Abstract
Adaptive immunity in response to virus infection involves the generation of Th1 cells, cytotoxic T cells, and antibodies. This type of immune response is crucial for the clearance of virus infection and for long-term protection against reinfection. Type I interferons (IFNs), the primary innate cytokines that control virus growth and spreading, can influence various aspects of adaptive immunity. The development of antiviral immunity depends on many viral and cellular factors, and the extent to which type I IFNs contribute to the generation of adaptive immunity in response to a viral infection is controversial. Using two strains (Cantell and 52) of the murine respiratory Sendai virus (SeV) with differential abilities to induce type I IFN production from infected cells, together with type I IFN receptor-deficient mice, we examined the role of type I IFNs in the generation of adaptive immunity. Our results show that type I IFNs facilitate virus clearance and enhance the migration and maturation of dendritic cells after SeV infection in vivo; however, soon after infection, mice clear the virus from their lungs and efficiently generate cytotoxic T cells independently of type I IFN signaling. Furthermore, animals that are unresponsive to type I IFN develop long-term anti-SeV immunity, including CD8+ T cells and antibodies. Significantly, this memory response is able to protect mice against challenge with a lethal dose of virus. In conclusion, our results show that primary and secondary anti-SeV adaptive immunities are developed normally in the absence of type I IFN responsiveness.
Efficient recovery from a virus infection requires the participation of diverse mechanisms of the innate and adaptive immune responses. Type I interferons (IFNs), including IFN-α and -β, are produced by most cells upon virus infection and constitute the main innate antiviral response. Type I IFN production results from the recognition by cellular proteins of stimulatory viral elements, such as the virus genome, the replication intermediary double-stranded RNA, or the viral ribonucleoproteins (24, 26, 59). Viral genomic elements can bind to Toll-like receptors (TLRs) and stimulate a signaling pathway that culminates in the activation of the transcription factors IFN regulatory factor-3 (IRF3), nuclear factor-κB (NF-κB), and activator protein-1, which are necessary for the transcription of type I IFNs and other genes (27, 35, 36, 54). TLRs are expressed on the cell surface or in endosomal compartments of many cell populations (6, 18, 21, 55, 56). Nevertheless, the triggering of TLR signaling in the specialized plasmacytoid dendritic cells (pDCs) (17, 22, 51, 68) leads to the secretion of most of the type I IFNs produced in response to virus infection (22, 51). Type I IFN synthesis is also triggered by the binding of viral double-stranded RNA to the cellular helicases retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (mda-5), which can activate the transcription factors IRF3 and NF-κB (2, 69). Secreted type I IFNs bind to their receptor and trigger a signaling cascade that leads to the induction of genes that are essential for the innate control of virus replication and spreading, such as the genes coding for the cellular proteins MxA (52) and 2′-5′-oligoadenylate synthetase (35, 43). Type I IFNs also enhance the cytolytic activity of natural killer cells (7, 44, 63), which contribute to innate immunity by lysing virus-infected cells (62).
Antiviral adaptive immune responses involve the generation of CD4+ T helper 1 (Th1) cells that are able to secrete the cytokines interleukin-2 (IL-2) and gamma interferon (IFN-γ) (1, 41). These cytokines activate phagocytes, induce the generation of cytotoxic T cells (CTLs), and direct B cells to produce antibodies that are essential for the elimination of virus-infected cells and for long-term protection from reinfection with the same virus (1, 16, 33, 41). It has been shown that the development of many aspects of adaptive immunity can be modulated by type I IFNs. These cytokines influence the generation of B cells and significantly enhance the production of antibodies (10, 25). Type I IFNs regulate the synthesis of the proinflammatory cytokine IL-6 (38) and promote the development of Th1 immunity by modulating the expression of IL-15 (44, 50, 71) and IL-12 (29, 57). Type I IFNs also enhance the proliferation and survival of T cells (61) and facilitate clonal expansion and the generation of memory in response to viral infection (23).
The pleiotropic effects of type I IFNs in the development of adaptive immunity, together with the discovery of pDCs, have led to the belief that type I IFNs serve as essential bridges between innate and adaptive antiviral immunity (5, 12, 13, 28, 37, 60). Nevertheless, a different population of DCs, the conventional DCs (cDCs), leads the development of primary adaptive immune responses (11). cDCs mature upon encountering a pathogenic invader and change the expression pattern of multiple genes. This maturation allows cDCs to efficiently present antigens to T cells, thus initiating primary adaptive immune responses. Interestingly, in response to Sendai virus (SeV) or influenza virus infection, the pathways leading to the maturation of and type I IFN production by cDCs do not depend on TLR signaling (32) or type I IFN responsiveness (31), questioning the role of these elements in the generation of adaptive immunity against these viruses.
In this work, we used two strains (Cantell and 52) of the murine paramyxovirus SeV with different type I IFN induction capabilities, together with type I IFN receptor-deficient mice, to evaluate the role of type I IFNs in the generation of primary and memory adaptive immunity. Our results demonstrate that type I IFNs have an innate role in regulating SeV replication and promoting the maturation of DCs and their migration to the draining lymph nodes. Nevertheless, efficient primary as well as memory anti-SeV adaptive immunity is achieved in mice independently of type I IFN signaling.
MATERIALS AND METHODS
Viruses, mice, and cell lines.
SeV-Cantell (SeV-C) and SeV-52 were grown in embryonated chicken eggs as previously described (30, 31). C57BL/6 and Sv129/SvEv mice were purchased from Taconic (Germantown, NY). Type I IFN receptor-deficient mice on the 129S6/SvEv background were obtained from B&K Universal Limited (East Yorkshire, England). Age- and sex-matched animals were used in all experiments. The animals were bred and housed in pathogen-free conditions, and the experiments were performed according to institutionally approved protocols. NIH 3T3 and LLCMK2 cells were grown in tissue culture medium consisting of Dulbecco's minimum essential medium (Invitrogen, Carlsbad, CA), 10% fetal calf serum (heat inactivated; endotoxin level, 0.25 EU/ml; HyClone, Logan, UT), 1 mM sodium pyruvate, 2 mM l-glutamine (Invitrogen), and 50 mg/ml gentamicin (Invitrogen).
Reporter gene assays.
NIH 3T3 cells were transiently transfected with 2 μg of the full IFN-β promoter, the IRF positive regulatory domain (PRD) of the IFN-β promoter, or the NF-κB PRD promoter reporter construct (48), kindly provided by C. Horvath (Northwestern University, Evanston, IL) and D. Thanos (Columbia University, New York, NY), each driving firefly luciferase production, together with 0.2 μg pRL-TK constitutively expressing Renilla luciferase for normalization (Promega, Madison, WI). Transfection was performed using Lipofectamine/Lipofectamine Plus (Invitrogen) according to the manufacturer's instructions. After 24 h, the cells were infected with SeV or mock infected. Eight or twenty-four hours later, the cell extracts were assayed for expression of firefly and Renilla luciferase.
Quantitative real-time PCR.
RNA was extracted from lung homogenates obtained from animals infected with SeV or mock treated using the TRIzol reagent (Invitrogen). Quantitative real-time PCR was performed as previously described (70). In short, each sample was assayed in triplicate and the change values (n-fold) for each gene were calculated using the median threshold cycle. Primers for housekeeping genes used for normalization (rps11, GAPDH, and tubulin) were previously described (67). The primer sequences used for murine IFN genes were 5′-TCCTGAGCCAAAGTGTAGAG-3′ and 5′-GAGAACAAGTGCCTTTACAG-3′ for IFN-α and 5′-AGATGTCCTCAACTGCTCTC-3′ and 5′-AGATTCACTACCAGTCCCAG-3′ for IFN-β.
Mouse infection and lung virus titration.
Anesthetized mice were infected intranasally with 10 to 20 50% infectious doses (ID50) of SeV in phosphate-buffered saline (PBS) and weighed daily. In some experiments, the mice were infected with 103 ID50 of SeV as a lethal dose. For virus titration, the lungs were extracted, homogenized in PBS-gelatin (1%), and frozen in dry ice-ethanol for preservation. The presence of infectious particles was evaluated by infecting LLCMK2 cells with 1:10 dilutions of the lung homogenates at 37°C. After 1 h of infection, 175 μl of medium containing 2 μg/ml trypsin was added and the cells were further incubated for 72 h at 37°C. A total of 25 μl of medium was then removed from the plate and tested by hemagglutination of chicken red blood cells (RBCs) for the presence of virus particles. Viruses at 1:4 dilutions in 0.5% chicken RBCs were incubated for 30 min at 4°C. The hemagglutination of RBCs indicated the presence of virus particles.
In vivo CTL assay and tetramer staining.
Splenocytes from naive mice were pulsed at 4 × 107 cells/ml with 20 μM SeV NP324-332 peptide or mock treated for 15 min at room temperature. The cells were then labeled at 2 × 107 cells/ml with different concentrations of carboxy-fluorescein diacetate succinimidyl ester (CFSE) (2.5 μM for peptide-pulsed cells or 0.125 μM for mock-pulsed cells) (Molecular Probes, Eugene, OR) at 37°C for 30 min. Labeled peptide-pulsed and mock-pulsed cells were mixed 1:1, and 2 × 107 total cells were injected intravenously into infected and uninfected syngeneic mice. Spleens were harvested 20 h after the injections, and single cell suspensions were prepared and analyzed by flow cytometry. Percentages of specific killing were calculated as described previously (32). To determine the percentage of T cells bearing the specific anti-SeV-NP peptide 324-332 H-2Kb receptor, spleen, lung, lymph node, or bone marrow single-cell suspensions were obtained 5 to 120 days after infection with SeV and costained with anti-CD8-fluorescein isothiocyanate antibody and phycoerythrin-labeled H-2Kb tetramers carrying the SeV NP324-332 peptide (NIH tetramer core facility).
DC isolation from lymph nodes and staining.
Lung-draining lymph nodes were harvested from mice at different time points after infection. Tissues were incubated 30 min with collagenase (Liberase Blendzymes, Roche, Indianapolis, IN), followed by a 5-min incubation with EDTA. Single-cell suspensions were stained with anti-CD11c, anti-CD86, and anti-CCR7 antibodies conjugated to different fluorochromes (BD Biosciences). Flow cytometry was performed in a Cytomic FC500 Coulter station (Beckman Coulter, Miami, FL). Data were analyzed with Flowjo software.
Spleen cell culture and cytotoxicity.
Stimulator splenocytes from naive C57BL/6 mice were infected with SeV at a multiplicity of infection of 40 for 45 min at 37°C and cultured with splenocytes from immunized animals at a 1/10 stimulator/responder (s/r) ratio as described previously (30). Effector cells from secondary in vitro cultures were mixed with 51Cr-labeled infected or mock-infected EL4 target cells as described previously (30). Supernatants were harvested, and gamma radiation was measured. Killing of mock-infected EL-4 cells was subtracted from that observed with infected targets.
Cytokine and antibody detection.
Supernatants from in vitro restimulated cultures were collected 3 or 4 days later, and IFN-γ was measured by capture enzyme-linked immunosorbent assay. Detection of antiviral total immunoglobulin G (IgG), IgG1, and IgG2b antibodies in mouse serum was performed by capture enzyme-linked immunosorbent assay 2 weeks after infection.
Statistical analysis of results.
Statistical analysis was performed using a paired two sample t test.
RESULTS
SeV-C induces high levels of type I IFN and shows reduced virulence in mice compared to SeV-52.
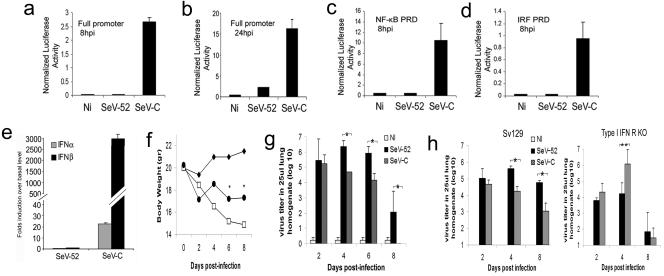
SeV-C induces the production of higher levels of type I IFNs by infected cells relative to other negative-stranded RNA viruses (31, 32). This is accomplished by a faster and more efficient activation of the different PRDs of the IFN-β promoter by SeV-C than by other viruses, such as SeV strain 52 (Fig. 1a through d). A stronger transcription of type I IFNs after SeV-C infection than that after SeV-52 infection is also observed in vivo when mice are infected with equivalent ID of these viruses. At 6 h postinfection, mRNA levels for IFN-α and -β are significantly higher in the lungs of C57BL/6 mice infected with SeV-C than in those infected with SeV-52 (Fig. 1e). In addition, animals infected with SeV-C show a reduced loss of body weight (Fig. 1f) and a faster clearance of the virus from the lungs relative to animals infected with an equivalent ID of SeV-52 (Fig. 1g). Similarly, a different strain of mice, Sv129, has a lung virus titer at least 1.5 log10 lower during infection with SeV-C than animals infected with SeV-52 at 4 and 8 days postinfection (Fig. 1 h). The innate antiviral properties of type I IFNs are responsible for the more rapid clearance of SeV-C from infected animals since SeV-C grows to a higher titer than and is cleared at the same rate as SeV-52 in animals with impaired type I IFN responsiveness (Fig. 1 h).
FIG. 1.
Reduced virulence of SeV-C compared to SeV-52 in mice. (a to d) Luciferase activity of whole-cell lysates from NIH 3T3 cells cotransfected with a plasmid constitutively expressing Renilla luciferase and a plasmid containing firefly luciferase reporter constructs driven by either the complete IFN-β promoter (a and b), four copies of the NF-κB PRD of the IFNβ promoter (c), or three copies of the IRF3/7 PRD of the IFN-β promoter (d) and infected with SeV for 8 h (a, c, and d) or 24 h (b). p.i., postinfection. (e) Quantification of IFN-α and -β gene transcripts by quantitative real-time PCR analysis from lung tissue of C57BL/6 mice 6 h after intranasal infection with the same ID50 of SeV-52 or SeV-C. (f) Weight loss of C57BL/6 mice treated with PBS (⧫) or infected with 20 ID50 of SeV-C (•) or SeV-52 (□) (n = 5). An asterisk indicates statistical difference, with a P value of ≤0.05. (g) Virus titer in the lungs of C57BL/6 mice treated with PBS or infected with 20 ID50 of SeV-C or SeV-52. An asterisk indicates statistical difference, with a P value of ≤0.05. (h) Virus titer in the lungs of wild-type Sv129 and type I IFN receptor-deficient (KO) mice infected intranasally with the same ID50 of SeV-52 or SeV-C. Results are representative of more than three independent experiments. An asterisk indicates statistical difference, with a P value of <0.005. The double asterisk indicates a P value of 0.07. Error bars indicate standard deviations.
Primary anti-SeV immunity is developed in the absence of type I IFN.
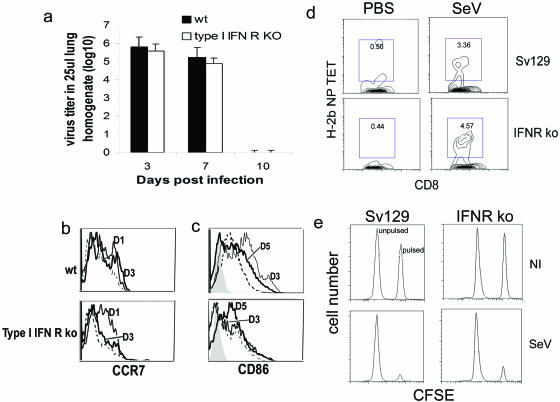
Despite type I IFNs having a role in facilitating the clearance of the virus from infected mice (Fig. 1), SeVs (both strains C and 52) are completely cleared from the lungs by day 10 postinfection in wild-type and type I IFN receptor-deficient animals (Fig. 2a and data not shown). This suggests that primary adaptive immunity able to eliminate the virus infection has developed in the absence of type I IFN signaling. The migration of mature DCs to the draining lymph nodes is essential for the generation of primary adaptive immunity (4). DCs expressing the chemokine receptor CCR7, expressed on recently migrating cells, can be detected in the lymph nodes, draining the lungs of wild-type as well as type I IFN unresponsive mice as early as 1 day postinfection with SeV. This migration is sustained up to at least 3 days postinfection in wild-type mice, while it is limited to earlier time points after infection in type I IFN receptor-deficient animals (Fig. 2b). DCs showing up-regulated CD86, indicating DC maturation, are also found in the lung-draining lymph nodes of wild-type and type I IFN receptor-deficient mice infected with SeV. Nevertheless, in the absence of type I IFN responsiveness, slower kinetics for the appearance of these cells and a reduced degree of maturation are observed (Fig. 2c).
FIG. 2.
Primary immune response against SeV. (a) Virus titers in the lungs of wild-type Sv129 and type I IFN receptor-deficient mice infected intranasally with SeV-52. Error bars indicate standard deviations. (b and c) Analysis of DC maturation (CD86 expression) and migration (CCR7 expression) to the lung draining lymph nodes extracted at different days postinfection; cells gated on the CD11c+ cells. The filled histogram corresponds to the isotype controls. The dashed line corresponds to lymph nodes from mock-infected animals. Continuous lines correspond to lymph nodes extracted from infected animals at the indicated day postinfection (D). Results are representative of two independent experiments. (d) H-2b NP peptide tetramer staining of CD8+ T cells specific for SeV in mice lungs 7 days after infection. (e) In vivo cytotoxicity 5 days postinfection. Lysis of splenocytes pulsed with SeV peptide and labeled with a high dose of CFSE or mock-pulsed and labeled with a low dose of CFSE was evaluated by flow cytometry 18 h after infusion into the infected mice. Results are representative of more than three independent experiments. KO, type I IFN receptor-deficient mice.
Although type I IFNs positively affect the migration and maturation of DCs, primary CD8+ T cells bearing the receptor against the SeV immunodominant peptide NP324-332 and CTLs with the ability to kill infected cells develop in type I IFN-unresponsive animals infected with SeV-52 or SeV-C within a week of infection (Fig. 2d and e and data not shown). The improvement in DC migration kinetics and CD86 up-regulation seen in type I IFN-competent animals corresponds with the reported adjuvant role of type I IFNs impacting these aspects of DC maturation in other systems (19, 34, 40, 58, 60). However, our results demonstrate that the enhancement of DC migration and maturation provided by type I IFNs is not essential for the development of primary adaptive immunity capable of eliminating SeV infection.
SeV strains with different abilities to induce type I IFN secretion induce equally potent adaptive immunity in mice.
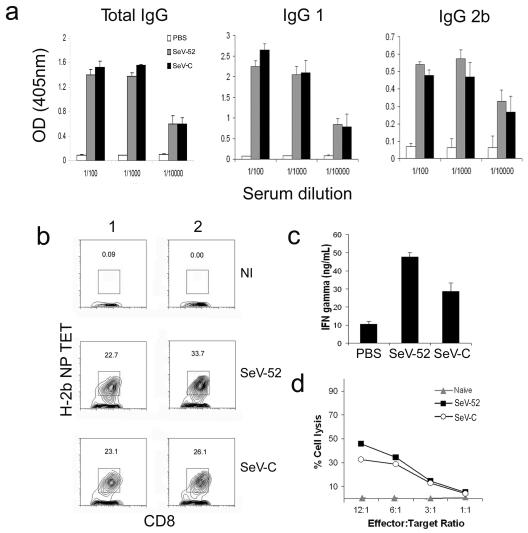
To study the role of type I IFNs in the development of memory adaptive immunity, we compared the long-term immunity of C57BL/6 mice immunized with equivalent ID of SeV-C and SeV-52 (Fig. 1g). Despite a reported role for type I IFNs in enhancing the production of antibodies and directing class switching by antigen-specific B cells (10, 25), similar levels of anti-SeV total IgGs, IgG1, and IgG2b antibodies were detected 2 weeks after infection in the sera from mice immunized with either virus (Fig. 3a).
FIG. 3.
Adaptive immunity against viruses inducing high and low levels of type I IFN. (a) Antibodies in the serum of C57BL/6 mice infected intranasally with the same ID50 of SeV-52 or SeV-C or treated with PBS as a control. Error bars indicate standard deviations. OD, optical density. (b) H-2b NP peptide tetramer staining of CD8+ T cells specific for SeV in mouse lungs 24 days after infection. Results from two different representative animals are shown. (c) IFN-γ in the supernatant of 4 day cultures of in vitro-restimulated splenocytes from animals infected with SeV. Error bars indicate standard deviations. (d) Cytotoxicity of in vitro-restimulated splenocytes from animals infected with SeV; cell lysis was determined using a Cr51 release assay. Results are representative of more than three independent experiments.
Likewise, in contrast to a documented role for type I IFN in enhancing the proliferation and survival of memory T cells (23), a comparable percentage of CD8+ T cells bearing the receptor against the SeV immunodominant peptide NP324-332 is found in the lungs of animals infected with SeV-52 or SeV-C (Fig. 3b) 24 days postinfection. Additionally, in vitro-restimulated splenocytes from animals infected with SeV-52 produce even higher levels of IFN-γ than do splenocytes from animals infected with SeV-C (Fig. 3c) and splenocytes from animals infected with SeV-52 or SeV-C generate equivalent CTLs (Fig. 3d). These results indicate that SeV-52 and SeV-C trigger the development of normal long-term adaptive immune responses despite a significant difference in their abilities to induce type I IFNs in mice.
Type I IFN-independent development of adaptive antiviral immunity.
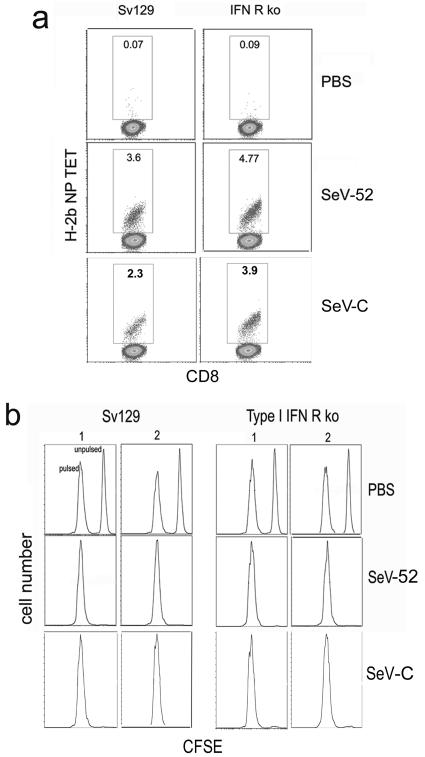
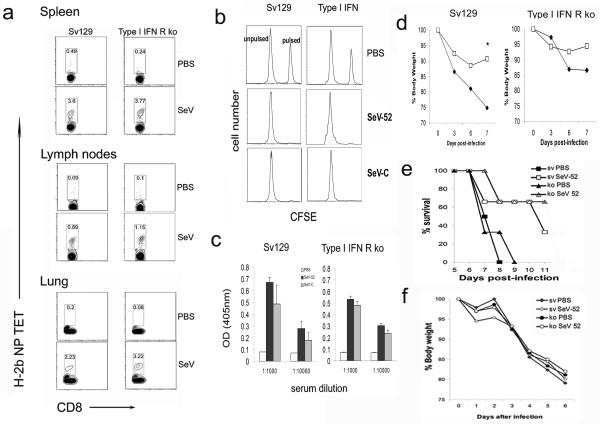
To more directly evaluate the contribution of type I IFNs in the development of adaptive immunity against SeV, we infected wild-type and type I IFN receptor-deficient mice with SeV-52 or SeV-C. Ten days after infection, equivalent CD8+ T cells bearing the receptor against the SeV immunodominant peptide NP324-332 are found in the lung-draining lymph nodes of control and type I IFN receptor-deficient animals (Fig. 4a) corresponding with a normal development of cytotoxic T cells in both groups of mice (Fig. 4b). These CD8+ T cells were still present in the lung, bone marrow, spleen, and lymph nodes of infected animals 4 months after the infection (Fig. 5a and data not shown), and efficient cytotoxic activity could be demonstrated 2 months after the virus infection had been completely cleared (Fig. 5b). Similarly, anti-SeV antibodies develop normally in type I IFN receptor-deficient mice (Fig. 5c). This long-term immunity is able to protect against reinfection, as animals challenged with a lethal dose of SeV 4 months after the primary infection show no signs of illness or virus growth in the lungs (Fig. 5d and data not shown). Moreover, mice adoptively transferred with 5 × 106 bone marrow cells taken from 70-day-infected wild-type or type I IFN receptor-deficient mice show a significantly improved rate of survival compared with that of animals that had received bone marrow cells from noninfected mice (Fig. 5e). Both groups of animals lost weight after challenge with a lethal dose of SeV, indicating equivalent infections (Fig. 5f). These data indicate that normal long-term protective antiviral adaptive immunity can be achieved in the absence of type I IFN responsiveness.
FIG. 4.
Type I IFN-independent development of anti-SeV adaptive immunity. (a) H-2b NP peptide tetramer staining of CD8+ T cells specific for SeV in wild-type Sv129 and type I IFN receptor-deficient mouse lungs 10 days after infection. (b) In vivo cytotoxicity 10 days postinfection. Lysis of splenocytes pulsed with SeV peptide and labeled with a high dose of CFSE or mock pulsed and labeled with a low dose of CFSE was evaluated by flow cytometry 18 h after infusion into the infected mice. Two different animals are shown. Results are representative of more than three independent experiments. ko, type I IFN receptor-deficient mice.
FIG. 5.
Long-term memory antiviral immunity is developed normally in the absence of type I IFN responsiveness. (a) H-2b NP peptide tetramer staining of CD8+ T cells specific for SeV-52 in lungs, spleen, and lymph nodes of Sv129 and type I IFN receptor-deficient mice, 120 days after infection. (b) In vivo cytotoxicity 60 days postinfection. Lysis of splenocytes pulsed with SeV peptide and labeled with a high dose of CFSE or mock pulsed and labeled with a low dose of CFSE was evaluated by flow cytometry 18 h after being infused into the infected mice. Results are representative of more than three independent experiments. (c) Antibodies in the serum of Sv129 and type I IFN receptor-deficient mice infected intranasally with the same ID50 of SeV-52 or SeV-C or treated with PBS as a control. Anti-SeV antibody was measured from the animals' serum 3 weeks after infection. Error bars indicate standard deviations. OD, optical density. (d) Weight of infected (□) or mock-infected (⧫) animals challenged 120 days after infection with a lethal dose of SeV. The asterisk indicates a P of 0.03. (e) Survival and (f) weight of Sv129 mice adoptively transferred with 5 × 106 bone marrow cells from infected (closed symbols) or PBS-treated (open symbols) animals and challenged with a lethal dose of SeV-52. Groups of four animals were used in panels d through f. ko, type I IFN receptor-deficient mice.
DISCUSSION
Because of the crucial role of adaptive immunity in the clearance of a virus infection and in the establishment of long-term protective immunity, it is essential to have an accurate understanding of the factors that affect the development of the antiviral adaptive immune response. The role of type I IFNs in generating an antiviral state is well known and constitutes a major innate antiviral mechanism. The effect of these cytokines in multiple aspects of adaptive immunity (9, 10, 14, 25, 38, 42, 44, 45, 71), together with the discovery of pDCs specialized in secreting large amounts of type I IFNs in response to virus infection (21), has led to the hypothesis that type I IFNs are an essential bridge between innate and adaptive antiviral immunities (5, 12, 13, 28, 37, 60). This hypothesis has been supported by studies demonstrating that a number of viruses, including the lymphocytic choriomeningitis virus (LCMV) and the vesicular stomatitis virus, have an increased susceptibility to infection in mice that are unresponsive to type I IFNs (20, 53, 64, 65). In fact, type I IFNs have been proposed as “endogenous adjuvants” based on their ability to enhance the up-regulation of costimulatory (CD80 and CD86) and major histocompatibility complex molecules on DCs (15, 25, 34, 50, 60). However, it has been demonstrated that type I IFNs alone cannot induce complete DC maturation, including the secretion of proinflammatory cytokines (19, 31, 47), that is necessary for the generation of efficient antiviral immunity. In addition, the in vitro maturation of conventional DCs (including cytokine secretion) in response to infection with influenza virus or with SeV is not affected by the lack of type I IFN responsiveness (31). Thus, the role of type I IFNs in the onset of antiviral adaptive immunity is unclear and constitutes the focus of our study.
While many studies on antiviral immunity are performed in the mouse model system, most of these studies use viruses that are adapted to mice rather than using true mouse pathogens (3, 20, 49, 53, 65). The studies presented here investigated the role of innate and adaptive immune systems in a natural virus host. The data show that although type I IFNs facilitate the clearance of SeV infection in mice, these cytokines are dispensable for the elimination of the infection and for the development of primary and long-term protective adaptive immunities. These results are in stark contrast to experiments that show an increased susceptibility to the mouse viral pathogen LCMV of mice unresponsive to type I IFNs (46, 64). In this system, it has been suggested that uncontrolled early virus titers may lead to the exhaustion of adaptive effector components, culminating in deficient virus clearance (46, 66). Additionally, type I IFNs have been shown to play a role in sustaining the survival of LCMV-specific CD8+ T cells (23, 42). Nevertheless, neither of these effects seems necessary for the clearance of and immunity to SeV.
Notably, these viruses infect different organs; SeV is a respiratory virus, while LCMV produces a systemic infection. This could be a possible determinant for the need of type I IFN in the induction of adaptive immunity. Also, the mechanisms involved in the triggering of DC maturation by SeV and LCMV are different. In contrast to the type I IFNs and TLR-independent DC maturation to SeV (31, 32), type I IFNs are needed for the complete maturation of DCs in response to LCMV infection (39) and TLR signaling is necessary for the generation of normal anti-LCMV immune response (72). Significantly, the LCMV Armstrong strain, used in most of these studies, does not infect DCs efficiently (8) when compared with SeV (31). These differences in the mechanisms utilized by SeV and LCMV for the induction of DC maturation could be reflected in a different role for type I IFNs in the development of efficient adaptive immunity after infection with these two mouse viruses.
The role for pDCs in the onset of antiviral adaptive immunity remains speculative. These cells are specialized for the secretion of large amounts of type I IFNs in response to TLR activation by viral products (21), suggesting that pDCs might be essential for the development of innate and adaptive antiviral immunity. Nevertheless, the induction of cDC maturation and immunity by SeV does not depend on TLR signaling (2, 69) but rather on an intracellularly triggered pathway, most probably mediated by the enzymes RIG-I and mda-5 (31). Interestingly, a role for type I IFNs in sustaining DC migration to the draining lymph nodes and in improving DC maturation is observed after SeV infection without a relevant impact on the development of adaptive immunity to SeV. It is actually noticeable that type I IFN reduces the efficiency of the adaptive immunity against SeV. It is possible that viruses with a weak ability to stimulate the TLR-independent pathway for the induction of DC maturation, such as the Newcastle disease virus that reportedly requires type I IFN to sustain DC maturation (19) or LCMV, may rely on exogenous type I IFN to achieve complete DC maturation and migration to the draining lymph nodes to initiate the adaptive immune response.
In conclusion, our results show that the elements contributing to the development of adaptive immunity in mice differ according to the virus used. Mice infected with SeV, which strongly activates DC maturation independently of TLR signaling (32) and secreted type I IFNs (31), do not require the effects of type I IFNs to generate efficient adaptive immunity.
Acknowledgments
This work was supported by grants 1R01AI41111 and U19AI062623 from the National Institute of Allergy and Infectious Diseases to T.M.M. and by funds granted by the Charles H. Revson Foundation to C.B.L.
We thank Thomas A. Kraus for his assistance with the reporter assays, Luis Muñoz for invaluable technical support, and the Quantitative PCR Shared Research Facility at the Mount Sinai School of Medicine.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Andrejeva, J., K. S. Childs, D. F. Young, T. S. Carlos, N. Stock, S. Goodbourn, and R. E. Randall. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc. Natl. Acad. Sci. USA 101:17264-17269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angel, J., M. A. Franco, H. B. Greenberg, and D. Bass. 1999. Lack of a role for type I and type II interferons in the resolution of rotavirus-induced diarrhea and infection in mice. J. Interferon Cytokine Res. 19:655-659. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Barchet, W., M. Cella, and M. Colonna. 2005. Plasmacytoid dendritic cells—virus experts of innate immunity. Semin. Immunol. 17:253-261. [DOI] [PubMed] [Google Scholar]
- 6.Barchet, W., A. Krug, M. Cella, C. Newby, J. A. Fischer, A. Dzionek, A. Pekosz, and M. Colonna. 2005. Dendritic cells respond to influenza virus through TLR7- and PKR-independent pathways. Eur. J. Immunol. 35:236-242. [DOI] [PubMed] [Google Scholar]
- 7.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 8.Borrow, P., C. F. Evans, and M. B. Oldstone. 1995. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69:1059-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brassard, D. L., M. J. Grace, and R. W. Bordens. 2002. Interferon-α as an immunotherapeutic protein. J. Leukoc. Biol. 71:565-581. [PubMed] [Google Scholar]
- 10.Braun, D., I. Caramalho, and J. Demengeot. 2002. IFN-α/β enhances BCR-dependent B cell responses. Int. Immunol. 14:411-419. [DOI] [PubMed] [Google Scholar]
- 11.Carbone, F. R., and W. R. Heath. 2003. The role of dendritic cell subsets in immunity to viruses. Curr. Opin. Immunol. 15:416-420. [DOI] [PubMed] [Google Scholar]
- 12.Colonna, M., G. Trinchieri, and Y. J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219-1226. [DOI] [PubMed] [Google Scholar]
- 13.Dalod, M., T. Hamilton, R. Salomon, T. P. Salazar-Mather, S. C. Henry, J. D. Hamilton, and C. A. Biron. 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon α/β. J. Exp. Med. 197:885-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan, G. S., H. W. Mittrucker, D. Kagi, T. Matsuyama, and T. W. Mak. 1996. The transcription factor interferon regulatory factor-1 is essential for natural killer cell function in vivo. J. Exp. Med. 184:2043-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallucci, S., M. Lolkema, and P. Matzinger. 1999. Natural adjuvants: endogenous activators of dendritic cells. Nat. Med. 5:1249-1255. [DOI] [PubMed] [Google Scholar]
- 16.Graham, M. B., V. L. Braciale, and T. J. Braciale. 1994. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 180:1273-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hertzog, P. J., L. A. O'Neill, and J. A. Hamilton. 2003. The interferon in TLR signaling: more than just antiviral. Trends Immunol. 24:534-539. [DOI] [PubMed] [Google Scholar]
- 18.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-α production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-α/β signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ida-Hosonuma, M., T. Iwasaki, T. Yoshikawa, N. Nagata, Y. Sato, T. Sata, M. Yoneyama, T. Fujita, C. Taya, H. Yonekawa, and S. Koike. 2005. The α/β interferon response controls tissue tropism and pathogenicity of poliovirus. J. Virol. 79:4460-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito, T., Y. H. Wang, and Y. J. Liu. 2005. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin. Immunopathol. 26:221-229. [DOI] [PubMed] [Google Scholar]
- 22.Kawai, T., S. Sato, K. J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, O. Takeuchi, and S. Akira. 2004. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061-1068. [DOI] [PubMed] [Google Scholar]
- 23.Kolumam, G. A., S. Thomas, L. J. Thompson, J. Sprent, and K. Murali-Krishna. 2005. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202:637-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar, A., J. Haque, J. Lacoste, J. Hiscott, and B. R. Williams. 1994. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κ B by phosphorylating I κ B. Proc. Natl. Acad. Sci. USA 91:6288-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14:461-470. [DOI] [PubMed] [Google Scholar]
- 26.Lepe-Zuniga, J. L., J. Rotbein, and J. U. Gutterman. 1989. Production of interferon-α induced by dsRNA in human peripheral blood mononuclear cell cultures: role of priming by dsRNA-induced interferons-γ and -β. J. Interferon Res. 9:445-456. [DOI] [PubMed] [Google Scholar]
- 27.Levy, D. E., D. S. Kessler, R. Pine, and J. E. Darnell, Jr. 1989. Cytoplasmic activation of ISGF3, the positive regulator of interferon-α-stimulated transcription, reconstituted in vitro. Genes Dev. 3:1362-1371. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Y. J. 2004. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 22:81-127. [DOI] [PubMed]
- 29.Lohoff, M., D. Ferrick, H. W. Mittrucker, G. S. Duncan, S. Bischof, M. Rollinghoff, and T. W. Mak. 1997. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity 6:681-689. [DOI] [PubMed] [Google Scholar]
- 30.Lopez, C. B., A. Fernandez-Sesma, J. L. Schulman, and T. M. Moran. 2001. Myeloid dendritic cells stimulate both Th1 and Th2 immune responses depending on the nature of the antigen. J. Interferon Cytokine Res. 21:763-773. [DOI] [PubMed] [Google Scholar]
- 31.Lopez, C. B., A. Garcia-Sastre, B. R. Williams, and T. M. Moran. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 187:1126-1136. [DOI] [PubMed] [Google Scholar]
- 32.Lopez, C. B., B. Moltedo, L. Alexopoulou, L. Bonifaz, R. A. Flavell, and T. M. Moran. 2004. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol. 173:6882-6889. [DOI] [PubMed] [Google Scholar]
- 33.Lopez, C. B., T. M. Moran, J. L. Schulman, and A. Fernandez-Sesma. 2002. Antiviral immunity and the role of dendritic cells. Int. Rev. Immunol. 21:339-353. [DOI] [PubMed] [Google Scholar]
- 34.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 35.Malmgaard, L. 2004. Induction and regulation of IFNs during viral infections. J. Interferon Cytokine Res. 24:439-454. [DOI] [PubMed] [Google Scholar]
- 36.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-α genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenna, K., A. S. Beignon, and N. Bhardwaj. 2005. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J. Virol. 79:17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitani, Y., A. Takaoka, S. H. Kim, Y. Kato, T. Yokochi, N. Tanaka, and T. Taniguchi. 2001. Cross talk of the interferon-α/β signalling complex with gp130 for effective interleukin-6 signalling. Genes Cells 6:631-640. [DOI] [PubMed] [Google Scholar]
- 39.Montoya, M., M. J. Edwards, D. M. Reid, and P. Borrow. 2005. Rapid activation of spleen dendritic cell subsets following lymphocytic choriomeningitis virus infection of mice: analysis of the involvement of type 1 IFN. J. Immunol. 174:1851-1861. [DOI] [PubMed] [Google Scholar]
- 40.Montoya, M., G. Schiavoni, F. Mattei, I. Gresser, F. Belardelli, P. Borrow, and D. F. Tough. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99:3263-3271. [DOI] [PubMed] [Google Scholar]
- 41.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen, K. B., W. T. Watford, R. Salomon, S. R. Hofmann, G. C. Pien, A. Morinobu, M. Gadina, J. J. O'Shea, and C. A. Biron. 2002. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 297:2063-2066. [DOI] [PubMed] [Google Scholar]
- 43.Nilsen, T. W., P. A. Maroney, and C. Baglioni. 1982. Synthesis of (2′-5′)oligoadenylate and activation of an endoribonuclease in interferon-treated HeLa cells infected with reovirus. J. Virol. 42:1039-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogasawara, K., S. Hida, N. Azimi, Y. Tagaya, T. Sato, T. Yokochi-Fukuda, T. A. Waldmann, T. Taniguchi, and S. Taki. 1998. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature 391:700-703. [DOI] [PubMed] [Google Scholar]
- 45.Ohteki, T., H. Yoshida, T. Matsuyama, G. S. Duncan, T. W. Mak, and P. S. Ohashi. 1998. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor-α/β+ (NK1+ T) cells, natural killer cells, and intestinal intraepithelial T cells. J. Exp. Med. 187:967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75:8407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pollara, G., M. Jones, M. E. Handley, M. Rajpopat, A. Kwan, R. S. Coffin, G. Foster, B. Chain, and D. R. Katz. 2004. Herpes simplex virus type-1-induced activation of myeloid dendritic cells: the roles of virus cell interaction and paracrine type I IFN secretion. J. Immunol. 173:4108-4119. [DOI] [PubMed] [Google Scholar]
- 48.Poole, E., B. He, R. A. Lamb, R. E. Randall, and S. Goodbourn. 2002. The V proteins of simian virus 5 and other paramyxoviruses inhibit induction of interferon-beta. Virology 303:33-46. [DOI] [PubMed] [Google Scholar]
- 49.Price, G. E., A. Gaszewska-Mastarlarz, and D. Moskophidis. 2000. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J. Virol. 74:3996-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santini, S. M., C. Lapenta, M. Logozzi, S. Parlato, M. Spada, T. Di Pucchio, and F. Belardelli. 2000. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191:1777-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sato, S., M. Sugiyama, M. Yamamoto, Y. Watanabe, T. Kawai, K. Takeda, and S. Akira. 2003. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κ B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171:4304-4310. [DOI] [PubMed] [Google Scholar]
- 52.Schwemmle, M., K. C. Weining, M. F. Richter, B. Schumacher, and P. Staeheli. 1995. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology 206:545-554. [DOI] [PubMed] [Google Scholar]
- 53.Shresta, S., J. L. Kyle, H. M. Snider, M. Basavapatna, P. R. Beatty, and E. Harris. 2004. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J. Virol. 78:2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 55.Stockinger, S., B. Reutterer, B. Schaljo, C. Schellack, S. Brunner, T. Materna, M. Yamamoto, S. Akira, T. Taniguchi, P. J. Murray, M. Muller, and T. Decker. 2004. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J. Immunol. 173:7416-7425. [DOI] [PubMed] [Google Scholar]
- 56.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 101:3516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taki, S., T. Sato, K. Ogasawara, T. Fukuda, M. Sato, S. Hida, G. Suzuki, M. Mitsuyama, E. H. Shin, S. Kojima, T. Taniguchi, and Y. Asano. 1997. Multistage regulation of Th1-type immune responses by the transcription factor IRF-1. Immunity 6:673-679. [DOI] [PubMed] [Google Scholar]
- 58.Tamir, A., W. J. Jordan, M. Ritter, N. Habib, R. I. Lechler, G. R. Foster, and G. Lombardi. 2005. Interferon-α2a is sufficient for promoting dendritic cell immunogenicity. Clin. Exp. Immunol. 142:471-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.tenOever, B. R., S. Sharma, W. Zou, Q. Sun, N. Grandvaux, I. Julkunen, H. Hemmi, M. Yamamoto, S. Akira, W. C. Yeh, R. Lin, and J. Hiscott. 2004. Activation of TBK1 and IKKɛ kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J. Virol. 78:10636-10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tough, D. F. 2004. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk. Lymphoma 45:257-264. [DOI] [PubMed] [Google Scholar]
- 61.Tough, D. F., P. Borrow, and J. Sprent. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272:1947-1950. [DOI] [PubMed] [Google Scholar]
- 62.Trinchieri, G.1989. Biology of natural killer cells. Adv. Immunol. 47:187-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trinchieri, G., and D. Santoli. 1978. Antiviral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J. Exp. Med. 147:1314-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van den Broek, M. F., U. Muller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van den Broek, M. F., U. Muller, S. Huang, R. M. Zinkernagel, and M. Aguet. 1995. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol. Rev. 148:5-18. [DOI] [PubMed] [Google Scholar]
- 66.White, L. J., J. G. Wang, N. L. Davis, and R. E. Johnston. 2001. Role of alpha/beta interferon in Venezuelan equine encephalitis virus pathogenesis: effect of an attenuating mutation in the 5′ untranslated region. J. Virol. 75:3706-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wurmbach, E., T. Yuen, B. J. Ebersole, and S. C. Sealfon. 2001. Gonadotropin-releasing hormone receptor-coupled gene network organization. J. Biol. Chem. 276:47195-47201. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto, M., S. Sato, K. Mori, K. Hoshino, O. Takeuchi, K. Takeda, and S. Akira. 2002. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J. Immunol. 169:6668-6672. [DOI] [PubMed] [Google Scholar]
- 69.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 70.Yuen, T., E. Wurmbach, R. L. Pfeffer, B. J. Ebersole, and S. C. Sealfon. 2002. Accuracy and calibration of commercial oligonucleotide and custom cDNA microarrays. Nucleic Acids Res. 30:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, X., S. Sun, I. Hwang, D. F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8:591-599. [DOI] [PubMed] [Google Scholar]
- 72.Zhou, S., E. A. Kurt-Jones, L. Mandell, A. Cerny, M. Chan, D. T. Golenbock, and R. W. Finberg. 2005. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 35:822-830. [DOI] [PubMed] [Google Scholar]