Abstract
Human cytomegalovirus (HCMV) is a ubiquitous human pathogen that infects 40 to 90% of adult human populations. HCMV infections are often asymptomatic in healthy individuals but can cause severe organ and life-threatening disease in immunocompromised patients. The antiviral antibody response to HCMV infection is complex and is known to include virus-neutralizing antibody production against surface glycoproteins encoded by HCMV. We have investigated the human antibody response to a complex of HCMV surface glycoproteins composed of glycoprotein M (gM)/gN, the gene products of the UL100 and UL73 open reading frames. Mouse monoclonal antibodies generated against gM/gN have previously been shown to neutralize HCMV infection of human fibroblasts in vitro. To determine whether human antibodies reactive with the gM/gN complex possess virus-neutralizing properties, we isolated human antibodies reactive with gM/gN from pooled human HCMV hyperimmune globulin by affinity purification using recombinant gM/gN. The affinity-purified human anti-gM/gN antibodies reacted specifically by immunofluorescence with HCMV-infected human fibroblasts and with cells transiently expressing gM/gN, but not with cells transfected with plasmids encoding other immunogenic HCMV proteins. The anti-gM/gN antibodies also reacted specifically only with gM/gN in immunoblot assays using lysates of transfected cells expressing specific HCMV proteins. Last, human anti-gM/gN antibodies efficiently neutralized infectious HCMV in vitro with a capacity comparable to that of human anti-gB antibodies. These data indicated that gM/gN can elicit a virus-neutralizing antibody response in humans infected with HCMV and therefore should be considered a potential candidate for inclusion in prophylactic CMV vaccines.
Human cytomegalovirus (HCMV) is a betaherpesvirus that infects a large proportion of the human population (10). Infection is usually asymptomatic in immunocompetent individuals but may be life threatening in immunocompromised hosts, including allograft recipients and patients with AIDS, and in newborn infants infected with HCMV in utero (10). Characterization of the immune response to HCMV in the healthy host has resulted in the identification of preventive or therapeutic strategies to limit disease in the immunocompromised host (2, 34).
Although the immunologic responses against several of the major HCMV surface glycoproteins, including glycoprotein B (gB) and gH, have been extensively characterized, only limited studies of the responses to the gM/gN complex have been reported (13, 18, 30-32, 41-43). The gM/gN complex is the most abundant glycoprotein component of the HCMV virion envelope (44). gM is a 42- to 45-kDa (kilodalton type) III membrane protein containing seven potential membrane-spanning domains (21). The gM open reading frame (ORF), UL100, is conserved among members of the herpesvirus family, having been reported in herpes simplex virus type 1, pseudorabies virus (PRV), Epstein-Barr virus, and equine herpesvirus type 1 among others (3, 7, 17, 20, 23, 36). In many herpesviruses, gM is nonessential for in vitro replication, although deletion of gM results in less efficient viral replication and reduced virulence in animal models (12, 25). However, gM is essential for in vitro replication of HCMV, and the UL100 ORF exhibits little predicted amino acid sequence variation among HCMV strains (22, 24). In HCMV, gM forms a heterodimeric complex with gN, the gene product of UL73 (23, 24). The gN homolog is also conserved among herpesviruses and has been reported to complex with gM in PRV and other herpesviruses (14, 19, 20). From calculations of the predicted primary amino acid sequence, HCMV gN is a small (15- to 18-kDa) type I membrane protein which in the mature virion is extensively modified by both N-linked and O-linked carbohydrates, resulting in a mature virion form of the protein with an apparent molecular mass of 39 to 53 kDa as estimated by its migration on denaturing sodium dodecyl sulfate (SDS)-polyacrylamide gels (5, 11, 23, 24). Sequence analysis of the UL73 gene indicates that this glycoprotein exhibits significant amino acid sequence variation among HCMV isolates and can be classified into four distinct genotypes (11). Sequence analysis of the UL73 gene from multiple virus strains has suggested that this variability results from positive selection pressure on this gene, implying a role of gN polymorphism in immune evasion (27). In other herpesviruses, such as PRV, varicella-zoster virus, and bovine herpesvirus type 1, gN is nonessential for in vitro replication, and sequence variation in the gN homologs derived from wild-type isolates of these viruses has not been reported (14, 35, 45).
Mouse monoclonal antibodies (MAbs) specifically reactive against the HCMV gM/gN complex have been shown previously to neutralize virus infection in vitro (5, 23). Earlier studies have also shown that individuals seropositive for HCMV generate antibodies against the gM/gN complex (23). In the following study we have investigated the virus-neutralizing activity of human anti-gM/gN antibodies isolated by affinity purification from a preparation of pooled human immunoglobulin G (IgG) antibodies. Our findings indicate that human anti-gM/gN antibodies can neutralize infectious virus with an efficiency similar to that of anti-gB antibodies isolated from the same preparation of pooled IgG antibodies. In addition, the anti-gM/gN antibodies also efficiently neutralized a heterologous strain of HCMV, Toledo, even though the anti-gM/gN antibodies were isolated using recombinant gM/gN derived from strain AD169.
MATERIALS AND METHODS
Cells and viruses.
The human embryonic kidney cell line HEK293T and the monkey cell line Cos-7 were grown in Dulbecco modified Eagle medium supplemented with 5% newborn calf serum and penicillin/streptomycin. Primary human foreskin fibroblasts were maintained in medium 199 supplemented with 5% newborn calf serum. HCMV strain AD169 was originally obtained from the American Type Culture Collection (www.atcc.org) and were propagated on human foreskin fibroblasts as previously described (4). The Toledo strain of HCMV was obtained from Stuart Adler, Medical College of Virginia, Richmond. The clinical isolate TR was provided by Jay Nelson (Oregon Health Sciences University, Portland). Titers of infectivity in viral stocks were determined in fibroblasts using a previously described indirect immunofluorescence assay with a monoclonal antibody against the HCMV immediate-early 1 (IE1) protein (1).
Antibodies.
Murine monoclonal antibodies reactive with pp65 (UL83), pp150 (UL32), gB (UL55), gM (UL100), IE1 (UL123), and the gM/gN complex (UL100/UL73) have been described in previous publications (1, 5, 6, 8, 28, 38, 43). A murine MAb (9E10; Developmental Hybridoma Bank, University of Iowa) and a rabbit polyvalent antiserum (Affinity Bioreagents, Boulder, Colo.) reactive with the myc epitope were used to immunoprecipitate and/or detect myc-tagged proteins. The source of human antibodies reactive with HCMV was a commercially available preparation of human IgG antibodies derived from pooled human serum samples from HCMV-immune donors, which has been formulated for intravenous administration to humans (Cytogam; MedImmune, Gaithersburg, Md.). The human IgG was diluted to approximately 10 mg/ml of IgG antibodies in Dulbecco's phosphate-buffered saline (DPBS) (pH 7.4) prior to its use for affinity isolation of anti-gM/gN antibodies.
Plasmids and transfections.
Expression plasmids encoding the UL83 (pp65), UL32 (pp150), UL55 (gB), UL100 (gM), and UL73 (gN) open reading frames were constructed using standard techniques. These particular HCMV genes were derived from HCMV AD169 and have been described in previous publications (23, 37, 38). The coding sequence of each of these genes was cloned into expression vector pcDNA3.1 or pEF-1myc/his (Invitrogen, Carlsbad, Calif.). Following calcium chloride-mediated transfection of approximately 2 μg of DNA into a 35-mm dish of subconfluent HEK293T cells, expression was confirmed by immunofluorescent reactivity with a specific MAb (9, 38). For imaging studies, Cos-7 cells were transfected with the respective plasmid and processed for image analysis as has been described previously (38). Secondary anti-mouse antibodies conjugated with either fluorescein isothiocyanate (FITC) or Texas Red were obtained from Southern Biotechnology Associates (Birmingham, Ala.).
Affinity isolation of anti-gM/gN antibodies.
Expression plasmids encoding UL83 (pp65), UL55 (gB), or UL100 (gM) plus UL73 (gN) were used to transfect HEK293T cells in 100-mm2 plates using either TransIT LT-1 transfection reagent (Mirus Corp, Madison, WI) or Lipofectamine Plus reagent (Invitrogen) according to the manufacturer's instructions. Several plates were not transfected and served as negative-control plates. Plates were fixed 2 or 3 days posttransfection using freshly prepared 4% (wt/vol) paraformaldehyde in DPBS for 2 h at ambient temperature, washed three times with DPBS, and blocked for 1 h at 37°C with 10% (vol/vol) fetal calf serum in DPBS prior to absorption. The human IgG antibodies obtained from the manufacturer were diluted to approximately 10 mg/ml in DPBS and absorbed sequentially on dishes containing nontransfected HEK293T cells and then on dishes containing HEK293T cells transfected with plasmids encoding UL83, UL55, or UL100/UL73, with each absorption for 1 h at 37°C. After absorption, plates were washed three times with DPBS, bound antibodies were eluted from plates using 0.4 M acetic acid for 5 min at room temperature, and the eluate was immediately neutralized using a saturated Tris solution to a final pH of 7.0 to 8.0. Eluted antibodies were concentrated in dialysis tubing (12- to 14-kDa size exclusion limit) by dehydration using polyethylene glycol powder and stored at 4°C.
Specificity of affinity-purified antibodies assayed by immunofluorescence.
Cos-7 cells were seeded into 24-well plates containing 13-mm glass coverslips and then transfected with approximately 1 μg of plasmid encoding UL83, UL55, UL100, UL73, or an equal mixture of UL100/UL73 as described above. In addition, cells were also transfected separately with plasmids encoding UL32 (pp150), UL75 (gH), or myc-tagged TRL10 (gpTRL10). At 48 h posttransfection, the cells were washed twice with DPBS, fixed with 4% (wt/vol) paraformaldehyde, washed three times with DPBS, permeabilized with permeabilizing solution (0.1% NP-40, 0.01% SDS in DPBS) for 5 min, washed again, and blocked for 1 hour with DPBS containing 10% (vol/vol) goat serum. To evaluate protein expression, cells were incubated with primary mouse MAbs 65-8 (anti-pp65), 7-17 (anti-gB), IMP-91 (anti-gM), 14-16A (anti-gM/gN complex), 36-14 (anti-pp150), and 14-4b (anti-gH), and with anti-myc MAb 9E10 (for detection of myc-tagged gpTRL10) as described in previous studies (1, 5, 6, 8, 28, 40, 43). Primary incubations were performed at 37°C for 1 h. After the slides were washed with DPBS, they were incubated with FITC-conjugated goat anti-mouse IgG plus IgM at a 1:40 dilution for 1 h at 37°C. Hoechst stain at 1 μg/ml in DPBS was added for the final 10 min of incubation, the slides were washed with DPBS, and coverslips were applied using SlowFade (Molecular Probes, Eugene, Oreg.) mounting medium. Images were collected using a Leica Diavert fluorescence microscope fitted with a Photometrics charge-coupled device and processed using ImagePro software (Media Cybernetics Inc., Silver Spring, MD). All images were collected under similar exposure times and identical gain. After the specificity of the expression of recombinant protein was confirmed, transfected cells were incubated with affinity-purified human antibodies against UL83, UL55, or UL100/UL73 at a 1:2 dilution together with the respective mouse MAb for 1 h at 37°C. After the cells were washed, they were incubated with FITC-conjugated goat anti-human IgG and Texas Red-conjugated goat anti-mouse IgG or IgM at a 1:50 dilution for 1 h at 37°C, washed, and mounted, and images were obtained as described above.
To quantify antigen-specific antibodies present in the human IgG preparation, slides were prepared from HEK293T cells transfected with UL83, UL55, and UL100/UL73 by spotting cells 48 h posttransfection into individual wells of 10-well coated slides. The slides were dried, fixed in acetone for 20 min, and then reacted for 1 h at 37°C with specific MAbs or the human IgG diluted serially from 1:50 to 1:30,000. After the slides were washed extensively, they were incubated with either FITC-conjugated goat anti-human IgG or FITC-conjugated goat anti-mouse IgG or IgM for 1 h at 37°C, washed, counterstained with 0.02% Evans blue, and mounted as described above. End-point dilutions of antigen detection using human IgG were determined visually using a fluorescence microscope (BX21; Olympus, Melville, NY).
Estimation of affinity-purified antibody concentration.
Serial dilutions of human IgG at known protein concentrations were vacuum aspirated onto a nitrocellulose filter using a standard filter hybridization manifold. Affinity-purified antibodies against UL55 (gB) and UL100/UL73 (gM/gN) were also applied to the same nitrocellulose filter. The filter was blocked for 1 h in BLOTTO (Tris-buffered saline containing 0.02% Tween 20 [TTBS], 5% [wt/vol] skim milk powder, and 0.02% sodium azide) and then incubated with goat anti-human IgG antibodies (Southern Biotechnology Associates, Birmingham, Ala.) at a 1:500 dilution in BLOTTO for 2 h at 37°C. After the filter was washed three times with TTBS, it was incubated with 125I-labeled protein A for 1 h at 37°C and washed again three times with TTBS. The filter was air dried and exposed using X-Omat film at −80°C. Autoradiographs of the affinity-purified antibodies were compared to those of the human IgG standard curve using densitometry to estimate the concentration of affinity-purified antibody.
Immunoprecipitation and Western blotting.
HEK293T cells were transfected with pEF1-myc/his constructs of pp65, gB, pp150, and gM/gN as described above. Cells were lysed in radioimmunoprecipitation assay buffer (0.1% SDS, 1% deoxycholate, 1% NP-40 in TBS, pH 7.4) at 4°C, and subsequent steps were performed at 4°C. Cells were homogenized by shearing through an 18-gauge needle and precleared by rotating incubation with 10% goat serum and formalin-fixed Staphylococcus aureus strain Cowan 1 bacteria (Pansorbin; Calbiochem, San Diego, CA) for 1 hour followed by centrifugation to remove Pansorbin. Precleared supernatants were incubated rotating overnight at 4°C with rabbit anti-myc antibody, followed by incubation for 1 hour with goat anti-rabbit antibody and then for 1 hour with Pansorbin. Following centrifugation, the precipitates were washed three times with cold radioimmunoprecipitation assay buffer. The precipitates were then incubated at room temperature in SDS sample buffer (5% 2-mercaptoethanol, 2% SDS, 8 M urea, 0.1 M Tris, pH 8.0). During the later part of these studies, we utilized magnetic beads conjugated with anti-myc antibodies to precipitate myc-tagged proteins according to the manufacturer's instructions (Miltenyi Biotec, Auburn, Calif.). All samples except gM/gN were boiled for 5 min. Samples were separated on a 12% polyacrylamide gel containing urea at a final concentration of 3 M, transferred to nitrocellulose membranes, blocked with BLOTTO for 1 h at room temperature, and then incubated with either anti-myc murine MAb or affinity-purified human anti-gM/gN in BLOTTO overnight at 4°C. The membranes were then washed three times with TTBS and incubated with either goat anti-mouse IgG or goat anti-human IgG conjugated to horseradish peroxidase at a 1:10,000 dilution in TTBS for 1 hour at room temperature. After the membranes were washed extensively, they were developed using West Pico chemiluminescence detection kit (Pierce Chemical Co., Rockford, Ill.) according to the manufacturer's instruction and exposed on Kodak BioMax film.
Virus-neutralizing antibody activity.
Neutralization assays were performed as described previously (1). For neutralization assays, titered virus stocks were added to Dulbecco modified Eagle medium supplemented with 5% newborn calf serum containing antiviral antibodies for 1 h at room temperature. Guinea pig serum was added to some samples as a source of complement to a final concentration of 5% (vol/vol) for 1 h at room temperature. Absorbed antibodies were tested with and without complement at a final concentration of 1:2, 1:10, or 1:50. Antibody was not added to some tubes to serve as a negative control. Human IgG (Cytogam) was also tested at concentrations of 1:50 and 1:100. For positive controls, mouse monoclonal antibodies 7-17 (anti-gB) and 14-16A (anti-gM/gN) were added to viral solutions at concentrations of 1:2, 1:10, and 1:50. After incubation, solutions were added to human foreskin fibroblast cultures grown to confluence in 96-well microtiter plates for 2 h at 37°C. All samples were tested in quadruplicate. After the viral solutions were removed, cells were incubated for another 16 to 20 h in fresh medium, then washed, fixed with ethanol, stained with anti-IE1 MAb p63-27 and FITC-conjugated anti-mouse IgG secondary antibodies. Following extensive washing, the plates were counterstained with Evans blue as described above. The number of infected cells per well was quantified using a fluorescence microscope adapted for reading microtiter plates (Leitz Diavert; Leica Corp., Nutley, NJ). The number of fluorescent cells in each well was compared to the number of cells infected by virus-containing medium with and without complement but lacking antibody.
RESULTS
Affinity purification of anti-gB and anti-gM/gN antibodies from pooled human IgG antibodies.
Although previous studies from our laboratories have demonstrated that serum samples from individuals with HCMV infections contained IgG antibodies reactive with gM and the gM/gN complex, it remains unclear whether these antibodies included virus-neutralizing antibodies. To determine whether anti-gM/gN antibodies contributed to the virus-neutralizing antibody activity in human serum, we affinity purified anti-HCMV antibodies from a pooled human polyclonal IgG preparation. Initially, we determined whether this preparation contained antibodies reactive with several envelope glycoproteins and two immunogenic tegument proteins. Glycoproteins gB (UL55), gM/gN (UL100/UL73), gH (UL75), and gpTRL10 (TRL10) and tegument proteins pp65 (UL83) and pp150 (UL32) were transiently expressed in Cos-7 cells, and expression was confirmed by immunofluorescence with murine monoclonal antibodies reactive with each protein. In the case of gpTRL10, this protein was myc tagged, and its expression was demonstrated by its reactivity with an anti-myc antibody. The pooled IgG preparation contained antibodies reactive with the HCMV-encoded proteins, including gB, gM/gN, pp65, pp150, gH, and gpTRL10 (Fig. 1). We attempted to quantify the reactivity for a subset of these HCMV proteins by end-point dilution of the reactivity as measured in an immunofluorescence assay that utilized transiently expressed HCMV proteins in HEK293T cells. We could detect reactivity against transiently expressed pp65, pp150, gH, and gM/gN proteins at an end-point dilution of 1:3,000 which corresponds to approximately 16 μg/ml of antibody (data not shown). In contrast, this pooled preparation of IgG antibodies contained higher titers of anti-gB antibodies, and reactivity could be detected when this IgG preparation was diluted to 1:30,000 (1.6 μg/ml; data not shown).
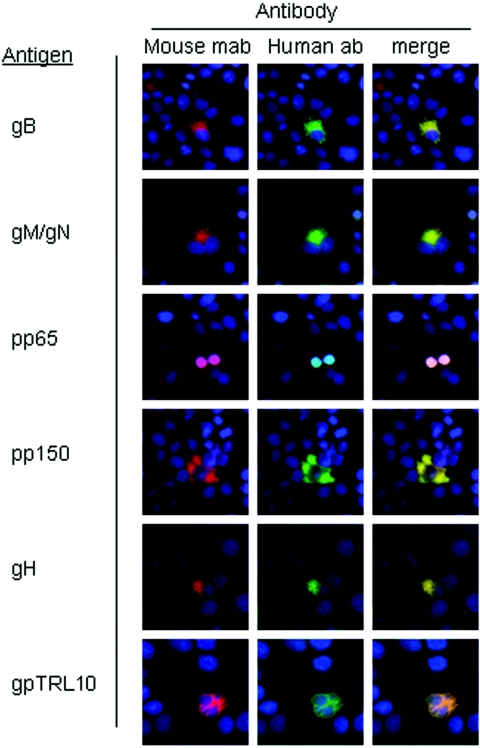
FIG. 1.
Pooled human immunoglobulins contain antibodies reactive with HCMV virion proteins. Cos-7 cells were transfected with plasmids containing HCMV glycoproteins and phosphoproteins, and protein expression was demonstrated by reactivity with mouse monoclonal antibodies (Mouse mab) specific for each protein (Antigen) followed by a Texas Red-conjugated anti-mouse secondary antibody (red signal). Cells were coincubated with pooled human antibodies (Human ab) and detected using a FITC-conjugated anti-human secondary antibody (green signal). The appearance of yellow in each of the merged images (merge) shows colocalization of red and green signals in all rows, indicating reactivity of pooled human antibodies against each of the transfected HCMV proteins. The cell nuclei were stained with Hoechst dye (blue).
Utilizing this preparation of pooled human IgG antibodies, we next devised an affinity purification scheme for isolating gM/gN antibodies from the population of antibodies present in this polyclonal antibody preparation. This approach was selected because both gB and the gM/gN complex were abundantly expressed on the surfaces of both virus-infected HF cells and following transient expression of these glycoproteins in Cos-7 cells (data not shown). Glycoproteins gB and gM/gN and the tegument protein pp65 were transiently expressed in HEK293T cells, and the monolayers were fixed in 4% paraformaldehyde 48 h posttransfection. Following fixation, the monolayers were washed extensively with DPBS. The IgG preparation was diluted to a concentration of 10 mg/ml and preabsorbed on similarly fixed but nontransfected HEK293T cells to limit any nonspecific reactivity in the preparation used for absorption. Following this step, the IgG preparation was sequentially absorbed on cells transiently expressing pp65, then gB, and finally, gM/gN. After each absorption, the plates were washed extensively with DPBS, and the bound antibody eluted with a low-pH buffer. Following neutralization, the purified antibodies were concentrated and characterized for their reactivity against these HCMV-encoded proteins and against HCMV pp150.
Affinity-purified anti-gM/gN antibodies absorbed from pooled human IgG antibodies are specific for the gM/gN complex.
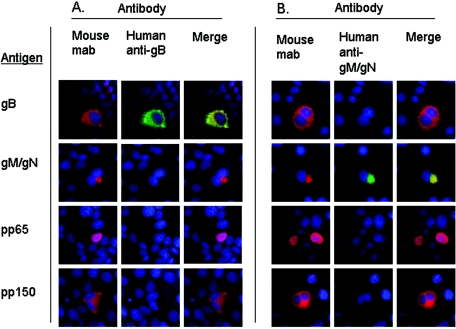
Initially, the specificity of the affinity-purified anti-gB and anti-gM/gN antibodies was determined in an immunofluorescence assay using Cos-7 cells transfected with plasmids that encoded gB, gM/gN, pp65, or pp150. The affinity-purified anti-gM/gN antibodies were reactive only with gM/gN transiently expressed in Cos-7 cells and nonreactive with transiently expressed gB, pp65, or pp150 in the same assay, indicating that this preparation of anti-gM/gN antibodies was specific for the gM/gN complex (Fig. 2B). Interestingly, the signal derived from the affinity-purified anti-gM/gN antibodies colocalized nearly completely with the signal derived from MAb 14-16A, which is specific for the gM/gN complex (Fig. 2B) (23). Similarly, the affinity-purified anti-gB antibodies also reacted specifically only with cells transiently expressing gB (Fig. 2A). These results indicated that the methodology for affinity purification of anti-gM/gN and anti-gB specific antibodies from a preparation of human IgG antibodies yielded a population of human antibodies specific for the respective antigen.
FIG. 2.
Affinity-purified human anti-gB and anti-gM/gN antibodies react specifically against gB or gM/gN. HCMV gB, gM/gN, pp65, or pp150 (Antigen) were expressed by transient transfection of Cos-7 cells with plasmids encoding each respective HCMV ORF. Protein expression was confirmed using mouse monoclonal antibodies specific for each protein (Mouse mab), followed by incubation with Texas Red-conjugated anti-mouse secondary antibodies (red signal). Cells were coincubated with affinity-purified human antibodies against either gB (Human anti-gB) (A) or gM/gN (Human anti-gM/gN) (B), and reactivity was detected using a FITC-conjugated anti-human secondary antibody (green signal). Merged images (Merge) show that human anti-gB reacts only with cells transfected with gB antigen (A) and human anti-gM/gN reacts only with cells transfected with gM/gN antigens (B). Hoechst nuclear stain (blue) shows the location of cell nuclei.
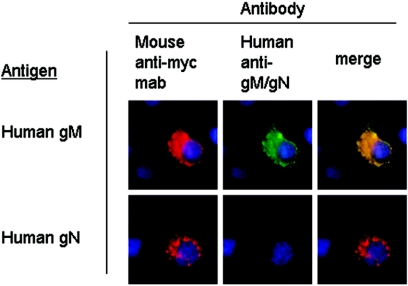
Further studies indicated that these affinity-purified antibodies were reactive with gM expressed in the absence of gN but were nonreactive with gN when only this component of the gM/gN glycoprotein complex was transiently expressed in Cos-7 cells (Fig. 3). Consistent with observations by others, the anti-myc staining of gN-expressing cells appeared limited to perinuclear compartments, without cell surface expression (24). Because the anti-gM/gN antibodies were affinity purified utilizing nonpermeabilized cells, this finding argued that the antigenicity of cell surface gN was limited to forms of this protein complexed with gM, perhaps secondary to a requirement for glycosylation of gN that occurs subsequent to complex formation with gM. In addition, our results suggested that utilization of gM/gN expressed on cell surfaces for affinity purification of anti-gM/gN antibodies yielded a population of antibodies that were specifically reactive with gM and the gM/gN complex.
FIG. 3.
Affinity-purified human anti-gM/gN antibodies recognize transfected gM alone but not gN. Cos-7 cells were transfected with plasmids expressing either myc-tagged HCMV gM or gN, and protein expression was detected by reactivity with a mouse anti-myc monoclonal antibody followed by a Texas Red-conjugated anti-mouse secondary antibody (Mouse anti-myc mab, red signal). Cells were coincubated with the affinity-purified human anti-gM/gN antibodies used in Fig. 2, and reactivity was detected using a FITC-conjugated anti-human secondary antibody (Human anti-gM/gN, green signal). Merged images (merge) indicate that the human anti-gM/gN antibody recognized gM when expressed alone (yellow), but it had no detectable reactivity against gN (red) when this gene was expressed in the absence of gM.
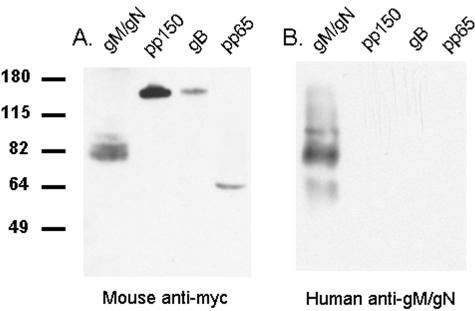
To further define the specificity of the affinity-purified anti-gM/gN antibodies, we analyzed the reactivity of the anti-gM/gN antibodies for transiently expressed recombinant proteins in an immunoblot assay. Previous studies have demonstrated that separation of the gM/gN complex from other virus-encoded proteins by conventional SDS-polyacrylamide gel electrophoresis (PAGE) is limited because of the insolubility of the complex and its propensity to aggregate (23). To overcome these obstacles, we tagged the gM and gN molecules with the myc epitope and expressed the myc-tagged proteins by transient transfection of HEK293T cells. Following immunoprecipitation with a rabbit anti-myc antibody, gM/gN complexes were separated by SDS-PAGE in urea-containing gels, transferred to nitrocellulose membranes, and detected using mouse anti-myc antibodies. Membranes containing myc-tagged pp65, gB, and pp150 and gM/gN were then prepared in this manner following precipitation of lysates of HEK293T cells transfected with expression plasmids encoding myc-tagged forms of these proteins. Probing the filter with anti-myc antibodies demonstrated that recombinant proteins with predicted molecular masses were expressed in these cells (Fig. 4A). The anti-myc antibodies detected only the uncleaved precursor form of gB, suggesting that introduction of the myc tag on the C terminus of this glycoprotein could have altered normal intracellular trafficking of gB or alternatively that a majority of gB following transient expression in these cells was found as the uncleaved form as reported by other investigators (Fig. 4A) (15, 33). In this experiment, the anti-myc antibodies detected a protein from cells cotransfected with the gM- and gN-expressing plasmids that migrated between 80 and 90 kDa, a mass consistent with an aggregated form of gM or perhaps, an incompletely reduced gM/gN complex that could contain an incompletely modified form(s) of gN (5, 23, 24). Overexposure of this membrane revealed additional minor bands that migrated in the range of 55 to 65 kDa, but this reactivity was very weak, suggesting that in this experiment the myc-tagged, mature form of gN was only minimally reactive with anti-myc antibodies (data not shown). These recombinant antigens were then used to confirm the specificity of the affinity-purified anti-gM/gN antibodies by probing a second membrane that had been simultaneously prepared using the same lysates of recombinant proteins. The affinity-purified human anti-gM/gN antibodies reacted only with proteins expressed in cells transfected with plasmids encoding the gM/gN complex, thus providing additional evidence of their specificity for the gM/gN complex (Fig. 4B). Interestingly, anti-gM/gN antibodies present in the affinity-purified preparation recognized three different species, (i) the mature form of gN migrating between 55 and 65 kDa, (ii) a protein with an estimated mass of 80 to 90 kDa that likely represents aggregated or dimeric forms of gM or alternatively gM complexed with incompletely modified forms of gN, and (iii) a high-molecular-weight form with an estimated mass of 100 kDa that could represent aggregated gM or the unreduced complex of gM and gN (Fig. 4B). In this experiment, the human affinity-purified antibodies failed to react with the monomeric gM (mass, 45 kDa) suggesting that most of gM was either complexed with gN or in an aggregated form. These results suggested that affinity-purified antibodies prepared using gM/gN expressed on the cell surface contained significant antibody reactivity directed at fully processed glycoprotein components of the complex, in particular the mature, glycosylated form of gN that is found in the gM/gN complex.
FIG. 4.
Affinity-purified human anti-gM/gN is specific for the gM/gN complex. Myc-tagged HCMV proteins gM/gN, pp150, gB, and pp65 were transiently expressed in HEK293T cells following transfection of plasmids encoding each of these HCMV ORFs. Proteins were purified by immunoprecipitation using a rabbit anti-myc antibody and Staphylococcus aureus protein A, separated on 12% SDS-polyacrylamide gels, and electrophoretically transferred to nitrocellulose membranes. The presence of transfected proteins was confirmed by incubation of one membrane with a mouse anti-myc monoclonal antibody (Mouse anti-myc) (A), followed by a horseradish peroxidase-conjugated anti-mouse secondary antibody and developed by enhanced chemiluminescence. A second membrane, prepared using the same precipitated proteins, was incubated with the affinity-purified human anti-gM/gN and developed as described above for panel A (Human anti-gM/gN) (B). The positions of molecular mass standards (in kilodaltons) are indicated to the left of the gel. The presence of transiently expressed gM/gN (82 kDa), pp150 (150 kDa), gB (160 kDa), and pp65 (65 kDa) is shown in panel A. Panel B shows only bands in the lane containing gM/gN protein but no signal in the lanes containing pp150, gB, or pp65, indicating that affinity-purified human anti-gM/gN antibodies react specifically with components of the gM/gN complex.
We further characterized the nature of anti-gM/gN antibodies prepared by affinity purification of antibodies from human immunoglobulin preparations by determining the reactivity of these antibodies for gN. This question was addressed because the only well-described virus-neutralizing antibody (MAb 14-16A) directed at the gM/gN complex is specific for the gN component of the complex and because the results described above suggested that anti-gN antibodies were present in preparations for affinity-purified anti-gM/gN antibodies (23). To explore this question, we expressed myc-tagged gN (gNmyc) alone or as a complex with wild-type gM, and following precipitation by anti-myc antibody-conjugated magnetic beads, separation by SDS-PAGE, and transfer to nitrocellulose membranes, we probed the membrane with a preparation of affinity-purified human anti-gM/gN antibodies that had been shown to be specific for gM/gN by immunofluorescence assays (Fig. 2). For a control for reactivity by the affinity-purified antibodies for gM, we included gMmyc in these experiments. We monitored protein expression by immunofluorescence using anti-myc antibodies and antibody 14-16A to ensure that individual proteins and the gM/gN complex were expressed in the transfected cells. In each case, a transfection efficiency of over 50% was noted for each transiently expressed protein, including transiently expressed gNmyc (data not shown). As noted above, all transiently expressed proteins were precipitated with magnetic beads conjugated with anti-myc antibodies prior to SDS-PAGE and transfer to nitrocellulose. In lysates from cells transfected with gNmyc alone, the anti-myc MAb (9E10) was reactive with a protein migrating around 20 kDa, the predicted mass of gNmyc (Fig. 5a). In lysates from cells expressing gM/gNmyc, anti-myc MAb 9E10 detected two major proteins consisting of the 20-kDa protein and a protein migrating between approximately 50 and 60 kDa, which was consistent with glycosylated gN, as well as a series of high-molecular-weight bands migrating at and above 90 kDa, which likely represented aggregated forms of gN (Fig. 5a). Finally, in lysates from cells transfected with gMmyc, the anti-myc MAb detected only a high-molecular-weight protein migrating at approximately 100 kDa, suggesting that under the conditions of this experiment, most of the gM was present as high-molecular-weight aggregates. These data were consistent with earlier studies that demonstrated that processing and posttranslational modifications of gN required coexpression with gM and that in the absence of gM, gN was not glycosylated (23, 24). It was of interest to note in this experiment that coexpression of gM and gNmyc resulted in the generation of both the glycosylated form of gNmyc migrating between 50 and 60 kDa and the nonglycosylated form migrating at 20 kDa (Fig. 5a, lane gM/gNmyc). This result suggested that in this experiment, cotransfection of two separate plasmids encoding gM and gNmyc resulted in the expression of both gNmyc complexed with gM and noncomplexed gNmyc, suggesting that gNmyc was expressed in excess of gM under these conditions.
FIG. 5.
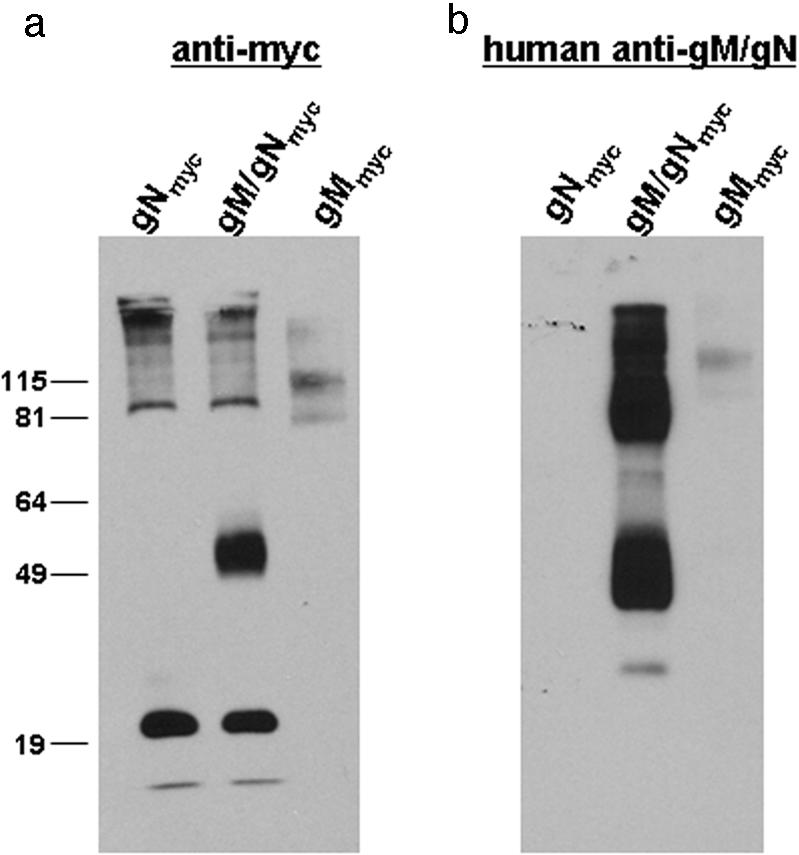
Myc-tagged gN or gM were transiently expressed in HEK293T cells as described in Materials and Methods. Similarly, wild-type gM was coexpressed with myc-tagged gN (gNmyc) by cotransfection of plasmids encoding each of these HCMV ORFs. The cells were lysed, and myc-tagged proteins were collected with paramagnetic beads conjugated with anti-myc antibodies (Miltenyi Biotec, Auburn, Calif.). After the proteins were washed, they were eluted and separated by SDS-PAGE in 12% gels. The proteins were transferred to a nitrocellulose membrane and probed with (a) an anti-myc MAb (9E10) or (b) affinity-purified human anti-gM/gN antibodies, and antibody binding was detected using an ECL kit (Pierce, Rockford, Ill). The positions of molecular mass standards (in kilodaltons) are indicated to the left of the gel in panel a. The most rapidly migrating bands in panel a represent the dye front of the original gel.
To determine the reactivity of the human anti-gM/gN affinity-purified antibodies for gM and gN, we stripped the same membrane and then reprobed this membrane with human anti-gM/gN antibodies. Human anti-gM/gN antibodies failed to react with gNmyc when this protein was expressed in the absence of gM (Fig. 5b). In contrast, the affinity-purified human anti-gM/gN antibodies reacted strongly with a protein that migrated between approximately 45 and 60 kDa and with a high-molecular-weight form migrating at and above 81 kDa (Fig. 5b). Proteins with similar rates of migrations were detected by the anti-myc MAb, suggesting that these forms contained gNmyc (Fig. 5a and b). It is likely that the broader bands of reactivity at 45 to 60 kDa and above 81 kDa, as well as a minor band at 37 kDa, seen in the immunoblot reacted with human anti-gM/gN antibodies might represent forms of gM and gN observable only when the two proteins are expressed in complex (Fig. 5b). It was of interest that in this experiment the monomeric form of gM at 45 kDa was not detected with the human anti-gM/gN antibodies when gMmyc was expressed alone but may be represented in the broad band at and above 45 kDa in the lysate containing gM/gN complex (Fig. 5b). Instead, higher-molecular-weight forms migrating around 82 kDa and 115 kDa could be detected in lysates from cells transfected with gMmyc alone (Fig. 5b). The finding that gM was present in only higher-molecular-weight aggregates was consistent with the explanation that this protein aggregated as a result of its overexpression in the absence of gN. This result was also consistent with results presented in Fig. 4, and it should be noted that even in the presence of urea during SDS-PAGE, gM readily aggregates. Together, these data provide additional evidence of the specificity of the affinity-purified anti-gM/gN antibodies and suggested that reactivity of these antibodies was primarily directed at glycosylated gN and gM, findings that were consistent with results obtained in immunofluorescence assays (Fig. 2 and 3).
Affinity-purified anti-gM/gN antibodies neutralize laboratory and clinical isolates of HCMV.
Mouse MAb 14-16A against gM/gN has previously been shown to neutralize infectious HCMV in vitro (5, 23). Affinity-purified human anti-gM/gN antibodies were tested in neutralization assays using HCMV laboratory strain AD169 as has been described previously (1). The anti-gM/gN MAb 14-16A was included in all neutralization assays as a positive control. Serial dilutions of the preparation of human IgG antibodies were also tested for neutralizing activity. The pooled human IgG antibodies produced 100% neutralization of input virus at 1:10 and 1:50 dilutions, both in the presence and absence of complement (Table 1). However, it should be noted that at these dilutions, the pooled human IgG antibodies contained approximately 5 mg/ml and 1 mg/ml of human IgG, a concentration approximately 1,000-fold higher than the concentration of MAb and approximately 10,000-fold higher than the concentration of IgG in the affinity-purified anti-gM/gN and anti-gB antibodies (see below). The anti-gM/gN MAb 14-16A neutralized 55% and 29% of infection without added complement at 1:2 and 1:10 dilutions, respectively, and neutralized 100% and 99% of infection with complement at 1:2 and 1:10 dilutions, respectively (Table 1). Affinity-purified human antibodies against gM/gN neutralized 99%, 40%, and 0% without complement at dilutions of 1:2, 1:10, and 1:5, respectively, and neutralized 94%, 58%, and 0% with complement at 1:2, 1:10, and 1:50 dilutions, respectively (Table 1). The affinity-purified anti-gB antibodies neutralized 100% of virus at a dilution of 1:2, 96% of input virus at a dilution of 1:10, and 45% of input virus at a dilution of 1:50 (Table 1). The neutralizing activity of the affinity-purified gB antibodies was not enhanced following the addition of exogenous complement (Table 1).
TABLE 1.
Affinity-purified human anti-gM/gN antibodies neutralize infectious HCMV AD169
| Antibody | % Reduction in infectivity at an antibody dilution ofa:
|
||
|---|---|---|---|
| 1:2 | 1:10 | 1:50 | |
| Anti-gBb | 100 (100) | 96 (94) | 45 (39) |
| Anti-gM/gNb | 99 (94) | 40 (58) | 0 (0) |
| Anti-gN MAbc (14-16a) | 55 (100) | 29 (99) | NT |
| Human IgGc | NT | 100 (100) | 100 (100) |
Infectivity reduction of HCMV AD169 in a microtiter neutralization assay (1). The percentages of reduction in input infectivity are shown as the percentages of reduction in the absence (values not shown in parentheses) or presence (values in parentheses) of 5% guinea pig serum as a source of complement. Dilutions were made in Dulbecco's modified Eagle medium containing 10% fetal calf serum. NT, not tested.
Affinity-purified anti-gB and anti-gM/gN human antibodies were prepared as described in Materials and Methods at concentrations of approximately 1 μg/ml (anti-gB) and 0.5 μg/ml (anti-gM/gN).
The anti-gN mouse MAb 14-16A and the pooled human IgG containing anti-HCMV antibodies were used as positive controls in these neutralization assays (5).
The reported sequence variation between the UL73 regions from different HCMV strains raised the possibility that virus-neutralizing antibodies directed against the gM/gN complex could be strain specific (16, 27). To determine whether the reported sequence variability in gN could influence the neutralizing capacity of anti-gM/gN antibodies prepared from a pooled immunoglobulin preparation, we compared the neutralizing activity of similarly prepared affinity-purified anti-gM/gN antibodies against the laboratory strain of HCMV AD169 and clinical isolates TR and Toledo (29). Affinity-purified anti-gM/gN antibodies were prepared by methodology similar to that described above and were shown to be specific for gM/gN as determined by reactivity in an immunofluorescence assay as described in the legend to Fig. 2. Using these anti-gM/gN antibodies, we could demonstrate neutralizing activity against the Toledo strain of HCMV, a clinical isolate with significant amino acid sequence divergence in gN compared to AD169 (Table 2). MAb 14-16A also had significant neutralizing activity against the Toledo strain of HCMV (Table 2). We then tested several different preparations of affinity-purified human anti-gM/gN antibodies for neutralizing activity against a recently derived clinical isolate, TR. In this experiment, we determined neutralizing activity only in the presence of complement and noted that at least one preparation efficiently neutralized the TR virus (Table 2). The results of these experiments suggested that anti-gM/gN antibodies have broad neutralizing activity, although it must be stressed that these do not represent quantitative comparisons, and assays using equivalent amounts of IgG would be required to detect differences in neutralizing capacity against different viruses.
TABLE 2.
Affinity-purified human anti-gM/gN antibodies neutralize unrelated HCMV strains
| Virusa | Antibody | % Reduction in input infectivity at the following dilutionb:
|
|||
|---|---|---|---|---|---|
| With complement | Without complement | ||||
| Expt 1 | 1:20 | 1:40 | 1:20 | 1:40 | |
| AD169 | Anti-gM/gN | 59 | 68 | 73 | 70 |
| Toledo | Anti-gM/gN | NT | 50 | NT | 50 |
| AD169 | Anti-gM/gN MAbc | NT | 96 | NT | NT |
| Toledo | Anti-gM/gN MAb | NT | 96 | NT | NT |
| Expt 2 | 1:18 | 1:54 | 1:18 | 1:54 | |
| TR | Anti-gM/gNad | 46 | 29 | NT | NT |
| Anti-gM/gNb | 45 | 50 | NT | NT | |
| Anti-gM/gNc | 64 | 64 | NT | NT | |
| Human IgG | 98 | 84 | NT | NT | |
| Anti-gN MAb (14-16a) | 77 | 69 | NT | NT | |
Strains of HCMV used in this assay included AD169 (gN genotype 1), Toledo (gN genotype 4a), and TR (gN genotype 3a) (27).
Virus neutralization expressed as percent reduction in input infectivity as quantified in a microneutralization assay (1). Dilutions of antibodies are indicated, and neutralizing activity was determined in the presence of 5% guinea pig serum as the source of complement or in the absence of complement. NT, not tested.
The neutralizing activity of monoclonal antibody 14-16 (anti-gN) was carried out in the presence of complement.
Three different preparations of affinity-purified anti-gM/gN antibodies (indicated by subscript letters a, b, and c) were tested in experiment 2, and each differed from the preparation tested in experiment 1. Each preparation was characterized for specificity by immunofluorescence assays using cells expressing gB, pp150, and gM/gN. The concentration of IgG in these preparations was not determined. Note that in each experiment, antibodies were prepared from the same starting pool of human antibodies using the protocol described in Materials and Methods. The human anti-CMV immune IgG (human IgG) is the starting material used for preparation of affinity-purified antibodies, and MAb 14-16a is a murine MAb reactive with gN (5). Both were used as positive controls in these assays.
To determine the concentrations of anti-gB and anti-gM/gN antibodies required for neutralization of HCMV, we first estimated the concentration of IgG antibodies present in the affinity-purified preparations of anti-gM/gN and anti-gB antibodies using a dot blot assay in which the amount of IgG in the affinity-purified antibodies was determined by comparison with a standard curve generated with known concentrations of human IgG. After autoradiography, densitometry was used to determine the concentration of anti-gM/gN antibodies in the dilutions used for the neutralization assay. The concentration of human IgG in the affinity-purified anti-gM/gN antibodies was 0.5 μg/ml ± 10%, indicating that the dilutions used for the neutralization assays corresponded to human anti-gM/gN antibody concentrations of approximately 0.25 μg/ml, 0.05 μg/ml, and 0.01 μg/ml at 1:2, 1:10, and 1:50 dilutions, respectively. This result indicated that anti-gM/gN antibodies neutralized approximately 50% of input virus when used at a concentration of 0.05 μg/ml. The affinity-purified anti-gB antibody concentration was approximately 1.0 μg/ml ± 10%, indicating that at dilutions of 1:10 to 1:50 that neutralized 50% of input virus, this preparation contained between 0.10 and 0.02 μg/ml of anti-gB antibodies. These results indicated that human antibodies against gM/gN have potent neutralizing capacity in vitro against HCMV and have a similar specific neutralizing capacity as human anti-gB antibodies (5, 23).
DISCUSSION
Previous studies have shown that a murine MAb reactive with the gM/gN complex can efficiently neutralize HCMV (23). The findings in the current study have extended these results and demonstrated that human antibodies specific for the gM/gN complex neutralized HCMV. In this study we have also shown that anti-gM/gN antibodies that are produced following infection with HCMV neutralized both laboratory and clinical strains of HCMV. Our results also indicated that the neutralizing activity of anti-gM/gN antibodies was similar to the activity of antibodies directed against gB, a previously defined major target of the virus-neutralizing antibody response (18, 26, 31, 43). Although the absolute quantity of anti-gM/gN antibodies in human convalescent-phase sera cannot be estimated in the current study, our findings suggested that antibodies reactive with the gM/gN complex could represent a significant component of the virus-neutralizing antibody activity in serum following HCMV infection. Because it is believed that virus-neutralizing antibodies play an important role in resistance to HCMV infection and disease, our findings could have implications for the design and composition of protective vaccines. Whether the gM/gN complex is sufficiently immunogenic to induce levels of anti-gM/gN antibodies that can provide a protective virus-neutralizing antibody response following HCMV infection is unknown; however, readily detectable antibody responses against gM/gN can be found in healthy individuals following HCMV infections (23). Taken together, our results suggest that gM/gN can induce virus-specific antibodies with potent virus-neutralizing activity and that these antibodies could contribute to protective responses generated following HCMV infection.
A potential limitation in the interpretation of the findings from this study was that the affinity-purified gM/gN antibodies could have contained antibodies against other viral proteins and that these antibodies were enriched during the affinity purification of the anti-gM/gN antibodies. Thus, the neutralizing activity that we assigned to the gM/gN antibodies could have been secondary to antibodies against other viral proteins that contaminated our preparation of affinity-purified anti-gM/gN antibodies. Several findings from our analysis of affinity-purified gM/gN antibodies argue that these antibodies were specific for the gM/gN complex. These included the specificity of the reactivity of the anti-gM/gN antibodies for transiently expressed gM/gN and the lack of reactivity for other immunogenic HCMV-encoded proteins, such as gB, pp65, or pp150, in an immunofluorescence assay. In addition, when transiently expressed myc-tagged gM/gN, gB, pp65, or pp150 were initially immunoprecipitated and then transferred to nitrocellulose membranes, only the gM/gN complexes and not other immunogenic HCMV proteins were detected when the membrane was probed with the affinity-purified anti-gM/gN antibodies. Because gB, pp65, and pp150 have been reported to be the most immunogenic proteins encoded by HCMV, the lack of reactivity of the anti-gM/gN antibodies for these viral proteins in these two different assays indicated that these antibodies were specific for gM/gN and that this antibody preparation likely did not contain significant quantities of antibodies with reactivity against other less immunogenic viral proteins.
It was of particular interest that anti-gM/gN antibodies reacted in immunofluorescence assays with either gM expressed alone or with the gM/gN complex. No reactivity was detected for gN expressed in the absence of gM, a finding confirmed by Western blots of transiently expressed forms of gN and gM/gN (Fig. 5). These findings suggested that affinity-purified antibodies contained reactivity for determinants expressed on gM and the gM/gN complex. Previous studies have demonstrated that antibodies present in human convalescent-phase sera were reactive with gM, but as noted previously it was unclear whether these antibodies contributed to the virus-neutralizing activity detected in human convalescent-phase sera. More recently, DNA immunization of small animals with expression plasmids encoding either gM or gM complexed with gN, but not plasmids encoding gN alone, generated virus-neutralizing antibodies (S. Lu [University of Massachusetts, Worcester, Mass.], personal communication). Interestingly, in this study the quantity of virus-neutralizing antibodies was increased in animals immunized with plasmids encoding the gM/gN complex compared to those given gM alone. Consistent with the results of this study in mice, previous analysis using a panel of human sera and cells transfected with gM or gM plus gN expression plasmids demonstrated that 62% of human convalescent-phase sera were reactive with gM/gN but only 32% of the same sera were shown to be reactive with gM (23). In the current study of the reactivity of the affinity-purified anti-gM/gN antibodies, it appeared that the majority of the reactivity was directed at gM and the glycosylated gN present in the gM/gN complex on the basis of the lack of reactivity for gN when expressed in the absence of gM by immunofluorescence and immunoblotting assays, a finding that was consistent with findings from previous studies. Although it is unclear why gN (UL73) is poorly immunogenic, it should be noted that nearly 80% of the mass of the mature form of gN is carbohydrate and that in the absence of gM, gN is not glycosylated but remains aggregated in the endoplasmic reticulum. This latter finding indicates that in the absence of gM, gN cannot traffic to the cell surface and/or cytoplasmic compartments other than the endoplasmic reticulum, and its presentation to the immune system in a native conformation is likely limited. A second possibility is that gM/gN complex formation could result in the expression of conformational determinants on gN and/or gM resulting from the covalent and noncovalent interactions between gM and gN. These could include interactions secondary to the extensive terminal carbohydrate modifications on gN. Thus, it is possible that either gM or gN could acquire conformational determinants as a result of their interactions and that these determinants represent the antibody binding sites on the gM/gN complex. Consistent with this explanation is the finding that the virus-neutralizing MAb, 14-16A, is reactive only with the glycosylated form of gN complexed with gM (23). In addition, affinity-purified human anti-gM/gN antibodies likely react with several modified forms of gN, including the mature fully glycosylated form, on the basis of the broadly migrating species detected by immunoblotting, but these antibodies do not react with forms of gN when this protein is expressed in the absence of gM (Fig. 5). Regardless of the explanation for the lack of antibodies directed specifically against gN (or more accurately, the nonglycosylated product of the UL73 ORF), our results suggest that virus-neutralizing antibody binding sites are expressed on the glycosylated gN and the gM/gN complex and that antibodies reactive with these sites can neutralize virus infectivity.
The source of antibodies used for affinity isolation of gM/gN antibodies was a preparation of IgG antibodies pooled from CMV-immune donors with demonstrated reactivity for HCMV-infected cell antigens in an enzyme immunoassay (39). The donor population from which this pooled preparation of IgG antibodies was derived was almost certainly infected with many different strains of HCMV, including HCMV with the different gN genotypes that have been previously described (11). Thus, it is not surprising that the starting material for our preparations of gM/gN-specific affinity-purified antibodies neutralized both the laboratory strain AD169 and unrelated clinical isolates, Toledo and TR. Although the gM proteins of Toledo and AD169 are over 99% identical, the gN protein of Toledo (genotype 4a) shares only 76% identity with AD169 gN (genotype 1), and all changes in the amino acid sequence are in the ectodomain of gN, a type I glycoprotein (11). Similarly, TR gN (genotype 3a) is about 83% identical with AD169 gN, with all variations occurring in the ectodomain (data not shown). Thus, it was somewhat surprising that the affinity-purified anti-gM/gN antibodies derived from this immunoglobulin preparation by absorption on gM/gN from AD169 (gN genotype 1) would neutralize an unrelated strain. However, our results can be explained by several different possibilities. First, it is possible that the dominant binding sites on gN for virus-neutralizing antibodies could be common to all gN genotypes. This explanation is consistent with the virus-neutralizing activity of MAb 14-16A against multiple viral strains (23). Another possible explanation is that virus-neutralizing activity of the affinity-isolated anti-gM/gN antibodies is directed at determinants on gM, and because of the sequence conservation of this protein, antibodies affinity purified on AD169 could be expected to recognize the gM/gN complex encoded by Toledo and likely by TR. Thus, the isolation of anti-gM/gN antibodies using strain AD169 could be expected to yield both antibodies reactive specifically with AD169 and antibodies reactive with the gM/gN complexes from other HCMV genotypes secondary to sequences conserved in gM. Last, it must be emphasized that this question can be definitively addressed by generation of AD169 isogenic viruses that contain genes encoding gN proteins from different genotypes and utilizing these viruses in comparative studies of neutralizing antibody activity, a project currently under way. Although at this time the biological relevance of a potential HCMV gN genotype-specific antibody response and a broader activity of virus-neutralizing gM/gN antibodies is unclear, it could be of major significance if gM/gN antibody responses are shown to be protective in vivo and if this glycoprotein complex is selected for inclusion in vaccines to limit HCMV disease. To resolve these possible explanations, it will be necessary to experimentally define the antibody binding sites on the gM/gN complex, a task that has thus far been limited by the lack of available MAbs reactive with this complex of glycoproteins.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (M.M., grant MA929/6-1) and the HHS, NIH (W.J.B., grants AI49537 and AI150189).
REFERENCES
- 1.Andreoni, M., M. Faircloth, L. Vugler, and W. J. Britt. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23:157-167. [DOI] [PubMed] [Google Scholar]
- 2.Arvin, A. M., P. Fast, M. Myers, S. Plotkin, and R. Rabinovich. 2004. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 39:233-239. [DOI] [PubMed] [Google Scholar]
- 3.Baines, J. D., and B. Roizman. 1993. The UL10 gene of herpes simplex virus 1 encodes a novel viral glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J. Virol. 67:1441-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britt, W. J. 1984. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 135:369-378. [DOI] [PubMed] [Google Scholar]
- 5.Britt, W. J., and D. Auger. 1985. Identification of a 65,000 dalton virion envelope of human cytomegalovirus. Virus Res. 4:31-36. [DOI] [PubMed] [Google Scholar]
- 6.Britt, W. J., and L. Vugler. 1987. Structural and immunological characterization of the intracellular forms of an abundant 68,000 Mr human cytomegalovirus protein. J. Gen. Virol. 68:1897-1907. [DOI] [PubMed] [Google Scholar]
- 7.Cai, J. S., H. K. Jang, Y. Izumiya, Y. Tsushima, K. Kato, A. M. Damiani, T. Miyazawa, C. Kai, E. Takahashi, and T. Mikami. 1999. Identification and structure of the Marek's disease virus serotype 2 glycoprotein M gene: comparison with glycoprotein M genes of Herpesviridae family. J. Vet. Med. Sci. 61:503-511. [DOI] [PubMed] [Google Scholar]
- 8.Chee, M., S. Rudolf, B. Plachter, B. Barrell, and G. Jahn. 1989. Identification of the major capsid protein gene of human cytomegalovirus. J. Virol. 63:1345-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crumpacker, C. S., and S. Wadhwa. 2005. Cytomegalovirus, p. 1786-1800. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, vol. 2. Elsevier, Philadelphia, Pa. [Google Scholar]
- 11.Dal Monte, P., S. Pignatelli, M. Mach, and M. P. Landini. 2001. The product of human cytomegalovirus UL73 is a new polymorphic structural glycoprotein (gpUL73). J. Hum. Virol. 4:26-34. [PubMed] [Google Scholar]
- 12.Dijkstra, J. M., V. Gerdts, B. G. Klupp, and T. C. Mettenleiter. 1997. Deletion of glycoprotein gM of pseudorabies virus results in attenuation for the natural host. J. Gen. Virol. 78:2147-2151. [DOI] [PubMed] [Google Scholar]
- 13.Furebring, C., A. Speckner, M. Mach, I. Sandlie, L. Norderhaug, C. A. Borrebaeck, H. Turesson, and M. Ohlin. 2002. Antibody-mediated neutralization of cytomegalovirus: modulation of efficacy induced through the IgG constant region. Mol. Immunol. 38:833-840. (Erratum, 39:121.) [DOI] [PubMed] [Google Scholar]
- 14.Jons, A., J. M. Dijkstra, and T. C. Mettenleiter. 1998. Glycoproteins M and N of pseudorabies virus form a disulfide-linked complex. J. Virol. 72:550-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kari, B., and R. Gehrz. 1993. Structure, composition and heparin binding properties of a human cytomegalovirus glycoprotein complex designated gC-II. J. Gen. Virol. 74:255-264. [DOI] [PubMed] [Google Scholar]
- 16.Klein, M., K. Schoppel, N. Amvrossiadis, and M. Mach. 1999. Strain-specific neutralization of human cytomegalovirus isolates by human sera. J. Virol. 73:878-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klupp, B. G., R. Nixdorf, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein M inhibits membrane fusion. J. Virol. 74:6760-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kniess, N., M. Mach, J. Fay, and W. J. Britt. 1991. Distribution of linear antigenic sites on glycoprotein gp55 of human cytomegalovirus. J. Virol. 65:138-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koyano, S., E. C. Mar, F. R. Stamey, and N. Inoue. 2003. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J. Gen. Virol. 84:1485-1491. [DOI] [PubMed] [Google Scholar]
- 20.Lake, C. M., S. J. Molesworth, and L. M. Hutt-Fletcher. 1998. The Epstein-Barr virus (EBV) gN homolog BLRF1 encodes a 15-kilodalton glycoprotein that cannot be authentically processed unless it is coexpressed with the EBV gM homolog BBRF3. J. Virol. 72:5559-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehner, R., H. Meyer, and M. Mach. 1989. Identification and characterization of a human cytomegalovirus gene coding for a membrane protein that is conserved among human herpesviruses. J. Virol. 63:3792-3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehner, R., T. Stamminger, and M. Mach. 1991. Comparative sequence analysis of human cytomegalovirus strains. J. Clin. Microbiol. 29:2494-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mach, M., B. Kropff, P. Dal Monte, and W. Britt. 2000. Complex formation by human cytomegalovirus glycoproteins M (gpUL100) and N (gpUL73). J. Virol. 74:11881-11892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mach, M., B. Kropff, M. Kryzaniak, and W. Britt. 2005. Complex formation by glycoproteins M and N of human cytomegalovirus: structural and functional aspects. J. Virol. 79:2160-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLean, C., L. Robertson, and F. Jamieson. 1993. Characterization of the UL10 gene product of herpes simplex virus type 1 and investigation of its role in vivo. J. Gen. Virol. 74:975-983. [DOI] [PubMed] [Google Scholar]
- 26.Marshall, G. S., G. P. Rabalais, G. G. Stout, and S. L. Waldeyer. 1992. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J. Infect. Dis. 165:381-384. [DOI] [PubMed] [Google Scholar]
- 27.Pignatelli, S., P. Dal Monte, G. Rossini, S. Chou, T. Gojobori, K. Hanada, J. J. Guo, W. Rawlinson, W. Britt, M. Mach, and M. P. Landini. 2003. Human cytomegalovirus glycoprotein N (gpUL73-gN) genomic variants: identification of a novel subgroup, geographical distribution and evidence of positive selective pressure. J. Gen. Virol. 84:647-655. [DOI] [PubMed] [Google Scholar]
- 28.Plachter, B., W. Britt, R. Vornhagen, T. Stamminger, and G. Jahn. 1993. Analysis of proteins encoded by IEI regions 1 and 2 of human cytomegalovirus using monoclonal antibodies generated against recombinant antigens. Virology 193:642-652. [DOI] [PubMed] [Google Scholar]
- 29.Quinnan, G. V., H. Masur, A. H. Rook, G. Armstrong, W. R. Frederick, J. Epstein, J. F. Manischewitz, A. M. Macher, L. Jackson, J. Ames, H. A. Smith, M. Parker, G. R. Pearson, J. Parrillo, C. Mitchell, and S. E. Straus. 1984. Herpes infections in the acquired immune deficiency syndrome. JAMA 252:72-77. [PubMed] [Google Scholar]
- 30.Rasmussen, L. 1990. Immune response to human cytomegalovirus infection. Curr. Top. Microbiol. Immunol. 154:222-254. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen, L., C. Matkin, R. Spaete, C. Pachl, and T. C. Merigan. 1991. Antibody response to human cytomegalovirus glycoproteins gB and gH after natural infection in humans. J. Infect. Dis. 164:835-842. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen, L., M. Nelson, M. Neff, and T. Merigan. 1988. Characterization of different human cytomegalovirus glycoproteins which are targets for virus neutralizing antibody. Virology 163:308-318. [DOI] [PubMed] [Google Scholar]
- 33.Reis, B., E. Bogner, M. Reschke, A. Richter, T. Mockenhaupt, and K. Radsak. 1993. Stable constitutive expression of glycoprotein B (gpUL55) of human cytomegalovirus in permissive astrocytoma cells. J. Gen. Virol. 74:1371-1379. [DOI] [PubMed] [Google Scholar]
- 34.Revello, M. G., and G. Gerna. 2002. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev. 15:680-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross, J., M. Williams, and J. I. Cohen. 1997. Disruption of the varicella-zoster virus dUTPase and the adjacent ORF9A gene results in impaired growth and reduced syncytia formation in vitro. Virology 234:186-195. [DOI] [PubMed] [Google Scholar]
- 36.Rudolph, J., C. Seyboldt, H. Granzow, and N. Osterrieder. 2002. The gene 10 (UL49.5) product of equine herpesvirus 1 is necessary and sufficient for functional processing of glycoprotein M. J. Virol. 76:2952-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez, V., P. C. Angeletti, J. A. Engler, and W. J. Britt. 1998. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J. Virol. 72:3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snydman, D. R. 1993. Review of the efficacy of cytomegalovirus immune globulin in the prophylaxis of CMV disease in renal transplant recipients. Transplant. Proc. 25:25-26. [PubMed] [Google Scholar]
- 40.Spaderna, S., H. Blessing, E. Bogner, W. Britt, and M. Mach. 2002. Identification of glycoprotein gpTRL10 as a structural component of human cytomegalovirus. J. Virol. 76:1450-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speckner, A., D. Glykofrydes, M. Ohlin, and M. Mach. 1999. Antigenic domain 1 of human cytomegalovirus glycoprotein B induces a multitude of different antibodies which, when combined, results in incomplete virus neutralization. J. Gen. Virol. 80:2183-2191. [DOI] [PubMed] [Google Scholar]
- 42.Urban, M., M. Klein, W. J. Britt, E. Hassfurther, and M. Mach. 1996. Glycoprotein H of human cytomegalovirus is a major antigen for the neutralizing humoral immune response. J. Gen. Virol. 77:1537-1547. [DOI] [PubMed] [Google Scholar]
- 43.Utz, U., W. Britt, L. Vugler, and M. Mach. 1989. Identification of a neutralizing epitope on glycoprotein gp58 of human cytomegalovirus. J. Virol. 63:1995-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu, S. X., X. P. Zhu, and G. J. Letchworth. 1998. Bovine herpesvirus 1 glycoprotein M forms a disulfide-linked heterodimer with the UL49.5 protein. J. Virol. 72:3029-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]