400 mg of fluconazole will have an AUC of 400 mg · h/liter (42, 85). Given this information, the CLSI Subcommittee for Antifungal Testing used the dose/MIC ratio as a surrogate for AUC/MIC in analyzing the relationship between drug dose, organism MIC, and clinical outcome for fluconazole treatment of mucosal candidiasis (85). The data showed a clear relationship between the dose/MIC ratio and clinical outcome (Table 6) (85). When the fluconazole dose/MIC ratio exceeded a value of 25, clinical treatment success was observed in 91% to 99% of patients (Table 6). When this PD value fell below 25, treatment failures were reported in 26% to 35% of cases (85). Thus, the dose/MIC ratio of ∼25 was predictive of outcome in humans, as was the AUC/MIC ratio in animal model PD studies (2, 3). Notably, the dose/MIC magnitude of ∼25 was supportive of the susceptibility breakpoint guidelines suggested in the CLSI M27 document (85).
TABLE 6.
Relationship between dose/MIC ratio and clinical response in fluconazole treatment of mucosal and invasive candidiasis
| Dose/MIC | % Clinical success (n/N)a
|
||||
|---|---|---|---|---|---|
| Rex et al. (85)b | Clancy et al. (18)c | Lee et al. (38)c | Takakura et al. (100)c | Total | |
| ≥400 | 99 (115/116) | 89 (8/9) | 98 (123/125) | ||
| 100-300 | 98 (129/132) | 60 (6/10) | 95 (135/142) | ||
| 50-75 | 92 (34/37) | 0 (0/1) | 79 (19/24) | 86 (53/62) | |
| 25-37.5 | 91 (30/33) | 0 (0/1) | 67 (4/6) | 70 (144/206) | 72 (178/246) |
| 6.26-12.5 | 74 (35/47) | 20 (1/5) | 64 (16/25) | 68 (52/77) | |
| <6.25 | 65 (30/46) | 0 (0/6) | 0 (0/2) | 55 (6/11) | 55 (36/65) |
Abbreviations: n, number of successful treatment events; N, number of total patient-episode-isolate events.
Mucosal infection study.
Invasive candidiasis (candidemia) study.
Subsequent analysis of several smaller patient series provides additional support for this concept as it applies to oropharyngeal candidiasis (2). Application of this PD analysis to patients with candidemia has been more problematic, largely due to lack of data from patients with invasive candidiasis who were treated with fluconazole and in which MICs were high enough to demonstrate a relationship between drug dose, MIC, and outcome (2, 3, 85). Three such studies have now been published, the results of which are compared with those of mucosal infection in Table 6. Although the rate of clinical success in these three studies was considerably lower than that in patients with mucosal infection, a strong relationship was observed between MIC, fluconazole dose, and outcome. Taken together, these three studies show that clinical success was observed in 70% of patients (181/257) when the dose/MIC ratio was 25 or greater and was 47% (23/49) when the PD value fell below 25 (Table 6). Combining the mucosal and invasive candidiasis databases shows a clear decrement in clinical response with a decreasing dose/MIC ratio (Table 6).
Looking at fluconazole MICs in the critical range of 8 to 32 μg/ml in light of the target dose/MIC ratio of 25, one can see that a dose of 800 mg/day would be required to treat an isolate for which the fluconazole MIC was 32 μg/ml and 200 mg/day would be required for an isolate for which the fluconazole MIC was 8 μg/ml (Table 7). The dose-dependent nature of the clinical response is very apparent in this critical MIC range, which spans the S and SDD susceptibility categories. In further support of the dose-dependent nature of response in candidemia are the experiences of Graninger et al. (24) and Voss and de Pauw (105), both of which demonstrated increased rates of successful therapy for candidemia when the dose of fluconazole was increased from 400 to 800 mg/day in successive cohorts of patients.
TABLE 7.
Analysis of the fluconazole dose/MIC ratio highlights important ranges for MICs and daily dosages
| Dose (mg/day) | MIC (μg/ml) | Dose/MIC | Anticipated % successa |
|---|---|---|---|
| 800 | 8 | 100 | 95 |
| 16 | 50 | 86 | |
| 32 | 25 | 72 | |
| 400 | 8 | 50 | 86 |
| 16 | 25 | 72 | |
| 32 | 12.5 | 68 | |
| 200 | 8 | 25 | 72 |
| 16 | 12.5 | 68 | |
| 32 | 6.25 | 55 |
Data from Table 6.
DEVELOPMENT OF DISK INTERPRETIVE BREAKPOINTS
The CLSI has standardized an agar disk diffusion test method for fluconazole and Candida spp. (51). The method employs a 25-μg disk and Mueller-Hinton agar supplemented with 2% glucose and 0.5 μg of methylene blue per ml (10, 11). In contrast to the 48-h incubation requirement for the BMD test, disk diffusion test results may be determined after only 24 h of incubation (50, 51). Several studies have shown good correlation between fluconazole BMD MICs and disk diffusion test zone diameters (10, 67, 72). Fluconazole disk tests have been used to great advantage in conducting antifungal resistance surveillance in the ARTEMIS DISK Global Antifungal Surveillance Study (30, 75). Previously, we documented that fluconazole disk testing can be performed with a high degree of accuracy as part of routine laboratory testing (72).
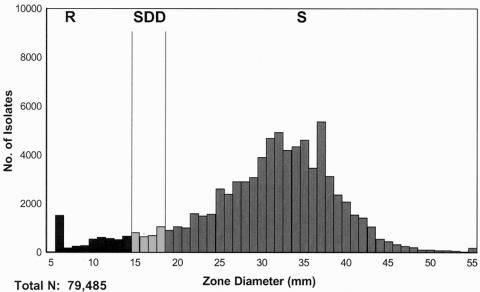
Fluconazole disk testing has been performed in more than 115 laboratories in 35 countries between 2001 and the present as part of the ARTEMIS program (72, 75). The frequency distribution of fluconazole zone diameters for 79,485 isolates of Candida spp. is shown in Fig. 2. The frequency distribution of zone diameters is similar to that of the MIC distribution (Table 1), with the vast majority of isolates showing large zones of inhibition indicative of susceptibility to fluconazole.
FIG. 2.
Fluconazole zone diameter distributions for all Candida spp.: 79,485 isolates tested against fluconazole. Isolates were obtained from 115 institutions in 34 countries from 2001 through 2003. Interpretive breakpoints: susceptible (S), ≥19 mm; susceptible-dose dependent (SDD), 15 to 18 mm; resistant (R), ≤14 mm. (Reprinted from reference 75.)
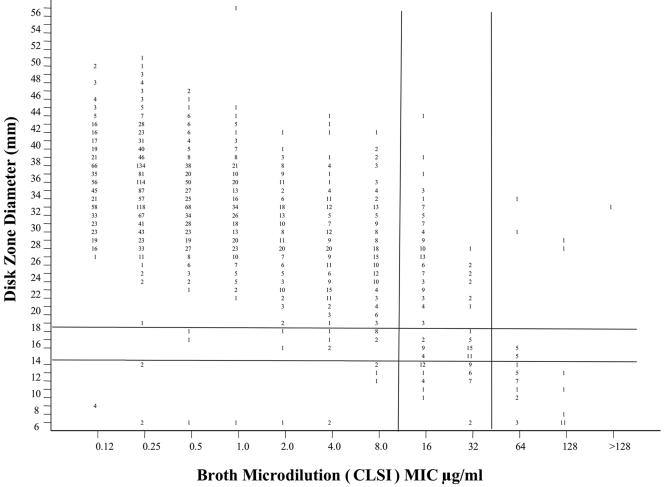
The relationship between fluconazole MICs and zone diameters is shown in Fig. 3 for 2,949 clinical isolates of Candida. These isolates represent the same species distribution as that shown in Table 1. Using the MIC breakpoints for fluconazole of ≤8 μg/ml, 16 to 32 μg/ml, and ≥64 μg/ml to represent the S, SDD, and R categories, respectively, one can then derive zone diameter breakpoints by the error-rate bounded method (45), whereby the number of discrepancies between the zone diameter and MIC categories is minimized (Fig. 3 and Table 8). The overall categorical agreement between the disk diffusion and BMD MIC results was determined for fluconazole with the MIC interpretive categories used as the reference. Major errors (ME) were identified as a classification of resistant by the disk diffusion test and susceptible by BMD, very major errors (VME) were identified as a classification of susceptible by the disk diffusion method and resistant by BMD, and minor errors (M) occurred when the result of one of the tests was susceptible or resistant and that of the other test was SDD.
FIG. 3.
Scatterplot showing the relationship between fluconazole MICs and zone diameters for 2,949 isolates of Candida species. The proposed interpretive breakpoints for each test method are indicated by the horizontal and vertical lines. (Adapted from reference 72.)
TABLE 8.
Categorical agreement between fluconazole BMD MIC and disk test results for 2,949 clinical isolates of Candidaa
| Methodb | % by interpretive categoryc
|
% Agreementd | % Errorse
|
||||
|---|---|---|---|---|---|---|---|
| S | SDD | R | VME | ME | M | ||
| BMD | 91.6 | 6.7 | 1.7 | ||||
| Disk | 94.1 | 2.2 | 3.7 | 92.8 | 0.1 | 0.4 | 6.6 |
Compiled from reference 72.
BMD and disk diffusion testing were performed in accordance with CLSI M27-A2 (50) and M44-A (51), respectively.
Interpretive categories: S, MIC of ≤8 μg/ml (≥19 mm); SDD, MIC of 16 to 32 μg/ml (15 to 18 mm); R, MIC of ≥64 μg/ml (≤14 mm).
Percent categorical agreement between disk diffusion and MIC test results.
VME, very major error; ME, major error; M, minor error.
With zone diameter breakpoints of ≥19 mm (S), 15 to 18 mm (SDD), and ≤14 mm (R), the overall categorical agreement between the disk diffusion test results and the MIC test results was excellent (92.8%), with very few VME or ME (Table 8). On the basis of these findings, it appears that the disk diffusion test is a useful method for testing the activity of fluconazole against Candida spp.
CLINICAL IMPORTANCE OF ANTIFUNGAL SUSCEPTIBILITY TESTING AS IT PERTAINS TO FLUCONAZOLE AND CANDIDA
Candidiasis is clearly a very important infectious disease (29, 34, 58, 79). Candidemia alone occurs at a rate of 8 to 10 infections per 100,000 population per year (29, 34, 79) and is associated with an excess mortality of 30 to 50% (27, 107). It is estimated that the excess cost attributable to candidemia in the United States approaches $1 billion per year (46, 60, 111). Although much of this cost can be attributed to length of stay in hospital, the cost of antifungal therapy, especially the newer lipid formulations of amphotericin B, the echinocandins, and the extended-spectrum triazoles, is not inconsequential (41, 46, 60, 111). Selection of the optimal antifungal therapeutic strategy is becoming increasingly complex and is often complicated further by concerns of emerging antifungal resistance (9, 41, 59).
In light of these concerns, one might expect that antifungal susceptibility testing would be useful as an aid in selecting the most appropriate antifungal agent, especially for treating more serious forms of candidiasis. However, routine use of antifungal susceptibility testing has been relatively uncommon, and its clinical utility is often limited by delays in the availability of results (9, 28, 31, 57, 59). Given the advances in standardization of antifungal susceptibility testing and the proposed interpretive breakpoints for fluconazole described herein, several authors and consensus groups have recommended routine fluconazole susceptibility testing of Candida spp. from sterile sites after identification of the clinical isolate (9, 31, 59, 85, 86).
Recent studies examining the clinical utility of “real-time” antifungal susceptibility testing in the treatment of candidemia have shown that when such testing is available on site, physicians find the results helpful and not infrequently alter therapy on the basis of the results (9, 28). At the University of Alabama, Baddley et al. (9) found that the most common change was a switch from amphotericin B to fluconazole. Furthermore, physicians felt reassured in continuing patients on fluconazole if the MIC demonstrated susceptibility of the Candida isolate. On the other hand, detection of resistance to fluconazole is clearly desirable, and early recognition of resistance in clinical isolates may contribute to successful clinical and microbiologic outcomes (86, 101, 112). Hadley et al. (28) reported the use of real-time antifungal susceptibility testing in modifying treatment decisions in several patients with invasive fungal infections. They documented resistance to fluconazole in two patients with C. albicans infection and noted that although fluconazole would normally have been an optimal choice for this species to avoid toxicities and excess costs, a report of resistance supported continued use of amphotericin B. Thus, it would appear that routine antifungal susceptibility testing can serve as an adjunct in the treatment of candidemia in the same way that antibacterial testing aids in the treatment of bacterial infections (9, 28, 86).
It is now apparent that antifungal susceptibility testing of fluconazole against Candida is being incorporated into clinical laboratory practice as BMD panels and fluconazole disks become available commercially (62, 67, 72, 75). In the United States, the number of clinical laboratories participating in the College of American Pathologists (CAP) antifungal proficiency testing program has increased from 50 laboratories in 1997 to more than 100 laboratories at present (76). The majority of these laboratories use commercial MIC tests, either YeastOne (Sensititre, Cleveland, OH) or Etest (AB BIODISK, Solna, Sweden), and more than a third of laboratories perform more than 100 MIC tests per year for clinical purposes. The performance of these laboratories in testing fluconazole against Candida spp. is excellent and, as seen in Table 9, is easily comparable to the level of performance accuracy achieved by CAP participants in testing a fastidious bacterium such as Streptococcus pneumoniae (76).
TABLE 9.
Comparative performance in CAP proficiency challenges: antibacterial versus antifungal susceptibility testinga
| Challenge strain | Antimicrobial agent | No. of results | % Correct |
|---|---|---|---|
| D-10, 2003 (S. pneumoniae) | Ceftriaxone | 1,087 | 43 |
| Penicillin | 1,932 | 89 | |
| Trimethoprim-sulfamethoxazole | 165 | 94 | |
| F-01, 2003 (C. albicans) | Fluconazole | 96 | 94 |
| F-07, 2003 (C. parapsilosis) | Fluconazole | 78 | 91 |
| F-17, 2003 (C. krusei) | Fluconazole | 68 | 100 |
| CAP, 2003 (all Candida spp.) | Fluconazole | 242 | 95 |
Data compiled from Pfaller et al. (76).
Fluconazole disk diffusion testing of Candida spp. is also increasing worldwide. The ARTEMIS DISK Global Antifungal Surveillance Program monitors the performance of 127 participating laboratories in 39 countries (75). These laboratories all perform fluconazole disk diffusion testing routinely for clinical purposes on isolates of Candida spp. and other opportunistic yeasts and report results to a central database (75). External validation of this routine laboratory testing by a central reference laboratory has documented excellent performance (72, 74). The simplicity and flexibility of disk diffusion testing makes it a very appealing method for use in the clinical laboratory (72, 74, 75). Results are clearly available in real time (i.e., 24 h), and innovative efforts to integrate this form of antifungal testing into the work flow of the clinical laboratory are ongoing (101). Thus, fluconazole disk diffusion testing is being performed in clinical laboratories throughout the world as an aid in selecting the most efficacious, nontoxic, and cost-effective therapy for treatment of candidiasis.
SUMMARY AND CONCLUSIONS
We have validated CLSI interpretive breakpoints for in vitro susceptibility testing of fluconazole and Candida spp.: S MIC (zone diameter), ≤8 μg/ml (≥19 mm); SDD, 16 to 32 μg/ml (15 to 18 mm); R, ≥64 μg/ml (≤14 mm). These interpretive breakpoints are supported by (i) consideration of mechanisms of resistance to fluconazole, (ii) analysis of the MIC population distribution, (iii) consideration of cross-resistance patterns, (iv) analysis of parameters associated with success in pharmacodynamic models, and (v) the results from clinical efficacy studies. The clinical data supporting these breakpoints have been expanded to include more cases with invasive candidiasis and more infections with isolates with higher fluconazole MICs. The strength of the correlation of these breakpoints with clinical outcome is consistent with that from other fungal and bacterial infections: a result of S is associated with a higher success rate than a result of R, but host factors also influence outcome.
This “blueprint” for establishing interpretive breakpoints should be applicable to other antifungal agents, such as voriconazole (77) and the echinocandins; however, the dose/MIC ratio used herein as a surrogate for the AUC/MIC ratio cannot be used for other agents that have less-predictable dose/AUC relationships.
Antifungal susceptibility testing of fluconazole and Candida is becoming recognized as a useful aid in optimizing treatment of candidiasis. Standardized MIC and disk diffusion testing can be performed accurately in the routine clinical laboratory, providing real-time results for difficult clinical infections.
Acknowledgments
Linda Elliott and Susan Shaffer provided excellent support in the preparation of the manuscript.
This work was supported in part by Pfizer, Inc., Pfizer Global Pharmaceuticals, New York, N.Y.
REFERENCES
- 1.Anaissie, E. J., D. P. Kontoyiannis, C. Huls, S. E. Vartivarian, C. Karl, R. A. Prince, J. Bosso, and G. P. Bodey. 1995. Safety, plasma concentrations, and efficacy of high-dose fluconazole in invasive mold infections. J. Infect. Dis. 172:599-602. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D. 2003. Clinical pharmacodynamics of antifungals. Infect. Dis. Clin. N. Am. 17:635-649. [DOI] [PubMed] [Google Scholar]
- 3.Andes, D. 2003. In vivo pharmacodynamics of antifungal drugs in treatment of candidiasis. Antimicrob. Agents Chemother. 47:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D., and M. van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoniadou, A., H. A. Torres, R. E. Lewis, J. Thornby, G. P. Bodey, J. P. Tarrand, X. Y. Han, K. V. Rolston, A. Safdar, I. I. Raad, and D. P. Kontoyiannis. 2003. Candidemia in a tertiary care center: in vitro susceptibility and its association with outcome of initial antifungal therapy. Medicine 82:309-321. [DOI] [PubMed] [Google Scholar]
- 6.Arikan, S., M. Akova, M. Hayran, O. Ozdemir, M. Erman, D. Gur, and S. Unal. 1998. Correlation of in vitro fluconazole susceptibility with clinical outcome for severely ill patients with oropharyngeal candidiasis. Clin. Infect. Dis. 26:903-908. [DOI] [PubMed] [Google Scholar]
- 7.Arthington-Skaggs, B. A., W. Lee-Yang, M. A. Ciblak, J. P. Frade, M. E. Brandt, R. A. Hajjeh, L. H. Harrison, A. N. Sofair, and D. W. Warnock. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC endpoint determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 46:2477-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asmundsdottir, L. R., H. Erlendsdottir, and M. Gottfredsson. 2002. Increasing incidence of candidemia: results from a 20-year nationwide study in Iceland. J. Clin. Microbiol. 40:3489-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baddley, J. W., M. Patel, M. Jones, G. Cloud, A. C. Smith, and S. A. Moser. 2004. Utility of real-time antifungal susceptibility testing for fluconazole in the treatment of candidemia. Diagn. Microbiol. Infect. Dis. 50:119-124. [DOI] [PubMed] [Google Scholar]
- 10.Barry, A., J. Bille, S. Brown, D. Ellis, J. Meis, M. Pfaller, R. Rennie, M. Rinaldi, T. Rogers, and M. Traczewski. 2003. Quality control limits for fluconazole disk susceptibility tests on Mueller-Hinton agar with glucose and methylene blue. J. Clin. Microbiol. 41:3410-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry, A. L., M. A. Pfaller, R. P. Rennie, P. C. Fuchs, and S. D. Brown. 2002. Precision and accuracy of fluconazole susceptibility testing by broth microdilution, Etest, and disk diffusion methods. Antimicrob. Agents Chemother. 46:1781-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borst, A., M. T. Raimer, D. W. Warnock, C. J. Morrison, and B. A. Arthington-Skaggs. 2005. Rapid acquisition of stable azole resistance by Candida glabrata isolates obtained before the clinical introduction of fluconazole. Antimicrob. Agents Chemother. 49:783-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgess, D. S., R. W. Hastings, K. K. Summers, T. C. Hardin, and M. G. Rinaldi. 2000. Pharmacodynamics of fluconazole, itraconazole, and amphotericin B against Candida albicans. Diagn. Microbiol. Infect. Dis. 36:13-18. [DOI] [PubMed] [Google Scholar]
- 14.Cameron, M. L., W. A. Schell, S. Bruch, J. A. Bartlett, H. A. Waskin, and J. R. Perfect. 1993. Correlation of in vitro fluconazole resistance of Candida isolates in relation to therapy and symptoms of individuals seropositive for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 37:2449-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cartledge, J. D., J. Midgley, M. Petrou, D. Shanson, and B. G. Gazzard. 1997. Unresponsive HIV-related oro-oesophageal candidosis—an evaluation of two new in-vitro azole susceptibility tests. J. Antimicrob. Chemother. 40:517-523. [DOI] [PubMed] [Google Scholar]
- 16.Chen, Y.-C., S.-C. Chang, K.-T. Luh, and W.-C. Hsieh. 2003. Stable susceptibility of Candida blood isolates to fluconazole despite increasing use during the past 10 years. J. Antimicrob. Chemother. 52:71-77. [DOI] [PubMed] [Google Scholar]
- 17.Chryssanthous, E. 2001. Trends in antifungal susceptibility among Swedish Candida species bloodstream isolates from 1994-1998: comparison of the Etest and the Sensititre YeastOne Colorimetric Antifungal Panel with the NCCLS M27-A reference method. J. Clin. Microbiol. 39:4181-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clancy, C. J., V. L. Yu, A. J. Morris, D. R. Snydman, and M. H. Nguyen. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49:3171-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuenca-Estrella, M., T. M. Diaz-Guerra, E. Mellado, A. Monzon, and J. L. Rodriguez-Tudela. 1999. Comparative in vitro activity of voriconazole and itraconazole against fluconazole-susceptible and fluconazole-resistant clinical isolates of Candida species from Spain. Eur. J. Clin. Microbiol. Infect. Dis. 18:432-435. [DOI] [PubMed] [Google Scholar]
- 20.Cuenca-Estrella, M., L. Rodero, G. Garcia-Effron, and J. L. Rodriguez-Tudelo. 2002. Antifungal susceptibilities of Candida spp. isolated from blood in Spain and Argentina, 1996-1999. J. Antimicrob. Chemother. 49:981-987. [DOI] [PubMed] [Google Scholar]
- 21.Cuenca-Estrella, M., D. Rodriguez, B. Almirante, J. Morgan, A. M. Planes, M. Almela, J. Mensa, F. Sanchez, J. Ayats, M. Gimenez, M. Salvado, D. W. Warnock, A. Pahissa, and J. L. Rodriguez-Tudela. 2005. In vitro susceptibilities of bloodstream isolates of Candida species to six antifungal agents: results from a population-based active surveillance programme, Barcelona, Spain, 2002-2003. J. Antimicrob. Chemother. 55:194-199. [DOI] [PubMed] [Google Scholar]
- 22.Diekema, D. J., S. A. Messer, A. B. Brueggemann, S. L. Coffman, G. V. Doern, L. A. Herwaldt, and M. A. Pfaller. 2002. Epidemiology of candidemia: three-year results from the Emerging Infections and the Epidemiology of Iowa Organisms study. J. Clin. Microbiol. 40:1298-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghannoum, M. A., and L. B. Rice. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graninger, W., E. Presteril, B. Schneeweis, B. Teleky, and A. Georgopoulos. 1993. Treatment of Candida albicans fungaemia with fluconazole. J. Infect. 16:133-146. [DOI] [PubMed] [Google Scholar]
- 25.Grant, S. M., and S. P. Clissold. 1990. Fluconazole: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs 39:877-916. [DOI] [PubMed] [Google Scholar]
- 26.Groll, A. H., J. C. Gea-Banacloche, A. Glasmacher, and T. J. Walsh. 2003. Clinical pharmacology of antifungal compounds. Infect. Dis. Clin. N. Am. 18:159-191. [DOI] [PubMed] [Google Scholar]
- 27.Gudlaugsson, O., S. Gillespie, K. Lee, J. Vande Berg, J. Hu, S. Messer, L. Herwaldt, M. Pfaller, and D. Diekema. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172-1177. [DOI] [PubMed] [Google Scholar]
- 28.Hadley, S., J. A. Martinez, L. McDermott, B. Rapino, and D. R. Snydman. 2002. Real-time antifungal susceptibility screening aids management of invasive yeast infections in immunocompromised patients. J. Antimicrob. Chemother. 49:415-419. [DOI] [PubMed] [Google Scholar]
- 29.Hajjeh, R. A., A. N. Sofair, L. H. Harrison, G. M. Lyon, B. A. Arthington-Skaggs, S. A. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. A. Ciblak, L. E. Benjamin, L. T. Sanza, S. Huie, S. F. Yeo, M. E. Brandt, and D. W. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hazen, K. C., E. J. Baron, A. L. Colombo, C. Girmenia, A. Sanchez-Souza, A. del Palacio, C. de Bedout, D. L. Gibbs, and The Global Antifungal Surveillance Group. 2003. Comparison of the susceptibilities of Candida spp. to fluconazole and voriconazole in a 4-year global evaluation using disk diffusion. J. Clin. Microbiol. 41:5623-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hospenthal, D. R., C. K. Murray, and M. G. Rinaldi. 2004. The role of antifungal susceptibility testing in the therapy of candidiasis. Diagn. Microbiol. Infect. Dis. 48:153-160. [DOI] [PubMed] [Google Scholar]
- 32.Hsueh, P. R., L. J. Teng, P. C. Yang, S. W. Ho, and K. T. Luh. 2002. Emergence of nosocomial candidemia at a teaching hospital in Taiwan from 1982 to 2000: increased susceptibility of Candida species to fluconazole. Microb. Drug Resist. 8:311-319. [DOI] [PubMed] [Google Scholar]
- 33.Hsueh, P. R., Y. L. Lau, Y. C. Chuang, J. H. Wan, W. K. Huang, J. M. Shyr, J. J. Yan, K. W. Yu, J. J. Wu, W. C. Ko, Y. C. Yang, Y. C. Liu, L. J. Teng, C. Y. Liu, and K. T. Luh. 2005. Antifungal susceptibilities of clinical isolates of Candida species, Cryptococcus neoformans, and Aspergillus species from Taiwan: surveillance of multicenter antimicrobial resistance in Taiwan program data from 2003. Antimicrob. Agents Chemother. 49:512-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kao, A. S., M. E. Brandt, W. R. Pruitt, L. A. Conn, B. A. Perkins, D. S. Stephens, W. S. Baughman, A. L. Reingold, G. A. Rothrock, M. A. Pfaller, R. W. Pinner, and R. A. Hajjeh. 1999. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin. Infect. Dis. 29:1164-1170. [DOI] [PubMed] [Google Scholar]
- 35.Kartsonis, N. A., A. Saah, C. J. Lipka, A. Taylor, and C. A. Sable. 2004. Second-line therapy with caspofungin for mucosal or invasive candidiasis: results from the caspofungin compassionate-use study. J. Antimicrob. Chemother. 53:878-881. [DOI] [PubMed] [Google Scholar]
- 36.Klepser, M. E., E. J. Wolfe, R. N. Jones, C. H. Nightingale, and M. A. Pfaller. 1997. Antifungal pharmacodynamic characterization of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaCassin, F., F. Damond, C. Chochillon, P. Longuet, J. Lebras, J. L. Vidle, and C. LePort. 1996. Response to fluconazole by 23 patients with human immunodeficiency virus infection and oral candidiasis: pharmacological and mycological factors. Antimicrob. Agents Chemother. 40:1961-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, S. C., C. P. Fung, J. S. Huang, C. J. Tsai, K. S. Chen, H. Y. Chen, N. Lee, L. C. See, and W. B. Shieh. 2000. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe Candida infections treated with fluconazole. Antimicrob. Agents Chemother. 44:2715-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Ribot, J. L., R. K. McAtee, L. N. Lee, W. R. Kirkpatrick, T. C. White, D. Sanglard, and T. F. Patterson. 1998. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 42:2932-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Ribot, J. L., R. K. McAtee, S. Perea, W. R. Kirkpatrick, M. G. Rinaldi, and T. F. Patterson. 1999. Multiple resistant phenotypes of Candida albicans coexist during episodes of oropharyngeal candidiasis in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 43:1621-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lortholary, O., A. Charlemagne, F. Bastides, P. Chevalier, A. Datry, M. F. Gonzales, G. Michel, P. Tilleul, B. Veber, and R. Herbrecht. 2004. A multicenter pharmacoepidemiological study of therapeutic practices in invasive fungal infections in France during 1998-1999. J. Antimicrob. Chemother. 54:456-464. [DOI] [PubMed] [Google Scholar]
- 42.Louie, A., G. L. Drusano, P. Banerjee, Q. F. Liu, W. Liu, M. Kaw, H. Shayegonia, H. Taber, and M. H. Miller. 1998. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob. Agents Chemother. 42:1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maertens, J. A. 2004. History of the development of azole derivatives. Clin. Microbiol. Infect. 10(Suppl. 1):1-10. [DOI] [PubMed] [Google Scholar]
- 44.Marr, K. A., C. N. Lyons, T. Rustad, R. A. Bowden, and T. C. White. 1998. Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob. Agents Chemother. 42:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metzler, C. M., and R. M. DeHaan. 1974. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J. Infect. Dis. 130:588-594. [DOI] [PubMed] [Google Scholar]
- 46.Miller, L. G., R. A. Hajjeh, and J. E. Edwards, Jr. 2001. Estimating the cost of nosocomial candidemia in the United States. Clin. Infect. Dis. 32:1110. [DOI] [PubMed] [Google Scholar]
- 47.Muñoz, P., C. P. Fernandez-Turegano, L. Alcala, M. Rodriguez-Creixems, T. Pelaez, and E. Bouza. 2002. Frequency and clinical significance of bloodstream infections caused by C. albicans strains with reduced susceptibility to fluconazole. Diagn. Microbiol. Infect. Dis. 44:163-167. [DOI] [PubMed] [Google Scholar]
- 48.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing for yeasts: approved standard, M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 49.National Committee for Clinical Laboratory Standards. 2001. Development of in vitro susceptibility testing criteria and quality control parameters: approved guideline, 2nd ed., M23-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 50.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 2nd ed., M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 51.National Committee for Clinical Laboratory Standards. 2004. Methods for antifungal disk diffusion susceptibility testing of yeasts: approved guideline, M44-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 52.Nolte, F. S., T. Parkinson, and D. J. Falconer. 1997. Isolation and characterization of fluconazole and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob. Agents Chemother. 41:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orozco, A., L. Higgenbothom, C. Hitchcock, T. Parkinson, D. Falconer, A. S. Ibrahim, M. A. Ghannoum, and S. G. Filler. 1998. Mechanisms of fluconazole resistance in Candida krusei. Antimicrob. Agents Chemother. 42:2645-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ostroksy-Zeichner, L., A. M. L. Oude Lashof, B. J. Kullberg, and J. H. Rex. 2003. Voriconazole salvage treatment of invasive candidiasis. Eur. J. Clin. Microbiol. Infect. Dis. 22:651-655. [DOI] [PubMed] [Google Scholar]
- 56.Ostrosky-Zeichner, L., D. Kontoyiannis, J. Raffalli, K. M. Mullane, J. Vazquez, E. J. Anaissie, J. Lipton, P. Jacobs, J. H. Jansen van Rensburg, J. H. Rex, W. Lau, D. Facklam, and D. N. Buell. 2005. International open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 24:654-661. [DOI] [PubMed] [Google Scholar]
- 57.Pai, M. P., and S. L. Penland. 2003. Antifungal susceptibility testing in teaching hospitals. Ann. Pharmacol. 37:192-196. [DOI] [PubMed] [Google Scholar]
- 58.Pappas, P. G., J. H. Rex, J. Lee, R. J. Hamill, R. A. Larsen, W. Powderly, C. A. Kauffman, N. Hyslop, J. E. Mangino, S. Chapman, H. W. Horowtiz, J. E. Edwards, and W. E. Dismukes. 2003. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clin. Infect. Dis. 37:634-643. [DOI] [PubMed] [Google Scholar]
- 59.Pappas, P. G., J. H. Rex, J. D. Sobel, S. G. Filler, W. E. Dismukes, T. J. Walsh, and J. E. Edwards. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161-189. [DOI] [PubMed] [Google Scholar]
- 60.Pelz, R. K., P. A. Lipsett, S. M. Swoboda, M. Diener-West, N. R. Powe, R. G. Brower, T. M. Perl, J. M. Hammond, and C. W. Hendrix. 2000. Candida infections: outcome and attributable ICU costs in critically ill patients. J. Intensive Care Med. 15:255-261. [Google Scholar]
- 61.Perea, S., and T. F. Patterson. 2002. Antifungal resistance in pathogenic fungi: Clin. Infect. Dis. 35:1073-1080. [DOI] [PubMed] [Google Scholar]
- 62.Pfaller, M. A. 2005. Antifungal susceptibility testing methods. Curr. Drug Targets 6:929-943. [DOI] [PubMed] [Google Scholar]
- 63.Pfaller, M. A., D. J. Diekema, R. N. Jones, H. S. Sader, A. C. Fluit, R. J. Hollis, S. A. Messer, and the SENTRY Participant Group. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibility to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J. Clin. Microbiol. 39:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfaller, M. A., and D. J. Diekema. 2002. Role of sentinel surveillance of candidemia: trends in species distribution and antifungal susceptibility. J. Clin. Microbiol. 40:3551-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and D. J. Diekema. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 46:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfaller, M. A., D. J. Diekema, R. N. Jones, S. A. Messer, R. J. Hollis, and the SENTRY Participant Group. 2002. Trends in antifungal susceptibility of Candida spp. from pediatric and adult patients with bloodstream infections: SENTRY Antimicrobial Surveillance Program, 1997 to 2000. J. Clin. Microbiol. 40:852-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, and R. J. Hollis. 2003. Activities of fluconazole and voriconazole against 1,586 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and Etest methods: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J. Clin. Microbiol. 41:1440-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfaller, M. A., and D. J. Diekema. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10(Suppl. 1):11-23. [DOI] [PubMed] [Google Scholar]
- 69.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 70.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Cross-resistance between fluconazole and ravuconazole and the use of fluconazole as a surrogate marker to predict susceptibility and resistance to ravuconazole among 12,796 clinical isolates of Candida spp. J. Clin. Microbiol. 42:3137-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS Antifungal Surveillance Program conducted in 2001 and 2002. J. Clin. Microbiol. 42:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pfaller, M. A., K. C. Hazen, S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Comparison of results of fluconazole disk diffusion testing for Candida species with results from a central reference laboratory in the ARTEMIS Global Antifungal Surveillance Program. J. Clin. Microbiol. 42:3607-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2005. In vitro susceptibilities of clinical isolates of Candida species, Cryptococcus neoformans, and Aspergillus species to itraconazole: global survey of 9,359 isolates tested by Clinical and Laboratory Standards Institute broth microdilution methods. J. Clin. Microbiol. 43:3807-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfaller, M. A., L. Boyken, S. A. Messer, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2005. Comparison of results of voriconazole disk diffusion testing for Candida species with results from a central reference laboratory in the ARTEMIS Global Antifungal Surveillance Program. J. Clin. Microbiol. 43:5209-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pfaller, M. A., D. J. Diekema, M. Rinaldi, R. Barnes, H. Bijie, A. Veselov, N. Tiraboschi, E. Nagy, D. L. Gibbs, and the Global Antifungal Surveillance Group. 2005. Results from the ARTEMIS DISK Global Antifungal Surveillance Study: a 6.5-year analysis of the worldwide susceptibility of yeasts to fluconazole and voriconazole using standardized disk diffusion testing. J. Clin. Microbiol. 43:5848-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pfaller, M. A., and R. N. Jones, for the Microbiology Resource Committee of the College of American Pathologists. Performance accuracy of antibacterial and antifungal susceptibility test methods: report from the College of American Pathologists (CAP) Microbiology Surveys Program (2001-2003). Am. J. Clin. Pathol., in press. [DOI] [PubMed]
- 77.Pfaller, M. A., D. J. Diekema, J. H. Rex, D. Andes, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, E. M. Johnson, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, L. Steele-Moore, P. Troke, T. J. Walsh, and D. J. Warnock. 2006. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J. Clin. Microbiol. 44:819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Redding, S., J. Smth, G. Farinacci, M. Rinaldi, A. Fothergill, J. Rhine-Chalberg, and M. Pfaller. 1994. Resistance of Candida albicans to fluconazole during treatment of oropharyngeal candidiasis in a patient with AIDS: documentation by in vitro susceptibility testing and DNA subtype analysis. Clin. Infect. Dis. 18:240-242. [DOI] [PubMed] [Google Scholar]
- 79.Rees, J. R., R. W. Pinner, R. A. Hajjeh, M. E. Brandt, and A. L. Reingold. 1998. The epidemiologic features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of a population-based laboratory active surveillance. Clin. Infect. Dis. 27:1138-1147. [PubMed] [Google Scholar]
- 80.Revankar, S. G., O. P. Dib, W. R. Kirkpatrick, R. K. McAtee, A. W. Fothergill, M. G. Rinaldi, S. W. Redding, and T. F. Patterson. 1998. Clinical evaluation and microbiology of oropharyngeal infection due to fluconazole-resistant Candida in human immunodeficiency virus-infected patients. Clin. Infect. Dis. 26:960-963. [DOI] [PubMed] [Google Scholar]
- 81.Rex, J. H., J. E. Bennett, A. M. Sugar, P. G. Pappas, C. M. van der Horst, J. E. Edwards, R. G. Washburn, W. M. Scheld, A. W. Karchmer, A. P. Dine, M. J. Levenstein, and C. D. Webb. 1994. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N. Engl. J. Med. 331:1325-1330. [DOI] [PubMed] [Google Scholar]
- 82.Rex, J. H., M. G. Rinaldi, and M. A. Pfaller. 1995. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 39:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rex, J. H., M. A. Pfaller, A. L. Barry, P. W. Nelson, and C. D. Webb. 1995. Antifungal susceptibility testing of isolates form a randomized multicenter trial of fluconazole versus amphotericin B as treatment of nonneutropenic patients with candidemia. Antimicrob. Agents Chemother. 39:40-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 85.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rex, J. H., and M. A. Pfaller. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35:982-989. [DOI] [PubMed] [Google Scholar]
- 87.Rex, J. H., P. G. Pappas, A. W. Karchmer, J. Sobel, J. E. Edwards, S. Hadley, C. Brass, J. A. Vazquez, S. W. Chapman, H. W. Horowitz, M. Zervos, D. McKinsey, J. Lee, T. Babinchak, R. W. Bradsher, J. D. Cleary, D. M. Cohen, L. Danziger, M. Goldman, J. Goodman, E. Hilton, N. E. Hyslop, D. H. Kett, J. Lutz, R. H. Rubin, W. M. Scheld, M. Schuster, B. Simmons, D. K. Stein, R. G. Washburn, L. Mautner, T. C. Chu, H. Panzer, R. B. Rosenstein, and J. Booth. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin. Infect. Dis. 36:1221-1228. [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez-Tudela, J. L., J. V. Martinez-Suarez, F. Dronda, F. Laguna, F. Chaves, and E. Valencia. 1995. Correlation of in-vitro susceptibility test results with clinical response: a study of azole therapy in AIDS patients. J. Antimicrob. Chemother. 35:793-804. [DOI] [PubMed] [Google Scholar]
- 89.Rogers, T. E., and J. N. Galgiani. 1986. Activity of fluconazole (UK 49,858) and ketoconazole against Candida albicans in vitro and in vivo. Antimicrob. Agents Chemother. 30:418-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monad, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1996. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents Chemother. 40:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 93.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanglard, D., F. Ischer, D. Calabrese, P. A. Majcherczyk, and J. Bille. 1999. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 43:2753-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 96.Sanguinetti, M., B. Posteraro, B. Fiori, S. Ranno, R. Torelli, and G. Fadda. 2005. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob. Agents Chemother. 49:668-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.St.-Germain, G., M. Laverdieu, R. Pelletier, A. M. Bourgault, M. Libman, C. Lemieux, and G. Noel. 2001. Prevalence and antifungal susceptibility of 442 Candida isolates from blood and other normally sterile sites: results from a 2-year (1996 to 1998) multicenter surveillance study in Quebec, Canada. J. Clin. Microbiol. 39:949-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sugar, A. M. 1997. Making antifungal susceptibility testing a clinically useful tool. Clin. Infect. Dis. 24:248-249. [DOI] [PubMed] [Google Scholar]
- 100.Takakura, S., N. Fujihara, T. Saito, T. Kudo, Y. Iinuma, S. Ichiyama, and The Japan Invasive Mycosis Surveillance Study Group. 2004. Clinical factors associated with fluconazole resistance and short-term survival in patients with Candida bloodstream infection. Eur. J. Clin. Microbiol. Infect. Dis. 23:380-388. [DOI] [PubMed] [Google Scholar]
- 101.Tan, G. L., and E. M. Peterson. 2005. CHROMagar Candida medium for direct susceptibility testing of yeast from blood cultures. J. Clin. Microbiol. 43:1727-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tortorano, A. M., A. L. Rigoni, E. Biraghi, A. Prigitano, M. A. Viviani, and the FIMUA-FCMM Candidemia Study Group. 2003. The European Confederation of Medical Mycology (ECMM) survey of candidemia in Italy: antifungal susceptibility patterns of 261 non-albicans Candida isolates from blood. J. Antimicrob. Chemother. 52:679-682. [DOI] [PubMed] [Google Scholar]
- 103.Van't Wout, J., H. Mattie, and R. van Furth. 1989. Comparison of the efficacies of amphotericin B, fluconazole, and itraconazole against systemic Candida albicans infection in normal and neutropenic mice. Antimicrob. Agents Chemother. 33:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vindes, A., J. Peman, E. Canton, P. Ubeda, J. L. Lopez-Ribot, and M. Gobernado. 2002. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome, and risk factors for death. Eur. J. Clin. Microbiol. Infect. Dis. 21:767-774. [DOI] [PubMed] [Google Scholar]
- 105.Voss, A., and B. E. DePauw. 1999. High-dose fluconazole therapy in patients with severe fungal infections. Eur. J. Clin. Microbiol. Infect. Dis. 18:165-174. [DOI] [PubMed] [Google Scholar]
- 106.Walmsley, S., S. King, A. McGeer, Y. Ye, and S. Richardson. 2001. Oropharyngeal candidiasis in patients with human immunodeficiency virus: correlation of clinical outcome with in vitro resistance, serum azole levels, and immunosuppression. Clin. Infect. Dis. 32:1554-1561. [DOI] [PubMed] [Google Scholar]
- 107.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1988. Hospital acquired candidemia: attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642-2645. [DOI] [PubMed] [Google Scholar]
- 108.White, T. C. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.White, T. C. 1997. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14-α-demethylase in Candida albicans. Antimicrob. Agents Chemother. 41:1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wilson, L. S., C. M. Reyes, M. Stolpman, J. Speckman, K. Allen, and J. Beney. 2002. The direct cost and incidence of systemic fungal infections. Value Health 5:26-34. [DOI] [PubMed] [Google Scholar]
- 112.Wong-Beringer, A., J. Hindler, L. Brankovic, L. Muehlbauer, and L. Steele-Moore. 2001. Clinical applicability of antifungal susceptibility testing of non-Candida albicans species in hospitalized patients. Diagn. Microbiol. Infect. Dis. 39:25-31. [DOI] [PubMed] [Google Scholar]