Abstract
The life cycle of calicivirus is not fully understood because most of the viruses cannot be propagated in tissue culture cells. We studied the mechanism of calicivirus entry into cells using feline calicivirus (FCV), a cultivable calicivirus. From the cDNA library of Crandell-Rees feline kidney (CRFK) cells, feline junctional adhesion molecule 1 (JAM-1), an immunoglobulin-like protein present in tight junctions, was identified as a cellular-binding molecule of the FCV F4 strain, a prototype strain in Japan. Feline JAM-1 expression in nonpermissive hamster lung cells led to binding and infection by F4 and all other strains tested. An anti-feline JAM-1 antibody reduced the binding of FCV to permissive CRFK cells and strongly suppressed the cytopathic effect (CPE) and FCV progeny production in infected cells. Some strains of FCV, such as F4 and F25, have the ability to replicate in Vero cells. We found that regardless of replication ability, FCV bound to Vero and 293T cells via simian and human JAM-1, respectively. In Vero cells, an anti-human JAM-1 antibody inhibited binding, CPE, and progeny production by F4 and F25. In addition, feline JAM-1 expression permitted FCV infection in 293T cells. Taken together, our results demonstrate that feline JAM-1 is a functional receptor for FCV, simian JAM-1 also functions as a receptor for some strains of FCV, and the interaction between FCV and JAM-1 molecules may be a determinant of viral tropism. This is the first report concerning a functional receptor for the viruses in the family Caliciviridae.
The family Caliciviridae comprises a diversity of pathogens in humans and animals. Studies of the life cycle of the virus have been quite limited because of the lack of cell culture methods for most caliciviruses (13). Porcine enteric calicivirus in the genus Sapovirus is known to propagate in vitro with the addition of an intestinal content fluid filtrate, and Chang et al. (6) recently identified bile acids as molecules responsible for culture in cells. Studies with virus-like particles showed that ABH histo-blood group antigens are ligands of noroviruses belonging to the genus Norovirus (24). Studies in vivo also showed that these antigens are critical for human susceptibility to norovirus infection (17, 22). Because noroviruses are not cultivable in cell culture (9), it has not been established whether ABH histo-blood antigens function as receptors for them.
Feline calicivirus (FCV) is a member of the genus Vesivirus and causes upper respiratory tract disease and acute mouth ulceration, occasionally associated with chronic stomatitis and acute arthritis in cats (15, 21). It is comparatively easy to study the life cycle of FCV, because the virus replicates efficiently in cell culture without specific supplementation. Kreutz et al. (20) investigated the binding of FCV to feline and nonfeline cells. They reported that FCV bound to feline cells but also showed poor binding ability to nonfeline cells. It was also demonstrated that when nonfeline cells were transfected with genomic RNA of FCV, infectious virus could be recovered from the cells (20). These data suggest that FCV host specificity is restricted in the early stage of FCV infection in cells.
In this study, to elucidate the cellular determinant(s) of FCV host specificity, we applied an improved expression cloning method (28, 29) to identify cell surface molecules that interact with FCV. As a result, feline junctional adhesion molecule 1 (JAM-1) was identified as a binding receptor for FCV. JAM-1 is a member of the immunoglobulin (Ig) superfamily expressed by various cells and is involved in regulation of cell-cell interactions in the immune system and apical tight junction formation (10, 23). We here demonstrate that feline JAM-1 (fJAM-1) possesses the characteristics required of a functional receptor for FCV.
MATERIALS AND METHODS
Cells.
Crandell-Rees feline kidney (CRFK) cells and human embryonic kidney 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma, St. Louis, MO) supplemented with 10% fetal calf serum (FCS). Nonpermissive hamster lung (HmLu-1) cells and African green monkey kidney (Vero) cells were cultured in DMEM with 5% FCS. Murine myeloma P3X63Ag8U.1 (P3U1) cells were grown in RPMI 1640 medium (Sigma) with 10% FCS. Plat-E cells, a 293T-derived murine leukemia virus-based packaging cell line (25), were kindly provided by T. Kitamura (Institute of Medical Science, University of Tokyo, Tokyo, Japan) and maintained in DMEM supplemented with 10% FCS, 1-μg/ml puromycin, and 10-μg/ml blasticidin. Table 1 lists the cell lines used in this study.
TABLE 1.
Cell lines used in this study
| Cell line | Origin | Susceptibility to FCV |
|---|---|---|
| CRFK | Feline kidney | Permissive |
| Vero | Monkey kidney | Nonpermissive for most strains |
| Permissive for F4 and F25 | ||
| 293T | Human embryonic kidney | Nonpermissive |
| HmLu-1 | Hamster lung | Nonpermissive |
| P3U1 | Murine myeloma | Nonpermissive |
| Plat-E | Human embryonic kidney | Nonpermissive |
Viruses.
The FCV strains used in this study were F4, F25 (33), and 91-40 (32), which were isolated from cats in Japan with respiratory symptoms, and the F9 vaccine strain, originally isolated from cats in the United States showing clinical signs of rhinitis and conjunctivitis (4). Viral titers were measured by endpoint titration of CRFK cells. Fifty microliters of 10-fold dilutions of each virus fraction and a 50-μl CRFK cell suspension (∼1.5 × 104 cells) were mixed in 96-well plates and incubated at 37°C. Four wells were used for each dilution. Cells were observed for the cytopathic effect (CPE) for 3 days, and the 50% tissue culture infectious dose was calculated by the Behren-Kärber method. Virus titration was performed three times, and mean values were calculated. Statistical analysis was carried out by the Student t test. P values of <0.05 were considered statistically significant.
For binding assays, these strains were propagated and concentrated as follows. CRFK cells grown in 690-cm2 roller bottles were infected with virus; the supernatant was harvested at 24-h postinfection, layered onto a 25% sucrose cushion, and ultracentrifuged at 120,000 × g for 2 h at 4°C. The pellet was suspended in phosphate-buffered saline (PBS) with Ca2+ and Mg2+. The suspension was centrifuged at 650 × g to remove insoluble matter and used for binding assays.
Expression cloning of fJAM-1.
Retrovirus-mediated expression cloning using a virus-coating dish was performed as described previously (28). A cDNA library was generated from poly(A)+ mRNA from the CRFK cell line in the retroviral vector pMX (19). The cDNA library was packaged in murine leukemia virus particles by transfection of Plat-E cells and used to infect P3U1 cells at a multiplicity of infection of ∼0.2. The dish, which was not tissue culture treated (Corning Glass Co., Corning, NY), was incubated at 4°C overnight with 10-μg/ml goat anti-mouse IgG (Rockland Immunochemicals, Gilbertsville, PA) in 4 ml of PBS. The dish was rinsed with PBS containing 2% FCS and incubated at 37°C for 30 min with an anti-FCV monoclonal antibody (MAb), 1D7 (35). The dish was washed four times with PBS containing 2% FCS. Ten milliliters of supernatant from CRFK cells infected with FCV F4 was applied to the antibody-coated dish and incubated at 20°C for 30 min. The medium was replaced by fresh viral fluid, and the incubation was repeated on three further occasions. At final replacement by fresh viral fluid, the dish was fixed with 0.1% paraformaldehyde at 20°C for 30 min. The dish was washed four times with PBS containing 2% FCS before the addition of 107 P3U1 cells transduced with the CRFK cDNA library in 4 ml PBS. The cells were incubated at 37°C for 2 h. Nonadherent cells were removed by being washed, and adherent cells were cultured for 5 days. Adherent cells formed colonies and were picked with a penicillin cup. From each of the clones, genomic DNA was isolated and subjected to amplification by PCR using LA Taq polymerase (TaKaRa, Tokyo, Japan) and pMX primers (18). Following amplification, one clone yielded a specific fragment, which was cloned in T vector (Invitrogen, Carlsbad, CA) and sequenced. The feline homolog of human JAM-1 (hJAM-1) was cloned into the BstXI site of pMX to produce pMX-fJAM-1. The fJAM-1 cDNA fragment was also cloned into the mammalian expression vector pcDNA3.1 (Invitrogen). pcDNA3.1-fJAM-1 was used for transfection of HmLu-1 cells and 293T cells using Mirus TransIT-LT1 (TaKaRa), and stably transduced HmLu-1 cells were selected with neomycin (Sigma). Cells transduced with empty pcDNA3.1 vector were used as a control.
Antibodies.
Antibodies used in this study are listed in Table 2. The antisera against fJAM-1 were generated by DNA immunization. BALB/c mice (6 weeks old; female) were intramuscularly injected with 100 μg pcDNA3.1-fJAM-1 per head at 3-week intervals. Control sera were obtained from normal BALB/c mice (11 weeks old; female). Both sera were filtered through a sterile 0.2-μm filter (Gelman Sciences, Ann Arbor, MI).
TABLE 2.
Antibodies used in this study
Detection of JAM-1 on cell surface by flow cytometry.
For flow cytometry of cell surface proteins, cells (106 per sample, monolayers dispersed with 0.02% EDTA in PBS) were washed once in a flow cytometry buffer (PBS containing 2% FCS and 0.1% NaN3) and incubated for 20 min on ice in 5 μl of anti-fJAM-1 antibody at fivefold dilution or anti-hJAM-1 antibody (R&D Systems, Minneapolis, MN) at 100 μg/ml. Normal mouse serum and normal goat IgG (R&D Systems) were used as control antibodies at the same concentrations, respectively. The cells were washed twice with 1 ml flow cytometry buffer and incubated for 20 min on ice with 5 μl flow cytometry buffer containing 10-fold dilutions of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse Ig or FITC-conjugated rabbit anti-goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA). The cells were washed twice, suspended in flow cytometry buffer, and analyzed with a FACSCalibur (Becton Dickinson, Franklin Lakes, MN).
Virus-binding assay by flow cytometry.
Cells were washed once in a flow cytometry buffer and incubated for 30 min on ice in 20 μl flow cytometry buffer containing concentrated FCV. In blocking experiments with the anti-fJAM-1 antibody or anti-hJAM-1 antibody, cells were incubated for 30 min on ice with the antibody before being subjected to the virus. The cells were washed twice and incubated for 20 min on ice with an anti-FCV MAb 2E1, which recognizes an epitope on the capsid protein (unpublished data). The cells were washed twice and resuspended in 5 μl flow cytometry buffer containing 10-fold dilutions of FITC-conjugated anti-mouse Ig and incubated for 20 min on ice. For the blocking experiment with the anti-fJAM-1 antibody, the second antibody was not required because the MAb 2E1 was labeled with Alexa Fluor 488 (Zenon, Oakville, Ontario, Canada), according to the manufacturer's instructions. The cells were washed and analyzed with a FACSCalibur.
Infection of fJAM-1-transfected HmLu-1 or 293T cells.
HmLu-1 cells stably transduced and 293T cells transiently transduced with pcDNA3.1-fJAM-1 or pcDNA3.1 only were inoculated with FCV at multiplicity of infection of 10, incubated at 37°C for 1.5 h, and washed with DMEM. Cells were harvested and fixed with ice-cold acetone 1, 12, 24, and 48 h after inoculation; and intracellular viral proteins were detected by indirect immunofluorescence assay (IFA) using an anti-FCV MAb, 8G1 (30). At 24 h postinfection, the supernatants of HmLu-1 cells transduced with pcDNA3.1-fJAM-1 or pcDNA3.1 were collected, and viral titers were measured as described above.
Inhibition experiments.
CRFK or Vero cells (1.5 × 104) per well in 96-well plates were plated 1 day before infection. CRFK cells were washed once with PBS and incubated with 50 μl anti-fJAM-1 antibody or control mouse serum at fivefold dilutions, and Vero cells were washed and incubated with 50 μl (100-μg/ml) anti-human JAM-1 antibody or normal goat IgG for 1 h at 37°C. After antibodies were removed, CRFK cells were inoculated with FCV at a multiplicity of infection of 0.1 and incubated for 1 h at 37°C. Vero cells were inoculated with the viruses at a multiplicity of infection of 1 and incubated under the same conditions. The inocula were removed, CRFK cells were monitored for CPE for 2 days, and Vero cells were monitored for CPE for 4 days. At 0 h and 2 days (CRFK cells) or 4 days (Vero cells) postinoculation, cell supernatants were collected and virus titration was performed as described above.
PCR cloning of simian JAM-1 cDNA from Vero cells.
Total RNA was isolated from Vero cells using an SV Total RNA Isolation system (Promega, Madison, WI), and first-strand cDNA was synthesized by SuperScript III reverse transcriptase (Invitrogen) and an oligo(dT) primer. Primers SCF (5′ATCGCGATGGGGACAAAGGC) and SCR (5′AGGCTCACACCAGGCATGAC) were designed according to the sequences of feline and human JAM-1 to identify simian JAM-1 (sJAM-1). An ∼900-bp DNA fragment was amplified by PCR using LA Taq polymerase (TaKaRa), and the sequence was determined.
Nucleotide sequence accession numbers.
Nucleotide sequences of fJAM-1 and sJAM-1 are available from the DNA Data Bank of Japan (DDBJ), accession numbers AB196140 and AB196141, respectively.
RESULTS
Identification of fJAM-1 as an FCV-binding protein.
To identify FCV receptor(s) based on the capacity to bind to FCV F4, expression cloning with a cDNA library from CRFK cells susceptible to FCV was attempted as described above. From one colony of library-induced P3U1 cells that was formed on FCV-coated dishes, a specific insert of ∼1.5 kb was amplified by PCR with pMX primers. Sequence analysis revealed that the amplified insert encoded the feline homolog of JAM-1. The nucleotide and deduced amino acid sequences of fJAM-1 cDNA (DDBJ accession number AB196140) are shown in Fig. 1. Reproducible binding to FCV F4 coated on dishes was observed when the cDNA of fJAM-1 was expressed in P3U1 cells (data not shown).
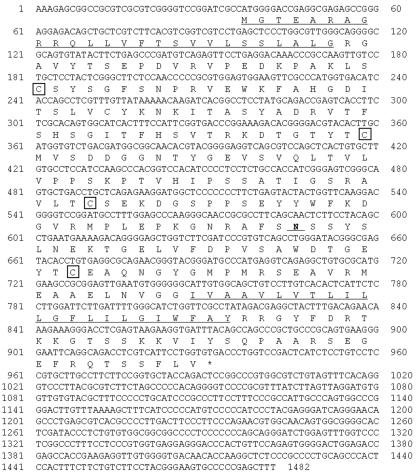
FIG. 1.
Nucleotide and deduced amino acid sequences of fJAM-1 cDNA. The putative hydrophobic signal peptide and transmembrane sequences are underlined. Four cystein residues that are predicted to form Ig-like domains are boxed. The putative N-linked glycosylation site is marked in boldface and underlined.
Detection of JAM-1 on the cell surface.
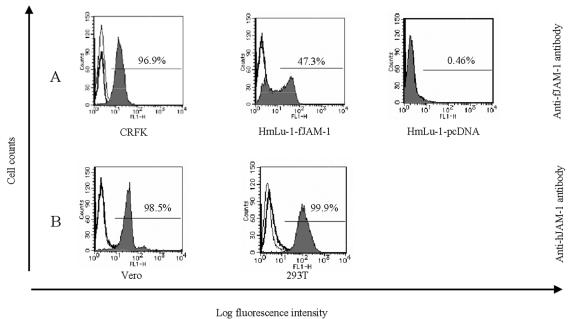
Using antibodies against feline and human JAM-1, we detected JAM-1 molecules by flow cytometry on CRFK, Vero, 293T, and HmLu-1 cells, which are nonpermissive to FCV infection and stably transduced with pcDNA3.1-fJAM-1 (HmLu-1-fJAM-1) or pcDNA3.1 only (HmLu-1-pcDNA). With anti-fJAM-1 antibody, fJAM-1 was detected on CRFK and HmLu-1-fJAM-1 cells, while the molecule was not detected in the cells transduced with vector only (Fig. 2A). Anti-hJAM-1 antibody reacted with sJAM-1 on Vero cells and hJAM-1 on 293T cells (Fig. 2B). There was no cross-reactivity between antibodies of feline and human JAM-1 (data not shown).
FIG. 2.
Detection of JAM-1 molecules on the cell surface by flow cytometry using anti-JAM-1 antibody. Cells used in this assay were Vero, 293T, CRFK, and HmLu-1 cells stably transduced with pcDNA3.1-fJAM-1 (HmLu-1-fJAM-1) or vector alone (HmLu-1-pcDNA). Cells were incubated with anti-JAM-1 antibody (shaded area), control antibody (thick line), or buffer only (thin line). Cells were incubated with a second antibody and analyzed by flow cytometry. (A) Detection of JAM-1 on cells using anti-fJAM-1 antibody. (B) Detection of JAM-1 on cells using anti-hJAM-1 antibody.
Binding of four strains of FCV to JAM-1.
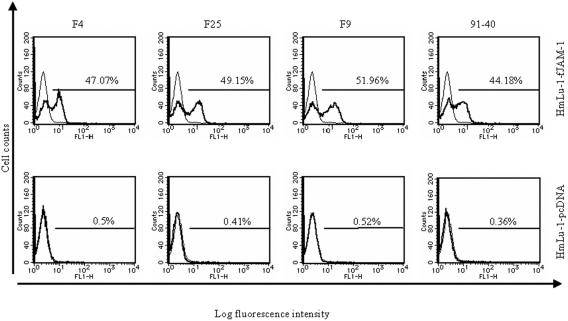
To confirm that FCV binds to fJAM-1, we used HmLu-1-fJAM-1 cells for FCV-binding assays by flow cytometry. FCV strains F4, F25, F9, and 91-40 bound specifically to HmLu-1-fJAM-1 cells, but not control cells, as shown in Fig. 3. Furthermore, FCV binding to CRFK cells was decreased by the anti-fJAM-1 antibody but not by control serum (Fig. 4A). These data suggest that FCV binds to CRFK cells, at least in part, via the fJAM-1 molecule.
FIG. 3.
FCV-binding assay by flow cytometry. HmLu-1-fJAM-1 or HmLu-1-pcDNA cells were incubated with four strains of FCV (thick line) or with buffer only (thin line). Viral antigens on cells were detected with an anti-capsid MAb by flow cytometry.
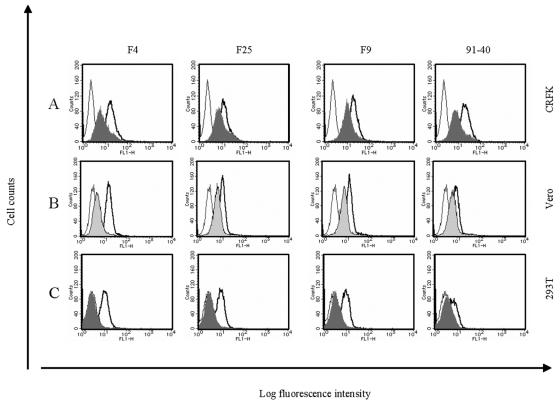
FIG. 4.
Binding inhibition assay by flow cytometry using anti-fJAM-1 and anti-hJAM-1 antibodies. (A) CRFK cells were incubated with anti-fJAM-1 antibody (shaded area) or control normal mouse serum (thick line) prior to incubation with FCV. In parallel, cells were incubated with buffer only (thin line). Vero (B) and 293T (C) cells were preincubated with anti-hJAM-1 antibody (shaded area) or normal goat IgG (thick line). FCV capsids were detected on the cells by flow cytometry.
Vero cells are reported to be resistant to infection by most FCV strains but are permissive to infection by FCV F4 and F25 (33). We found that, regardless of growth ability, FCV F9 and 91-40, as well as F4 and F25 strains, could bind to Vero cells and that the binding intensity was reduced by anti-hJAM-1 antibodies but not by normal goat IgG (Fig. 4B). While FCV did not infect 293T cells, we found that all strains of FCV examined bound to this cell line and that anti-hJAM-1 antibodies decreased the binding (Fig. 4C). These results suggest that FCV binds to Vero and 293T cells via sJAM-1 and hJAM-1, respectively.
fJAM-1 expression confers FCV susceptibility on nonpermissive cells.
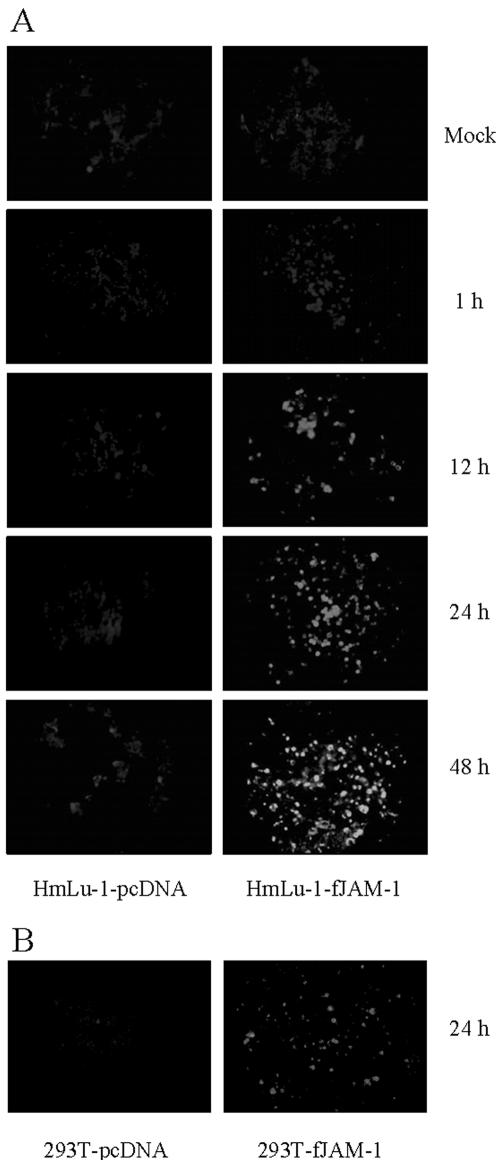
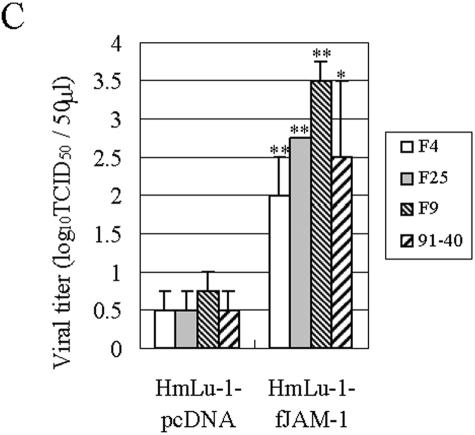
We investigated whether fJAM-1 expression could mediate FCV infection. fJAM-1-expressing HmLu-1 or 293T cells were inoculated with FCV F4 at a multiplicity of infection of 10; after the cells were washed with DMEM, viral antigens in the cells were examined by IFA at 1, 12, 24, and 48 h postinoculation. As shown in Fig. 5A, viral antigens were detected as early as 12 h postinoculation in HmLu-1-fJAM-1 cells. No viral capsid protein was detected in FCV-inoculated control HmLu-1-pcDNA cells. fJAM-1-expressing 293T cells showed similar results (Fig. 5B). When we used FCV F9, F25, and 91-40 for infection, results were same as for the F4 strain (data not shown). Furthermore, viral titers in supernatants of HmLu-1-fJAM-1 cells at 24 h postinfection were 102- to 103-fold higher than those of control cells (Fig. 5C). These data suggest that ectopic expression of fJAM-1 in nonpermissive cells renders them susceptible to infection with FCV.
FIG.5.
FCV infection in nonpermissive cells expressing fJAM-1. (A) HmLu-1-fJAM-1 or HmLu-1-pcDNA cells were inoculated with FCV F4 at a multiplicity of infection of 10 and fixed with acetone at 1, 12, 24, and 48 h postinfection. FCV antigens were detected by IFA. (B) 293T cells transduced with pcDNA3.1-fJAM-1 (293T-fJAM-1) or vector alone (293T-pcDNA) were inoculated with FCV F4 and fixed with acetone at 24 h postinfection. FCV antigens were detected by IFA. (C) Viral titers in supernatants of HmLu-1-fJAM-1 and HmLu-1-pcDNA cells at 24 h postinfection. Viral titers are expressed as the mean of three independent experiments. *, P < 0.05, and **, P < 0.01, compared to the supernatants of control cells.
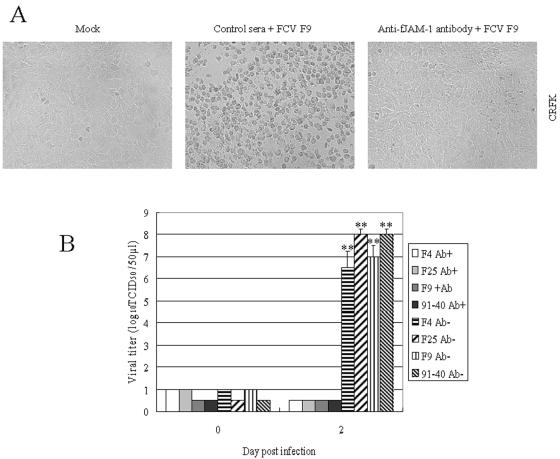
Inhibition of FCV infection by anti-JAM-1 antibody.
To ascertain that fJAM-1 is a receptor for FCV, we investigated the effect of the anti-fJAM-1 antibody on FCV infection in a permissive cell line, CRFK cells. In the presence of the antibody, CPE caused by FCV infection (appearance of round-shaped cells) was not observed (Fig. 6A), and viral titers in culture supernatants resulted in a 106- to 107-fold reduction compared to the control serum at 2 days postinfection (Fig. 6B). These results indicate that the anti-fJAM-1 antibody inhibits FCV infection at the attachment stage.
FIG. 6.
Effect of anti-fJAM-1 antibody on FCV infection in CRFK cells. CRFK cells were incubated with the anti-fJAM-1 antibody or control normal mouse serum prior to inoculation with FCV at a multiplicity of infection of 0.1. After antibody and inocula were removed, cells were added to fresh medium and incubated for 0 h or 2 days. Cells were observed, and virus titration was performed. (A) CRFK cells at 2 days postinfection or mock infection. (B) Viral titers in supernatants of CRFK cells at 0 h or 2 days postinfection. Viral titers are expressed as the mean of three independent experiments. **, P < 0.01, compared to the supernatants of CRFK cells incubated with anti-fJAM-1 antibody at 2 days postinfection.
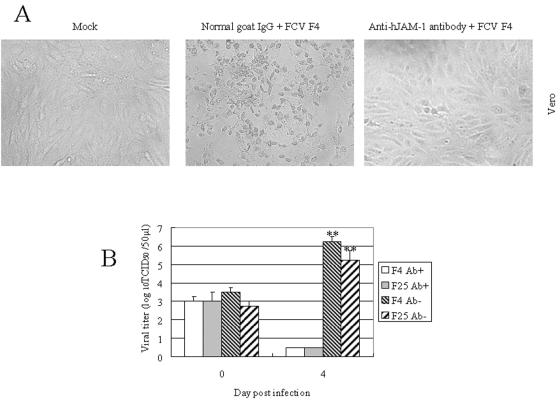
Furthermore, we used anti-hJAM-1 to examine the effect of the antibody on FCV infection in Vero cells that were sensitive to infection with FCV F4 and F25. CPE and viral production caused by F4 and F25 infection in Vero cells were strongly inhibited by the anti-hJAM-1 antibody (Fig. 7). These data show that some strains of FCV can productively infect the cells via the sJAM-1 molecule.
FIG. 7.
Effect of anti-hJAM-1 antibody on FCV infection in Vero cells. Vero cells were incubated with anti-hJAM-1 antibody or normal goat IgG prior to inoculation with FCV F4 and F25 strains at a multiplicity of infection of 1. After antibody and inocula were removed, cells were added to fresh medium and incubated for 0 h or 4 days. Cells were observed, and virus titration was performed. (A) Vero cells at 4 days postinfection or mock infection. (B) Viral titers in supernatants of Vero cells at 0 h or 4 days postinfection. Viral titers are expressed as the mean of three independent experiments. **, P < 0.01, compared to the supernatants of Vero cells incubated with anti-hJAM-1 antibody at 4 days postinfection.
DISCUSSION
We determined here that transduction of fJAM-1 molecules into FCV-nonpermissive cells renders them susceptible to binding and infection with FCV and that the anti-fJAM-1 antibody specifically inhibits viral binding to CRFK cells, resulting in the inhibition of viral replication within the cells. These data provide evidence that fJAM-1 is a functional receptor for FCV. Furthermore, FCV bound to sJAM-1 and hJAM-1, and the viral growth of some strains in Vero cells was inhibited by the anti-hJAM-1 antibody, demonstrating that sJAM-1 can also be an FCV receptor. To our knowledge, this is the first report concerning a functional receptor for the viruses in the family Caliciviridae.
JAM-1 is a member of the Ig superfamily and is expressed in various tissues and cell types, including leukocytes and platelets, as well as endothelial and epithelial cells, and is specifically localized at apical tight junctions (10, 23). However, FCV locally infects the respiratory tract, except for strains that cause virulent systemic disease (16, 27), suggesting that JAM-1 is a critical determinant of FCV host specificity and that tissue-specific molecular components other than JAM-1 may result in local infection of FCV in vivo. Consistent with this hypothesis, CRFK cells treated with neuraminidase, followed by O-glycanase treatment, demonstrated decreased FCV-binding ability (20). Therefore, such carbohydrates expressed in specific tissues or cells may enhance JAM-1-mediated FCV infection. Of course, it is possible that FCV uses an alternative receptor in vivo besides JAM-1, which has a tissue-specific distribution.
The majority of FCV strains, as represented by F9 and 91-40, have no growth ability in Vero cells, but binding to the cells was detected (Fig. 4B). When Vero cells are transfected with genomic RNA from these viruses, infectious virus can be recovered from Vero cells (20). Hence, these strains may be restricted in Vero cells at the early stage, probably after attachment, such as penetration and uncoating. The full genome sequences of F4 (infectious in Vero cells) and F9 (noninfectious in Vero cells) have been determined (5, 26, 36). Studies using infectious cDNA clones of FCV F4 and F9 strains would clarify the difference between F4 and F9 in terms of growth ability in Vero cells.
As JAM-1 is highly conserved among cats, monkeys, and humans (Fig. 8), it is possible that FCV binds to JAM-1 in these three animals via a conserved region(s). Although FCV has no growth ability in 293T cells, it seems unlikely that 293T cells have a postentry resistance mechanism against FCV, because ectopic expression of fJAM-1 in 293T cells supported FCV infection (Fig. 5B). Therefore, hJAM-1, at least expressed on 293T cells, can be recognized by FCV but cannot support virus infectivity. However, we found two amino acid residues conserved between fJAM-1 and sJAM-1 in extracellular domains of JAM-1, i.e., S91 and K155 in fJAM-1, but not in hJAM-1 (Fig. 8). The two residues in fJAM-1 may play an important role in FCV infectivity. Further studies are required to determine at which stage(s) the strains of FCV that cannot replicate in Vero and 293T cells are restricted after attachment. There has been no report in the past that FCV has the ability to infect monkeys or humans, suggesting that unidentified factors in addition to receptors may affect host species restriction.
FIG. 8.
A comparison of JAM-1 amino acid sequences of cats, monkeys, and humans. The putative signal peptide and transmembrane domain are underlined. Simian and human JAM-1 had a high identity of 96.7%, while fJAM-1 had 75.6% sequence identity with hJAM-1 and 76.6% sequence identity with sJAM-1. Two amino acid residues (S91 and K155) in extracellular domains of JAM-1 that were conserved between cats and monkeys, but not in humans, are boxed.
JAM-1 is also a receptor for reovirus (2), which infects most children and can cause mild gastrointestinal or respiratory illness. Reovirus induces activation of nuclear factor-κB and apoptosis, and receptor binding and disassembly of the viruses are required to elicit apoptosis (8, 11). However, the attachment and internalization of FCV do not induce apoptosis in cells. Instead, viral replication and protein synthesis trigger caspase activation and apoptosis (1, 31). Thus, the stages of FCV infection eliciting an apoptotic response are thought to be distinct from those of reovirus.
As well as JAM-1, coxsackievirus-adenovirus receptor and nectin, which are components of the apical tight junction complex of Ig-like proteins, regulate tight junctions (7, 14, 23). Some viruses are reported to use these Ig-like proteins as receptors. Adenovirus and coxsackieviruses use the coxsackievirus-adenovirus receptor (3); alphaherpeviruses and poliovirus use the nectin family (12, 34) for host cell entry. Targeting Ig-like proteins of apical tight junction complexes in infection may support efficient propagation in vitro, and possibly in vivo. Studies of FCV interaction with JAM-1 molecules may help determine how the viruses invade tight junction barriers of mucosal membranes.
Acknowledgments
We thank Toshio Kitamura (Department of Hematopoietic Factors and Department of Clinical Oncology, Institute of Medical Science, Advanced Clinical Research Center, The University of Tokyo, Tokyo, Japan) for providing Plat-E cells and the pMX vector.
This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
REFERENCES
- 1.Al-Molawi, N., V. A. Beardmore, M. J. Carter, G. E. N. Kass, and L. O. Roberts. 2003. Caspase-mediated cleavage of the feline calicivirus capsid protein. J. Gen. Virol. 84:1237-1244. [DOI] [PubMed] [Google Scholar]
- 2.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 4.Bittle, J. L., C. J. York, J. W. Newberne, and M. Martin. 1960. Serologic relationship of new feline cytopathogenic viruses. Am. J. Vet. Res. 21:546-547. [Google Scholar]
- 5.Carter, M. J., I. D. Milton, J. Meanger, M. Bennett, R. M. Gaskell, and P. C. Turner. 1992. The complete nucleotide sequence of a feline calicivirus. Virology 190:443-448. [DOI] [PubMed] [Google Scholar]
- 6.Chang, K. O., S. V. Sosnovtsev, G. Belliot, Y. Kim, L. J. Saif, and K. Y. Green. 2004. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc. Natl. Acad. Sci. USA 101:8733-8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, C. J., J. T. C. Shieh, R. J. Pickles, T. Okegawa, J. T. Hsieh, and J. M. Bergelson. 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98:15191-15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly, J. L., and T. S. Dermody. 2002. Virion disassembly is required for apoptosis induced by reovirus. J. Virol. 76:1632-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duizer, E., K. J. Schwab, F. H. Neill, R. L. Atmar, M. P. G. Koopmans, and M. K. Estes. 2004. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 85:79-87. [DOI] [PubMed] [Google Scholar]
- 10.Ebnet, K., A. Suzuki, S. Ohno, and D. Vestweber. 2004. Junctional adhesion molecules (JAMs): more molecules with dual functions? J. Cell Sci. 117:19-29. [DOI] [PubMed] [Google Scholar]
- 11.Forrest, J. C., and T. S. Dermody. 2003. Reovirus receptors and pathogenesis. J. Virol. 77:9109-9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 13.Green, K. Y., R. M. Chanock, and A. Z. Kapikian. 2001. Human caliciviruses, p. 841-874. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 14.Honda, T., K. Shimizu, T. Kawakatsu, M. Yasumi, T. Shingai, A. Fukuhara, K. Ozaki-Kuroda, K. Irie, H. Nakanishi, and Y. Takai. 2003. Antagonistic and agonistic effects of an extracellular fragment of nectin on formation of E-cadherin-based cell-cell adhesion. Genes Cells 8:51-63. [DOI] [PubMed] [Google Scholar]
- 15.Hoover, E. A., and D. E. Kahn. 1975. Experimentally induced feline calicivirus infection: clinical signs and lesions. J. Am. Vet. Med. Assoc. 166:463-468. [PubMed] [Google Scholar]
- 16.Hurley, K. F., P. A. Pesavento, N. C. Pedersen, A. M. Poland, E. Wilson, and J. E. Foley. 2004. An outbreak of virulent systemic feline calicivirus disease. J. Am. Vet. Med. Assoc. 224:241-249. [DOI] [PubMed] [Google Scholar]
- 17.Hutson, A. M., R. L. Atmar, D. Y. Graham, and M. K. Estes. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 185:1335-1337. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura, T., and Y. Morikawa. 2000. Isolation of T-cell antigens by retrovirus-mediated expression cloning. Methods Mol. Biol. 134:143-152. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura, T., M. Onishi, S. Kinoshita, A. Shibuya, A. Miyajima, and G. P. Nolan. 1995. Efficient screening of retroviral cDNA expression libraries. Proc. Natl. Acad. Sci. USA 92:9146-9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreutz, L. C., B. S. Seal, and W. L. Mengeling. 1994. Early interaction of feline calicivirus with cells in culture. Arch. Virol. 136:19-34. [DOI] [PubMed] [Google Scholar]
- 21.Levy, J. K., and A. Marsh. 1992. Isolation of calicivirus from the joint of a kitten with arthritis. J. Am. Vet. Med. Assoc. 201:753-755. [PubMed] [Google Scholar]
- 22.Lindesmith, L., C. Moe, S. Marionneau, N. Ruvoen, X. Jiang, L. Lindblad, P. Stewart, J. Le Pendu, and R. Baric. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548-553. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Y., A. Nusrat, F. J. Schnell, T. A. Reaves, S. Walsh, M. Pochet, and C. A. Parkos. 2000. Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci. 113:2363-2374. [DOI] [PubMed] [Google Scholar]
- 24.Marionneau, S., N. Ruvoën, B. Le Moullac-Vaidye, M. Clement, A. Cailleau-Thomas, G. Ruiz-Palacois, P. Huang, X. Jiang, and J. Le Pendu. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morita, S., T. Kojima, and T. Kitamura. 2000. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7:1063-1066. [DOI] [PubMed] [Google Scholar]
- 26.Oshikamo, R., Y. Tohya, Y. Kawaguchi, K. Tomonaga, K. Maeda, N. Takeda, E. Utagawa, C. Kai, and T. Mikami. 1994. The molecular cloning and sequence of an open reading frame encoding for non-structural proteins of feline calicivirus F4 strain isolated in Japan. J. Vet. Med. Sci. 56:1093-1099. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen, N. C., J. B. Elliott, A. Glasgow, A. Poland, and K. Keel. 2000. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet. Microbiol. 73:281-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimojima, M., T. Miyazawa, Y. Ikeda, E. L. McMonagle, H. Haining, H. Akashi, Y. Takeuchi, M. J. Hosie, and B. J. Willett. 2004. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science 303:1192-1195. [DOI] [PubMed] [Google Scholar]
- 29.Shimojima, M., T. Miyazawa, Y. Sakurai, Y. Nishimura, Y. Tohya, Y. Matsuura, and H. Akashi. 2003. Usage of myeloma and panning in retrovirus-mediated expression cloning. Anal. Biochem. 315:138-140. [DOI] [PubMed] [Google Scholar]
- 30.Shin, Y. S., Y. Tohya, R. Oshikamo, Y. Kawaguchi, K. Tomonaga, T. Miyazawa, C. Kai, and T. Mikami. 1993. Antigenic analysis of feline calicivirus capsid precursor protein and its deleted polypeptides produced in a mammalian cDNA expression system. Virus Res. 30:17-26. [DOI] [PubMed] [Google Scholar]
- 31.Sosnovtsev, S. V., E. A. Prikhod'ko, G. Belliot, J. I. Cohen, and K. Y. Green. 2003. Feline calicivirus replication induces apoptosis in cultured cells. Virus Res. 94:1-10. [DOI] [PubMed] [Google Scholar]
- 32.Tajima, T., E. Nakata, H. Kawamura, M. Mochizuki, and T. Uchino. 1993. Virus isolation from feline respiratory disease from November 1990 to May 1991. J. Anim. Clin. Res. Found. 1:19-23. (In Japanese.) [Google Scholar]
- 33.Takahashi, E., S. Konishi, and M. Ogata. 1971. Studies on cytopathogenic viruses from cats with respiratory infections. II. Characterization of feline picornaviruses. Jpn. J. Vet. Sci. 33:81-87. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi, K., H. Nakanishi, M. Miyahara, K. Mandai, K. Satoh, A. Satoh, H. Nishioka, J. Aoki, A. Nomoto, A. Mizoguchi, and Y. Takai. 1999. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 145:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tohya, Y., K. Masuoka, E. Takahashi, and T. Mikami. 1991. Neutralizing epitopes of feline calicivirus. Arch. Virol. 117:173-181. [DOI] [PubMed] [Google Scholar]
- 36.Tohya, Y., Y. Taniguchi, E. Takahashi, E. Utagawa, N. Takeda, K. Miyamura, S. Yamazaki, and T. Mikami. 1991. Sequence analysis of the 3′-end of feline calicivirus genome. Virology 183:810-814. [DOI] [PMC free article] [PubMed] [Google Scholar]