Abstract
Novel chlamydiae are newly recognized members of the phylum Chlamydiales that are only distantly related to the classic Chlamydiaceae, i.e., Chlamydia and Chlamydophila species. They also exibit an obligate biphasic intracellular life cycle within eukaryote host cells. Some of these new chlamydiae are currently considered potential emerging human and/or animal pathogens. Parachlamydia acanthamoebae and Simkania negevensis are both emerging respiratory human pathogens, Waddlia chondrophila could be a novel abortigenic bovine agent, and Piscichlamydia salmonis has recently been identified as an agent of the gill epitheliocystis in the Atlantic salmon. Fritschea spp. and Rhabdochlamydia spp. seem to be confined to arthropods, but some evidence for human exposure exists. In this review, we first summarize the data supporting a pathogenic potential of the novel chlamydiae for humans and other vertebrates and the interactions that most of these chlamydiae have with free-living amoebae. We then review the diagnostic approaches to infections potentially due to the novel chlamydiae, especially focusing on the currently available PCR-based protocols, mammalian cell culture, the amoebal coculture system, and serology.
INTRODUCTION
Chlamydiae are obligate intracellular parasites of vertebrates, of some arthropod species, and of several free-living amoebae (reviewed in references 14, 32, and 68). They exhibit a peculiar two-stage developmental cycle that includes an extracellular infectious elementary body and an intracellular vegetative reticulate body. A further infective stage, the crescent body, was recently described for the Parachlamydiaceae (41), a new family within the order Chlamydiales.
The family Chlamydiaceae comprises two genera, Chlamydia and Chlamydophila (29). While Chlamydia species seem to infect only mammals such as humans, rodents, and swine, host specificity for Chlamydophila species is less strict, including for amphibians, reptiles, birds, and mammals (14). Both Chlamydia and Chlamydophila species comprise important human pathogens (88). Chlamydia trachomatis is a common cause of urogenital infection in humans and the agent of trachoma, one of the leading infectious causes of blindness worldwide. Chlamydophila pneumoniae is another important human pathogen, causing mainly respiratory infections. C. pneumoniae might also be involved in the pathogenesis of atherosclerotic cardiovascular diseases (8) and neurodegenerative syndromes (91). The other species are mainly veterinary pathogens, though some of them may cause rare but severe anthropozoonotic infections, such as psittacosis due to Chlamydophila psittaci or zoonotic abortion due to Chlamydophila abortus (14, 68, 83).
Historically, the term “chlamydia-like organisms” has been applied to refer to any intracellular microorganism that, like Chlamydiaceae, exhibited a two-stage developmental cycle. Since phylogenetic molecular analyses performed on some of these chlamydia-like organisms have showed this group to be polyphyletic, the term “chlamydia-like organisms” is only descriptive and retains no taxonomic value. Other authors (55, 56) proposed the term “environmental chlamydiae” to refer to chlamydial organisms that fall outside the family Chlamydiaceae, with the latter being designated “pathogenic chlamydiae.” However, such a distinction seems inadequate, since a growing body of evidence supports the pathogenic role of some of these chlamydiae (32, 40). By analogy to the term “amoeba-resistant microorganisms” (43), the term “amoeba-resisting chlamydiae” could also be applied to the species that may infect and survive within amoebae, i.e., Parachlamydiaceae, Simkania negevensis, and Waddlia chondrophila (see below). Indeed, most of these new species exhibited symbiotic or lytic interaction with amoebae. Nevertheless, since not all new species of chlamydiae have been tested for their ability to resist destruction by free-living amoebae, the more general term “novel chlamydiae” should be preferred to designate all the chlamydiae not belonging to the Chlamydiaceae.
These chlamydiae, which have recently been discovered and assigned to new families, are currently being investigated for their role as emerging pathogens. Simkania negevensis (family Simkaniaceae) (32) and Parachlamydia acanthamoebae (family Parachlamydiaceae) (40) could represent important respiratory pathogens in humans, while Waddlia chondrophila (family Waddliaceae) seems to be a new agent of abortion in ruminants (21, 53).
Molecular studies performed on human and animal samples have demonstrated the wide biodiversity and broad host range of chlamydiae. Thus, additional Parachlamydiaceae species, such as Neochlamydia sp., and a large variety of new 16S rRNA gene phylotypes have been detected in humans (15, 17), cats (93), Australian marsupials (7, 20), reptiles (6, 90), and fishes (24), as well as in various environmental samples (16, 18).
Moreover, new members of the Chlamydiales infecting invertebrates have recently been characterized. These include Fritschea bemisiae and Fritschea eriococci (family Simkaniaceae), which infect homopteran insects (28, 92), and Rhabdochlamydia porcellionis (64) and Rhabdochlamydia crassificans (19), which infect the woodlouse Porcellio scaber (Crustacea: Isopoda) and the cockroach Blatta orientalis (Insecta: Blattodea), respectively. The presence of chlamydiae in arthropods is interesting, since arthropods were not previously considered to play a role in the epidemiology of chlamydial infections, with the only exception being flies as a vector of the agent of trachoma (25, 26). To date, if we exclude cases of possible Rhabdochlamydia-related uveitis in humans (see below), there is no hint that these invertebrate-associated chlamydiae may be pathogenic to humans.
Evidence for a much larger biodiversity within Chlamydiales was afforded by several molecular studies performed on humans, animals, and environmental samples (Table 1). Here we report the present knowledge on the pathogenicity of all Chlamydiales except Chlamydiaceae, and we review the diagnostic methods currently available to diagnose human infections potentially due to these emerging pathogens.
TABLE 1.
Detection of novel Chlamydiales from human and animal samples
| Host(s)a | Sample(s)b | Clinical syndrome(s) | Detectionc | Chlamydia(e)d | Reference(s) |
|---|---|---|---|---|---|
| Human | Respiratory | Respiratory infections | PCR, serology | P. acanthamoebae | 5, 40, 45, 48, 51, 71 |
| PCR | Parachlamydiaceae | 15, 17 | |||
| Isolation, PCR, serology | S. negevensis | 31, 33, 34, 39, 60, 66, 67, 81 | |||
| PCR | Novel lineages | 73 | |||
| Ocular | Conjunctivitis/keratitis, uveitis | Isolation, PCR | Parachlamydiaceae | 37, 73 | |
| Uveitis | PCR | Rhabdochlamydia sp. | 14, 73 | ||
| —e | PCR | Novel lineages | 73 | ||
| Blood, artery | — | PCR | Parachlamydiaceae | 73 | |
| — | PCR | Simkania sp. | 73 | ||
| — | PCR | Novel lineages | 73 | ||
| Mammals (Placentals) | |||||
| Cat | Conjunctival swab | Conjunctivitis/keratitis | PCR | Neochlamydia sp. | 93 |
| Swine | Artery | — | PCR | Parachlamydiaceae | 73 |
| Bovine | Fetus | Abortion | Isolation, PCR, serology | Waddlia chondrophila | 21, 22, 53 |
| Fruit bat | Urine | — | Isolation, PCR | Waddlia malaysiensis | 9, 10 |
| Australian marsupials | Conjunctival swab | Conjunctivitis | PCR | P. acanthamoebae | 7 |
| PCR | Parachlamydiaceae | 7 | |||
| PCR | Novel lineages | 7 | |||
| Urogenital, kidney, bladder | Cystitis, nephritis | PCR | Parachlamydiaceae | 7, 20 | |
| PCR | Waddlia sp. | 7 | |||
| Ocular | Conjunctivitis | PCR | Novel lineages | 7, 20 | |
| Respiratory | — | ||||
| Birds (chicken) | Urogenital | — | PCR | Novel lineages | 73 |
| Reptiles | |||||
| Green sea turtle | Heart tissue | — | PCR | Neochlamydia sp. | 6 |
| Various species | Various tissues | Granulomatous inflammation | PCR | Parachlamydia/Simkania | 90 |
| Fishes (various species) | Gills | Epitheliocystis | PCR | Neochlamydia sp. | 73 |
| PCR | Novel lineages | 73 | |||
| PCR, LPS | Piscichlamydia salmonis | 24 | |||
| LPS | Uncharacterized chlamydia-like | 52 | |||
| Insects | |||||
| Bemisia tabaci (Homoptera) | Bacteriocyte | Lower fecundity and plant pathogenicityf | PCR | Fritschea bemisiae | 28, 92 |
| Eriococcus spurius (Homoptera) | Bacteriocyte | PCR | Fritschea eriococci | 28, 92 | |
| Blatta orientalis (Blattoidea) | Fat body | Abdominal swelling | PCR | Rhabdochlamydia crassificans | 19 |
| Crustacean, Porcellio scaber (Isopoda) | Hepatopancreas | Apparently healthy | PCR | Rhabdochlamydia porcellionis | 64 |
| Mollusc, Crassostrea gigas (Bivalvia) | Ctenidia, mantle | — | LPS | Uncharacterized chlamydia-like | 84 |
To date, only C. pneumoniae has been detected in amphibians. Molecular evidence for the presence of novel chlamydial lineages was obtained from different environmental samples, such as soils, marine sediments, freshwater, wastewater, and water conduit systems (reviewed in references 14 and 18).
Note that the urethral and genital tracts open into a urogenital sinus in marsupials, whereas they open into a cloaca in birds and reptiles.
PCR detection targeted mainly ribosomal operon fragments. Isolation was on mammalian cells for Simkania negevensis and Waddlia species and on amoebal coculture for Parachlamydiaceae (see also Tables 2 and 4). LPS indicates that positive reactivity was found using anti-chlamydial LPS antibodies.
Taxa without specific names refer to uncharacterized chlamydiae reputed to belong to these families on the basis of 16S rRNA gene sequences. Novel lineages refer to uncharacterized chlamydiae that are not in any of the described Chlamydiales families. Uncharacterized chlamydia-like refers to chlamydia-like organisms for which precise molecular data are lacking.
—, not applicable.
Compared to the chlamydia-free sister-species Bemisia argentifolii.
PATHOGENIC POTENTIAL OF NOVEL CHLAMYDIAE
Human Infections
Parachlamydiaceae.
Humans are commonly exposed to Parachlamydiaceae, as demonstrated (i) by the presence of two strains of Parachlamydia acanthamoebae within amoebae isolated from the nasal mucosa of two healthy volunteers (1, 74, 77) and (ii) by the amplification of Parachlamydia sp. DNAs from nose and/or throat swabs (17, 73, 80).
There is much evidence supporting the role of P. acanthamoebae as an emerging respiratory pathogen (reviewed in reference 40). First, Parachlamydia strain Hall's coccus was discovered in an amoeba isolated from the source of an outbreak of fever (65), and a serological study identified an association with an acute infection (5). In another serological study, a fourfold increase in antibody titers against P. acanthamoebae was observed in 2 of 500 patients with pneumonia (4). In addition, 8 of 371 (2.2%) patients with community-acquired pneumonia exhibited antibodies directed against P. acanthamoebae, compared to 0 out of 511 healthy subjects (P = 0.001) (71). Two patients described in that study presented pneumonia and serological evidence of acute P. acanthamoebae infection (71). The first patient suffered from an adult-onset Kawasaki disease (acute vasculitis following respiratory infection). The second patient was a renal transplant recipient chronically treated with cyclosporine and corticosteroids. This seroconversion in an allograft recipient treated with immunosuppressive drugs (71) and the fact that another case of probable Parachlamydia pneumonia was identified in a human immunodeficiency virus-infected subject with only 80 CD4 cells/mm3 (51) suggest that P. acanthamoebae is an opportunistic human respiratory pathogen. Recently, significant antibody titers against P. acanthamoebae have been detected in 5 of 37 (13.5%) polytraumatized intensive care patients, while serology was negative in 100 blood donors (P = 0.001) (48). In that work, which highlights the high prevalence of P. acanthamoebae infection in intensive care unit patients, seroconversion was associated with aspiration pneumonia. The temperature-dependent release of P. acanthamoebae (45) further supports the role of this amoeba-resisting chlamydia in this clinical setting. The amplifications of Parachlamydia sp. DNA from bronchoalveolar lavage fluid and sputum are additional hints of a potential pathogenicity (15, 17, 51). Finally, P. acanthamoebae may enter and multiply within human macrophages (44, 49, 50). These studies suggest that human exposure to Parachlamydiaceae spp. may lead to bronchitis, community-acquired pneumonia, and aspiration pneumonia. No animal model of infection has yet been established, and further studies are needed to better define the role played by Parachlamydiaceae as agents of pneumonia.
The pathogenic role of Neochlamydia hartmannellae (57) is unknown and remains to be determined. However, this microorganism could play a role in ocular infections, as Neochlamydia sp. strain UWC22 has been recovered within an Acanthamoeba isolated from a contact lens of a patient with keratitis (36, 37). Whether this role is direct, with a pathogenesis similar to that of infections due to other ocular chlamydiae such as C. trachomatis, or whether the internalized neochlamydiae may enhance the pathogenicity of the amoebae remains to be elucidated. Its presence within Acanthamoeba may also be coincidental, since Acanthamoeba keratitis is a well-established clinical entity that occurs especially in patients wearing contact lenses (70).
The role of other Parachlamydiaceae, such as Protochlamydia amoebophila (11), is still unknown. Some Parachlamydiaceae might be involved in bronchitis, atherosclerosis, uveitis, and urogenital infection, since 16S rRNA gene sequences related to Parachlamydia were also amplified from mononuclear cells taken from a patient with bronchitis (80), as well as from arterial, aqueous humor, and cervical samples (73).
Simkania negevensis.
Simkania negevensis is another chlamydia that resists destruction by free-living amoebae and that may use the amoebae as an environmental reservoir (59, 61). Its role as an emerging human respiratory pathogen is suspected (32). Epidemiological studies using PCR, cell culture, and serology have documented not only the worldwide presence of this microorganism (31, 33, 39, 58) but also its association with bronchiolitis in infants (39, 60, 81) and with lower respiratory tract infections in adults (34, 66, 67, 81). More recently, S. negevensis DNA has also been amplified from human arterial biopsy specimens (32).
Uncharacterized chlamydia lineages.
In addition, various 16S rRNA gene sequences not specifically belonging to any known chlamydia species have been obtained by PCR and sequencing from different human samples, including arteries and aqueous humors (73). It is noteworthy that some of the DNA sequences amplified from aqueous humors of patients suffering from uveitis showed a high level of similarity with the 16S rRNA gene sequences of the arthropod parasite Rhabdochlamydia (reviewed in reference 14).
Infections in Other Vertebrates
Molecular approaches have shown that all classes of vertebrates are exposed to chlamydiae (Table 1) (reviewed in reference 14). Further studies are necessary to reliably identify chlamydial species and to clarify the pathogenic potential of each novel chlamydia in animals, since most of these studies are based only on DNA amplification plus sequencing and since most sequenced DNA fragments are less than 300 nucleotides in length (7, 73, 90, 93).
Parachlamydiaceae.
Parachlamydiaceae could have some importance as ocular pathogens of cats (93), urogenital and ocular pathogens of various species of Australian marsupials (7, 20), and systemic pathogens of reptiles (6, 90). Neochlamydia sequences have been obtained from artery samples of swine and gills of fishes (73).
Simkaniaceae.
The presence of Simkania-related organisms in vertebrates is indicated by PCR studies: Soldati et al. obtained 23S rRNA gene sequences with some similarity to Simkania genes from various tissues of reptiles (90), and Simkania-like 16S rRNA gene sequences were also obtained from other animal species, but their affiliation to the Simkaniaceae clade is not clearly established (73, 80).
Waddliaceae.
Waddlia chondrophila (85) is a new agent of bovine abortion. This chlamydia has been isolated from two aborted fetuses, in the United States (21) and in Germany (53). A recent serological study revealed a significant statistical association between anti-Waddlia antibody titers and cows that have aborted (22). Future research on the mechanisms leading to abortion and on the abortive potential of Waddlia are warranted, given the veterinary and socioeconomic impact of abortion in cattle. More importantly, the role of W. chondrophila as an emerging agent of bovine abortion should lead to the evaluation of its role as a potential zoonotic agent, since C. abortus, another ruminant abortigenic chlamydia, was shown to cause zoonotic abortion in humans (83). The Waddlia 16S rRNA gene has also been detected in urogenital samples from healthy Australian marsupials (Potorous gilbertii) (7). Moreover, a new species, Waddlia malaysiensis, has been isolated from urine samples from the Malaysian fruit bat Eonycteris spelaea (9, 10). This may have important epidemiological implications given the important role of bats as vectors of various zoonotic pathogens.
Piscichlamydia.
Piscichlamydia salmonis is a recently identified chlamydial organism detected in the gill tissues of Atlantic salmon and thus indicated as the probable etiologic agent of the gill epitheliocystis in this fish species (24). Gill epitheliocystis is an infectious disease caused by chlamydia-like organisms, occurring worldwide and affecting several marine and freshwater bony fish species, most of which have important economic value. Limited molecular studies have indicated chlamydiae, not necessarily belonging to the same Piscichlamydia lineage, as etiologic agents of this disease (73).
Uncharacterized chlamydia lineages.
New 16S rRNA gene phylotypes, presumably representing distinct lineages, have been detected in various sample types from several mammals, birds, and fishes (7, 20, 73).
INTERACTIONS BETWEEN CHLAMYDIAE AND AMOEBAE
Free-living amoebae such as Acanthamoeba and Hartmannella play a key role as reservoirs (Fig. 1) and/or vectors for a variety of amoeba-resisting microorganisms (reviewed in reference 43), including the chlamydiae. Acanthamoeba is a suitable host for most Parachlamydiaceae (1, 76) and S. negevensis (59, 61), whereas Hartmannella, Balamuthia, Dictyostelium, and Naegleria (Table 2) are also able to sustain infection by some Parachlamydiaceae (57, 89), S. negevensis (75), and Waddlia chondrophila (78). Various interactions between chlamydiae and amoebae may take place, depending on the chlamydial and the amoebal species and strain and on environmental conditions. Thus, P. acanthamoebae is endosymbiotic at 25 to 30°C and lytic at 32 to 37°C for Acanthamoeba spp. (45). Since the temperature of the human nasal mucosa is generally lower (about 30°C) than that of the lower respiratory tract (generally between 35 and 37°C), P. acanthamoebae could reside symbiotically within amoebae present on the nasal mucosa and induce the lysis of the amoebae that reach the lower respiratory tract, inducing their own release and possibly starting an infectious process. Conversely, the virulence of the amoebae could be increased when harboring chlamydiae. Fritsche et al. (35) reported that the cytopathic effect on human embryonic tonsillar fibroblasts was enhanced by 66 to 70% when Acanthamoeba contained the Parachlamydia-related symbiont UWE25, which was recently designated as a new genus and species, Protochlamydia amoebophila (11).
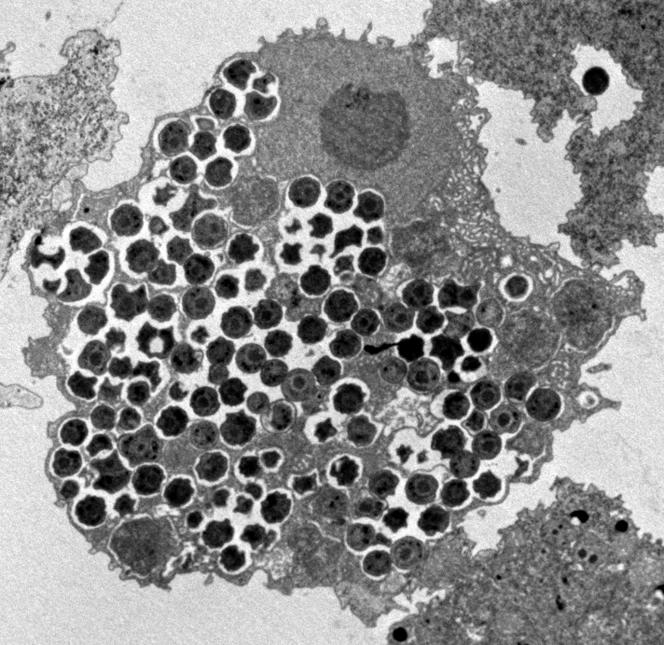
FIG. 1.
Parachlamydia acanthamoebae strain BN9 within Acanthamoeba polyphaga, as seen by electron microscopy. Magnification, ×3,500.
TABLE 2.
Known protist host range for Chlamydiales
| Chlamydial family and species | Known protist host (known host[s]) | Successful exptl infection | Features | Unsuccessful exptl infection | Reference(s) |
|---|---|---|---|---|---|
| Parachlamydiaceae | |||||
| Parachlamydia acanthamoebae | Acanthamoeba sp. | Acanthamoeba spp.a | Intravacuolar or free in the cytoplasm, cysts do not form or are chlamydia free, a third developmental stage has been described | Naegleria sp., Saccamoeba sp. | 1, 13, 41 |
| Neochlamydia hartmannellae | Hartmannella vermiformis | Hartmannella vermiformisa, Dictyostelium discoideuma | Free in the cytoplasm, increase of amoeba growth rate, developmental cycle not disturbed, release of uninfected spores | Acanthamoeba spp.b, Comandonia operculata, Naegleria spp., Willaertia magna | 12, 57 |
| Neochlamydia sp.c | Acanthamoeba sp. | Acanthamoeba sp., Dictyostelium discoideum | Hartmannella vermiformis | 36, 89 | |
| Protochlamydia amoebophila | Acanthamoeba sp. | Acanthamoeba spp.b, Dictyostelium discoideum | Increased cytopathic effect rate on mammalian cells, may decrease amoeba growth rate, cyst may contain chlamydial inclusion | Acanthamoeba spp. | 11, 12, 35, 36 |
| Other Parachlamydiaceaed | Acanthamoeba sp. | Acanthamoeba spp.b, Balamuthia mandrillaris, Hartmannella vermiformis, Dictyostelium discoideum, Naegleria sp., Willaertia magna | Continuous excretion of elementary bodies from all but H. vermiformis, Dictyostelium fruiting body development strongly disturbed | Acanthamoeba spp., Naegleria spp. | 12, 36, 76, 89 |
| Simkaniaceae Simkania negevensis | Amoeba? (human) | Acanthamoeba sp., Balamuthia mandrillarisf, Hartmannella vermiformisf, Naegleria clarki | Survives within cyst and cyst walls, with or without amoebal cytoplasme, production of Simkania-free cysts | Acanthamoeba spp., Hartmannella vermiformisf, Dictyostelium discoideumf, Naegleria spp., Willaertia magnaf | 32, 59, 61, 75 |
| Fritschea bemisiae | (Bemisia tabaci) | Acanthamoeba | 28 | ||
| Waddliaceae | |||||
| Waddlia chondrophila | (Bovine) | Hartmannella vermiformis, Acanthamoeba sp.g, Dictyostelium discoideumg, Hyperamoeaba-likeg, Vahlkampfia ovisg, Naegleria sp.g | Intravacuolar or free in the cytoplasm of Hartmannella, successful infection only after passage in Hartmannella, encystment inhibited for all but Vahlkampfia ovis | Acanthamoeba spp., Balamuthia mandrillaris, Echinamoeba sp., Vannella miroides, Comandonia operculata, Naegleria spp. | 78 |
| Chlamydiaceae | |||||
| Chlamydophila pneumoniae | (Land vertebrates) | Acanthamoeba sp. | Atypical inclusions at 10-14 days p.i., C. pneumoniae retains infectivity for HL cells | Not tested | 27 |
Various strains. When 18S rRNA genotyping of Acanthamoeba spp. was performed, strains belonged to the T4 lineage.
Various strains. When 18S rRNA genotyping of Acanthamoeba spp. was performed, strains belonged also to non-T4 lineages.
Neochlamydia strains UWC22 (human corneal sample) and TUME1 (water conduit).
Acanthamoeba endosymbiont strains UWE1 and k-cont. These strains could belong to distinct new species or genera within the Parachlamydiaceae.
Not in all strains tested.
Acanthamoeba-adapted Simkania.
Hartmannella-adapted Waddlia.
Additionally, the growth rate of the amoebae may be differentially affected by their parachlamydial symbionts. Thus, Hartmannella vermiformis infected by Neochlamydia hartmannellae grew more rapidly, while an Acanthamoeba sp. infected by Protochlamydia amoebophila (strain UWE25) grew more slowly, with respect to the aposymbiotic amoebae (12). Interestingly, this amoeba- parachlamydia couple (Acanthamoeba sp. strain UWC1/Protochlamydia amoebophila UWE25) was the same that showed the stronger cytopathic effect enhancement in the study by Fritsche et al. (35). Similarly, amoebal development stages, such as encystment (Acanthamoeba), enflagellation (Naegleria), or body fruiting (Dictyostelium), may be differentially affected depending on the infecting chlamydial strain (57, 75, 76, 78, 89). Notably, only Hartmannella strains were susceptible to infection at the first challenge with Waddlia chondrophila, whereas Waddlia grown in hartmannellae acquired the ability to infect several other free-living amoebae (78).
Whether chlamydiae and amoebae in some way influence each other's virulence deserves additional studies. However, it is likely that the ability of P. acanthamoebae to resist the microbicidal effector mechanisms of macrophages (44) has been acquired during their long history of coevolution with amoebae (43).
PROCESSING OF MICROBIOLOGICAL SPECIMENS
Little information is available on the optimal procedures for collecting and processing the novel chlamydiae. Handling of clinical samples for detection of chlamydiae should be performed in a biosafety level 2 security laboratory, given their pathogenic potential and the fact that various chlamydiae share the same ecological niche (the amoebae) with other amoeba-resisting bacteria of established pathogenicity, such as Legionella pneumophila.
The obligate intracellular nature of chlamydiae implies that an adequate specimen must contain infected host cells and/or a sufficient amount of extracellular elementary bodies. Since extracellular elementary bodies are unlikely to be present during persistent infection, the presence in the specimen of infected host cells is mandatory for both culture and nucleic acid amplification. For mucosal specimens, swabbing seems appropriate for the recovery of a sufficient amount of infected cells. Alginate swabs should be avoided since they may produce artifacts when Giemsa staining is performed. Cotton swabs are preferred. Sampling error may be reduced by scraping of mucosal surfaces, which increases the cellular yield compared to simple swabbing. However, this somewhat invasive procedure may induce bleeding and should be used only for selected indications.
Specific transport media such as the 2-sucrose phosphate or the sucrose-glutamate phosphate medium, originally developed for rickettsiae, may be used. Commonly available viral transport media generally contain antimicrobial agents and should therefore be avoided, since macrolides and tetracyclines inhibit the growth of both Parachlamydia (72) and Simkania (32) and tetracyclines are active against Waddlia species (10, 85). Bacterial overgrowth may be prevented by adding gentamicin (10 μg/ml) and/or vancomycin (100 μg/ml) to the transport medium. Although amphotericin B (1 to 5 μg/ml) is frequently used to prevent fungal overgrowth in cell culture, this compound should be avoided for amoebal coculture given the susceptibility of most free-living amoebae to amphotericin B.
Parachlamydiaceae, Simkania negevensis, and Waddlia spp. have been isolated and propagated in cell cultures or amoebae by using transport media and/or culture media containing penicillins (1, 10, 21, 53, 60). However, the use of penicillins should ideally be avoided given the susceptibility of Chlamydiaceae and the potential susceptibility of the other chlamydiae to penicillins and, by analogy with what is known for Chlamydiaceae, given the risk of induction of persistent nonmultiplying aberrant forms.
Microbiological specimens should be stored at 4 to 8°C and processed as soon as possible. If the time between collection and processing is >24 h, specimens should be frozen at −70°C. Freezing may result in a loss of chlamydial viability. This loss has been estimated at up to 20% for Chlamydiaceae and may be reduced by the addition of fetal bovine serum (2 to 10%) (2). No data are available for novel chlamydiae.
NUCLEIC ACID AMPLIFICATION
Nucleic acid amplification by PCR and related techniques is widely used in clinical microbiology laboratories in order to detect and/or identify any microorganisms that may be present in clinical samples. These molecular techniques are especially useful in epidemiological studies and as diagnostic tools, e.g., when used for the identification of microorganisms that are difficult to grow in culture. Additionally, PCR approaches that target phylogenetically informative genes such as the 16S rRNA gene contribute to the identification of new strains and species.
PCR techniques that target mainly the ribosomal operon have recently been developed to study the novel chlamydiae. However, sequences of other genes of additional diagnostic and phylogenetic value, such as the ATP/ADP translocase-encoding gene (42), are becoming available. At present, whole genome sequences are available for various Chlamydia and Chlamydophila strains, for Protochlamydia amoebophila strain UWE25 (55), and for S. negevensis (see the website http://www.tigr.org/tdb/mdb/mdbinprogress.html), whereas a 16.6-kbp genome portion, including the ribosomal operon and nine other genes, has been determined for Fritschea bemisiae (92). PCR tests that have been developed mainly for diagnostic purposes are summarized in the following section.
Species and Strain Identification
Species and strain identification may be achieved by (i) applying taxon-specific primers, either directly or as inner primers in a nested PCR; (ii) analysis of electrophoretic profiles after enzymatic restriction of amplicons (PCR-restriction fragment length polymorphism); or (iii) sequencing of PCR products. For the new chlamydiae, sequencing of the 16S rRNA gene remains the optimal method to infer strain affiliation, since this housekeeping gene carries some phylogenetic information and since 16S rRNA gene sequences are available for all type strains. Supporting this idea is the recent study of Maraha et al. (69), which, after sequencing of various 16S rRNA gene clones obtained with primers considered specific for C. pneumoniae, found that they were parachlamydiae.
The use of a primer set amplifying a small portion of the gene (e.g., 300 bp) may be ideal to screen a large numbers of samples, mainly due to the reduced cost of this approach. This approach may also be useful in the setting of degradation of nucleic acids, as in the case of paraffin-embedded tissues (6, 90). However, sequencing of larger gene portions or of other less conserved genes provides phylogenetic information that is necessary to reliably infer the phylogenetic relationship of the strain.
16S rRNA Genes
Universal/eubacterial primer sets have been used to amplify and sequence the 16S rRNA genes of uncharacterized chlamydia-like organisms first documented by morphological analyses and then described as Parachlamydiaceae (5, 36), as Waddlia chondrophila (85), and as Rhabdochlamydia porcellionis (64). These primer sets have in some instances also allowed identification of new chlamydial phylotypes from whole DNA extracts of environmental samples. However, their application to clinical samples is limited, as DNA from almost all bacteria, including normal microbiota, may be amplified.
Using the growing number of Chlamydiales sequences available, pan-chlamydia primer sets have been designed and applied to clinical and environmental samples to specifically detect chlamydiae (Table 3). Thus, using chlamydia-specific primers (For2/Rev2 [Table 3]), Meijer and Ossewaarde (73, 80) amplified 98 chlamydial 16S rRNA gene phylotypes, called CRG1 to CRG98. Other authors (7, 20, 93) have applied the chlamydial primer set 16SIGF/16SIGR described by Everett et al. (29) to amplify nearly the same region at the 5′ end of the 16S rRNA gene from clinical samples from various animals. Applying this primer set to koala samples, Devereaux et al. (20) determined various chlamydial sequences, named UKC (for uncultured koala chlamydia), and they noted that several of these sequences have a C or a T at primer position 9 (position 58 in P. acanthamoebae 16S rRNA gene numbering; accession no. Y07556). The same is true for several environmental clones sequenced by Horn and Wagner (56). Molecular studies that used the pan-chlamydia forward primer described by Ossewaarde and Meijer (80), ending at that position but with an A, may well have missed several chlamydial genotypes (20). The primers ccF and ccR, developed by Kahane et al. (61) to detect Simkania, amplify a 512-bp fragment from position 420 to 930 of the 16S rRNA gene and may also be considered pan-chlamydia primers. The degenerate primers CF1/CR6 have been used to amplify nearly complete 16S rRNA genes from all known chlamydiae (17). Species-specific primers targeting the 16S rRNA gene have also been designed. Kahane et al. developed an S. negevensis-specific PCR by using the primer set ZPF/ZPR, which amplifies a 398-bp fragment of the 16S rRNA gene (position 457 to 859 in the S. negevensis numbering; accession no. U68460) (39, 60). ZPF/ZPR may also be applied as an inner set in a nested PCR, using ccF/ccR as external primers (61).
TABLE 3.
Primer sequences and specificities
| Gene and primer set | Primer sequence (5′→3′)a | Amplicon (bp)b | Specificityd | Reference |
|---|---|---|---|---|
| 16S rRNA gene | ||||
| 16SIGF 16SIGR | CGG CGT GGA TGA GGC AT TCA GTC CCA GTG TTG GC | 298 | Chlamydiales 5′ 16S rRNA gene | 29 |
| ccF | CCT CGG GTT GTA AAG CAC TTT CGC | 512 | Chlamydialesf | 61 |
| ccR | CCC CGT CAA TTC TTT TGA GTT T | |||
| CF1 | CGT GGA TGA RGC ATG CRA GTC G | 1,445 | Chlamydiales | 17 |
| CR6 | GTC ATC RGC CYY ACC TTD SRC RYY TCT | |||
| FOR2 | CGT GGA TGA GGC ATG CAA GTC GA | 264 | Chlamydiales | 80 |
| REV2 | CAA TCT CTC AAT CCG CCT AGA CGT CTT AG | |||
| ZpF | AAA GGT AAC GAA TAA TTG CCT | 405 | S. negevensis | 60 |
| ZpR | GCA CAG TCG GGG TTG AGA CCG ACT | |||
| 23S rRNA gene | ||||
| 23SAPF2 | GAA CCT GAA ACC ART AGC | 92 | Chlamydiales | 90 |
| 23SAPR | CCT TTT GCA TGA TGA GCC AG | |||
| AFc | CAC AGG TAG GCA TGA TGA | 1,099 | S. negevensis | 30 |
| BR | CTA GCT GCG GGT AAA CG | |||
| IntF IntR | TTA GAT GCA CAA TGG ATA GTT GGA CCA TCA GCG CTC ATG TGC TCA | 338 | S. negevensis intron I | 30 |
| Other genes | ||||
| TS1F TS1R | ATG CTT TCG TTC TGG TCT AC CCT GCA CGG AGA CGG TTG AC | 180 | S. negevensis Hsp60 gene | 81 |
| Adp81Fe Adp84R | TAG TGA TCT GCT ACG GGA TTT TTG GAT TAG GAT ATT GCT TAA A | 81 | P. acanthamoebae ATP/ADP translocase gene | 51 |
| Adp_probe | 6-FAM-5′-AACCTTGTAGAAGTAACCTGGAAGAACCAGC-3′-TAMRA |
For degenerate primers, D is A, G, or C; R is A or G; S is C or G; and Y is T or C.
Lengths of amplicons may vary slightly depending on the chlamydia taxon.
The IntF/IntR set used in nested PCR on AF/AR amplicons. There are four mismatches with the F. bemisiae sequence for primers AF and IntF but no mismatch for primer AR. There are three to seven mismatches with sequences of other Chlamydiales for primers AF and AR.
Specificity was determined with the sequences available at the time of primer design. For pan-chlamydia primers, such specificity may not cover all the newly determined sequences.
This primer set was applied in a real-time PCR with an internal probe.
Primer ccF matches exactly the last seven nucleotides at the 3′ end but has two to five mismatches at the 5′ end for sequences other than S. negevensis.
23S rRNA Genes
Recently, Soldati et al. (90) designed a pan-chlamydia primer set, 23SAPF2/23SAPR, amplifying a 92-bp fragment of the 23S rRNA gene. This primer set, applied to 90 tissue samples from various species of reptiles suffering from various granulomatous diseases, successfully amplified C. pneumoniae DNA (n = 9; 10%) and DNAs of Parachlamydia/Simkania-related organisms (n = 49; 54.4%).
S. negevensis and F. bemisiae 23S rRNA genes contain a group I intron (30, 92). Everett et al. (30) designed Simkania-specific primer sets in order to amplify the region of the gene harboring this intron. A first primer set, AF/BR, amplifies a 1,100-bp region of the 23S rRNA gene flanking the intron, whereas an inner primer set, IntF/IntR, targeting the intron sequence, produces a 338-bp fragment (Table 3). During a first amplification, this nested PCR strategy might also detect intronless strains (yet unrecognized). It has been applied to detect S. negevensis from water samples (61). This nested PCR, however, did not detect F. bemisiae. Indeed, while complete overlapping for the AR primer exists for sequences of both Fritschea species, the F. bemisiae sequence has four nucleotide mismatches for the AF and IntF primers and a 16-bp nonoverlapping fragment for the IntR primer. Three to seven nucleotide mismatches are present for the AF/BR set with sequences of other Chlamydiales.
Other Genes
Other genes have been used as targets for amplification by PCR. Petrich et al. (81) developed a Simkania negevensis-specific PCR targeting the heat shock protein 60-encoding gene (groEL). This PCR was successfully applied both to peripheral blood mononuclear cells taken from patients with chronic obstructive pulmonary disease and to nasopharyngeal swabs from infants with respiratory illness and nursing home patients. Greub et al. used a real-time PCR targeting the tlc gene for the diagnosis of P. acanthamoebae infection (51). This gene encodes an energy parasite enzyme, the ADP/ATP translocase, which is present only in Rickettsiales, Chlamydiales, and plant plastids (42). Since the gene is not present in other bacterial genomes, the risk of false-positive PCRs due to amplification of DNAs of other bacterial species such as Neisseriaceae, streptococci, and other oropharyngeal colonizers that frequently contaminate respiratory samples is limited. The specificity of this PCR was increased by using a real-time system with a specific primer set and a specific probe. An additional advantage of this real-time PCR was its reliability in quantifying the number of P. acanthamoebae organisms (45, 49). However, the high specificity of this PCR prevents its use to detect Neochlamydia and any infection due to more distant members of the Chlamydiales.
Recently Griffiths et al. (51a) described insertion/deletion signatures, i.e., indels, characteristic for Chlamydiales, including Chlamydiaceae and Parachlamydia, Neochlamydia, Simkania, and Waddlia species. These indels are within five essential proteins: the RNA polymerase alpha subunit (RpoA), the elongation factors Tu and P (EF-Tu and EF-P), the DNA gyrase beta subunit (GyrB), and the lysyl-tRNA synthetase (LysRS). In addition to providing very interesting phylogenetic data, this work will probably have some practical applications also, as it describes new tools for the identification of chlamydiae beside the ribosomal operon.
False-Positive and False-Negative Results
A major problem of PCR-based diagnostic approaches is the risk of false-positive results. These are principally due to (i) vertical contamination of the sample by previously amplified products, (ii) the accidental presence of the nucleic acid target in the specimen, and (iii) unexpected low specificity of the PCR protocol.
Vertical contamination is especially frequent when a high number of reactions that target the same DNA region are carried out or when nested PCR is performed. Detection of vertical contamination may be facilitated by using multiple blank reaction tubes intermixed with sample reaction tubes and by confirming any positive results by a second PCR that targets a second gene (47). The risk of vertical contamination may be reduced by performing the extraction, amplification, and postamplification steps in separate rooms; by using hot-start polymerases; and by decontaminating workplaces (e.g., by UV irradiation and PCR reagents such as restriction enzyme and/or dUTP-uracil-DNA glycosylase) at regular time intervals.
False-positive amplification products may also result from the presence of the microorganism (or of part of its genomic material) in PCR reagents (water, deoxynucleoside triphosphates, or enzyme stocks). The presence of the target DNA in laboratory reagents has caused false-positive results for chlamydiae in at least two studies (69, 73). False-positive results may also occur due to the presence of parachlamydiae in the noses of healthy volunteers (1) and the presumable secondary contamination of lower respiratory tract samples with the subjects' oropharyngeal secretions.
An unexpectedly low specificity of the PCR protocol may be attributable to the low specificity of the primers used because of the presence in the specimen of as-yet-unrecognized species harboring the same target sequence (69) or may originate from suboptimal temperatures and magnesium concentrations.
Given the significant risk of false-positive results, a single positive PCR or nested PCR is only a first indirect hint of the presence of a chlamydia in the specimen. Confirmation of the presence of viable microorganisms by reverse transcription-PCR or culture may be important. On the other hand, false-negative results may result from inadequate sensitivity of the PCR protocol and from the presence of PCR inhibitors.
CELL CULTURE
The isolation and propagation of intracellular bacteria such as chlamydiae in a cell culture system are fundamental in demonstrating the presence of a viable organism in the clinical sample and characterizing the strain beyond its gene sequence. Considering the huge diversity within chlamydiae that is suggested by molecular studies, efforts should be made to adapt cell culture protocols to allow the recovery of these new species. Chlamydiaceae have been cultivated into embryonated eggs, but this culture system was relatively rapidly replaced by cell cultures, which are easier and more sensitive (2). Embryonated eggs are used today mainly for massive production of antigens or for propagation of fastidious strains (2). Egg culture has not yet been tested for any novel chlamydia.
The possibility of Chlamydiaceae entering into a persistent/cryptic state is a key pathogenic feature explaining the wide range of chronic diseases that most Chlamydiaceae seem to induce in their vertebrate hosts (3, 54). However, persistence complicates the laboratory diagnosis of chlamydial infection, at least when using culture-based approaches, potentially leading to false-negative results. It is yet unknown whether Parachlamydiaceae, Simkaniaceae, and Waddliaceae may also show a persistent state and which cell lines or amoebal strains may especially help in resuscitating such viable but nonculturable bacteria.
Mammalian Cells
As obligate intracellular parasites, chlamydiae are generally isolated and grown on monolayers of mammalian cells. To screen for the presence of bacteria in cell culture, standard stains such as Giemsa and Gimenez stains can be used. Immunofluorescence techniques, using homemade specific antibodies raised against each novel chlamydia, are employed in some laboratories.
Chlamydiaceae.
Various cell lines are routinely used for members of the Chlamydiaceae, with each species or biovar showing a relative specificity for a given cell type (2, 86). Following inoculation, centrifugation (1,000 to 3,000 × g, 1 h, 30 to 35°C) is generally used for Chlamydiaceae, as it increases the infectious rates by about 100- to 1,000-fold (79, 86). The culture is then incubated for 5 to 14 days, with some protocols requiring more than one passage. Penicillin and derivatives should be avoided because they may disturb the development of Chlamydiaceae by inducing aberrant nonreplicating developmental stages (3). Bacterial and fungal overgrowth is prevented by adding gentamicin (10 to 50 μg/ml), vancomycin (100 μg/ml), and amphotericin B (1 to 4 μg/ml). Since chlamydiae are “energy parasites” that utilize the host cell ATP pool (42, 79), inhibitors of eukaryotic metabolism such as cycloheximide (1 to 5 μg/ml) may be added to the growth medium. This favors chlamydial growth by limiting host cell utilization of energy. The importance of cycloheximide to promote the growth of Chlamydiaceae in cell culture is for some Chlamydiaceae much smaller than that of the initial centrifugation (86).
Simkania negevensis.
S. negevensis and Waddlia spp. have been grown in various cell lines (Table 4). S. negevensis was discovered as a cell culture contaminant (62). Vero cells are currently used to isolate S. negevensis from clinical samples (39, 60). However, successful culture has also been obtained using HeLa cells, HEp-2 cells, and the human macrophage cell line U937 (32, 62). To optimize the propagation of S. negevensis in Vero cells, Yamaguchi et al. (95) recently applied various protocols that included (i) centrifugation or sonication of the inoculum, (ii) pretreatment of the cell monolayer with polyethylene glycol or DEAE-dextran, and (iii) use of culture media of different compositions regarding fetal calf serum concentration and the addition of antibiotics (streptomycin and vancomycin, 100 μg/ml each) and cycloheximide (1 μg/ml). Those authors found that the number of inclusions was significantly higher when the inoculum was centrifuged (1,500 × g, 60 min, 35°C). Sonication had no effect. By contrast, pretreatment of Vero cells with either polyethylene glycol or DEAE-dextran decreased the number of inclusions, as did the addition of cycloheximide. Thus, ideal conditions may include centrifugation of the inoculum on untreated Vero cells grown in RPMI 1640 medium with 10% fetal calf serum, the addition of antibiotics, and no cycloheximide. The efficiency of this protocol when applied to clinical samples remains to be evaluated and to be compared to amoebal coculture (see below).
TABLE 4.
Mammalian cell culture of Chlamydiales species
| Chlamydial family and species | Known host(s) | First isolation | Successful propagation in mammalian cells | Reference(s) |
|---|---|---|---|---|
| Chlamydiaceae | ||||
| Chlamydia spp. | Mammals | Embryonated eggs | Various cell linesa | 2 |
| Chlamydophila spp.h | Land vertebrates | Embryonated eggs | Various cell linesa | 2 |
| Parachlamydiaceae | ||||
| Parachlamydia acanthamoebae | Acanthamoeba sp. | Acanthamoeba spp.b | Vero,c HeLa, NCI-H292, blood-derived human macrophagesd | 1, 13, 44, 49, 72 |
| Protochlamydia amoebophila | Acanthamoeba sp. | Acanthamoeba spp. | None | 11 |
| Neochlamydia hartmannellae | Hartmannella vermiformis | Hartmannella vermiformis | None | 57 |
| Simkaniaceae | ||||
| Simkania negevensis | Human, amoeba | Veroe | Vero, HEp-2, human U937, macrophages, Buffalo green monkey cells | 32, 60, 61, 75 |
| Fritschea spp. | Homopteran insects | None | 28, 92 | |
| Waddliaceae | ||||
| Waddlia chondrophilah | Bovine | Bovine turbinate cellsf | Bovine turbinate cells, McCoy cells, Buffalo green monkey cells, human diploid fibroblasts, murine P388D1 macrophages | 21, 53 |
| Waddlia malaysiensis | Fruit bat | Verog | Vero, MRC-5, A549, HEK, HEp-2, B-lymphoblastoid cell line, LLC-MK2, 3T3, BHK | 9, 10 |
| Rhabdochlamydiaceae | ||||
| Rhabdochlamydia spp. | Arthropods | None | 19, 64 | |
| Piscichlamydia salmonis | Salmonid fish | None | 24 |
Penicillin sensitive.
Different strains have been used (see Table 2). Sensitive to aminoglycosides in Acanthamoeba coculture (72).
Other authors reported on the failed propagation on this cell line, as well as on McCoy cells, murine P388D1 macrophages, and human embryonic lung fibroblasts (72).
Rapid induction of apoptosis (49).
Longer life cycle (about 2 weeks).
Vacuolization at 24 to 48 h p.i.; periodic acid-Schiff stain negative.
Cytopathic effect 5 to 7 days p.i.; periodic acid-Schiff stain positive.
Chlamydophila pneumoniae and Waddlia chondrophila have been cultivated experimentally in Acanthamoeba and various amoeba species, respectively (see Table 2).
Waddliaceae.
Waddlia chondrophila (strain WSU 86-1044) was first isolated from a fetal homogenized lung-liver pool by inoculating bovine turbinate cells incubated at 35°C in a penicillin-containing medium without centrifugation (21). The same strain was also propagated in P388D1 mouse macrophages without antibiotics in order to study its developmental cycle (63). Henning et al. (53) reported the isolation of an additional strain (2032/99) from bovine fetal heart on Buffalo green monkey cells, McCoy cells, and human diploid fibroblasts. Waddlia malaysiensis was isolated on Vero cells inoculated with pooled urine samples from Malaysian fruit bats, and the strain was successfully propagated in various human, simian, and rodent cell lines (9, 10) (Table 4).
A rapid cytopathic effect on bovine turbinate cells and Vero cells appeared at 36 h postinfection (p.i.) and 5 to 7 days p.i. for W. chondrophila (21) and W. malaysiensis (10), respectively. Thus, although a large number of cell lines may be appropriate for the growth of Waddlia and Simkania, Vero cells might be the preferred mammalian cell line, being permissive to representatives of both genera.
Parachlamydiaceae.
Mammalian cells are generally not used to grow Parachlamydiaceae. A first report on the culture of P. acanthamoebae (strain Berg17) in Vero cells (1, 77) has not been confirmed using another P. acanthamoebae strain (Bn9) (72). McCoy cells, P388D1 macrophages, and human embryonic fibroblasts were also not permissive to that strain (72). Successful infection of blood-derived human macrophages has, however, been obtained for another P. acanthamoebae strain (Hall's coccus) by Greub et al. (49). P. acanthamoebae strain UV-7 was propagated in Vero, HeLa, and H292 cells after an initial recovery in amoebal coculture (13). The Parachlamydiaceae strain CorvenA4, present in a human bronchoalveolar lavage sample, has been characterized by the 16S rRNA gene sequence only, since its isolation on both Vero and HeLa cells failed (15). Because Maurin et al. (72) found P. acanthamoebae susceptible to gentamicin in an Acanthamoeba system, the use of aminoglycosides should probably be discouraged since it might hamper the recovery of Parachlamydiaceae and some other new chlamydiae.
Other novel chlamydiae.
Chlamydiae that infect arthropods, i.e., Fritschea and Rhabdochlamydia, have not yet been tested for their ability to grow in vertebrate cells, and the vast majority of studies describing other novel chlamydiae have been limited to molecular approaches. Bodetti et al. (7) used HEp-2 cells to isolate chlamydiae from various Australian marsupial species that were swabbed at the urogenital sinus, conjunctiva, and nasopharynx. Inocula were centrifuged at 800 × g on cycloheximide-treated cells, and repeated centrifugations at 4 to 7 days p.i. were performed. By using a commercially available anti-chlamydial lipopolysaccharide (LPS) fluorescent monoclonal antibody, six isolates were detected, but only one was successfully subcultured. The specificity of anti-LPS monoclonal antibodies to Chlamydiaceae species (but see “Staining” below) and the unknown permissivity of HEp-2 cells to most other chlamydiae (S. negevensis is the only documented exception [32]) could explain the low rate of positive results obtained by standard cell culture protocols.
Amoebal Coculture
Free-living amoebae, mainly Acanthamoeba spp., have been shown to be natural hosts for several amoeba-resisting bacteria, including Parachlamydiaceae. These unicellular eukaryotes have also been successfully used in a cell culture system to isolate a variety of amoeba-resisting bacteria from clinical samples (43, 46).
The fact that C. pneumoniae (27) and S. negevensis (59, 75) are able to infect Acanthamoeba in vitro and the fact that free-living amoebae likely play a role as an environmental host of Simkania (61) support the use of Acanthamoeba to isolate novel chlamydiae from clinical samples. Of note, Neochlamydia hartmannellae (57) (but not other Neochlamydia spp. [36]), has been successfully grown within Hartmannella vermiformis but not within Acanthamoeba. Moreover, Waddlia chondrophila may grow in Acanthamoeba and other free-living amoebae, but only after subcultivation in H. vermiformis (78). Efforts to cultivate Fritschea in Acanthamoeba have failed (28). In addition, experimental studies have shown that (i) a wide diversity of free-living amoebae are able to sustain the growth of the new chlamydiae and (ii) not all strains of a free-living amoeba species are susceptible to infection by these agents. This should be taken into account when interpreting negative coculture results (Table 2) (35, 57, 75-78, 89). Thus, the use of more than one strain of Acanthamoeba and/or the use of several amoeba species (e.g., Acanthamoeba and Hartmannella) is recommended to increase the rate of isolation of chlamydiae, at least from clinical samples that give a positive PCR result. In addition to the possible problem of a persistent viable but noncultivable stage, false-negative culture may also occur due to the use of a cell that is not permissive to a given species (i.e., Neochlamydia and Acanthamoeba) or due to the presence of antibiotics in the amoebal coculture to which the bacterium is susceptible. False-positive results might occur if the cell line is contaminated with an endosymbiotic chlamydia (which will not be easily detected) or if the broth is not adequately filtered and/or heat sterilized.
Amoebal Enrichment
Since amoebae may harbor Parachlamydiaceae, S. negevensis (59, 61, 75), and Waddlia chondrophila (78), it may be useful to first isolate amoebae from clinical samples and then look within these amoebae for whether a chlamydia is present. Screening of environmental samples for the presence of amoebae may also contribute to the identification of a possible source of infection (e.g., humidifiers or air conditioning systems).
Amoebal enrichment may be performed using established protocols (43, 46, 87). Specimens are generally seeded on nonnutrient agar preinoculated with heat-inactivated Escherichia coli.
Stainings
Detection of bacteria that grow in both cell culture and amoebal culture may be performed using different staining methods (Gram, Giemsa, Gimenez, or immunofluorescence) or using fluorescent in situ hybridization (FISH). Since chlamydiae are inconsistently stained with Gram stain, alternative stains are preferred, such as Giemsa and Gimenez stains. With Giemsa stain, the bacteria stain blue. To obtain a better contrast, alternative staining approaches such as the Giemsa-May-Grünwald stains may be useful, since the cell nucleus is stained with eosin, whereas the chlamydiae appear as clear blue dots on the slightly stained cytoplasm background. This staining method, slightly modified to use eosin, a thiazine derivative, and a fixative methanol solution, is commercially available (Diff-Quick set; Dade, Düdingen, Switzerland) and may be easily implemented even in routine diagnostic laboratory procedures. However, the best staining for screening is probably the Gimenez method (38). The chlamydiae appear as reddish-staining bacteria (fuchsin) against a green background (malachite green). Briefly, the staining solution, which contains basic fuchsin (10 g/liter) in ethanol (10%) and phenol (1%), is diluted in a phosphate buffer (NaH2PO4-Na2HPO4) and applied to the sample for 1 or 2 min. Then, after washing with water, counterstaining is performed twice with malachite green (0.8% in water) for 15 to 30 seconds. After washing with tap water and dry blotting, the sample is ready to be examined.
The sensitivity of immunofluorescence for chlamydia detection is probably higher than that of standard staining techniques. However, its use for the detection of novel chlamydiae is limited by the specificity of anti-Chlamydiaceae antibodies (anti-LPS or anti-major outer membrane protein) and by the limited availability of antibodies directed against the new chlamydia species. It should be noted that reactivity against anti-Chlamydiaceae LPS antibodies has been reported for the distantly related fish pathogen Piscichlamydia salmonis (24) and for at least two uncharacterized chlamydia-like organisms infecting a fish (52) and an oyster (84).
FISH using rRNA-targeted oligonucleotide probes is widely used for the detection of uncultured bacteria in complex environmental microbial communities. It may also be a valuable tool for the rapid detection of novel chlamydiae in clinical specimens. Poppert et al. (82) developed a set of probes that may specifically detect and differentiate most Chlamydiaceae that are pathogenic to humans (C. pneumoniae, C. trachomatis, and C. psittaci) and a “Chlamydiales” probe that detects some Chlamydiaceae and Parachlamydiaceae (82). FISH-stained chlamydiae were demonstrable as early as 12 h p.i., and rRNA highlighted by FISH colocalized with chlamydial antigen, further supporting the validity of this approach. Its use on clinical specimens, however, has not yet been validated.
SEROLOGY
In addition to molecular approaches and culture, serology is increasingly used to assess the pathogenic roles of the novel chlamydiae, even though serology provides only an indirect hint of pathogenicity (5, 31, 48, 66, 71).
An enzyme-linked immunosorbent assay (ELISA) has been developed for the diagnosis of Simkania negevensis infection (60, 67). This ELISA, which may detect immunoglobulin A (IgA) and IgG antibodies (but not IgM), was initially designed to work with sera diluted 1:30 to 1:100 for IgA and IgG, respectively (67). With this ELISA, the presence of IgA was empirically considered as an indicator of recent infection, whereas that of IgG suggested recent or past infection. Using this ELISA at the mentioned serum dilutions, as many as 39 to 68% of healthy adults living in different parts of the world (Israel, Canada, the United States, and Denmark) were found to be seropositive (32). Of note, using immunofluorescence and a cutoff for positivity of as low as 1:8, Yamaguchi et al. did not find such a high prevalence, with a 7.5% seroprevalence in adults and a maximum seropositivity of 15% in elderly subjects (94). Thus, the clinical significance of antibody reactivity against Simkania should be interpreted with caution, especially when using ELISA. Yamaguchi et al. also showed limited cross-reactivity between Simkania and C. pneumoniae (94).
Immunofluorescence, which is considered the gold standard for Chlamydiaceae (23), has been used for Parachlamydiaceae in different studies, supporting the role of these organisms in community-acquired pneumonia (4, 5, 71) and in aspiration pneumonia (48). Whole heat-inactivated bacteria were used as the antigen. Cutoffs for seropositivity used in these studies ranged from an antibody titer of >1:50 (71) to ≥1:128 (4).
To use the same dilutions as those used for the diagnosis of Chlamydia-related infections (23), it seems preferable in the future to use serial dilutions starting at 1:8 and not at 1:25 for the diagnosis of infections due to the new chlamydiae. Since among 100 sera taken from healthy blood donors, none exhibited an antibody titer of ≥1:50, we suggest considering an anti-IgG titer of ≥1/64 as evidence of prior infection. Based on our own experience and the criteria used by Marrie et al. (71), we suggest considering the following serological results as indicative of a recent infection: (i) a single IgM titer of ≥1:32, (ii) seroconversion (IgG increase from 0 to ≥1:64 in paired specimens), and (iii) a ≥4-fold rise in the IgG titer between acute- and convalescent-phase sera. Since no definite cutoffs have been established for immunofluorescence for Simkania and Waddlia spp., we propose to also use these cutoffs for these chlamydiae.
Confirmation of positive immunofluorescence results by Western blotting has been reported for sera that tested positive for Parachlamydia (48) and for Simkania (94). For Parachlamydia, proteins of approximately 60 kDa and 48 kDa were shown to exhibit the strongest antigenicity, whereas for Simkania, a strong and specific signal was observed at 64 kDa. More important, this 64-kDa signal was shown to be specific for Simkania, still being present after serum adsorption with C. pneumoniae. The degrees of cross-reactivity between the different novel chlamydia species and between every member of the Chlamydiales remain to be determined. Indeed, false-positive results may be caused mainly by serological cross-reactivities. Therefore, Western blotting and cross-adsorption, which may be of some help in confirming the specificity of any seropositive test result, should ideally be performed.
CONCLUSION
In conclusion, there is increasing evidence of the pathogenicity of Simkania negevensis (32) and Parachlamydia acanthamoebae (40), especially as agents of lower respiratory tract infection. The pathogenic potentials of additional novel chlamydiae remain to be defined (14). Consequently, there is an urgent need for efficient and standardized diagnostic approaches. Based on the published reports, a combined diagnostic strategy using both molecular approaches and serology as an initial diagnostic screening work-up seems appropriate. Positive results may then be confirmed by cell and amoebal coculture. Future diagnostic challenges include the identification of the best PCR protocols, confirmation of the serological cutoff levels that we have proposed here, and the determination of the optimal growth conditions for each novel chlamydia. We hope that the present review will help stimulate clinical diagnostic microbiology laboratory and research teams to work in this exciting emerging field.
Acknowledgments
We thank Philip Tarr (Infectious Diseases Division, Lausanne, Switzerland) and Nicola Casson (Institute of Microbiology, Lausanne, Switzerland) for reviewing the manuscript.
Research done in G. Greub’s laboratory is partially supported by Swiss National Science Foundation (SNSF) grant no. 3200BO-105885.
REFERENCES
- 1.Amann, R. I., N. Springer, W. Schönhuber, W. Ludwig, E. N. Schmid, K. D. Müller, and R. Michel. 1997. Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl. Environ. Microbiol. 63:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes, R. C. 1989. Laboratory diagnosis of human chlamydial infections. Clin. Microbiol. Rev. 2:119-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty, W. L., R. P. Morrison, and G. I. Byrne. 1994. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 58:686-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson, R. F., W. J. Drozanski, T. J. Rowbotham, I. Bialkowska, D. Losos, J. C. Butler, H. B. Lipman, J. F. Plouffe, and B. S. Fields. 1995. Serologic evidence of infection with 9 Legionella-like amoebal pathogens in pneumonia patients, abstr. C-200. Abstr. 95th Gen. Meet. Am. Soc. Microbiol. 1995. American Society for Microbiology, Washington, D.C.
- 5.Birtles, R. J., T. J. Rowbotham, C. Storey, T. J. Marrie, and D. Raoult. 1997. Chlamydia-like obligate parasite of free-living amoebae. Lancet 349:925-926. [DOI] [PubMed] [Google Scholar]
- 6.Bodetti, T. J., E. Jacobson, C. Wan, L. Hafner, A. Pospischil, K. Rose, and P. Timms. 2002. Molecular evidence to support the expansion of the host range of Chlamydophila pneumoniae to include reptiles as well as humans, horses, koalas and amphibians. Syst. Appl. Microbiol. 25:146-152. [DOI] [PubMed] [Google Scholar]
- 7.Bodetti, T. J., K. Viggers, K. Warren, R. Swan, S. Conaghty, C. Sims, and P. Timms. 2003. Wide range of Chlamydiales types detected in native Australian marsupials. Vet. Microbiol. 96:177-187. [DOI] [PubMed] [Google Scholar]
- 8.Boman, J., and M. Hammerschlag. 2002. Chlamydia pneumoniae and atherosclerosis: critical assessment of diagnostic methods and relevance to treatment studies. Clin. Microbiol. Rev. 15:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chua, K. B. 2003. A novel approach for collecting samples from fruit bats for isolation of infectious agents. Microb. Infect. 5:487-490. [DOI] [PubMed] [Google Scholar]
- 10.Chua, K. B., J. E. Corkill, P. S. Hooi, C. Winstanley, and C. A. Hart. 2005. Waddlia malaysiensis: an obligate intracellular bacterium isolated from a fruit bat (Eonycteris spelaea). Emerg. Infect. Dis. 11:271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collingro, A., E. R. Toenshoff, M. W. Taylor, T. R. Fritsche, M. Wagner, and M. Horn. 2005. ‘Candidatus Protochlamydia amoebophila,’ an endosymbiont of Acanthamoeba spp. Int. J. Syst. Evol. Microbiol. 55:1863-1866. [DOI] [PubMed] [Google Scholar]
- 12.Collingro, A., J. Walochnik, C. Baranyi, R. Michel, M. Wagner, M. Horn, and H. Aspöck. 2004. Chlamydial endocytobionts of free-living amoebae differentially affect the growth rate of their hosts. Eur. J. Protistol. 40:57-60. [Google Scholar]
- 13.Collingro, A., S. Poppert, E. Heinz, S. Schmitz-Esser, A. Essig, M. Schweikert, M. Wagner, and M. Horn. 2005. Recovery of environmental chlamydia strain from activated sludge by co-cultivation with Acanthamoeba sp. Microbiology 151:301-309. [DOI] [PubMed] [Google Scholar]
- 14.Corsaro, D., and D. Venditti. 2004. Emerging chlamydial infection. Crit. Rev. Microbiol. 30:75-106. [DOI] [PubMed] [Google Scholar]
- 15.Corsaro, D., D. Venditti, A. Le Faou, P. Guglielmetti, and M. Valassina. 2001. A new chlamydia-like 16S rDNA sequence from a clinical sample. Microbiology 147:515-516. [DOI] [PubMed] [Google Scholar]
- 16.Corsaro, D., D. Venditti, and M. Valassina. 2002. New chlamydial lineages from freshwater samples. Microbiology 148:343-344. [DOI] [PubMed] [Google Scholar]
- 17.Corsaro, D., D. Venditti, and M. Valassina. 2002. New parachlamydial 16S rDNA phylotypes detected in human clinical samples. Res. Microbiol. 153:563-567. [DOI] [PubMed] [Google Scholar]
- 18.Corsaro, D., M. Valassina, and D. Venditti. 2003. Increasing diversity within chlamydiae. Crit. Rev. Microbiol. 29:37-78. [DOI] [PubMed] [Google Scholar]
- 19.Corsaro, D., V. Thomas, G. Goy, D. Venditti, R. Radek, and G. Greub. Rhabdochlamydia crassificans, sp. nov., comb. nov., and intracellular bacterial pathogen of the cockroach Blatta orientalis (Insecta: Blattodea), and proposal for Rhabdochlamydiaceae, fam. nov., a new family of chlamydiae infecting arthropods. Syst. Appl. Microbiol., in press. [DOI] [PubMed]
- 20.Devereaux, L. N., A. Polkinghorne, A. Meijer, and P. Timms. 2003. Molecular evidence for novel chlamydial infections in the koala (Phascolarctos cinereus). Syst. Appl. Microbiol. 26:245-253. [DOI] [PubMed] [Google Scholar]
- 21.Dilbeck, P. M., J. F. Evermann, T. B. Crawford, A. C. S. Ward, C. W. Leathers, C. J. Holland, C. A. Mebus, L. L. Logan, F. R. Rurangirwa, and T. C. McGuire. 1990. Isolation of a previously undescribed rickettsia from an aborted bovine fetus. J. Clin. Microbiol. 28:814-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dilbeck-Robertson, P., M. M. McAllister, D. Bradway, and J. F. Evermann. 2003. Results of a new serologic test suggest an association of Waddlia chondrophila with bovine abortion. J. Vet. Diagn. Investig. 15:568-569. [DOI] [PubMed] [Google Scholar]
- 23.Dowell, S. F., R. W. Peeling, J. Boman, G. M. Carlone, B. S. Fields, J. Guarner, M. R. Hammerschlag, L. A. Jackson, C.-C. Kuo, M. Maass, T. O. Messmer, D. F. Talkington, M. L. Tondella, and S. R. Zaki. 2001. Standardizing Chlamydia pneumoniae assay: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada). Clin. Infect. Dis. 33:492-503. [DOI] [PubMed] [Google Scholar]
- 24.Draghi, A., II, V. L. Popov, M. M. Kahl, J. B. Stanton, C. C. Brown, G. J. Tsongalis, A. B. West, and S. Frasca, Jr. 2004. Characterization of “Candidatus Piscichlamydia salmonis” (order Chlamydiales), a chlamydia-like bacterium associated with epitheliocystis in farmed atlantic salmon (Salmo salar). J. Clin. Microbiol. 42:5286-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emerson, P. M., R. L. Bailey, O. S. Mahdi, G. E. L. Walraven, and S. W. Lindsay. 2000. Transmission ecology of the fly Musca sorbens, a putative vector of trachoma. Trans. R. Soc. Trop. Med. Hyg. 94:28-32. [DOI] [PubMed] [Google Scholar]
- 26.Emerson, P. M., S. W. Lindsay, N. Alexander, M. Bah, S.-M. Dibba, H. B. Faal, K. O. Lowe, K. P. McAdam, A. A. Ratcliffe, G. E. Walraven, and R. L. Bailey. 2004. Role of flies and provision of latrines in trachoma control: cluster-randomised controlled trial. Lancet 363:1093-1098. [DOI] [PubMed] [Google Scholar]
- 27.Essig, A., M. Keinemann, U. Simnacher, and R. Marre. 1997. Infection of Acanthamoeba castellanii by Chlamydia pneumoniae. Appl. Environ. Microbiol. 63:1396-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Everett, K. D. E., M. L. Thao, M. Horn, G. E. Dyszynski, and P. Baumann. 2005. Novel chlamydiae in whiteflies and scale insects: endosymbionts ‘Candidatus Fritschea bemisiae’ strain Falk and ‘Candidatus Fritschea eriococci’ strain Elm. Int. J. Syst. Evol. Microbiol. 55:1581-1587. [DOI] [PubMed] [Google Scholar]
- 29.Everett, K. D. E., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 30.Everett, K. D. E., S. Kahane, R. M. Bush, and M. G. Friedman. 1999. An unspliced group I intron in 23S rRNA links Chlamydiales, chloroplasts, and mitochondria. J. Bacteriol. 181:4734-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman, M. G., A. Galil, S. Greenberg, and S. Kahane. 1999. Seroprevalence of IgG antibodies to the chlamydia-like microorganism ‘Simkania Z’ by ELISA. Epidemiol. Infect. 122:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman, M. G., B. Dvoskin, and S. Kahane. 2003. Infections with the chlamydia-like microorganism Simkania negevensis, a possible emerging pathogen. Microb. Infect. 5:1013-1021. [DOI] [PubMed] [Google Scholar]
- 33.Friedman, M. G., B. Dvoskin, J. Hartley, D. Greenberg, C.-H. Chiu, M. Hammerschlag, D. Lieberman, P. Roblin, M. Gelling, S. Ben-Dror, and S. Kahane. 2000. Seropositivity to the novel microorganism Simkania negevensis in Israel, North America and Great Britain, p. 324. In P. Saikku (ed.), Proceedings of the Fourth Meeting of the European Society for Chlamydia Research, Helsinki, Finland, 20-23 August 2000. Società Editrice Esculapio, Bologna, Italy.
- 34.Friedman, M. G., N. Birkebael, B. Dvoskin, P. L. Andersen, S. Kahane, and L. Ostegaard. 2000. Serologic evidence for infection with Simkania negevensis in Denmark and its possible association with chronic cough, p. 258. In P. Saikku (ed.), Proceedings of the Fourth Meeting of the European Society for Chlamydia Research, Helsinki, Finland, 20-23 August 2000. Società Editrice Esculapio, Bologna, Italy.
- 35.Fritsche, T. R., D. Sobek, and R. K. Gautom. 1998. Enhancement of in vitro cytopathogenicity by Acanthamoeba spp. following acquisition of bacterial endosymbionts. FEMS Microbiol. Lett. 166:231-236. [DOI] [PubMed] [Google Scholar]
- 36.Fritsche, T. R., M. Horn, M. Wagner, R. P. Herwig, K.-H. Schleifer, and R. K. Gautom. 2000. Phylogenetic diversity among geographically dispersed Chlamydiales endosymbionts recovered from clinical and environmental isolates of Acanthamoeba spp. Appl. Environ. Microbiol. 66:2613-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fritsche, T. R., R. K. Gautom, S. Seyedirashti, D. L. Bergeron, and T. D. Lindquist. 1993. Occurrence of bacterial endosymbionts in Acanthamoeba spp. isolated from corneal and environmental specimens and contact lens. J. Clin. Microbiol. 31:1122-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gimenez, D. F. 1964. Staining rickettsiae in yolk-sac cultures. Stain Technol. 39:135-140. [DOI] [PubMed] [Google Scholar]
- 39.Greenberg, D., A. Banerji, M. G. Friedman, C.-H. Chiu, and S. Kahane. 2003. High rate of Simkania negevensis among Canadian Inuit infants hospitalized with lower respiratory tract infections. Scand. J. Infect. Dis. 35:506-508. [DOI] [PubMed] [Google Scholar]
- 40.Greub, G., and D. Raoult. 2002. Parachlamydiaceae: potential emerging pathogens. Emerg. Infect. Dis. 8:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greub, G., and D. Raoult. 2002. Crescent bodies of Parachlamydia acanthamoeba and its life cycle within Acanthamoeba polyphaga: an electron micrograph study. Appl. Environ. Microbiol. 68:3076-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greub, G., and D. Raoult. 2003. History of the ADP/ATP-translocase-encoding gene, a parasitism gene transferred from a Chlamydiales ancestor to plants 1 billion years ago. Appl. Environ. Microbiol. 69:5530-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greub, G., and D. Raoult. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greub, G., B. Desnues, D. Raoult, and J.-L. Mege. 2005. Lack of microbicidal response in human macrophages infected with Parachlamydia acanthamoebae. Microb. Infect. 7:714-719. [DOI] [PubMed] [Google Scholar]
- 45.Greub, G., B. La Scola, and D. Raoult. 2003. Parachlamydia acanthamoebae is endosymbiotic or lytic for Acanthamoeba polyphaga depending on the incubation temperature. Ann. N. Y. Acad. Sci. 990:628-634. [DOI] [PubMed] [Google Scholar]
- 46.Greub, G., B. La Scola, and D. Raoult. 2004. Amoebae-resisting bacteria isolated from human nasal swabs by amoebal coculture. Emerg. Infect. Dis. 10:470-477. [DOI] [PubMed] [Google Scholar]
- 47.Greub, G., H. Lepidi, C. Rovery, J. P. Casalta, G. Habib, F. Collard, P. E. Fournier, and D. Raoult. 2005. Diagnosis of infectious endocarditis in patients undergoing valve surgery. Am. J. Med. 118:230-238. [DOI] [PubMed] [Google Scholar]
- 48.Greub, G., I. Boyadjiev, B. La Scola, D. Raoult, and C. Martin. 2003. Serological hint suggesting that Parachlamydiaceae are agents of pneumonia in polytraumatized intensive care patients. Ann. N. Y. Acad. Sci. 990:311-319. [DOI] [PubMed] [Google Scholar]
- 49.Greub, G., J. L. Mege, and D. Raoult. 2003. Parachlamydia acanthamoebae enters and multiplies within human macrophages and induces their apoptosis. Infect. Immun. 71:5979-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greub, G., J.-L. Mege, J. P. Gorvel, D. Raoult, and S. Méresse. 2005. Intracellular trafficking of Parachlamydia acanthamoebae. Cell Microbiol. 7:581-589. [DOI] [PubMed] [Google Scholar]
- 51.Greub, G., P. Berger, L. Papazian, and D. Raoult. 2003. Parachlamydiaceae as rare agents of pneumonia. Emerg. Infect. Dis. 9:755-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51a.Griffiths, E., A. K. Petrich, and R. S. Gupta. 2005. Conserved indels in essential proteins that are distinctive characteristics of Chlamydiales and provide novel means for their identification. Microbiology 151:2647-2657. [DOI] [PubMed] [Google Scholar]
- 52.Groff, J. M., S. E. LaPatra, R. J. Munn, M. L. Anderson, and B. I. Osburn. 1996. Epitheliocystis infection in cultured white sturgeon (Acipenser transmontanus): antigenic and ultrastructural similarities of the causative agent to the chlamydiae. J. Vet. Diagn. Investig. 8:172-180. [DOI] [PubMed] [Google Scholar]
- 53.Henning, K., G. Schares, H. Granzow, U. Polster, M. Hartmann, H. Hotzel, K. Sachse, M. Peters, and M. Rauser. 2002. Neospora caninum and Waddlia chondrophila strain 2032/99 in a septic stillborn calf. Vet. Microbiol. 85:285-292. [DOI] [PubMed] [Google Scholar]
- 54.Hogan, R. J., S. A. Mathews, S. Mukhopadhyay, J. T. Summersgill, and P. Timms. 2004. Chlamydial persistence: beyond the biphasic paradigm. Infect. Immun. 72:1843-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horn, M., A. Collingro, S. Schmitz-Esser, C. L. Beier, U. Purkhold, B. Fartmann, P. Brandt, G. J. Nyakatura, M. Droege, D. Frishman, T. Rattei, H.-W. Mewes, and M. Wagner. 2004. Illuminating the evolutionary history of chlamydiae. Science 304:728-730. [DOI] [PubMed] [Google Scholar]
- 56.Horn, M., and M. Wagner. 2001. Evidence for additional genus-level diversity of Chlamydiales in the environment. FEMS Microbiol. Lett. 204:71-74. [DOI] [PubMed] [Google Scholar]
- 57.Horn, M., M. Wagner, K.-D. Müller, E. N. Schmid, T. R. Fritsche, K. H. Schleifer, and R. Michel. 2000. Neochlamydia hartmannellae gen. nov., sp. nov. (Parachlamydiaceae), an endoparasite of the amoeba Hartmannella vermiformis. Microbiology 146:1231-1239. [DOI] [PubMed] [Google Scholar]
- 58.Johnsen, S., N. Birkebaek, P. L. Andersen, C. Emil, J. S. Jensen, and L. Ostergaard. 2005. Indirect immunofluorescence and real time PCR for detection of Simkania negevensis infection in Danish adults with persistent cough and in healthy controls. Scand. J. Infect. Dis. 37:251-255. [PubMed] [Google Scholar]
- 59.Kahane, S., B. Dvoskin, M. Mathias, and M. G. Friedman. 2001. Infection of Acanthamoeba polyphaga with Simkania negevensis and S. negevensis survival within amoebal cysts. Appl. Environ. Microbiol. 67:4789-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kahane, S., D. Greenberg, M. G. Friedman, H. Haikin, and R. Dagan. 1998. High prevalence of “Simkania Z,” a novel Chlamydia-like bacterium, in infants with acute bronchiolitis. J. Infect. Dis. 177:1425-1429. (Erratum, 178:1553.) [DOI] [PubMed] [Google Scholar]
- 61.Kahane, S., N. Platzner, B. Dvoskin, A. Itzhaki, and M. G. Friedman. 2004. Evidence for the presence of Simkania negevensis in drinking water and in reclaimed wastewater in Israel. Appl. Environ. Microbiol. 70:3346-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kahane, S., R. Gonen, C. Sayada, J. Elion, and M. G. Friedman. 1993. Description and partial characterization of a new chlamydia-like microorganism. FEMS Microbiol. Lett. 109:329-334. [DOI] [PubMed] [Google Scholar]
- 63.Kocan, K. M., T. B. Crawford, P. M. Dilbeck, J. F. Evermann, and T. C. McGuire. 1990. Development of a rickettsia isolated from an aborted bovine fetus. J. Bacteriol. 172:5949-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kostanjsek, R., J. Strus, D. Drobne, and G. Avgustin. 2004. ‘Candidatus Rhabdochlamydia porcellionis,’ gen. nov., sp. nov., an intracellular bacterium from hepatopancreas of the terrestrial isopod Porcellio scaber. Int. J. Syst. Evol. Microbiol. 54:543-549. [DOI] [PubMed] [Google Scholar]
- 65.Lewis, D. M., J. Dutkiewicz, W. G. Sorenson, M. Mamolen, and J. E. Hall. 1990. Microbiological and serological studies of an outbreak of ‘humidifier fever’ in a print shop. Biodeterioration Res. 3:467-477. [Google Scholar]
- 66.Lieberman, D., B. Dvoskin, D. V. Lieberman, S. Kahane, and M. G. Friedman. 2002. Serological evidence of acute infection with the chlamydia-like microorganism Simkania negevensis (Z) in acute exacerbation of chronic obstructive pulmonary disease. Eur. J. Clin. Microbiol. Infect. Dis. 21:307-309. [DOI] [PubMed] [Google Scholar]
- 67.Lieberman, D., S. Kahane, D. Lieberman, and M. G. Friedman. 1997. Pneumonia with serological evidence of acute infection with the Chlamydia-like microorganism “Z.” Am. J. Respir. Crit. Care Med. 156:578-582. [DOI] [PubMed] [Google Scholar]
- 68.Longbottom, D., and L. J. Coulter. 2003. Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 218:217-244. [DOI] [PubMed] [Google Scholar]
- 69.Maraha, B., H. Berg, M. Kerver, S. Kranendonk, J. Hamming, J. Kluytmans, M. Peeters, and A. van der Zee. 2004. Is the perceived association between Chlamydia pneumoniae and vascular diseases biased by methodology? J. Clin. Microbiol. 42:3937-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marrie, T. J., D. Raoult, B. La Scola, R. J. Birtles, E. de Carolis, and Canadian CAP Study Group. 2001. Legionella-like and other amoebal pathogens as agents of community-acquired pneumonia. Emerg. Infect. Dis. 7:1026-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maurin, M., A. Bryskier, and D. Raoult. 2002. Antibiotic susceptibilities of Parachlamydia acanthamoebae in amoebae. Antimicrob. Agents Chemother. 46:3065-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meijer, A., and J. M. Ossewaarde. 2002. Description of a wider diversity within the order Chlamydiales than currently classified. International Chlamydia Conference, Antalya, Turkey, 16-21 June 2002. [Also available online at http://www.chlamydiae.com.]
- 74.Michel, R., B. Haurôder-Philippczyk, K.-D. Müller, and I. Weishaar. 1992. Observations on Acanthamoebae from nasal mucosa infected by obligate intracellular parasites. Zbl. Bakt. Hyg. Abstr. 325:56. [Google Scholar]
- 75.Michel, R., K. D. Müller, L. Zöller, J. Walochnick, M. Hartmann, and E. N. Schmid. 2005. Free-living amoebae serve as host for the chlamydia-like bacterium Simkania negevensis. Acta Protozool. 44:113-121. [Google Scholar]
- 76.Michel, R., K.-D. Müller, and R. Hoffmann. 2001. Enlarged Chlamydia-like organisms as spontaneous infection of Acanthamoeba castellanii. Parasitol. Res. 87:248-251. [DOI] [PubMed] [Google Scholar]
- 77.Michel, R., K.-D. Müller, E. N. Schmid, and I. Weishaar. 1994. Acanthamoebae isolated from human nasal mucosa harboured cocci of a new type as obligate intracellular parasites. Trop. Med. Parasitol. 45(Suppl. II):190. [Google Scholar]
- 78.Michel, R., M. Steinert, L. Zöller, B. Hauröder, and K. Henning. 2004. Free-living amoebae may serve as hosts for the Chlamydia-like bacterium Waddlia chondrophila isolated from an aborted bovine foetus. Acta Protozool. 43:37-42. [Google Scholar]
- 79.Moulder, J. W. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol. Rev. 55:143-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ossewaarde, J. M., and A. Meijer. 1999. Molecular evidence for the existence of additional members of the order Chlamydiales. Microbiology 145:411-417. [DOI] [PubMed] [Google Scholar]
- 81.Petrich, A., M. Smieja, K. Luinstra, T. Sinha, S. Chong, R. Leigh, M. Loeb, and J. Mahony. 2002. Development of a PCR assay to determine prevalence of Simkania negevensis DNA in specimens from patients with respiratory diseases, p. 471-474. In J. Schacter et al. (ed.), Chlamydial infections. Proceedings of the Tenth International Symposium on Human Chlamydial Infections. International Chlamydia Symposium, San Francisco, Calif.
- 82.Poppert, S., A. Essig, R. Marre, M. Wagner, and M. Horn. 2002. Detection and differentiation of chlamydiae by fluorescence in situ hybridization. Appl. Environ. Microbiol. 68:4081-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pospischil, A., R. Thoma, M. Hilbe, P. Grest, and J.-O. Gebbers. 2002. Abortion in woman caused by caprine Chlamydophila abortus (Chlamydia psittaci serovar 1). Swiss Med. Wkly. 132:64-66. [DOI] [PubMed] [Google Scholar]
- 84.Renault, T., and N. Cochennec. 1995. Chlamydia-like organisms in ctenidia and mantle cells of the Japanese oyster Crassostrea gigas from the French Atlantic coast. Dis. Aquat. Organ. 23:153-159. [DOI] [PubMed] [Google Scholar]
- 85.Rurangirwa, F. R., P. M. Dilbeck, T. B. Crawford, T. C. McGuire, and T. F. McElwain. 1999. Analysis of the 16S rRNA gene of microorganism WSU 86-1044 from an aborted bovine foetus reveals that it is a member of the order Chlamydiales: proposal of Waddliaceae fam. nov., Waddlia chondrophila gen. nov., sp. nov. Int. J. Syst. Bacteriol. 49:577-581. [DOI] [PubMed] [Google Scholar]
- 86.Schiller, I., A. Schifferli, P. Gysling, and A. Pospischil. 2004. Growth characteristics of porcine chlamydial strains in different cell culture systems and comparison with ovine and avian chlamydial strains. Vet. J. 168:74-80. [DOI] [PubMed] [Google Scholar]
- 87.Schuster, F. L. 2002. Cultivation of pathogenic and opportunistic free-living amoebas. Clin. Microbiol. Rev. 15:342-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Senn, L., M. Hammerschlag, and G. Greub. 2005. Therapeutic approaches to Chlamydia infection. Expert Opin. Pharmacother. 6:2281-2290. [DOI] [PubMed]
- 89.Skriwan, C., M. Fajardo, S. Hageler, M. Horn, M. Wagner, R. Michel, G. Krohne, M. Schleicher, J. Hacker, and M. Steinert. 2002. Various bacterial pathogens and symbionts infect the amoeba Dictyostelium discoideum. Int. J. Med. Microbiol. 291:615-624. [DOI] [PubMed] [Google Scholar]
- 90.Soldati, G., Z. H. Lu, L. Vaughan, A. Polkinghorne, D. R. Zimmermann, J. B. Huder, and A. Pospischil. 2004. Detection of mycobacteria and chlamydiae in granulomatous inflammation of reptiles: a retrospective study. Vet. Pathol. 41:388-397. [DOI] [PubMed] [Google Scholar]
- 91.Stratton, C. W., and S. Sriram. 2003. Association of Chlamydia pneumoniae with central nervous system disease. Microb. Infect. 5:1249-1253. [DOI] [PubMed] [Google Scholar]
- 92.Thao, M. L., L. Baumann, J. M. Hess, B. W. Falk, J. C. K. Ng, P. J. Gullan, and P. Baumann. 2003. Phylogenetic evidence for two new insect-associated chlamydia of the family Simkaniaceae. Curr. Microbiol. 47:46-50. [DOI] [PubMed] [Google Scholar]
- 93.von Bomhard, W., A. M. Polkinghorne, Z. H. Lu, L. Vaughan, A. Vogtlin, D. R. Zimmermann, B. Spiess, and A. Pospischil. 2003. Detection of novel chlamydiae in feline ocular disease. Am. J. Vet. Res. 64:1421-1428. [DOI] [PubMed] [Google Scholar]
- 94.Yamaguchi, T., T. Yamazaki, M. Inoue, C. Mashida, K. Kawagoe, M. Ogawa, S. Shiga, Y. Nakagawa, T. Kishimoto, I. Kurane, K. Ouchi, and T. Ohzeki. 2005. Prevalence of antibodies against Simkania negevensis in a healthy Japanese population determined by the microimmunofluorescence test. FEMS Immunol. Med. Microbiol. 43:21-27. [DOI] [PubMed] [Google Scholar]
- 95.Yamaguchi, T., T. Yamazaki, M. Inoue, M. Ogawa, S. Shiga, Y. Nakagawa, T. Kishimoto, K. Ouchi,., and T. Ohzeki. 2004. Factors improving the propagation of Simkania negevensis strain Z in cell culture. Jpn. J. Infect. Dis. 57:103-106. [PubMed] [Google Scholar]