Abstract
We have previously shown that hepatitis C virus (HCV) core protein modulates multiple cellular processes, including those that inhibit tumor necrosis factor alpha (TNF-α)-mediated apoptosis. In this study, we have investigated the signaling mechanism for inhibition of TNF-α-mediated apoptosis in human hepatoma (HepG2) cells expressing core protein alone or in context with other HCV proteins. Activation of caspase-3 and the cleavage of DNA repair enzyme poly(ADP-ribose) polymerase were inhibited upon TNF-α exposure in HCV core protein-expressing HepG2 cells. In vivo protein-protein interaction studies displayed an association between TNF receptor 1 (TNFR1) and TNFR1-associated death domain protein (TRADD), suggesting that the core protein does not perturb this interaction. A coimmunoprecipitation assay also suggested that HCV core protein does not interfere with the TRADD-Fas-associated death domain protein (FADD)-procaspase-8 interaction. Further studies indicated that HCV core protein expression inhibits caspase-8 activation by sustaining the expression of cellular FLICE (FADD-like interleukin-1β-converting enzyme)-like inhibitory protein (c-FLIP). Similar observations were also noted upon expression of core protein in context to other HCV proteins expressed from HCV full-length plasmid DNA or a replicon. A decrease in endogenous c-FLIP by specific small interfering RNA induced TNF-α-mediated apoptotic cell death and caspase-8 activation. Taken together, our results suggested that the TNF-α-induced apoptotic pathway is inhibited by a sustained c-FLIP expression associated with the expression of HCV core protein, which may play a role in HCV-mediated pathogenesis.
Apoptosis is a key element in a host organism's defense against viral infections, inhibiting viral spread and persistence (27, 33). To circumvent host defense, viruses have evolved mechanisms that antagonize host cell death signals so that virus propagation can continue unabated in infected cells. A block of apoptosis could be critical in the establishment of lifelong persistence in its human host. Alterations in cell survival contribute to the pathogenesis of a number of human diseases, including viral oncogenesis (30). Hepatitis C virus (HCV) core protein exhibits a gene regulatory role and the potential to suppress the onset of apoptotic cell death (20, 22, 23). We and others have shown that HCV core protein suppresses apoptosis mediated by tumor necrosis factor alpha (TNF-α) (13, 21). Since HCV core protein is the first viral protein to be expressed after infection, presumably the virus has adapted this protein to antagonize apoptosis and possibly other important biological events. Therefore, further investigation is necessary to delineate the mechanism of core protein-mediated apoptotic inhibition.
HCV core protein specifically interacts with the cytoplasmic tail of the lymphotoxin-β receptor, a member of the TNF family, and TNF receptor 1 (TNFR1) (14, 36). Since lymphotoxin-β receptor is involved in apoptotic signaling, this strongly suggests that core protein may have an immunomodulatory function and may play a critical role in the establishment of HCV persistence and in disease pathogenesis (1). HCV core protein suppresses host immune responses, in particular, the generation of virus-specific cytotoxic T lymphocytes (11). A direct binding of HCV core to gC1qR on T cells leads to impaired Lck/Akt activation and T-cell function (34). Since TNF-mediated DNA fragmentation is suppressed in core-induced Hep191 cells, these findings suggest that expression of HCV core at physiological levels upregulates inhibitor of caspase-activated DNase and consequently inhibits apoptotic cell death (25). Elevated levels of TNF-α in serum have been found in patients with hepatitis C (31, 32). Furthermore, it has been shown that liver-infiltrating cytotoxic T lymphocytes and, to a lesser extent, hepatocytes produce TNF-α during HCV infections (3, 8, 12). Activation of TNF-α has a pivotal role in the inflammatory process of chronic hepatitis C, and TNF-α levels correlate with the degree of inflammation. TNF-α is also suggested to be a possible link between HCV infection and diabetes (7).
TNF-α signals through two distinct receptors belonging to the TNF receptor superfamily. Many of the best-characterized signaling pathways of TNF, such as the induction of apoptosis and activation of the transcription factor NF-κB, are initiated by TNFR1. However, TNFR2 also appears to play a direct role in a limited number of TNF responses (17, 28). The intracellular portion of TNFR1 contains a death domain, which is required for apoptosis signaling and NF-κB activation. The silencer of death domain (SODD) is a negative regulatory protein that is normally associated with the death domain of TNFR1 (5). SODD inhibits the intrinsic self-aggregation properties of the death domain to maintain TNFR1 in an inactive, monomeric state. This inhibition is relieved by TNF-mediated receptor aggregation, which triggers the rapid release of SODD from the death domain of TNFR1. The uncomplexed death domains of TNFR1 are then able to bind the adapter protein TNFR1-associated death domain protein (TRADD), which in turn recruits TNF receptor-associated factor 2, receptor-interacting protein, and Fas-associated death domain protein (FADD) to form an active TNFR1 signaling complex. The signaling complex activates signaling cascades leading to apoptosis, Jun N-terminal protein kinase/stress-activated protein kinase activation, and NF-kB activation, respectively (5). Consideration of apoptosis-associated proteins within the context of their interactions with other molecules in a particular signal pathway is important. Many of these pathways require or are regulated by specific protein-protein interactions beginning at the level of the receptor-signaling complex, as is the case with TNFR1, and continuing throughout the process to the final stages of apoptosis. If HCV core protein can directly interact with the cellular signaling domains of one or more members of the TNF receptor family in infected cells to inhibit the function of these receptors, then this inhibitory effect may account for the suppression of an early innate response to virus infection. In this study, we have investigated the signaling molecules responsible for HCV core protein-mediated inhibition of TNF-α-induced apoptosis in HepG2 cells.
MATERIALS AND METHODS
Cells and transfections.
HepG2 cells were transfected with pBabe core or pCI-neo-HCV full-length (FL) plasmid DNA using Lipofectamine (Life Technologies, Inc.), and stable cell colonies were selected using puromycin or neomycin as previously described (22). Pooled cells transfected with HCV genomic region were used to avoid artifactual results from clonal variation. Parental HepG2 cells were used in parallel as a positive control. HepG2 cells and their transfected derivatives were maintained in Dulbecco's modified Eagle medium containing 10% fetal calf serum and a lower dose (1 μg of puromycin/ml or 400 μg of G418/ml) of the selection antibiotic. For maintaining expression of full-length and subgenomic HCV replicons in Huh-7 cells, antibiotic was added to cell culture medium. Cells harboring replicons displayed expression of both core and/or NS5a proteins, based on the replicon used, by indirect immunofluorescence.
TNF-α-mediated apoptosis.
TNF-α (Calbiochem) was used in a dose-dependent manner to determine its apoptotic effect on HepG2 cells. Approximately 5 × 104 cells were exposed to TNF-α for 24 to 72 h before being harvested. The apoptotic sensitivity of cells stably transfected with HCV core or full-length cDNA to TNF-α-mediated apoptosis was determined by enzyme-linked immunosorbent assay (ELISA) (Roche) following the manufacturer's protocol. Huh-7 cells expressing HCV replicon were treated with actinomycin D (50 ng/ml) prior to addition of TNF-α for apoptosis, followed by a similar experimental procedure.
siRNA.
A 21-nucleotide small interfering RNA (siRNA) duplex targeted to cellular FADD-like interleukin-1β-converting enzyme (c-FLIP) (Santa Cruz Biotechnology, CA) and random sequence control siRNA were purchased. The siRNA was introduced into cells by transfection using Lipofectamine 2000 (Invitrogen). Transfected cells were treated with 20 ng of TNF-α/ml to determine the role of c-FLIP in mediating apoptosis. After 48 h of transfection, cells were treated with TNF-α (20 ng/ml) for 2 h and lysed for detection of caspase-8 activation by Western blot analysis with a specific antibody.
In vivo coimmunoprecipitation assay.
HepG2 cells were infected with recombinant vaccinia virus expressing T7 polymerase (vvT7) and transfected with HCV core plasmid DNA using Lipofectamine 2000 (Invitrogen). To understand the potential role for core protein to disrupt the interaction between TNFR1-TRADD, HepG2 cells were transfected with a TNFR1 construct containing an N-terminal FLAG-tagged region (FLAG-TNFR1) and a C-terminal hemagglutinin (HA)-TRADD construct (TRADD-HA). Empty-vector DNA was used in the place of core plasmid DNA as a control. HepG2 cells were also transfected with TRADD-HA and AU1-tagged FADD (FADD-AU1) in the presence or absence of a FLAG-tagged core construct (FLAG-core) to determine if core protein interrupts TRADD-FADD-procaspase-8 interaction. Cells were lysed after 24 h of transfection with TNTG buffer (30 mM Tris [pH 8.0], 150 mM NaCl, 1% Triton X-100, 10% glycerol) supplemented with a cocktail of protease inhibitors. After sonication, cell debris was removed by centrifugation, and anti-FLAG (for TNFR1-TRADD interaction) or anti-AU1 antibody (for TRADD-FADD-procaspase-8 interaction) and protein G-Sepharose agarose beads were added to the cell lysates and mixed for 14 h. The beads were washed with TNTG buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis for Western blotting.
Western blot analysis.
Cells were washed with phosphate-buffered saline and lysed in TNTG buffer. After freeze-thaw, cell debris was removed by centrifugation and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred onto a nitrocellulose membrane, incubated with specific antibodies, and detected by chemiluminescence (Amersham). Cellular actin or β-tubulin was detected similarly in the reprobed blot for relative quantitation of proteins in each lane.
Antibodies.
Anti-caspase-3 and anti-HA antibodies (Santa Cruz Biochemical, CA), anti-AU1 antibody (Covance, CA), anti-FLAG and anti-β-tubulin antibodies (Sigma, St. Louis, MO), anti-poly(ADP-ribose) polymerase (anti-PARP) and c-FLIP antibodies (ALEXIS), anti-caspase-8 (BD Pharmingen), and anti-human actin immunoglobulin M and anti-immunoglobulin M-horseradish peroxidase (Oncogene Science) were procured.
RESULTS
HCV core protein inhibits TNF-α-induced apoptosis and impairs downstream signaling pathway in HepG2 cells.
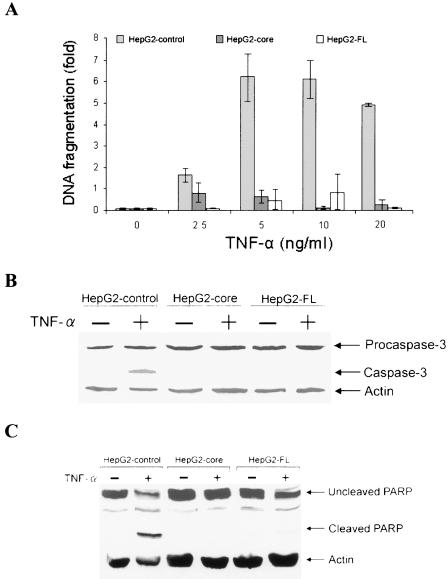
HepG2 cells stably transfected with core (HepG2-core) or full-length plasmid DNA (HepG2-FL) were used to determine TNF-α-mediated apoptotic cell death. Empty vector-transfected HepG2 cells were used as a control for comparison. An ∼6-fold increase in DNA fragmentation was observed at >5 ng of TNF-α-treated HepG2 control cells/ml than was apparent with HepG2-core- or HepG2-FL-expressing cells by cell death ELISA (Fig. 1A). Thus, the results from cell death ELISA suggested that TNF-α-induced apoptosis is inhibited by core protein alone or in context with other HCV proteins in HepG2 cells. To determine whether core protein expression interferes with the TNF-α-induced apoptotic signaling pathway, cell lysates were analyzed for caspase-3 activation by Western blotting with a specific antibody. Active caspase-3 consists of ∼17-kDa and ∼12-kDa subunits, which are derived from ∼32-kDa proenzyme. The antibody to caspase-3 recognizes ∼32-kDa procaspase-3 and the ∼17-kDa subunit protein. Following TNF-α treatment, activation of caspase-3 was observed with HepG2 control cells but not with HepG2-core or HepG2-FL cells (Fig. 1B). Densitometric scanning of the Western blot suggested ∼42% caspase-3 cleavage in TNF-α-treated control cells compared to that of HepG2-core or HepG2-FL. Analysis of the integrity of death substrate PARP in control HepG2 cells also suggested the cleavage of the native ∼116-kDa PARP to the signature ∼85-kDa proteolytic fragment after 48 h of TNF-α treatment (Fig. 1C). However, HepG2 cells transfected with HCV core or FL cDNA did not exhibit PARP cleavage. Densitometric scanning of Western blot suggested ∼36% PARP cleavage in TNF-α-treated control cells compared to that of HepG2-core or HepG2-FL. These results suggested that activation of caspase-3 and the cleavage of DNA repair enzyme PARP were inhibited upon TNF-α exposure in the presence of HCV core protein.
FIG. 1.
HCV core protein inhibits TNF-α-mediated apoptosis in HepG2 cells. (A) Comparison of the fold differences of DNA fragmentation as an index of apoptotic cell death in TNF-α-treated empty vector-transfected HepG2 cells (control) and cells stably transfected with core or FL cDNA of HCV. Cells (5 × 104) were treated with indicated concentrations of TNF-α for 48 h, and cellular DNA fragmentation was measured by a cell death ELISA (Roche). A similar level of DNA fragmentation was observed between 5 to 20 ng of TNF-α/ml. (B) Western blot analysis for the expression status of caspase-3 in control HepG2 cells treated with TNF-α (20 ng/ml) and cells stably transfected with core or FL cDNA for 48 h. The ∼17-kDa band (right) indicates the active form of caspase 3. The level of cellular actin was used as an internal control for comparison of protein load. (C) Similar Western blot analysis for PARP cleavage, as described in the legend to panel B. An ∼85-kDa signature peptide (right) indicates the cleavage product from PARP. The molecular weights of the protein bands were ascertained from the migration of standard protein molecular weight markers (Invitrogen).
HCV core protein does not interfere with TNFR1-TRADD or TRADD-FADD-procaspase-8 interaction.
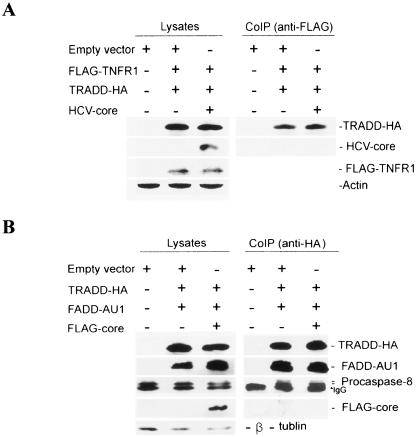
A coimmunoprecipitation assay was performed to determine whether there is an interruption in apoptotic pathway downstream of the TNFR1 for association with HCV core protein. HepG2 cells were infected with vvT7 and cotransfected with empty vector or FLAG-TNF-R1, TRADD-HA, and core expression plasmid under the control of a T7 promoter. After 24 h, cell lysates were immunoprecipitated with a mouse monoclonal antibody to FLAG for TNFR1 and immunoblotted with a rabbit antiserum to HCV core protein to determine whether core protein interferes with TNFR1-TRADD interaction. HCV core protein was not detected by immunoprecipitation of FLAG-TNFR1 and TRADD-HA (Fig. 2A). On the other hand, TNFR1 coprecipitated with TRADD, suggesting that core protein does not interfere with the TNFR1-TRADD interaction. The level of exogenous HCV core, FLAG-TNFR1, and TRADD-HA expression in HepG2 cell lysates was shown by Western blot analysis (Fig. 2A). Taken together, these data suggested that HCV core protein does not interact with TNFR1 or TRADD. We also examined whether HCV core protein interferes with signaling pathways downstream of TNFR1-TRADD interaction, such as TRADD-FADD-procaspase-8 association. For this, HepG2 cells were transfected with HA-tagged TRADD and AU1 epitope-tagged FADD or with the FLAG-HCV core. Core protein was not detected from in vivo coimmunoprecipitation of TRADD-HA, FADD-AU1, and procaspase-8. The levels of exogenously expressed TRADD-HA, FADD-AU1, and FLAG-core and endogenous expression of procaspase-8 in HepG2 cell lysates are shown (Fig. 2B). These results suggested that HCV core protein expression does not perturb TRADD-FADD-procaspase-8 interaction for the TNF-α-mediated apoptotic signaling pathway.
FIG. 2.
HCV core protein does not interfere with TNFR1-TRADD or TRADD-FADD-procaspase-8 interaction. (A) In vivo coimmunoprecipitation of TNFR1 and TRADD in the presence of HCV core protein. HepG2 cells were infected with vvT7 and cotransfected with empty vector or with FLAG-TNFR1, TRADD-HA, and core expression plasmids under the control of a T7 promoter. After 24 h of transfection, cell lysates were immunoprecipitated with a mouse monoclonal antibody to FLAG for TNFR1 and immunoblotted with an HA-specific antibody for TRADD, FLAG for TNFR1, and a rabbit antiserum for the core protein. The expression of HCV core protein and FLAG-TNFR1 was verified by immunoblotting cell lysates. The level of cellular actin was used as an internal control for comparison. (B) In vivo coimmunoprecipitation of TRADD-FADD-procaspase-8 in the presence of HCV core protein. HepG2 cells were infected with vvT7 and cotransfected with TRADD-HA, FADD-AU1, and FLAG-core expression plasmids. Anti-HA antibody and protein G-Sepharose beads were added to cell lysates for immunoprecipitation of the TRADD-FADD-procaspase-8 complex. The immunoprecipitate was subjected to Western blot analysis for detection of TRADD-HA, FADD-AU1, procaspase-8, and FLAG-core with specific antibodies. The presence of TRADD-HA, FADD-AU1, FLAG-core proteins, and endogenous procaspase-8 in cell lysates was verified by immunoblotting. The level of cellular tubulin was used as an internal control for comparison. The molecular weight of the protein bands were ascertained from the migration of standard protein molecular weight markers (Bio-Rad).
HCV core protein inhibits caspase-8 activation.
The binding of TNF-α to TNFR1 results in activation of the caspase cascade. Caspase-8 is located upstream of the caspase cascade and is activated by TNF-α. Therefore, to determine whether core protein expression affects this protease cascade, we examined the activation of caspase-8 in HepG2-core, HepG2-FL, and empty vector-transfected HepG2 control cells following TNF-α treatment. Procaspase-8 cleavage was inhibited in HepG2-core and HepG2-FL-transfected cells between 0 to 24 h of TNF-α treatment, while procaspase-8 was cleaved in HepG2 control cells after 12 h of TNF-α treatment (Fig. 3). Densitometric scanning of the Western blot suggested ∼36% caspase-8 cleavage in TNF-α-treated control cells compared to that of HepG2-core or HepG2-FL. These results indicated that HCV core protein plays an inhibitory role in caspase-8 activation in TNF-α-induced HepG2 cells and that the suppression is likely to occur above or at the upstream of caspase-8 signaling pathway.
FIG. 3.
HCV core protein inhibits activation of procaspase-8 in TNF-α-treated cells. HepG2 cells stably transfected with HCV core or FL plasmid DNA were treated with TNF-α (20 ng/ml) for 0, 12, or 24 h. Empty vector-transfected HepG2 cells were similarly treated as a control. Cell lysates were analyzed for detection of caspase-8 by Western blotting. The level of cellular actin was used as an internal control for comparison. The molecular weights of the protein bands were ascertained from the migration of standard protein molecular weight markers (Bio-Rad).
c-FLIP expression is sustained in TNF-α-treated HCV core-expressing hepatocytes.
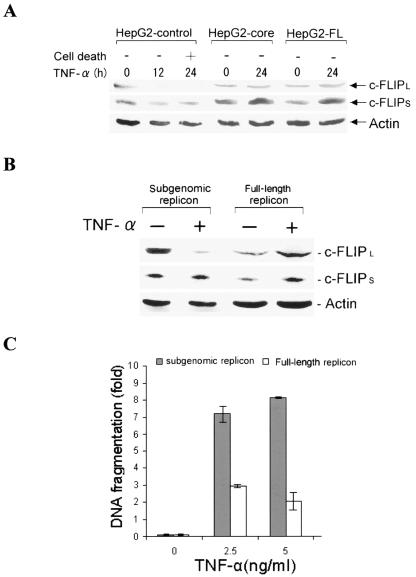
Inhibition of caspase-8 cleavage by HCV core protein prompted us to examine whether a cellular antiapoptotic inhibitor relates to inhibition of caspase-8 cleavage by HCV core protein. c-FLIP is a cellular inhibitor of caspase-8 cleavage, and it blocks caspase-8 activation by interfering with the activation of procaspase-8. To investigate whether there is a difference in c-FLIP expression level in TNF-α-treated HepG2-core, HepG2-FL, and HepG2 control cells, we performed Western blot analysis using an anti-c-FLIP antibody after 24 h of incubation. c-FLIP appears as two variants, c-FLIPS and c-FLIPL. In HepG2 control cells, expression of c-FLIPL could not be detected; however, the expression level of c-FLIPL was sustained in HepG2-core- or HepG2-FL-expressing cells (Fig. 4A). On the other hand, the level of c-FLIPS increased to a smaller extent in TNF-α-treated HepG2-core and HepG2-FL cells but not in HepG2 control cells. The kinetics of c-FLIPL degradation in HepG2 control cells coincided with caspase-8 cleavage by TNF-α (Fig. 3).
FIG. 4.
c-FLIPL expression level is sustained in TNF-α-treated HCV core-expressing hepatocytes. (A) Western blot analysis for expression of c-FLIP from TNF-α-treated HepG2 cells stably transfected with empty vector (control), HCV core, or FL cDNA. The expression level of c-FLIPL in TNF-α-treated HepG2 control cells could not be detected after 24 h, while its expression was sustained in HepG2-core or HepG2-FL cells. The level of c-FLIPS increased upon TNF-α treatment of HepG2-control, HepG2-core, or HepG2-FL cells. (B) Western blot analysis for endogenous c-FLIP expression levels in Huh-7 cells harboring subgenomic or full-length HCV replicon, following TNF-α treatment. (C) Comparison of the fold differences of DNA fragmentation as an index of apoptotic cell death after 24 h in TNF-α- and actinomycin D (50 ng/ml)-treated Huh-7 cells harboring subgenomic or full-length HCV replicon.
The difference in c-FLIP expression level between TNF-α-treated HepG2-core, HepG2-FL, and HepG2 control cells led us to further examine c-FLIP status in a replicon system with and without TNF-α treatment. For this, Huh-7 cells harboring subgenomic (lacking structural protein expression) or full-length HCV replicon were treated with TNF-α for 24 h in the presence of actinomycin D. Cell lysates were subjected to Western blot analysis with an anti-c-FLIP antibody. The expression of both c-FLIPL and c-FLIPS increased twofold in TNF-α-treated Huh-7 cells harboring the full-length replicon, compared to untreated cells (Fig. 4B). However, TNF-α-treated Huh-7 cells expressing the subgenomic replicon displayed a decrease in c-FLIPL and similar levels of c-FLIPS, compared to untreated cells. Cell death ELISA suggested that Huh-7 cells harboring the full-length replicon exhibited a higher level of protection from TNF-α-mediated apoptosis than cells harboring the subgenomic replicon (Fig. 4C). Taken together, c-FLIP appeared to play an important role in the inhibition of TNF-α-mediated apoptosis in hepatocytes expressing HCV core protein alone or in context with other HCV proteins.
Knockdown of c-FLIP induces TNF-α-mediated apoptosis in HCV core-expressing cells.
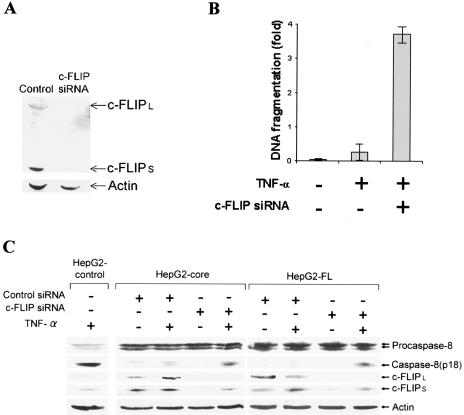
We examined whether the knockdown of endogenous c-FLIP by siRNA induces TNF-α-mediated apoptotic cell death in HepG2 cells expressing HCV core protein. We knocked down c-FLIP expression by an siRNA duplex, which specifically interacts with the mRNAs for both long and short forms of c-FLIP. The depletion of c-FLIP was verified by immunoblot analysis (Fig. 5A). Analysis for apoptotic cell death indicated that knockdown of c-FLIP increases DNA fragmentation by ∼7 fold compared to the negative control (Fig. 5B). Our results suggested that sustained c-FLIP expression in HCV core-expressing HepG2 cells inhibited TNF-α-mediated apoptosis. In addition, knockdown of c-FLIPL and c-FLIPS by siRNA displayed TNF-α-induced caspase-8 activation in HCV core-expressing HepG2 cells, while nonspecific siRNA did not exhibit a similar effect (Fig. 5C). These results suggested that sustained c-FLIP expression inhibits caspase-8 activation and apoptosis in HCV core-expressing HepG2 cells (Fig. 6).
FIG. 5.
Knockdown of c-FLIP in HCV core-expressing cells causes TNF-α-mediated apoptosis. (A) HepG2-core cells were transfected with c-FLIP-specific or control siRNA (100 nM). Cell lysates were subjected to Western blot analysis for detection of cFLIP expression levels after 48 h. The level of cellular actin was used as an internal control for comparison. (B) Downregulation of cFLIP correlates with TNF-α-mediated apoptosis. HepG2-core cells transfected with siRNA (100 nM) to cFLIP were treated with TNF-α (20 ng/ml) and analyzed for apoptotic cell death by ELISA (Roche). (C) Western blot analysis of HepG2 control, HepG2-core, or HepG2-FL cells transfected with siRNA (100 nM) to cFLIP and treated with TNF-α (20 ng/ml). Cell lysates were analyzed for expression of caspase-8, c-FLIPL, c-FLIPS, and actin with specific antibodies. Molecular weights of the identified protein bands were validated from the positions of prestained molecular weight markers (Bio-Rad).
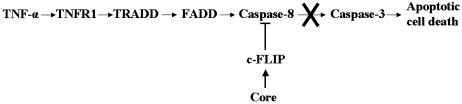
FIG. 6.
A schematic diagram depicting the signaling pathway for TNF-α-mediated apoptotic cell death and the inhibitory role of HCV core protein. TNFR1 associates with TRADD, which recruits FADD for activation of caspase-8, and initiates the apoptotic signaling pathway. HCV core protein appears to inhibit TNF-α-mediated apoptosis by sustained c-FLIP expression, thus impairing activation of caspase-8 for downstream signaling of executioner caspases.
DISCUSSION
Our results demonstrated that the TNF-α-induced apoptotic pathway is inhibited in HCV core protein-expressing HepG2 cells by a sustained expression of c-FLIP, an endogenous caspase-8 inhibitor. In the absence of c-FLIP, active caspase-8 is released from the death-inducing signaling complex (DISC) and mediates apoptosis if downstream signals are set on apoptosis execution (19). c-FLIP appears as two variants. c-FLIPS only contains two N-terminal death effector domains that are very similar to the prodomains of caspase-8 and -10. In contrast, c-FLIPL is identical in length to caspase-8 and also contains N-terminal tandem death effector domains. c-FLIPL is an inhibitor of the extrinsic apoptotic signaling pathway. TNF-α exerts a variety of effects that are mediated mainly by TNF-R1 and can induce apoptosis by activation of procaspase-8. c-FLIP is able to modulate procaspase-8 activation, preventing the induction of apoptosis mediated by death receptors (4, 19, 6). Overexpression of c-FLIP mediates resistance to the effects of TNF-α. Thus, c-FLIP, as a modulator of caspase-8, regulates life and death in various types of normal cells and tissues and renders resistance to death receptor-mediated apoptosis in many types of cancer cells.
Krueger et al. (10) suggested that a small amount of c-FLIP protein allows for the processing of procaspase-8, leading to the formation of active caspase-8 heterotetramer composed of the p18 and p10 subunits. In the presence of large amounts of c-FLIPL, procaspase-8 is recruited into the DISC, and cleavage is blocked after generation of the p43 cleavage products of both caspase-8 and c-FLIPL. In the presence of large amounts of c-FLIPS, procaspase-8 is recruited into the DISC but remains unprocessed. c-FLIP protein expression is regulated by both transcriptional gene regulation and posttranslational modification (9, 18, 26). In the present study, we have observed that c-FLIPL expression is sustained in the presence of HCV core protein alone or in context with other HCV proteins following TNF-α treatment, and c-FLIPS expression is moderately enhanced. We have also observed protection of TNF-α-mediated apoptotic cell death in Huh-7 cells harboring a full-length or subgenomic replicon. Huh-7 cells harboring the full-length replicon were more resistant to TNF-α than cells harboring the subgenomic replicon lacking the HCV structural region. Both c-FLIPL and c-FLIPS were upregulated in TNF-α-treated Huh-7 cells harboring the full-length replicon, while c-FLIPL was downregulated in subgenomic replicon-harboring cells. Together, these results suggest that sustained c-FLIP expression significantly inhibits caspase-8 cleavage in TNF-α-treated HepG2 cells expressing HCV core protein. HCV core protein does not associate with c-FLIP, and we do not know the mechanism for c-FLIP modulation by HCV core protein at this time. It appears that de novo c-FLIP protein synthesis is upregulated by expression of core protein and required for cell survival.
c-FLIP has been observed to be upregulated through mitogen-activated protein kinase, phosphatidylinositol 3 (PI3) kinase, or the NF-κB pathway (2, 9, 15, 29, 35). However, we did not observe the inhibition of c-FLIP expression or induction of apoptotic cell death by TNF-α upon concurrent treatment with mitogen-activated protein kinase 2 (MAPP2), mitogen-activated kinase kinase kinase 1 (MEKK-1), and PI3 kinase inhibitors (PD98095 and LY293002), respectively. Therefore, these two pathways may not be responsible for the upregulation of c-FLIP gene in our experimental systems. c-FLIP may be upregulated by the NF-κB pathway (9, 15). Genomic variation in HCV core protein may determine the functional regulation of NF-κB (24). HCV core protein from genotype 1a suppresses NF-κB-mediated gene upregulation in the presence of TNF-α. Therefore, HCV core protein appeared to upregulate c-FLIP gene expression by some mechanism other than mitogen-activated protein kinase, PI3 kinase, or the NF-κB signaling pathway. c-FLIP expression level is also regulated by proteolytic degradation, including the ubiquitin-dependent pathway. Perez and White (18) suggested that adenovirus E1A protein induces apoptotic cell death by inducing degradation of c-FLIPS through an ubiquitin-dependent pathway. HCV core protein binds to proteasome activator PA28γ, suggesting that it affects cellular proteolytic machinery to prevent cellular protein degradation, including that of c-FLIP (16). We have observed that the expression of c-FLIP is significantly reduced in TNF-α-treated HepG2 control cells, while c-FLIP expression is sustained in the presence of core protein or in context with other HCV proteins. An in vitro reporter assay suggested that the core protein upregulates c-FLIP promoter activity in HepG2 cells (unpublished data). Further studies are in progress to unravel the precise mechanism of HCV core protein-mediated c-FLIP regulation. Strategies to overcome c-FLIP-induced blockade of TNF-α-mediated apoptosis might be a critical target for therapeutic intervention of chronic HCV infection.
Acknowledgments
We thank Ralf Bartenschlager for providing the subgenomic and full-length HCV replicons, P. Chaudhary and D. V. Goeddel for providing TRADD plasmid DNA, Harris Perlman for providing c-FLIPL cDNA, Arvind Patel for providing rabbit antiserum to HCV core protein, and Lin Cowick for preparation of the manuscript.
This work was supported by research grant CA85486 from the National Institutes of Health.
REFERENCES
- 1.Clarke, B. 1997. Molecular virology of hepatitis C virus. J. Gen. Virol. 78:2397-2410. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert, S., A. Loranger, and N. Marceau. 2004. Keratins modulate c-Flip/extracellular signal-regulated kinase 1 and 2 antiapoptotic signaling in simple epithelial cells. Mol. Cell. Biol. 24:7072-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Amaro, R., C. Garcia-Monzon, L. Garcia-Buey, R. Moreno-Otero, J. L. Alonso, E. Yague, J. P. Pivel, M. Lopez-Cabrera, E. Fernandez-Ruiz, and F. Sanchez-Madrid. 1994. Induction of tumor necrosis factor alpha production by human hepatocytes in chronic viral hepatitis. J. Exp. Med. 179:841-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J. L. Bodmer, M. Schroter, K. Burns, C. Mattmann, D. Rimoldi, L. E. French, and J. Tschopp. 1997. Inhibition of death receptor signals by cellular FLIP. Nature 388:190-195. [DOI] [PubMed] [Google Scholar]
- 5.Jiang, Y., J. D. Woronicz, W. Liu, and D. V. Goeddel. 1999. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science 283:543-546. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka, T. 2005. The caspase-8 modulator c-FLIP. Crit. Rev. Immunol. 25:3158. [DOI] [PubMed] [Google Scholar]
- 7.Knobler, H., and A. Schattner. 2005. TNF-α, chronic hepatitis C and diabetes: a novel triad. QJM 98:1-6. [DOI] [PubMed] [Google Scholar]
- 8.Koziel, M. J., D. Dudley, N. Afdhal, A. Grakoui, C. M. Rice, Q. L. Choo, M. Houghton, and B. D. Walker. 1995. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J. Clin. Investig. 96:2311-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreuz, S., D. Siegmund, P. Scheurich, and H. Wajant. 2001. NF-κB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol. Cell. Biol. 21:3964-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krueger, A., S. Baumann, P. H. Krammer, and S. Kirchhoff. 2001. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol. Cell. Biol. 21:8247-8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Large, M. K., D. J. Kittlesen, and Y. S. Hahn. 1999. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J. Immunol. 162:931-938. [PubMed] [Google Scholar]
- 12.Lohr, H. F., J. F. Schlaak, G. Gerken, B. Fleischer, H. P. Dienes, and K. H. Meyer zum Buschenfelde. 1994. Phenotypical analysis and cytokine release of liver-infiltrating and peripheral blood T lymphocytes from patients with chronic hepatitis of different etiology. Liver 14:161-166. [DOI] [PubMed] [Google Scholar]
- 13.Marusawa, H., M. Hijikata, T. Chiba, and K. Shimotohno. 1999. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-kB activation. J. Virol. 73:4713-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto, M., T.-Y. Hsieh, N. Zhu, T. VanArsdale, S. B. Hwang, K.-S. Jeng, A. E. Gorbalenya, S.-Y. Lo, J.-S. Ou, C. F. Ware, and M. M. C. Lai. 1997. Hepatitis C virus core protein interacts with cytoplasmic tail of lymphotoxin-β receptor. J. Virol. 71:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Micheau, O., S. Lens, O. Gaide, K. Alevizopoulos, and J. Tschopp. 2001. NF-κB signals induce the expression of c-FLIP. Mol. Cell. Biol. 21:5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriishi, K., T. Okabayashi, K. Nakai, K. Moriya, K. Koike, S. Murata, T. Chiba, K. Tanaka, R. Suzuki, T. Suzuki, T. Miyamura, and Y. Matsuura. 2003. Proteasome activator PA28γ-dependent nuclear retention and degradation of hepatitis C virus core protein. J. Virol. 77:10237-10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata, S. 1997. Apoptosis by death factor. Cell 88:355-365. [DOI] [PubMed] [Google Scholar]
- 18.Perez, D., and E. White. 2003. E1A sensitizes cells to tumor necrosis factor alpha by downregulating c-FLIPS. J. Virol. 77:2651-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peter, M. E. 2004. The flip side of FLIP. Biochem. J. 382:e1-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray, R. B., K. Meyer, and R. Ray. 1996. Suppression of apoptosis cell death by hepatitis C virus core protein. Virology 226:176-182. [DOI] [PubMed] [Google Scholar]
- 21.Ray, R. B., K. Meyer, R. Steele, A. Shrivasava, B. B. Aggarwal, and R. Ray. 1998. Inhibition of tumor necrosis factor (TNF-α) mediated apoptosis by hepatitis C virus core protein. J. Biol. Chem. 273:2256-2259. [DOI] [PubMed] [Google Scholar]
- 22.Ray, R. B., K. Meyer, and R. Ray. 2000. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology 271:197-204. [DOI] [PubMed] [Google Scholar]
- 23.Ray, R. B., and R. Ray. 2001. Hepatitis C virus core protein: intriguing properties and functional relevance. FEMS Microbiol. Lett. 202:149-156. [DOI] [PubMed] [Google Scholar]
- 24.Ray, R. B., R. Steele, A. Basu, K. Meyer, M. Majumder, A. K. Ghosh, and R. Ray. 2002. Distinct functional role of hepatitis C virus core protein on NF-κB regulation is linked to genomic variation. Virus Res. 87:21-29. [DOI] [PubMed] [Google Scholar]
- 25.Sacco, R., T. Tsutsumi, R. Suzuki, M. Otsuka, H. Aizaki, S. Sakamoto, M. Matsuda, N. Seki, Y. Matsuura, T. Miyamura, and T. Suzuki. 2003. Antiapoptotic regulation by hepatitis C virus core protein through up-regulation of inhibitor of caspase-activated DNase. Virology 317:24-35. [DOI] [PubMed] [Google Scholar]
- 26.Salon, C., B. Eymin, O. Micheau, L. Chaperot, J. Plumas, C. Brambilla, E. Brambilla, and S. Gazzeri. 2005. E2F1 induces apoptosis and sensitizes human lung adenocarcinoma cells to death-receptor-mediated apoptosis through specific downregulation of c-FLIPshort. Cell Death Differ. 29:1-13. [DOI] [PubMed] [Google Scholar]
- 27.Shen, Y., and T. E. Shenk. 1995. Viruses and apoptosis. Curr. Opin. Genet. Dev. 5:105-111. [DOI] [PubMed] [Google Scholar]
- 28.Smith, C. A., T. Farrah, and R. G. Goodwin. 1994. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 76:959-962. [DOI] [PubMed] [Google Scholar]
- 29.Suhara, T., T. Mano, B. E. Oliveira, and K. Walsh. 2001. Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J. Biol. Chem. 276:6893-6896. [DOI] [PubMed] [Google Scholar]
- 30.Thompson, C. B. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267:1456-1462. [DOI] [PubMed] [Google Scholar]
- 31.Tilg, H., A. Wilmer, W. Vogel, M. Herold, B. Nolchen, G. Judmaier, and C. Huber. 1992. Serum levels of cytokines in chronic liver diseases. Gastroenterology 103:264-274. [DOI] [PubMed] [Google Scholar]
- 32.Torre, D., C. Zeroli, M. Giola, G. Ferrario, G. P. Fiori, G. Bonetta, and R. Tambini. 1994. Serum levels of interleukin-1 alpha, interleukin-1 beta, interleukin-6, and tumor necrosis factor in patients with acute viral hepatitis. Clin. Infect. Dis. 18:194-198. [DOI] [PubMed] [Google Scholar]
- 33.White, E. 1996. Life, death, and the pursuit of apoptosis. Genes Dev. 10:1-15. [DOI] [PubMed] [Google Scholar]
- 34.Yao, Z. Q., A. Eisen-Vandervelde, S. N. Waggoner, E. M. Cale, and Y. S. Hahn. 2004. Direct binding of hepatitis C virus core to gC1qR on CD4+ and CD8+ T cells leads to impaired activation of Lck and Akt. J. Virol. 78:6409-6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh, J. H., S. C. Hsu, S. H. Han, and M. Z. Lai. 1998. Mitogen-activated protein kinase kinase antagonized fas-associated death domain protein mediated apoptosis by induced FLICE-inhibitory protein expression. J. Exp. Med. 16:1795-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu, N., A. Khoshnan, R. Schneider, M. Matsumoto, G. Dennert, C. Ware, and M. M. C. Lai. 1998. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor I and enhances TNF-mediated apoptosis. J. Virol. 72:3691-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]