Abstract
NF-κB is an inducible transcription factor mediating innate immune responses whose activity is controlled by the multiprotein IκB kinase (IKK) “signalsome”. The core IKK consists of two catalytic serine kinases, IKKα and IKKβ, and a noncatalytic subunit, IKKγ. IKKγ is required for IKK activity by mediating kinase oligomerization and serving to couple the core catalytic subunits to upstream mitogen-activated protein 3-kinase cascades. We have discovered an alternatively spliced IKKγ mRNA isoform, encoding an in-frame deletion of exon 5, termed IKKγ-Δ. Using a specific reverse transcription-PCR assay, we find that IKKγ-Δ is widely expressed in cultured human cells and normal human tissues. Because IKKγ-Δ protein is lacking a critical coiled-coil domain important in protein-protein interactions, we sought to determine its signaling properties by examining its ability to self associate, couple to activators of the canonical pathway, and mediate human T-cell leukemia virus type 1 (HTLV-1) Tax-induced NF-κB activity. Coimmunoprecipitation and confocal colocalization assays indicate IKKγ-Δ has strong homo- and heterotypic association with wild-type (WT) IKKγ and, like IKKγ WT, associates with the IKKβ kinase. Similarly, IKKγ-Δ mediates IKK kinase activity and downstream NF-κB-dependent transcription in response to tumor necrosis factor (TNF) and the NF-κB-inducing kinase-IKKα signaling pathway. Surprisingly, however, in contrast to IKKγ WT, IKKγ-Δ is not able to mediate HTLV-1 Tax-induced NF-κB-dependent transcription, even though IKKγ-Δ binds and colocalizes with Tax. These observations suggest that IKKγ-Δ is a functionally distinct alternatively spliced mRNA product differentially mediating TNF-induced, but not Tax-induced, signals converging on the IKK signalsome. Differing levels of IKKγ-Δ expression, therefore, may affect signal transduction cascades coupling to IKK.
Nuclear factor-κB (NF-κB) is an inducible transcription factor that controls the expression of inducible inflammatory and antiapoptotic genes (49, 51). NF-κB responds to a diverse series of inflammatory activators, including UV light, double-stranded RNA, cytokines, vasoactive peptides, and viral oncogenes through several distinct intracellular pathways (reviewed in reference 36). Because of its central role as an integrator of stress and inflammatory stimuli, the pathways controlling NF-κB have been intensively investigated.
Currently, it is thought that NF-κB activation is controlled by two distinct pathways, termed the canonical (23) and noncanonical pathways (9, 43). The canonical pathway controls nuclear translocation of the prototypical NF-κB complex, composed of 65-kDa Rel A-50-kDa NF-κB1 heterodimers. Under normal conditions, the Rel A · NF-κB1 complex is sequestered and inactivated in the cytoplasm by the IκB inhibitors, proteins which inactivate Rel A DNA binding and nuclear translocation by masking its nuclear localization sequence (reviewed in reference 1). NF-κB activators induce IκB phosphorylation on serine residues 32 and 36 on its NH2-terminal regulatory domain (reviewed in reference 23). Phospho-IκB is then specifically bound by the Skp1-Cullin-F-Box-type E3 ubiquitin ligase, E3RS, initiating IκB ubiquitination and proteolysis through the proteasome (4, 23, 24) and calpain pathways (15). Nuclear translocated NF-κB binds high-affinity chromatin sites and activates the expression of a diverse gene network (50) by inducing assembly of active promoters (2) and recruiting coactivators to target gene promoters (44).
The cytoplasmic IκB kinase (IKK), also known as the “signalsome,” is the rate-limiting kinase responsible for inducible IκBα phosphorylation (23) and is composed of core catalytic kinases and scaffolding proteins. The catalytic core contains the ubiquitous helix-loop-helix proteins IKKα and IKKβ (33, 54), associated with the noncatalytic regulatory protein, IKKγ, in a precise stoichiometric relationship of 2 catalytic subunits (either an IKKβ homodimer or an IKKα-IKKβ heterodimer) and 2 subunits of IKKγ (32). In spite of significant sequence similarity between the two α/β catalytic kinase subunits, IKKβ has ∼30-fold higher activity toward IκBα (17, 54) and its phosphorylation is the final common step in IKK activation (11, 33). IKKγ, known also as the NF-κB essential modulator (NEMO) (57), the IKK-associated protein (32), and the 14.7K interacting protein (27), is essential for inducible IKK activity, as IKKγ-deficient cells are unable to activate the canonical NF-κB pathway in response to all stimuli tested, including interleukin-1, tumor necrosis factor (TNF), phorbol myristate acetate, double-stranded RNA, or human T-cell leukemia virus type 1 (HTLV-1) Tax oncoprotein (21, 40, 41, 57). IKKγ plays multiple roles in IKK activation through its ability to organize the assembly of IKKs into the activated high-molecular-weight complex (38, 56) and to serve as an adapter molecule to recruit upstream kinases that phosphorylate the catalytic subunits (40, 58, 59). Through these activities, IKKγ forms a molecular bridge between IKK, its upstream activators, and its substrate.
Consistent with its role as a signaling integrator, IKK activity is induced by several discrete mechanisms. In response to the type I cytokine TNF, IKKγ recruits inactive cytosolic IKK to a submembranous complex formed on the cytoplasmic effector domains of the liganded TNF receptor (59). Here, an ordered activation process is initiated by the upstream mitogen-activated protein kinase kinase kinases (MAP3Ks). The MAP3Ks that activate IKK include the NF-κB-inducing kinase (NIK) (28, 30, 34), a kinase that activates IKKβ in a directional manner through IKKα (35), and the transforming growth factor β-associated kinase-1. In a separate mechanism, the Tax oncogenic protein from HTLV-1 directly associates with IKKγ, resulting in Tax recruitment into the IKK signalsome and kinase activation (21). Together, these observations indicate that oligomerization and MAP3K-induced sequential IKKα/β phosphorylation are important processes regulating IKK activity.
In this study, we have identified a 43-kDa IKKγ alternate-splice product that results in an in-frame deletion of exon 5, encoding a protein that we term IKKγ-Δ, that is widely expressed all cell types examined. IKKγ-Δ strongly associates with IKKγ wild type (WT) in coimmunoprecipitation assays; reasoning that enhanced self-association could influence its response to IKK inducing signals, we explored whether there were functional differences between these two IKKγ isoforms. Experiments involving coexpression of the catalytic kinases IKKα/β and the MAP3K NIK indicate that IKKγ-Δ efficiently mediates IKK and NF-κB activation. In striking contrast, IKKγ-Δ is unable to mediate Tax-inducible IKK activation, even though it associates with Tax. These findings suggest that IKKγ-Δ is a functionally distinct alternate-splice product, which is HTLV Tax resistant and unable to form productive Tax-IKK complexes with cellular proteins.
MATERIALS AND METHODS
Cell culture, treatment, and transfections.
Human HepG2, HeLa S3, A549, K562, U937, and Hep3B cells were obtained from ATCC and cultured as recommended by the supplier (ATCC, Rockville, MD). mRNA was isolated from normal human tissues collected from discarded surgical specimens in accordance with UTMB IRB-approved protocol. Human CD4+ lymphocytes were isolated from Ficoll gradient-purified peripheral mononuclear cells using commercial anti-CD4+ magnetic beads (Miltenyi Biotech). Tax-transformed, IKKγ-deficient 5R cells were maintained in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum (FBS), penicillin-streptomycin, and 15% filtered conditioned medium (57). 8321 Jurkat T-cell lines were cultured in Iscove's modified Dulbecco's medium, supplemented with 10% FBS (20% FBS for the stables), 50 μM β-mercaptoethanol, and 15 μg/ml of gentamicin (16). IKKγ-deficient mouse embryonic fibroblasts (E8i cells) were cultured as described previously (42). Cells were transiently transfected using Lipofectamine (Invitrogen) into triplicate 60-mm plates with indicated plasmids. The total amount of DNA was kept constant by inclusion of empty vector pcDNA3. After 48 h, cells were stimulated with TNF-α (30 ng/ml, 6 h) and harvested for the measurement of reporter activity. Relative induction was calculated by dividing normalized reporter treatment values by those of the control.
Plasmid construction.
The NF-κB-LUC reporter consists of the trimerized angiotensinogen NF-κB sequences driving the expression of the firefly luciferase reporter gene (19). The bacterial expression vector encoding glutathione S-transferase (GST)-IκBα (1 to 51) was constructed by PCR amplification of the human IκBα cDNA using the upstream primer 5′-GTG ATA GGA ATT CTC CAG GCG GCC GAG CGC CCC-3′, and the downstream primer 5′-ACC TAA GCT TCT AGA GGC GGA TCT CC TGC AGC-3′to incorporate EcoRI and HindIII restriction sites (underlined). IκBα (1 to 51) was restricted with EcoRI and HindIII and cloned into pGEX-KG restricted with the same sites (12). The eukaryotic expression vector pcDNA3-FLAG containing an N-terminal FLAG epitope downstream of a strong Kozak initiation sequence was produced by ligating a duplex oligonucleotide, 5′-AGC TCG TCT ACC ATG GAC TAC AAA GAC GAT GAC GAT AAG GGA TCC AAG GAA AAG CTT GAT ATC GATC-3′ encoding the initiator methionine (underlined) upstream of the FLAG epitope and unique downstream restriction sites into the Hind III/XbaI-digested pcDNA3 vector (Invitrogen). pcDNA-FLAG-IKKγ (WT) and pcDNA-FLAG-IKKγΔ were constructed by PCR using the upstream primer 5′-TAA GGGA TCC ATG AAT AGG CAC CTC TTG GAA GAG CC-3′ and the downstream primer 5′-ATA TCAA GCT TCT ACT CAA TGC ACT CCA TGA CAT GTA TCT GC-3′ to amplify the IKKγ and IKKγ-Δ cDNAs and incorporate BamHI and HindIII restriction sites (underlined) flanking the initiation and stop codons (bold), respectively. The cDNAs were digested with BamHI/HindIII, purified, and cloned into pcDNA3-FLAG. To construct pEF6-FLAG-IKKγ-Δ, the T7 and SP6 primers were used to PCR amplify pcDNA-FLAG-IKKγ-Δ. The purified PCR product was then TA cloned into the pEF6/V5-His-TOPO vector (Invitrogen). Plasmids were purified by ion-exchange chromatography prior to transfection; all constructions were confirmed by automated sequencing. The eukaryotic expression vectors pRK-IKKα, pRK-IKKβ, pRK-IKKβ (K44A), pRKmyc-NIK, and pRKmyc-NIK (T559A) were gifts of D. Goeddel, Tularik, South San Francisco, CA (54). pCMV-Tax was a gift of Warner Greene, The Gladstone Foundation, San Francisco, CA (46). The hemagglutinin (HA)-tagged IKKγ WT expression vector was previously described (21).
Stable transfectants.
For HeLa stable transfectants, cells were transfected with 20 μg of pcDNA-FLAG-IKKγ or pcDNA-FLAG-IKKγΔ expression plasmid and selected for antibiotic resistance to G418 (400 μg/ml). For 5R cell stable transfectants, 5R cells were transfected with 20 μg of pEF6-IKKγΔ expression plasmid DNA and selected for antibiotic resistance in the presence of 10 μg/ml blasticidin S (Invitrogen Corp., San Diego, CA). 8321 is a CD3+ derivative of Jurkat T-cell clone 3T8 generated by ICR191 mutagenesis and subsequent negative enrichment for cells that do not respond to phorbol myristate acetate that also lack functional IKKγ expression (16). To make 8321 cells stably expressing either IKKγ or IKKγ-Δ stable cell line, 8321 cells were transfected with pEF6-FLAG-IKKγ and pEF6-FLAG-IKKγ-Δ and selected for blasticidin S (12 μg/ml) resistance. Transfectants were cloned and identified by screening with Western immunoblots using horseradish peroxidase-conjugated anti-FLAG antibody.
Reverse transcription (RT)-PCR cloning of IKKγ.
Total RNA was isolated using RNAqueous (Ambion, Austin, TX). First-strand cDNA synthesis was performed using 5 μg HeLa S3 total RNA, 0.5 μg oligo(dT)12-18, and 50 units of SuperScriptII reverse transcriptase (Invitrogen) in a final volume of 20 μl of 20 mM Tris-HCl, pH 8.4, 50 mM KCl, 5 mM MgCl2, 10 mM dithiothreitol, and 0.5 mM concentrations of each deoxynucleoside triphosphate. PCR amplification of IKKγ cDNA was performed using 100 pmol of each primer (5′-ATGAATAGGCACCTCTGGAAGAGC-3′, 5′-CTACTCAATGCACTCCATGACATG-3′) in a final volume of 100 μl of Tris-HCl, pH 8.4, 1.5 mM MgCl2, 50 mM KCl, 0.2 mM concentrations of deoxynucleoside triphosphates, and 2.5 U of AmpliTaq polymerase (Applied Biosystems). After an initial denaturation at 95°C for 2 min, the reaction mixture was subjected to 40 cycles of the following program: denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and primer extension for 8 min at 72°C. Two amplified DNAs (1,107 bp and 1,260 bp) were purified by polyacrylamide gel electrophoresis and TA cloned into pCRII (Invitrogen). Nucleotide sequences for representative clones were determined using fluorescent tagged terminator cycle sequencing and analyzed on an Applied Biosystems model 310 Genetic Analyzer.
IKKγ exon 5 assay.
Normal human tissue was obtained from the UTMB Tumor Bank representing discarded material from surgical specimens obtained with our IRB-approved protocol. Total RNA was isolated using Totally RNA (Ambion). First-strand cDNA synthesis was performed using 5 μg of total RNA as described above. PCR amplification was performed using Fail Safe buffer D (Epicenter) and 45 pmol of each primer (5′-AGCCCAGGTGACGTCCTTGCTC-3′, 5′-CTTCAGCTTATCGATCACCTCCTG-3′). After denaturation at 95°C for 2 min, the reaction mixtures were incubated for 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 4 min. The last primer extension was continued for 10 min at 72°C to ensure completion of DNA synthesis. Amplified DNA (wild type, 427 bp; exon 5 deletion, 274 bp) was analyzed by electrophoresis on polyacrylamide gels. Primers for detection of human GAPDH (5′-GTCATCCATGACAACTTTGGTATCG-3′, 5′-CAGGTTTTTCTAGACGGCAGGTC-3′), and human polymerase beta (5′-CGGGGGAATCACCGACATGCTC-3′, 5′-TCCAGTTTACGTAATTTTCCAGTTGC-3′) were included as positive controls. The respective amplified target DNAs were 269 bp for GAPDH and 222 bp (wild type) and 166 bp (exon 2 deletion) for polymerase beta (7).
2D electrophoresis (2DE).
Isoelectric focusing (IEF) was performed with 11-cm precast IPG strips (pH 3 to 10, or 5 to 8 as indicated; Bio-Rad). Two-hundred-microliter aliquots of protein were loaded onto an IPG strip and allowed to rehydrate overnight. IEF was performed at 20°C with the following parameters: 50 V, 11 h; 250 V, 1 h; 500 V, 1 h; 1,000 V, 1 h; 8,000 V, 2 h; 8,000 V, 6 h. After IEF, the IPG strip was stored at −80°C until the two-dimensional (2D) sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). For the 2D SDS-PAGE, the IPG strips were incubated in 4 ml of equilibration buffer (6 M urea, 2% SDS, 50 mM Tris-HCl, pH 8.8, 20% Glycerol) plus 10 μl/ml Tris(2-carboxyethyl) phosphine for 15 min at 22°C with shaking. The samples were then incubated in equilibration buffer with 25 mg/ml iodoacetamide for 15 min at 22°C with shaking. Electrophoresis was performed at 150 V for 2.25 h at 4°C with precast 8 to 16% polyacrylamide gels in Tris-glycine buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3). After electrophoresis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes and used for Western immunoblots.
Preparation of subcellular extracts.
S100 cytosolic and particulate fractions were prepared as previously described (14, 15). In brief, cells were incubated in hypotonic buffer (20 mM HEPES, pH 7.4, 10 mM potassium acetate, 1.5 mM magnesium acetate) for 5 min on ice. Lysis was completed in a Dounce homogenizer and verified by microscopic examination. Nuclei and unbroken cells were removed by low-speed centrifugation. The low-speed supernatants, containing cytoplasm and membrane proteins were centrifuged at 100,000 × g for 1 h at 4°C in a Beckman SW 55Ti rotor. The resultant S100 supernatants were taken as cytosolic extract, and pellets (the particulate) were resuspended in hypotonic buffer with 1% (vol/vol) IGEPAL. Assay of enrichment of cytoplasmic and nuclear markers is shown in Results (see Fig. 6A). All extracts (cytosol, particulate, cytoplasmic, and nuclear extracts) were normalized for protein amounts determined by Coomassie G-250 staining (Bio-Rad, Hercules, CA).
FIG. 6.
IKKγ-Δ mediates TNF-induced target gene expression. (A) IKKγ expression in IKKγ-deficient 8321 cells. 8321 cells stably transfected with empty vector (8321EMPTY), IKKγ WT (8321IKKγ-WT), or IKKγ-Δ (8321IKKγ-Δ) were isolated, and IKKγ expression was confirmed by Western blotting. Top panel, Western blot using anti-FLAG (α-FLAG); Middle panel, Western blot using anti-IKKγ (α-IKKγ); bottom panel, Western blot using anti-β-actin (α-βActin). 8321EMPTY cells are deficient in IKKγ expression, whereas 8321IKKγ-WT and 8321IKKγ-Δ express similar amounts of epitope-tagged IKKγ isoform. (B) Northern blot hybridization of NF-κB-dependent gene expression in IKKγ-complemented 8321 cells. 8321EMPTY, 8321IKKγ-WT, or 8321IKKγ-Δ cells were stimulated with TNF (20 ng/ml, T) for 1.5 h. Graph represents hybridization intensities quantitated by exposure to PhosphorImager cassette where IκBα intensity normalized to that of thymosin β is plotted. Inset, autoradiogram of Northern blot hybridization to IκBα probe (IκBα) or Thymosin β (Thy). The experiment was repeated twice with similar results. C, control; −−, absent. (C) HeLa cells stably expressing IKKγ WT or IKKγ-Δ were TNF stimulated as in Fig. 5B. Shown is Northern blot hybridization to IκBα probe (top) or thymosin B (bottom). IκBα mRNA is induced to a greater degree in HeLa IKKγ-Δ-expressing cells.
IKK immunoprecipitation-kinase assays.
The GST-IκBα (1 to 51) substrate was expressed by isopropyl-β-d-thiogalactopyranoside (IPTG) induction in XL1-Blue bacteria and purified to homogeneity by glutathione-agarose (Sigma) chromatography (12). HepG2 cells and 5R cells were transfected with pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ and IKKβ expression vectors. Forty-eight hours later, cells were harvested and cytoplasmic extracts prepared. Five hundred micrograms of cytoplasmic extracts was incubated for 1 to 2 h at 4°C with 10 μg of anti-FLAG M2 (Sigma) in TB buffer (150 mM NaCl, 5 mM EDTA, 50 mM Tris-HCl [pH 7.5], 0.05% IGEPAL CA-630). Immune complexes were precipitated with protein A-agarose (Sigma) overnight at 4°C. The immunoprecipitates were washed with TB buffer followed by a final wash in kinase buffer (20 mM HEPES, pH 7.5, 10 mM MgCl2, 50 mM NaCl, 20 mM β-glycerophosphate, 100 μM Na3VO4, 20 μM ATP, 10 μg/ml aprotinin, 2 mM dithiothreitol). The immunoprecipitates were then incubated for 30 min at 30°C with 1 μCi of [γ-32P]ATP and 2 μg of GST-IκBα (1 to 51) substrate in 1× kinase buffer. Reactions were stopped by adding 4× SDS-PAGE sample buffer and boiling for 5 min. Products were separated by 10% SDS-PAGE, electrophoretically transferred to an Immobilon-P transfer membrane (Millipore), and exposed to BioMax film (Kodak). The membranes were subsequently probed with anti-FLAG antibody to determine the amounts of expressed IKKγ WT and IKKγ-Δ present.
Northern blots.
Total cellular RNA was extracted by acid guanidium-phenol extraction (Tri Reagent; Sigma). RNA (20 μg) was denatured, fractionated by electrophoresis on a 1.2% agarose-formaldehyde gel, capillary transferred to a nitrocellulose membrane (Zeta-ProbeGT; Bio-Rad), and prehybridized as described previously (48). The IκBα and thymosin B probes were produced by asymmetric PCR with plasmid templates as previously described (14, 60). The probes were purified by size exclusion chromatography on MicroSpin TMG-25 columns (Amersham Biosciences). The membrane was hybridized with 1 × 106 to 2 × 106 cpm/ml probe at 60°C overnight in 5% SDS hybridization buffer (50). The membrane was washed with a buffer containing 5% SDS and 1× saline-sodium citrate (0.15 M NaCl and 0.015 M sodium citrate) for 20 min at room temperature followed by 30 min at 60°C. The membrane was exposed to BioMax film and quantified by exposure to a PhosphorImager cassette.
Confocal colocalization microscopy.
Cells cultured on 25-mm coverslips (Thomas Scientific) were transfected with 0.5 μg of plasmid DNA. Twenty-four hours later, cells were fixed with 3.7% formaldehyde and then permeabilized with phosphate-buffered saline containing 0.1% Triton X-100. Where indicated, cells were stained with monoclonal anti-HA, anti-FLAG antibodies (Abs), followed with anti-mouse Alexa Fluor 594 or Alexa Fluor 488 (Molecular Probes). Subcellular localization of green fluorescent protein (GFP)-Tax was revealed by direct laser excitation. Coverslips were mounted onto glass slides with VECTASHIELD mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI) (Roche) and examined with a Zeiss Axiovert 135 laser-scanning microscope.
RESULTS
IKKγ-Δ encodes a deletion in the NH2-terminal coiled-coil domain and is widely expressed.
IKKγ is essential for inducible regulation of the IKK complex through its ability to induce oligomeric association of the catalytic IKKα and β subunits and couple them to upstream activators. IKKγ cDNA sequences independently cloned by three groups identified the predicted amino acid sequence to be of 419 amino acids (aa) encoded by an open reading frame of 1,257 bp (32, 40, 57). We therefore were surprised that RT-PCR using primers spanning exons 2 to 10 encoding the IKKγ open reading frame produced two bands under a variety of stringent conditions: one corresponding to the predicted 1.26-kb size and another of 1.1 kb. Multiple independent clones of the smaller product were sequenced and found to contain a 153-nucleotide (nt) “in-frame” deletion exactly spanning exon 5 of the human IKKγ gene (45), encoding a protein we term IKKγ-Δ. Exon 5 encodes amino acid residues 174 to 224 that form a COOH-terminal portion of the central IKKγ coiled-coil domain, a secondary structure motif responsible for protein-protein association, including its self-association (5, 29, 58).
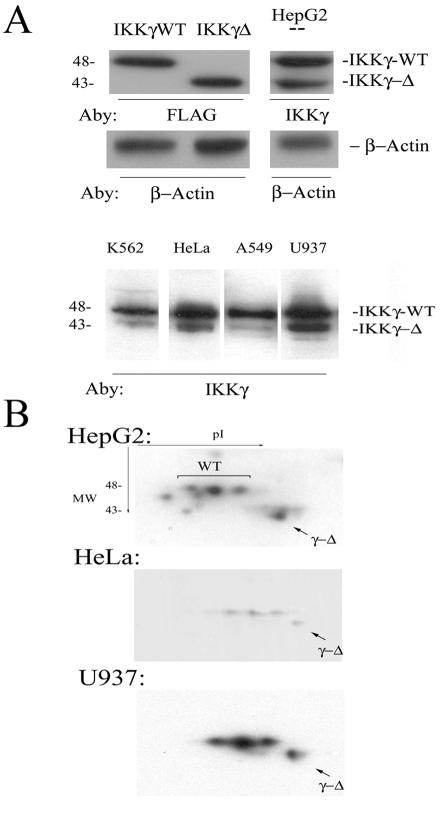
To determine whether the IKKγ-Δ expression was unique to HeLa, we next examined its expression patterns in cultured cells and normal human tissues. First, Western immunoblots were performed on cytoplasmic lysates of cultured cells to determine the distribution of IKKγ-Δ expression and the relative steady-state abundances of the isoforms. Because no antibody will uniquely identify IKKγ-Δ without also recognizing IKKγ WT, the abundance of IKK γ-Δ was determined by its unique migration in one-dimensional SDS-PAGE and 2DE (Fig. 1A, B, respectively). In HepG2 cells, two isoforms of IKKγ were identified, each comigrating with a respective transiently expressed epitope-tagged standard of 48 and 43 kDa (Fig. 1A, top panel). From this experiment, we estimated that IKKγ-Δ was expressed at a 1:4 ratio with the IKKγ WT isoform in HepG2 cells. Moreover, in K562, HeLa, A549, and U937 cells, the 43-kDa IKKγ-Δ isoform could be detected at various ratios with IKKγ WT, from <1:4 in A549 cells to ∼1:2 in U937 cells (Fig. 1A, lower panel). To more definitively separate the IKKγ isoforms, Western immunoblots of cytoplasmic proteins were performed after 2DE. Consistent with its multiple posttranslational modifications (6, 39, 47), IKKγ WT fractionated into three distinct isoforms based on pI (Fig. 1B). Interestingly, the IKKγ-Δ isoform focused as a single spot at a distinctly higher pI than the IKKγ WT, suggesting that it lacked posttranslational modifications similar to IKKγ WT (even though the identified IKKγ phosphoacceptor sites are outside the occluded exon 5).
FIG. 1.
IKKγ-Δ protein expression. (A) Western immunoblot. Cytoplasmic lysates were fractionated by one-dimensional SDS-PAGE and blotted onto PVDF membranes. Epitope-tagged IKKγ WT and IKKγ-Δ stably expressed in HeLa cells serve as markers and were identified by anti-FLAG in a Western blot (left panel). Two distinct IKKγ isoforms are identified in HepG2 cells that comigrate with the 48-kDa IKKγ WT and 43-kDa IKKγ-Δ standards (right). The blot was reprobed with β-actin as a loading control. Bottom panel, cytoplasmic lysates from K562, A549, and U937 cells were blotted with anti-IKKγ. The relative migration of IKKγ WT and IKKγ-Δ are indicated. (B) Western immunoblot in 2DE. Cytoplasmic lysates were subjected to isoelectric focusing over the pH range 5 to 8 and fractionated by 12 to 20% gradient SDS-PAGE in the second dimension. The proteins were blotted onto PVDF membranes and probed with anti-IKKγ antibody. The relative migration of IKKγ WT and IKKγ-Δ was determined in HeLa cell stable transfectants (data not shown). IKKγ-Δ focuses as a single spot at a more basic pI in a 1:4 abundance ratio with the IKKγ WT isoforms.
To compare relative steady-state transcript levels, primers were designed to amplify IKKγ exons 4 to 7 to produce a 427-nt product corresponding to IKKγ WT mRNA and a 274-nt product for IKKγ-Δ mRNA (missing exon 5). RNA from a variety of cultured cell lines was then assayed for relative abundance of IKKγ isoforms. As seen in Fig. 2A, IKKγ-Δ was expressed in A549, Bcr-Abl/HL-60, K562, and HL-60 cells at an ∼1:2 ratio relative to IKKγ WT, and was the predominant isoform detected in Hep3B and HeLa S3 cells (Fig. 2A, lanes 3, 6). Together, these data indicate that IKKγ-Δ is expressed and synthesized in transformed cell lines, but its steady-state abundance is generally less than that of IKKγ WT. Finally, to determine whether the IKKγ-Δ splice product was unique to transformed cell lines, mRNA from normal human tissue was assayed for expression of IKKγ splice products with the RT-PCR assay. IKKγ-Δ was the predominant transcript in breast and cervical tissue and was also detectable in the kidney, liver, testes, adrenal glands, colon, lung, and pancreas (Fig. 2B). Although the relative abundance of the protein isoforms has not been determined, these data do indicate that IKKγ-Δ transcripts are widely expressed in normal human tissues at various ratios with the full-length IKKγ WT transcript and is the predominant isoform expressed in the breast and cervix.
FIG. 2.

IKKγ-Δ mRNA expression. (A) IKKγ RT-PCR assay. Ethidium bromide staining of RT-PCR products fractionated by PAGE. Top panel, RT-PCR assay of various cellular RNA with IKKγ exon 4 and 6 primers. Bottom panel, housekeeping mRNA controls amplified from same source, GAPDH and DNA β-polymerase (β-Pol) are indicated. Lane 1, A549 human alveolar carcinoma cells; lane 2, human erythroleukemia (HL-60 cells) expressing Bcr-Abl; lane 3, human hepatocarcinoma Hep3B; lane 4, K562 erythroleukemia; lane 5, HL-60; lane 6, HeLa S3. (B) IKKγ expression in normal human tissues. Ethidium bromide staining of RT-PCR products fractionated by PAGE. Top panel, PCR products using IKKγ primers as in Fig. 2B. Bottom panel, PCR products for GAPDH and β-Pol. Lane 1, breast; lane 2, cervix; lane 3, kidney; lane 4, liver; lane 5, testicle; lane 6, adrenal gland; lane 7, colon; lane 8, lung; lane 9, pancreas.
Effect of exon 5 exclusion on IKKγ heterotypic association.
IKKγ-Δ, internally deleted of amino acid residues 174 to 224, corresponds to a coiled-coil domain of IKKγ previously known to mediated IKKγ homotypic association (58). Specifically, previous studies showed that internal deletion of amino acid residues 201 to 300 disrupted IKKγ self-association (58), suggesting to us that the alternatively spliced IKKγ-Δ may have association properties distinct from those of IKKγ WT. To test whether IKKγ-Δ associates with the more abundant IKKγ WT, either Myc epitope-tagged IKKγ WT or Myc-tagged IKKγ-Δ were transiently expressed with FLAG epitope-tagged IKKγ WT into HepG2 cells. In the crude lysates, equivalent amounts of Myc-tagged proteins were expressed (Fig. 3A). However, after immunoprecipitation with Myc Ab, the abundance of associated FLAG-IKKγ WT was significantly less in the cells transfected with Myc-IKKγ WT than the abundance in cells transfected with Myc-IKKγ-Δ (Fig. 3A, bottom panel). These data indicate that IKKγ-Δ binds more tightly to IKKγ WT (or with a greater stoichiometry) than IKKγ WT binds to itself.
FIG. 3.
IKKγ-Δ is heterotypic association competent. (A) Enhanced IKKγ-Δ heterotypic association. Eukaryotic expression vectors encoding FLAG epitope-tagged IKKγ WT were cotransfected into HepG2 cells with either Myc-tagged IKKγ WT or Myc-IKKγ-Δ. Forty-eight hours later, cells were lysed and fractionated by SDS-PAGE for Western immunoblotting (IB) with anti-Myc (α-Myc) antibody (top panel). Cytosolic extracts were then immunoprecipitated (IP) with anti-Myc. Immune complexes were then fractionated and association with FLAG-IKKγ WT was determined by Western immunoblotting with anti-FLAG (α-FLAG) antibody (bottom panel). Although FLAG-IKKγ WT was detected in both lanes, IKKγ-Δ immunoprecipitates contained a greater abundance of FLAG-IKKγ WT. (B) IKKγ-Δ heterotypic association in IKKγ-deficient background. E8i cells were transfected with HA-tagged IKKγ WT or FLAG-IKKγ-Δ as indicated. Lanes 1 to 3, lysates; lanes 4 to 6, IP with anti-HA Ab. Shown is a Western blot (WB) using anti-IKKγ Ab. ++, present;−−, absent. (C) Confocal colocalization microscopy. Top, FLAG-IKKγ-Δ and HA-IKKγ WT were cotransfected into E8i cells cultured on coverslips. Cells were stained with monoclonal anti-HA or polyclonal anti-FLAG Abs, followed by Alexa Fluor 594 anti-mouse Ab and Alexa Fluor 488 anti-rabbit Ab. Bottom, FLAG-IKKγ-Δ and HA-IKKγ WT were cotransfected into HeLa cells and stained as described above. Merged image (yellow in panels a and d) shows cytoplasmic colocalization.
To confirm that IKKγ-Δ coassociation was not dependent on levels of endogenous proteins, coimmunoprecipitation experiments were performed in IKKγ−/−-deficient E8i cells (42). In this experiment, E8i cells were transfected with HA-IKKγ WT or FLAG-IKKγ-Δ expression vectors. IKKγ WT-associated proteins were immunoprecipitated using anti-HA Ab, and assay of either IKKγ was detected using anti-IKKγ Ab that recognized both isoforms (Fig. 3B). Here again, strong binding of IKKγ-Δ to IKKγ WT was seen in the immunoprecipitates.
To exclude potential artifacts induced by biochemical fractionation, confocal colocalization experiments were performed using HA-tagged IKKγ WT and FLAG-IKKγ-Δ. Expression vectors encoding HA-IKKγ WT and FLAG-IKKγ-Δ were cotransfected into IKKγ−/−-deficient E8i cells (42). Cells were stained with monoclonal anti-HA or rabbit anti-FLAG Abs followed by Alexa Fluor 594 anti-mouse and Alexa Fluor 488 anti-rabbit Abs, producing red and green fluorescence, respectively. Consistent with previous studies, IKKγ WT was primarily distributed in a cytoplasmic distribution, with some nuclear staining (53). The IKKγ-Δ distribution was similar, being primarily cytoplasmic, with an apparently greater fraction distributed in the nucleus. The merged image shows strong cytoplasmic colocalization (Fig. 3C). Similar results were found in HeLa cells (Fig. 3C, bottom panel). Together, these data indicate that IKKγ-Δ is capable of homo- and heterotypic association in a cell type-independent manner.
IKKγ-Δ partitions into the nucleus and membrane compartment.
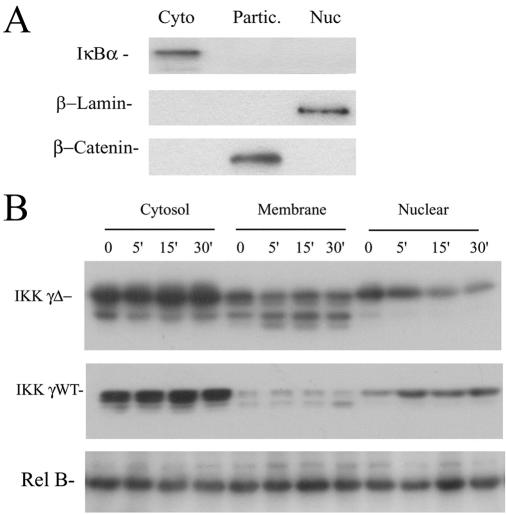
Previously, it has been shown that IKKγ is a dynamic molecule undergoing cytoplasmic-nuclear translocation (52). Inspection of our confocal microscopy experiments indicated that IKKγ-Δ apparently distributed into the nuclear compartment to a greater degree than IKKγ WT (Fig. 3C). To confirm this finding, and exclude artifactual distribution produced by transient overexpression, stable transfectants of epitope-tagged IKKγ WT and IKKγ-Δ in HeLa cells were generated. The cells were TNF stimulated and fractionated into cytoplasmic (100,000 × g supernatant), membrane-enriched particulate (100,000 × g pellet), or sucrose cushion-purified nuclear fractions using well-established protocols (14, 15). These subcellular fractions were first assayed for the presence of specific cytosolic (IκBα) membrane (β-catenin) and nuclear (lamin B) markers by Western immunoblotting. β-Catenin was chosen as a specific membrane marker because this protein forms intercellular adhesion complexes with E-cadherin in cell membranes (37). As seen in Fig. 4A, anti-β-catenin strongly stained the particulate fraction but not the cytoplasmic or nuclear subcellular fractions. Conversely, the cytoplasmic marker (IκBα) selectively stained the cytoplasmic compartment, and the nuclear marker (β-lamin) stained the nuclear fraction (Fig. 4A). Together, these observations indicate that the subcellular fractions were enriched in their respective marker proteins and could be used to determine relative partitioning of the IKKγ isoforms.
FIG. 4.
IKKγ-Δ partitions into nuclear and membrane compartments. (A) Characterization of subcellular fractionation. Unstimulated HeLa cells were fractionated into cytosolic (100,000 × g supernatant) (Cyto), membrane (100,000 × g pellet) (Partic.), and nuclear (Nuc) fractions as previously described (14). The abundance of specific cytoplasmic (IκBα), membrane (cytokeratin), and nuclear (lamin B) markers were determined by Western blotting. For example, the membrane fraction stains selectively with cytokeratins, not IκBa or lamin B, indicating that it is relatively free of detectable cytoplasmic or nuclear contamination. (B) Partitioning of IKK γ-Δ. Stably transfected HeLa cells expressing pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ were isolated, stimulated with TNF for indicated times (top, in min) and fractionated into cytosolic, particulate (membrane), and nuclear fractions. Shown is a Western immunoblot of equivalent cell amounts using anti-FLAG antibody. Top panel, IKKγ-Δ-expressing cells. Middle panel, IKKγ WT-expressing cells. Bottom panel, Rel B as a protein loading control. Rel B equally distributes into the cytoplasmic, membrane, and nuclear compartments (15). Relative to IKKγ WT, IKKγ-Δ has a greater distribution into the particulate fraction and shows distinct nuclear redistribution after TNF stimulation.
The relative abundance of IKKγ was determined by Western immunoblotting in these subcellular fractions (Fig. 4B). We noted that both IKKγ-Δ and IKKγ WT stable transfectants expressed multiple isoforms of apparently distinct size; these species probably represent differential posttranslational modifications, such as phosphorylation (39). Consistent with the confocal microscopy results, the majority of both IKKγ WT and IKKγ-Δ was cytosolic in location. In multiple independent clones, we noted that IKKγ-Δ had a relatively lower abundance in the cytosolic fraction and a greater proportion in the membrane-enriched particulate fraction relative to the distribution of IKKγ WT. Interestingly, although no detectable changes in the membrane or cytosolic fraction were produced by TNF stimulation, the nuclear abundance appeared to change upon cytokine stimulation, with IKKγ-Δ decreasing within 15 min of TNF stimulation (Fig. 4B). By contrast, the nuclear abundance of IKKγ WT increased after 5 min of TNF stimulation. Together, these data indicated that IKKγ-Δ partitions into cytoplasmic, membrane, and nuclear compartments.
IKKγ-Δ efficiently couples with the core signalsome kinase.
Previous studies have shown that IKKγ forms complexes with IKKβ, the major catalytic activity in the IKK responsible for IκBα phosphorylation (26, 38, 40). We next determined whether there were differences in the functional interaction of IKKγ WT and IKKγ-Δ with IKKβ. IKKγ-deficient 5R cells were transfected with NF-κB-LUC in the presence of either pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ and increasing amounts of IKKβ expression vector. In the presence of IKKγ WT, cotransfection of IKKβ increased reporter activity 2.3-fold at the lowest amount of IKKβ, which was maintained over a broad concentration range (Fig. 5A). Note that approximately equivalent expression levels of the FLAG-IKKγ WT and FLAG-IKKγ-Δ proteins are produced by these expression vectors (cf. Fig. 2A). In contrast, in the presence of IKKγ-Δ, IKKβ increased reporter activity 5-fold relative to its control value, with values consistently above those produced by IKKγ WT at any dose. These data suggested that IKKγ-Δ efficiently couples with IKKβ kinase to activate NF-κB-dependent transcription.
FIG. 5.
IKKγ-Δ couples with IKKβ catalytic kinases. (A) IKK γ-Δ potentiates the effect of IKKβ. 5R cells were transfected with NF-κB-LUC in the presence of either pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ and increasing amounts of IKKβ expression vector. For each transfection condition, normalized reporter activity was converted to relative activation over the control. Open boxes, no IKKβ; shaded boxes, IKKβ expression vector at the indicated doses. Results were repeated a minimum of three times. Although the relative activation is shown, similar findings were obtained for the raw luciferase values, where IKKγ-Δ induced greater absolute amounts of NF-κB-LUC activity for all concentrations of IKKβ (not shown). *, P < 0.05, two-tailed t test. (B) IP-kinase assays of IKKβ-IKKγ isoform complexes. Top panel, autoradiogram of IP-kinase assay. Cytoplasmic lysates of 5R cells transiently transfected with pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ in the absence (−−) or presence (++) of IKKβ were immunoprecipitated with anti-FLAG antibodies and used to phosphorylate GST-IκBα (1 to 51) in vitro. Radiolabeled GST-IκBα (1 to 51) was fractionated by SDS-PAGE and exposed for autoradiography. A doublet is seen due to NH2-terminal proteolysis of the GST molecule during preparation. Middle panel, anti-FLAG Western immunoblot (WB). Immunoprecipitates were subjected to Western immunoblotting to control for IKKγ recovery in the immunoprecipitation. Relative location of IKKγ isoforms are indicated at the right. Bottom panel, anti-IKKβ Western immunoblot. Indistinguishable amounts of IKKβ were detected in the immunoprecipitates, indicating that the kinase activity was increased. (C) IKKγ-Δ potentiates the effect of IKKβ in the HepG2 background. HepG2 cells were transfected with NF-κB-LUC in the presence of either pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ and increasing amounts of IKKβ expression vector as in Fig. 5A. *, P < 0.05, two-tailed t test. (D) IKK IP-kinase assay. Top panel, autoradiogram of IP-kinase assay. Cytoplasmic lysates of HepG2 cells transiently transfected with IKKβ in the presence of either pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ were assayed for IKK activity by IP-kinase as described for Fig. 5B. Bottom panel, the blots were probed with anti-FLAG. con, control.
To confirm that IKKγ-Δ associated with IKKβ and that the differences in NF-κB-dependent reporter activity were indeed due to bone fide changes in IKK kinase activity, immunoprecipitation (IP)-kinase assays were performed on transiently transfected 5R cells. In this experiment, cytoplasmic lysates of 5R cells transiently transfected with pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ in the absence or presence of IKKβ were immunoprecipitated with anti-FLAG antibodies; the IP-enriched IKK complexes were used to measure IKK activity by phosphorylating GST-IκBα (1 to 51) in vitro. In the case of IKKγ WT, no IKK activity was seen in its absence (Fig. 5B), yet strong induction of IKK activity was produced when IKKβ was cotransfected. We noted that in the presence of IKKγ-Δ, IKKβ induced significantly higher IKK activity than that induced in the presence of IKKγ WT. To exclude the trivial possibility that different abundances of IKKβ were recovered, the immunoprecipitates were assayed for total IKKβ abundance by Western immunoblotting and were found to be indistinguishable (Fig. 5B, bottom panel). These data indicate that IKKγ-Δ associates with IKKβ and strongly mediates its activation.
The composition of the IKK varies in a cell-type specific fashion where different abundances of IKKα/β are found (32). To confirm that the different behavior of the widely distributed IKKγ-Δ was a general phenomenon, not restricted to the Tax-transformed 5R cells, we compared their activities in the well-studied HepG2 cells that exhibit highly TNF-inducible NF-κB activation (3, 13, 14). However, in response to IKKβ, IKKγ-Δ mediated highly inducible NF-κB transcription in a similar manner as that seen in the 5R cells (Fig. 5C). For example, 1 μg of pcDNA-FLAG-IKKγ-Δ produced 28-fold activation of NF-κB-dependent luciferase activity, whereas the same amount of pcDNA-FLAG-IKKγ-WT induced only a 5-fold activation (Fig. 5C). IP-kinase assays confirmed enhanced IKKβ kinase activity in cells transfected with IKKγ-Δ over that produced by IKKγ WT in both untreated and TNF-α-stimulated condition (Fig. 5D). Together, these findings indicate that IKKγ-Δ effectively couples with IKKβ, mediating both kinase activity and NF-κB activation.
IKKγ-Δ efficiently couples TNF stimulation to endogenous NF-κB gene expression.
The 8321 cell, a clonal derivative of 3T8 Jurkat cells, was generated by ICR191 mutagenesis and lacks functional IKKγ expression (16). To determine the signal transducing properties of IKKγ WT and IKK γ-Δ, stably transfected 8321IKKγ-WT and 8321IKKγ-Δ cells were isolated. The expression of the respective FLAG-IKKγ isoform was confirmed by Western blotting, where the FLAG- and IKKγ-reactive species exactly comigrated (Fig. 6A). The relative ability of the two IKKγ isoforms to mediate inducible NF-κB activity was determined by Northern blot analysis of endogenous IκBα mRNA transcripts, a well-established NF-κB-dependent gene (48, 49). Compared to 8321EMPTY cells, we noted that basal IκBα expression was slightly (∼2-fold) higher in both 8321IKKγ-WT and 8321IKKγ-Δ transfectants. Upon TNF stimulation of the 8321EMPTY cells, cells that lack NF-κB signaling, a rapid loss of both IκBα and internal control RNA transcripts was seen due to TNF's proapoptotic activity (Fig. 6B) (16). In cells stably expressing the IKKγ isoforms, TNF induced IκBα expression more strongly in 8321IKKγ-Δ cells than 8321IKKγ-WT and 8321EMPTY cells. In stably transfected HeLa cells expressing IKKγ WT and IKK γ-Δ, TNF-inducible IκBα mRNA was also seen (Fig. 6C). Together, these data indicate that IKK γ-Δ efficiently couples the TNF signaling pathway and the catalytic kinase, IKKβ, to NF-κB activation in a cell type-independent manner.
IKKγ-Δ efficiently couples IKKα and NIK pathways to NF-κB activation.
In certain cell types, the IKKα signaling pathway to canonical NF-κB activation is distinct from that of IKKβ (25). To determine whether IKKγ-Δ functionally coupled to the IKKα signaling pathway, 5R cells were transfected with either IKKγ WT or IKK γ-Δ in the presence of increasing concentrations of IKKα (Fig. 7A). In the presence of IKKγ WT, IKKα expression induced a maximum of two- to threefold activation in reporter activity. By contrast, in the presence of 0.5 μg (and greater) of IKKγ-Δ expression vector, IKKα induced a significantly increased five to sixfold increase in NF-κB dependent reporter activity. More dramatic findings were observed in the HepG2 cellular background, where IKKα produced a 38-fold activation in the presence of 2 μg transfected IKKγ-Δ relative to a 12-fold activation in the presence of IKKγ WT (Fig. 7B). Together, these data suggest that the IKKγ-Δ also couples IKKα to IKK activation more efficiently than does IKKγ WT.
FIG. 7.
IKKγ-Δ couples with upstream IKKα and NIK kinases. (A) IKKγ-Δ efficiently couples with IKKα. 5R cells were transfected with NF-κB-LUC in the presence of either pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ and increasing amounts of IKKα expression vector. Reporter activity and data analysis were as described for Fig. 5A. White bar, no IKKα; black bar, IKKα expression vector at the indicated amounts (bottom). *, P < 0.05, two-tailed t test. (B) IKKγ-Δ couples with IKKα in the HepG2 background. HepG2 cells were transfected with either pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ and IKKα expression vector as described for Fig. 6A. (C) IKK γ-Δ recruits IKKα/β into the membrane. Nondenaturing coimmunoprecipitation assays were performed with FLAG-IKKγ WT- and IKKγ-Δ-expressing cells. Membrane fractions were isolated and immunoprecipitated (IP) with anti-FLAG antibodies. Top panel, Western immunoblot (WB) of immune complexes probed with anti-IKKβ and IKKα antibodies. The relative migration of IKKβ and IKKα is indicated at right. Bottom panel, the immunoprecipitates were probed with anti-FLAG antibody. Equivalent amounts of IKKγ WT and IKKγ-Δ are present in the immunoprecipitates. (D) IKKγ-Δ mediates NIK-dependent NF-κB activation in 5R. 5R cells were transfected with NF-κB-LUC, pcDNA-FLAG-IKKγ-WT, or pcDNA-FLAG-IKKγ-Δ and increasing amounts of NIK expression vector (amounts shown at bottom). NIK preferentially induces NF-κB transcription in the presence of IKKγ-Δ. (E) IKKγ-Δ mediates NIK-dependent NF-κB activation in the HepG2 background. HepG2 cells were transfected as described for Fig. 6C. At any concentration of NIK, NF-κB transcriptional activity is greater in the presence of IKKγ-Δ than that seen with IKKγ WT. +, present; −−, absent.
To determine whether increased IKKα-mediated transcription was the result of enhanced recruitment of IKKα/β heterodimer to IKKγ-Δ complexes, we performed co-IP experiments. Because the site of activation of IKK is thought to be in the membrane fraction, membrane fractions were immunoprecipitated with the FLAG Ab and associated IKK was determined by Western blotting. In this experiment, the amount of membrane fraction was adjusted so that equivalent amounts of IKKγ WT and IKKγ-Δ were input in the immunoprecipitation reaction mixtures. We observed that the membrane-associated IKKγ-Δ had enhanced IKKα and β compared to that associated with IKKγ WT (Fig. 7C, upper). Controls for the presence of IKKγ indicate equivalent abundances of each isoform (Fig. 7C, lower panel), indicating that IKKγ-Δ has a greater affinity or stoichiometry for core IKK kinases, recruiting them into a preactivated state in the membrane-enriched particulate fraction.
IKKγ-Δ efficiently couples with NIK.
NIK/MEKK14, a member of the MAP3K family, has been reported to complex with the TNF receptor-associated factor 2, an early step in the IKK activation pathway downstream of the TNF and interleukin-1 receptors (30). To determine if IKKγ-Δ-containing IKK complexes mediated NIK-inducible NF-κB activation, a eukaryotic NIK expression vector was transiently transfected into 5R cells in the presence of saturating concentrations of IKKγ WT and IKKγ-Δ. In the presence of IKKγ WT, NIK activated NF-κB-dependent reporter activity only 1.3-fold, whereas in the presence of IKKγ-Δ, NIK potently activated NF-κB-LUC activity by ∼13-fold, significantly higher than IKKγ WT at all concentrations tested (Fig. 7D). These findings were replicated in the HepG2 background to exclude cell type-specific effects on NIK activation, where similar differences between IKKγ-Δ and IKKγ WT were found (Fig. 7E).
IKKγ-Δ is unable to mediate IKK activity induced by HTLV-1 Tax.
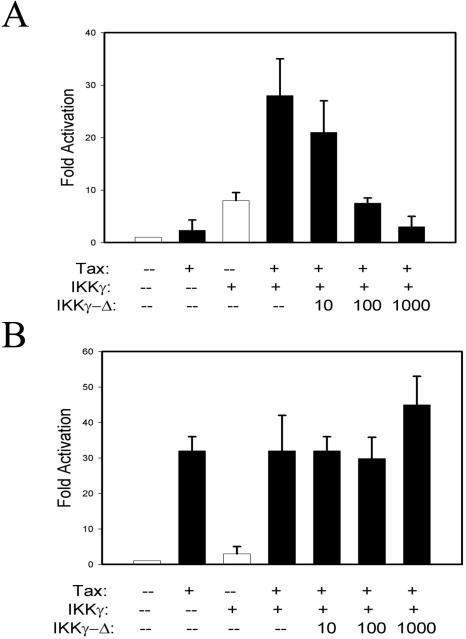
HTLV-1 Tax is a potent inducer of NF-κB signaling and affects many steps in its activation pathway. Of these, IKKγ-mediated recruitment to the IKK complex plays an important role (36). Because earlier studies indicated that IKKγ amino acid residues 196 to 419, overlapping the region of the alternatively spliced exon 5, functioned as a dominant-negative inhibitor of Tax-induced IKK activity (18), we sought to determine the functional ability of IKKγ-Δ in mediating Tax-dependent transcription. In this first experiment, Tax-transformed 5R cells were cotransfected with NF-κB-LUC in the presence of increasing concentrations of pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ expression plasmids. As shown in Fig. 8A, consistent with earlier studies, a low level of pcDNA-FLAG-IKKγ-WT was a potent activator of NF-κB-dependent reporter activity, producing a 17-fold induction of reporter activity (in presence of 10 ng of expression vector). At higher concentrations, reporter activity fell, a previously described phenomenon probably due to disruption of the precise stoichiometry of the IKK complex (57). Surprisingly, transfection of pcDNA-FLAG-IKKγ-Δ produced a distinct response, being unable to mediate NF-κB-dependent transcriptional activity, producing only twofold induction of NF-κB-dependent reporter activity at 100 and 500 ng of transfected expression vector and returning to control values at 1 μg (Fig. 8A). These data indicated that, in contrast to cytokine-induced IKK activation, IKKγ-Δ did not mediate Tax-inducible NF-κB activity.
FIG. 8.
IKKγ-Δ is unable to mediate HTLV-1 Tax-induced IKK activity. (A) IKKγ alternate-splice forms differentially mediate Tax-induced IKK activation. Triplicate plates of Tax-transformed 5R cells were transfected with NF-κB-LUC in the presence of increasing concentrations of pcDNA-FLAG-IKKγ-WT and pcDNA-FLAG-IKKγ-Δ. The total amount of DNA was kept constant with empty expression vector. Normalized luciferase activity was calculated for each triplicate result and plotted. pcDNA-FLAG-IKKγ-WT activated NF-κB-LUC activity 4-fold (5 ng), 17-fold (10 ng), 12-fold (100 ng), 3-fold (500 ng), and 1-fold (1000 ng). pcDNA-FLAG-IKKγ-Δ activated NF-κB-LUC activity 1-fold (5 ng), 0.8-fold (10 ng), 2-fold (100 ng), 2-fold (500 ng), and 1-fold (1,000 ng). The experiment was repeated three times with similar results. *, P < 0.05, two-tailed t test. (B) IKKγ WT, but not IKKγ-Δ, mediates Tax-inducible IKK activation. Triplicate plates of E8i cells were transfected with NF-κB-LUC in the presence of increasing amounts of either pcDNA-FLAG-IKKγ-WT or pcDNA-FLAG-IKKγ-Δ and pCMV-Tax. Empty plasmid was used to maintain identical DNA concentrations. Normalized reporter activity was expressed as change relative to plates transfected with empty Tax expression vector (pcDNA). (C) Subcellular localization of IKKγ isoforms and Tax. Transfected E8i cells were fixed, permeabilized, and stained with either monoclonal anti-HA (HA-IKKγ [a, b]) or monoclonal anti-FLAG (FLAG-IKKγ-Δ [d, e]) Ab followed with anti-mouse conjugated to Alexa Fluor 594 (red) (Molecular Probes). The subcellular localization of GFP-Tax (g, h) was directly revealed by laser excitation of its GFP tag (green). Nuclei were counterstained with DAPI (c, f, i) (blue). (D) Colocalization of IKKγ-Δ and Tax. E8i cells were transfected with of HA-IKKγ (a, b) or FLAG-IKKγ-Δ (d, e) (stained in red as described for panel A) with GFP-Tax (c, f) (green) resulted in trans-localization of GFP-Tax from nucleus to cytoplasm. Arrows indicate cytoplasmic colocalization of GFP-Tax and IKKγ or IKKγ-Δ. (E) Coassociation of IKKγ-Δ and Tax. HeLa cells were transfected with pCMV-Tax or pcDNA-FLAG-IKKγ-Δ as indicated. Whole-cell lysates were immunoprecipitated with anti-FLAG Ab and immunoblotted (IB) with anti-Tax. Migration of Tax is shown at right. Tax is captured by FLAG Ab only in lysates cotransfected with IKKγ-Δ. Bottom panel, lysates are immunoblotted with anti-Tax. +, present; −, absent; −−, absent.
To exclude the possibility that this finding was anomalous due to the extensively mutagenized 5R cells (57), a similar experiment was conducted in IKKγ-deficient embryonic fibroblasts (E8i cells) transfected with increasing concentrations of IKKγ-Δ in the absence or presence of HTLV-1 Tax expression vector. In the absence of IKKγ, Tax was unable to activate NF-κB, but potently activated at 10- and 100-ng amounts of transfected IKKγ, producing an ∼5- to 6-fold activation over that observed with IKKγ WT alone (Fig. 8B). In contrast, IKKγ-Δ only weakly mediated Tax-inducible NF-κB reporter activity, producing an ∼1.5-fold activation over that observed with IKKγ-Δ alone. Together, these data consistently suggested that IKKγ-Δ was functionally distinct from IKKγ WT, selectively lacking the ability to mediate Tax-dependent IKK activity.
IKKγ-Δ forms stable cytoplasmic complexes with HTLV-1 Tax.
The ability of Tax to activate IKK is mediated by its ability to directly interact with IKKγ and be recruited into the IKK complex (21, 21, 36). We therefore sought to determine whether Tax associated with IKKγ-Δ by confocal colocalization. Expression vectors encoding either FLAG-IKKγ WT, FLAG-IKKγ-Δ, or Tax alone and mixtures of the IKKγ isoforms in the presence of Tax were transfected into E8i cells. The subcellular localization of each was determined by confocal immunofluorescence microscopy (Fig. 8C). Expression of GFP-Tax alone indicates that the protein primarily localizes to the nucleus in punctate nuclear structures, with a fine granular distribution in the cytoplasm. By contrast, in the presence of either IKKγ WT or IKKγ-Δ, Tax is redistributed into cytoplasmic complexes where the merged signal indicates colocalization of the two proteins (Fig. 8D). Similar conclusions were produced by nondenaturing coimmunoprecipitation experiments, where strong association of Tax with IKKγ-Δ was observed (Fig. 8E). Together these data indicate that both IKKγ WT and IKKγ-Δ strongly associate with Tax, although only IKKγ WT mediates productive NF-κB activation.
IKKγ-Δ is a dominant-negative inhibitor of Tax-IKKγ WT activation.
As an independent test of stable Tax-IKKγ-Δ association, we reasoned that if IKKγ-Δ binds Tax, it should function as a dominant-negative inhibitor of Tax-IKKγ WT signaling. To examine this question, increasing concentrations of IKKγ-Δ were cotransfected with plasmids encoding Tax and IKKγ. In the absence of IKKγ-Δ, a significant transactivation of NF-κB-LUC reporter was produced (Fig. 9A). However, cotransfection of increasing amounts of IKKγ-Δ (from 10 to 1,000 ng) significantly diminished the NF-κB reporter activity (Fig. 9A). As a control for specificity, we examined the effect of IKKγ-Δ on Tax's ability to transactivate the HTLV-1-long terminal repeat (LTR)-LUC reporter. Tax initiates many signaling pathways to transactivate the HTLV-1 LTR mediated not only through NF-κB but also through the CREB and serum response elements (36). The same titration experiment indicated that IKKγ-Δ failed to affect Tax activation of the HTLV-1-LTR-LUC reporter (Fig. 9B), indicating that IKKγ-Δ was selectively inhibiting Tax-mediated NF-κB transactivation. Together, these data indicated that IKKγ-Δ was a functionally distinct IKKγ splice form, being able to mediate cytokine-induced NF-κB activity but not HTLV-1 Tax.
FIG. 9.
IKKγ-Δ is a dominant-negative inhibitor of Tax-IKKγ WT-mediated IKK activation. (A) Tax and IKKγ (10 ng) were cotransfected with NF-κB-LUC reporter in the presence (+) of increasing amounts of IKKγ-Δ (from 10 to 1,000 ng). The total amount of DNA was normalization by addition of pcDNA3. Shown is the normalized luciferase reporter activity. (B) Same transfection condition as in Fig. 5A except HTLV-1-LTR-LUC was used as a reporter. IKKγ-Δ fails to inhibit Tax activation of HTLV-1-LTR-LUC reporter. −−, absent.
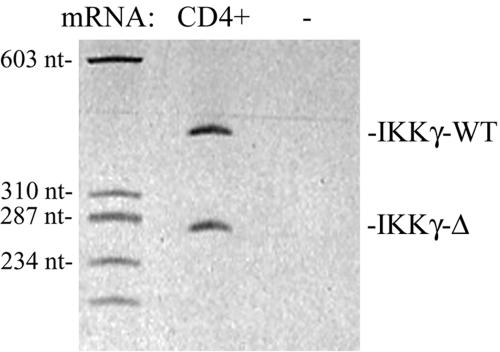
HTLV-1 was originally isolated from patients with cutaneous T-cell lymphoma or adult T-cell leukemia, and shows trophism for CD4+ lymphocytes. To determine whether IKKγ-Δ was expressed in this cell type, we performed RT-PCR assays for the IKKγ transcripts from normal human CD4+ lymphocytes. Here, nearly equivalent levels of IKKγ WT and IKKγ-Δ transcripts were found (Fig. 10).
FIG. 10.
IKKγ transcripts in human CD4+ lymphocytes. Shown is a PAGE gel of IKKγ RT-PCR assay. Lane 1, molecular weight standards. Lane 2, RT-PCR using CD4+ cDNA. Lane 3, RT-PCR without (−) cDNA input. Size (in nucleotides) of molecular weight standards shown at left. Locations of IKKγ-Δ and IKKγ WT transcripts are indicated at right.
DISCUSSION
NF-κB is a highly pathogen- and cytokine-inducible transcription factor that controls genes involved in the immune response, intercellular signaling, hepatic acute-phase response, and cellular survival (50). The rate-limiting step in NF-κB activation is controlled by the activity of the IKK, a multiprotein transducing complex that converts intracellular signals into the serine phosphorylation of the IκB inhibitors. Recent work has clearly shown that regulated signal transduction through IKK is dependent on noncatalytic proteins associated with the complex. Specifically, IKKγ lacks intrinsic kinase activity but is required for stimulus-dependent IKK activation, where its role is to mediate IKK oligomerization and serve as an adapter for upstream kinases (8, 38). In this report, we describe the presence and functional characteristics of a previously unknown alternatively spliced product of IKKγ, IKK γ-Δ, produced by exclusion of exon 5. Western blots and qualitative RT-PCR assays indicate that IKKγ-Δ expression is widely distributed. In fact, in normal human breast tissues and cultured HeLa and Hep3B cells, IKKγ-Δ is the predominant IKKγ transcript. Because IKKγ-Δ has distinct self-association properties, we investigated the hypothesis that it has unique signaling activity. Here we report our surprising findings that IKKγ-Δ mediates IKKβ and NIK-IKKα induces NF-κB transcription, it does not couple with HTLV-1 Tax. These observations suggest IKKγ-Δ differentially couples the IKK signalsome to upstream activators and reveals unexpected complexity in IKK regulation.
Previous work has shown that the IKKγ gene is carried by the 10-exon-containing gene located at chromosome Xq28 (22). In humans, mutations in the IKKγ gene that have been identified primarily encode truncations of the IKKγ protein; these mutations have been linked to the syndromes of incontinentia pigmenti and anhidrotic ectodermal dysplasia associated with immunodeficiency (10). Like IKKβ−/− knockout cells, cells from these patients do not activate NF-κB in response to cytokines (10, 57). Although human IKKγ transcripts containing an alternatively spliced first exon have been deposited in GenBank (AI24572, AF091453), these alternatively spliced transcripts encode IKKγ WT (22). Our data are the first to demonstrate the existence of a previously unsuspected IKKγ exon 5 deletion transcript that encodes a functionally distinct signaling protein. It is interesting to us that two different 50- and 52-kDa IKKγ isoforms were identified in an earlier study by affinity chromatography purification in HeLa cells (40). Although 10 distinct tryptic peptides were sequenced, none corresponded to exon 5, perhaps indicating that the exon 5-occluded IKKγ-Δ was present in this preparation. Because IKKγ-Δ has distinct signaling properties, our data suggest that the relative abundance of the two IKKγ isoforms may contribute to cell type-specific signaling responses to TNF and HTLV-1. This hypothesis will require further experimental verification.
Because of its role in linking IKK recruitment to upstream kinases, IKKγ is known to associate with many proteins in the IKK activation pathway, including upstream kinases, and also is the target for direct IKK activators. Mapping studies have shown that IKKγ binds to the IKKα and β subunits through a region in its NH2-terminal 119 aa, a region unaffected in the IKKγ-Δ splice variant (31, 32, 58). In contrast, the region deleted by exon 5 occlusion encodes amino acids 174 to 224, a coiled-coil domain shown to be important in IKKγ self-association (58). Coiled-coil domains are heptad repeats of apolar residues recognized to be one of the principal subunit oligomerization motifs in proteins (5). That IKKγ self-association is critical for IKK activation has been shown in elegant experiments where enforced oligomerization could be exogenously induced using IKKγ fusions with the FK506 binding domain. In these studies, oligomerization alone was sufficient for hormone-independent IKK activation (38). Our findings indicate that IKKγ-Δ has stronger self-association properties than the wild-type protein and may account for its enhanced ability to mediate IKK activation through both the core signalsome and the NIK-IKKα pathways. IKKγ-Δ may cause IKKα/β to associate more productively with themselves, producing enhanced autophosphorylation, or maintain the complex in a conformation that is more accessible to activating upstream MAP3K.
The IKK is a dynamic complex, undergoing changes in subcellular localization under basal and stimulated conditions. In this study, we find that IKKγ-Δ partitions into cytoplasmic, membrane, and nuclear compartments by biochemical fractionation and fluorescent microscopy studies. Consistent with our findings here, previous fluorescence microscopy studies have shown that IKKγ constitutively translocates into the nucleus (53). Similarly, in response to stimulation, IKKγ serves an important role in IKK activation through its ability to recruit the preactivated IKK complex into membrane complexes where it can associate with upstream kinases and intracellular domains of activated receptors (38). Of note, we find that IKKγ-Δ partitions to a greater extent than IKKγ WT into the particulate/membrane fraction of unstimulated cells (Fig. 3, 4, 8). Because cells expressing IKKγ-Δ signal to a consistently greater degree in response to TNF, IKKβ, IKKα, and NIK (Fig. 5 to 7), we suggest that this membrane-localizing property may make a greater fraction of IKKγ-Δ-associated IKK readily available for activation by receptor-associated kinases. More studies will need to be done to directly test this mechanism, including determining whether the membrane-associated IKKγ-Δ is associated with the core or upstream IKK-activating kinases and whether there are differences in the dynamics of IKKγ-Δ and IKKγ WT, developing strategies to disrupt the membrane partitioning of IKKγ-Δ, and determining its effect on IKK activation.
Tax is the transforming activity of HTLV-1 and is known to persistently activate NF-κB, producing cellular activation and increased expression of progeny virus in infected lymphocytes (reviewed in reference 20). In HTLV-1-infected cells, Tax is found to be stably associated with the IKK complex (21). Studies dissecting interacting domains of IKKγ bound by Tax identified two separate leucine zipper motifs in IKKγ, one located in the NH2 terminus (aa 100 to 140), and the second at the COOH terminus (aa 312 to 340), that were required for Tax interaction (55). Importantly, disruption of Tax-IKKγ interactions blocks the ability of Tax to activate the IKK (55). Interestingly, these regions are outside the occluded exon 5, and therefore, it was perhaps not unexpected to find that IKKγ-Δ associates with Tax both in colocalization and co-IP experiments (Fig. 8). What is surprising in our study is that IKKγ-Δ is unable to mediate IKK activation and, in fact, can function as a dominant-negative inhibitor. These findings suggest that, although binding to IKKγ is necessary for Tax-induced IKK activation, it is not sufficient.
To us, these findings suggest further analysis of the nonproductive Tax-IKKγ-Δ complex could provide important new insights into how Tax-IKKγ WT interaction results in productive IKK activation. Our data are consistent with a number of possibilities that will require further investigation: (i) IKKγ-Δ may not form a permissive structural conformation when it is bound to Tax; (ii) IKKγ-Δ may produce a nonproductive stoichiometry of the IKK complex in response to Tax binding; and/or (iii) IKKγ-Δ may not be permissive for recruitment of uncharacterized cellular factors that Tax otherwise normally recruits to activate IKK. Of relevance, others have found that separate domains of IKKγ, located outside the Tax binding domains, are required for mediating Tax-inducible IKK activity. Deletion studies indicate that the mid-molecule region of IKKγ (aa 195 to 300) are required for Tax-induced oligomerization, a necessary step in forming the activatable, high-molecular-weight signalsome (18). Our previous studies using monoclonal Abs that recognize specific IKKγ domains to disrupt IKK signaling identified an Ab, termed EA2-6, that selectively disrupted Tax-, but not TNF-inducible NF-κB activity (18). This study was the first to indicate that distinct domains of IKKγ are required for mediating these two signaling pathways to IKK activation. We also note that other studies have suggested that Tax may recruit upstream activating kinases, such as MLK, NIK, or MEKK1, into the signalsome to produce persistent IKK activation (20). In this regard, our data indicate that IKKγ-Δ effectively mediates NIK-inducible IKK activity (Fig. 7), indirectly excluding NIK as the cellular cofactor important in Tax-induced IKK activation. Whether IKKγ-Δ mediates MLK- or MEKK1-inducible IKK activity will need to be addressed in future studies. Finally, because of IKKγ-Δ's high level of endogenous expression in CD4+ lymphocytes, an important target cell for HTLV-1-induced transformation, it is interesting to postulate that modulated expression of IKKγ mRNA alternative splicing may have therapeutic implications for HTLV-1 treatments.
In summary, we have identified a ubiquitously expressed alternative splice product, IKK γ-Δ, that encodes a regulatory subunit of the IKK with distinct functional properties. IKK γ-Δ has strong self-association properties and distributes into the membrane/particulate fraction of cells. Although IKKγ-Δ mediates efficient coupling with the TNF- and NIK-IKKα pathways for NF-κB activation, IKKγ-Δ does not mediate Tax-induced IKK activation. Together, these findings suggest that the novel IKKγ-Δ splice product differentially couples signaling cascades to the NF-κB transcription factor. Moreover, its ability to differentially mediate IKK activation could potentially be exploited to disrupt oncogenic Tax signaling without affecting cytokine-induced NF-κB activation important in homeostatic responses to inflammation and antiapoptosis.
Acknowledgments
We thank Adrian Ting for the gift of 8321 cells; Warner Greene and David Goeddel for expression plasmids; and Deepthi Kolli and Roberto Garofalo for CD4+ lymphocyte isolation.
This work was supported by NIAID grant 40218 (A.R.B.). Grants NHLBI N01-HV-28184 (Alex Kurosky, UTMB), NIEHS P30 ES06676 (J. Halpert, UTMB), and NIAID PO1 AI062885 (A.R.B.) provided core laboratory support.
REFERENCES
- 1.Beg, A. A., and A. S. J. Baldwin. 1993. The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 7:2064-2070. [DOI] [PubMed] [Google Scholar]
- 2.Brasier, A. R., M. Jamaluddin, A. Casola, W. Duan, Q. Shen, and R. Garofalo. 1998. A promoter recruitment mechanism for tumor necrosis factor-α-induced IL-8 transcription in type II pulmonary epithelial cells: dependence on nuclear abundance of Rel A, NF-κB1 and c-Rel transcription factors. J. Biol. Chem. 273:3551-3561. [DOI] [PubMed] [Google Scholar]
- 3.Brasier, A. R., M. Lu, T. Hai, Y. Lu, and I. Boldogh. 2001. NF-κB inducible BCL-3 expression is an autoregulatory loop controlling nuclear p50/NF-kB1 residence. J. Biol. Chem. 276:32080-32093. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 5.Burkhard, P., S. Stetefeld, and S. V. Strelkov. 2001. Coiled coils: a highly versatile protein folding motif. Trends Cell Biol. 11:82-88. [DOI] [PubMed] [Google Scholar]
- 6.Carter, R. S., K. N. Pennington, B. J. Ungurait, and D. W. Ballard. 2003. In vivo identification of inducible phosphoacceptors in the IKKgamma/NEMO subunit of human IkappaB kinase. J. Biol. Chem. 278:19642-19648. [DOI] [PubMed] [Google Scholar]
- 7.Chan, Y., S. Ackerman, N. Shepherd, O. McBride, S. Widen, S. Wilson, and T. Wood. 1994. The human DNA polymerase b gene structure: evidence of alternative splicing in gene expression. Nucleic Acids Res. 22:2719-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chariot, A., A. Leonardi, J. Muller, M. Bonif, K. Brown, and U. Siebenlist. 2002. Association of the adaptor TANK with the I kappa B kinase (IKK) regulator NEMO connects IKK complexes with IKK epsilon and TBK1 kinases. J. Biol. Chem. 277:37029-37036. [DOI] [PubMed] [Google Scholar]
- 9.Choudhary, S., S. Boldogh, R. P. Garofalo, M. Jamaluddin, and A. R. Brasier. 2005. RSV influences NF-κB-dependent gene expression through a novel pathway involving MAP3K14/NIK expression and nuclear complex formation with NF-κB2. J. Virol. 79:8948-8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtois, G., A. Smahi, and A. Israel. 2001. NEMO/IKKg: linking NF-κB to human disease. Trends Mol. Med. 7:427-430. [DOI] [PubMed] [Google Scholar]
- 11.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandl, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 12.Guan, K. L., and J. E. Dixon. 1991. Eukaryotic proteins expressed in E. coli: An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192:262-267. [DOI] [PubMed] [Google Scholar]
- 13.Han, Y., and A. R. Brasier. 1997. Mechanism for biphasic Rel A:NF-kB1 nuclear translocation in tumor necrosis factor α-stimulated hepatocytes. J. Biol. Chem. 272:9823-9830. [DOI] [PubMed] [Google Scholar]
- 14.Han, Y., T. Meng, N. R. Murray, A. P. Fields, and A. R. Brasier. 1999. Interleukin-1-induced nuclear factor-κB-I κBa autoregulatory feedback loop in hepatocytes: a role for protein kinase Cα in post-transcriptional regulation of IκBα resynthesis. J. Biol. Chem. 274:939-947. [DOI] [PubMed] [Google Scholar]
- 15.Han, Y., S. A. Weinman, S. Boldogh, and A. R. Brasier. 1999. TNFα-inducible IκBα proteolysis and NF-κB activation mediated by cytosolic m-calpain. J. Biol. Chem. 274:787-794. [DOI] [PubMed] [Google Scholar]
- 16.He, K.-L., and A. T. Ting. 2002. A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Mol. Cell. Biol. 22:6034-6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huynh, Q. K., H. Boddupalli, S. A. Rouw, C. M. Koboldt, T. Hall, C. Sommers, S. D. Hauser, J. L. Pierce, R. G. Combs, B. A. Reitz, J. A. Diaz-Collier, R. A. Weinberg, B. L. Hood, B. F. Kilpatrick, and C. S. Tripp. 2000. Characterization of the recombinant IKK1/IKK2 heterodimer. Mechanisms regulating kinase activity. J. Biol. Chem. 275:25883-25891. [DOI] [PubMed] [Google Scholar]
- 18.Iha, H., K. V. Kibler, V. R. K. Yedavalli, J.-M. Peloponese, K. Haller, A. Miyazato, T. Kasai, and K.-T. Jeang. 2003. Segregation of NF-kappaB activation through NEMO/IKK gamma by Tax and TNFalpha: implications for stimulus-specific interruption of oncogenic signaling. Oncogene 22:8912-8923. [DOI] [PubMed] [Google Scholar]
- 19.Jamaluddin, M., T. Meng, J. Sun, I. Boldogh, Y. Han, and A. R. Brasier. 2000. Angiotensin II induces nuclear factor (NF)-kappaB1 isoforms to bind the angiotensinogen gene acute-phase response element: a stimulus-specific pathway for NF-kappaB activation. Mol. Endocrinol. 14:99-113. [DOI] [PubMed] [Google Scholar]
- 20.Jeang, K. T. 2001. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-κB. Cytokine Growth Factor Rev. 12:207-217. [DOI] [PubMed] [Google Scholar]
- 21.Jin, D. Y., V. Giordano, K. V. Kibler, H. Nakano, and K. T. Jeang. 1999. Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. J. Biol. Chem. 274:17402-17405. [DOI] [PubMed] [Google Scholar]
- 22.Jin, D.-Y., and K.-T. Jeang. 1998. Isolation of full length cDNA and chromosomal localization of human NF-κB modulator NEMO to Xq28. J. Biomed. Sci. 6:115-120. [DOI] [PubMed] [Google Scholar]
- 23.Karin, M. 1999. The beginning of the end: IκB kinase (IKK) and NF-κB activation. J. Biol. Chem. 274:27342. [DOI] [PubMed] [Google Scholar]
- 24.Karin, M., and Y. Ben Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 25.Lamb, P., H. M. Seidel, J. Haslam, L. Milocco, L. V. Kessler, R. B. Stein, and J. Rosen. 1995. STAT protein complexes activated by interferon-gamma and gp130 signaling molecules differ in their sequence preferences and transcriptional induction properties. Nucleic Acids Res. 23:3283-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Q., D. Van Antwerp, F. Mercurio, K.-F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science 284:321-325. [DOI] [PubMed] [Google Scholar]
- 27.Li, Y., J. Kang, J. Frieman, L. Tarassishin, J. Ye, A. Kovalenko, D. Wallach, and M. S. Horwitz. 1999. Identification of a cell protein (FIP-3) as a modulator of NF-κB activity and as a target of an adenovirus inhibitor of tumor necrosis factor α-induced apoptosis. Proc. Natl. Acad. Sci. USA 96:1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, X., Y. Mu, E. T. Cunningham, K. B. Marcu, R. Geleziunas, and W. G. Greene. 1998. Molecular determinants of NF-κB inducing kinase action. Mol. Cell. Biol. 18:5899-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lupas, A. 1996. Prediction and analysis of coiled-coil structures. Methods Enzymol. 266:513-525. [DOI] [PubMed] [Google Scholar]
- 30.Malanin, N. L., M. P. Boldin, A. V. Kovalenko, and D. Wallach. 1997. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature 385:540-544. [DOI] [PubMed] [Google Scholar]
- 31.May, M. J., F. D'Acquisto, L. A. Madge, J. Glockner, J. S. Pober, and S. Ghosh. 2000. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 289:1550-1554. [DOI] [PubMed] [Google Scholar]
- 32.Mercurio, F., B. W. Murray, A. Shevchenko, B. L. Bennett, D. B. Young, J. W. Li, G. Pascual, A. Motiwala, H. Zhu, M. Mann, and A. M. Manning. 1999. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol. Cell. Biol. 19:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:818-819. [DOI] [PubMed] [Google Scholar]
- 34.Nakano, H., M. Shindo, S. Sakon, S. Nishinaka, M. Mihara, H. Yagita, and K. Okumura. 1998. Differential regulation of IκB kinase a and b by two upstream kinases, NF-κB inducing kinase and mitogen-activated protein kinase/erk kinase kinase-1. Proc. Natl. Acad. Sci. USA 95:3537-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Mahony, A., X. Lin, R. Geleziunas, and W. C. Greene. 2000. Activation of the heterodimeric IκB kinase α (IKKα)-IKKβ complex is directional: IKKα regulates IKKβ under both basal and stimulated conditions. Mol. Cell. Biol. 20:1170-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pahl, H. 1999. Activators and target genes of Rel/NF-kB transcription factors. Oncogene 18:6853-6866. [DOI] [PubMed] [Google Scholar]
- 37.Pokutta, S., and W. I. Weis. 2002. The cytoplasmic face of cell contact sites. Curr. Opin. Struct. Biol. 12:255-262. [DOI] [PubMed] [Google Scholar]
- 38.Poyet, J.-L., S. M. Srinivasula, J.-H. Lin, T. Fernandes-Alnemri, S. Yamaoka, P. N. Tsichlis, and E. S. Alnemri. 2000. Activation of the IκB kinases by RIP via IKKγ/NEMO-mediated oligomerization. J. Biol. Chem. 275:37966-37977. [DOI] [PubMed] [Google Scholar]
- 39.Prajapati, S., and R. B. Gaynor. 2002. Regulation of Ikappa B kinase (IKK)gamma/NEMO function by IKKbeta-mediated phosphorylation. J. Biol. Chem. 277:24331-24339. [DOI] [PubMed] [Google Scholar]
- 40.Rothwarf, D. M., E. Zandi, G. Natoli, and M. Karin. 1998. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature 395:297-300. [DOI] [PubMed] [Google Scholar]
- 41.Rudolph, D., W. C. Yeh, A. Wakeham, B. Rudolph, D. Nallainathan, J. Potter, A. J. Elia, and T. W. Mak. 2000. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 14:854-862. [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt-Supprian, M., W. Bloch, G. Courtois, K. Addicks, A. Israel, K. Rajewsky, and M. Pasparakis. 2000. NEMO/IKKγ-deficient mice model incontinentia pigmenti. Mol. Cell 5:981-992. [DOI] [PubMed] [Google Scholar]
- 43.Senftleben, U., Y. Cao, G. Xiao, F. R. Greten, G. Krahn, G. Bonizzi, Y. Chen, Y. Hu, A. Fong, S. C. Sun, and M. Karin. 2001. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293:1495-1499. [DOI] [PubMed] [Google Scholar]
- 44.Sheppard, K., D. W. Rose, Z. K. Haque, R. Kurokawa, E. McInerney, S. Westin, D. Thanos, M. G. Rosenfeld, C. K. Glass, and T. Collins. 1999. Transcriptional activation by NF-κB requires multiple coactivators. Mol. Cell. Biol. 19:6367-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smahi, A., G. Courtois, P. Vabres, S. Yamaoaka, S. Heuertz, A. Munnich, A. Israel, N. S. Heiss, S. M. Klauch, et al. 2000. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incotntientia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature 405:466-472. [DOI] [PubMed] [Google Scholar]
- 46.Smith, M. R., and W. C. Greene. 1990. Identification of HTLV-I tax transactivator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 4:1875-1885. [DOI] [PubMed] [Google Scholar]
- 47.Tang, E. D., C. Y. Wang, Y. Xiong, and K. L. Guan. 2003. A role for NF-kappaB essential modifier/IkappaB kinase-gamma (NEMO/IKKgamma) ubiquitination in the activation of the IkappaB kinase complex by tumor necrosis factor-alpha. J. Biol. Chem. 278:37297-37305. [DOI] [PubMed] [Google Scholar]
- 48.Tian, B., and A. R. Brasier. 2003. Identification of an NF-κB dependent gene network. Recent Prog. Horm. Res. 58:95-130. [DOI] [PubMed] [Google Scholar]
- 49.Tian, B., D. Nowak, and A. R. Brasier. 2005. A TNF-induced gene expression program under oscillatory NF-κB control. BMC Genomics 6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian, B., Y. Zhang, B. A. Luxon, R. P. Garofalo, A. Casola, M. Sinha, and A. R. Brasier. 2002. Identification of NF-κB-dependent gene networks in respiratory syncytial virus-infected cells. J. Virol. 76:6800-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian, B., D. E. Nowak, M. Jamaluddin, S. Wang, and A. R. Brasier. 2005. Identification of direct genomic targets downstream of the NF-kappa B transcription factor mediating TNF signaling. J. Biol. Chem. 280:17435-17448. [DOI] [PubMed] [Google Scholar]
- 52.Verma, U. N., Y. Yamamoto, S. Prajapati, and R. B. Gaynor. 2003. Nuclear role of IKKgamma /NEMO in NF-kappa B-dependent gene expression. J. Biol. Chem. M309300200. [DOI] [PubMed]
- 53.Verma, U. N., Y. Yamamoto, S. Prajapati, and R. B. Gaynor. 2004. Nuclear role of IκB Kinase-γ/NF-κB essential modulator (IKKγ/NEMO) in NF-κB-dependent gene expression. J. Biol. Chem. 279:3509-3515. [DOI] [PubMed] [Google Scholar]
- 54.Woronicz, J., X. Gao, Z. Cao, M. Rothe, and D. V. Goeddel. 1998. IkB kinase-b: NF-κB activation and complex formation with IkB kinase a and NIK. Science 866-869. [DOI] [PubMed]
- 55.Xiao, G., E. W. Harhaj, and S. C. Sun. 2000. Domain-specific interaction with the Ikappa B kinase (IKK) regulatory subunit IKKgamma is an essential step in Tax-mediated activation of IKK. J. Biol. Chem. 275:34060-34067. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto, Y., D. W. Kim, Y. T. Kwak, S. Prajapati, U. Verma, and R. B. Gaynor. 2001. IKKgamma /NEMO facilitates the recruitment of the IkappaB proteins into the IkappaB kinase complex. J. Biol. Chem. 276:36327-36336. [DOI] [PubMed] [Google Scholar]
- 57.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 58.Ye, J., X. Xie, L. Tarassishin, and M. S. Horwitz. 2000. Regulation of the NF-κB activation pathway by isolated domains of FIP3/IKKγ, a component of the IκB-α kinase complex. J. Biol. Chem. 275:9882-9889. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, S. Q., A. Kovalenko, G. Cantarella, and D. Wallach. 2000. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKγ) upon receptor stimulation. Immunity 12:301-311. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, Y., B. A. Luxon, A. Casola, R. P. Garofalo, M. Jamaluddin, and A. R. Brasier. 2001. Expression of RSV-induced chemokine gene networks in lower airway epithelial cells revealed by cDNA microarrays. J. Virol. 75:9044-9058. [DOI] [PMC free article] [PubMed] [Google Scholar]