Abstract
Interferon (IFN) is one of the molecules released by virus-infected cells, resulting in the establishment of an antiviral state within infected and neighboring cells. IFN-induced antiviral response may be subject to modulation by the cellular signaling environment of host cells which impact the effectiveness of viral replication. Here, we show that cells with an activated Ras/Raf/MEK signaling cascade allow propagation of viruses in the presence of IFN. Ras-transformed (RasV12) and vector control NIH 3T3 cells were infected with vesicular stomatitis virus (VSV) or an IFN-sensitive vaccinia virus (delE3L) in the presence of alpha interferon. While IFN protected vector control cells from infection by both viruses, RasV12 cells were susceptible to viral infection regardless of the presence of IFN. IFN sensitivity was restored in RasV12 cells upon RNA interference (RNAi) knockdown of Ras. We further investigated which elements downstream of Ras are responsible for counteracting IFN-induced antiviral responses. A Ras effector domain mutant that can only stimulate the Raf kinase family of effectors was able to suppress the IFN response and allow VSV replication. IFN-induced antiviral mechanisms were also restored in RasV12 cells by treatment with a MEK inhibitor (U0126 or PD98059). Moreover, by using RNAi to MEK1 and MEK2, we determined that MEK2, rather than MEK1, is responsible for suppression of the IFN response. In conclusion, our results suggest that activation of the Ras/Raf/MEK pathway downregulates IFN-induced antiviral response.
The interferon (IFN) system is the first line of defense against viral infection. Upon infection by viruses, host cells release the antiviral cytokine IFN, which can act in an autocrine or paracrine manner, to activate intracellular antiviral defenses and restrict viral replication. To evade the host IFN system, many viruses are equipped with proteins that directly interrupt cellular antiviral responses induced by IFN (19, 37). However, there are other strategies for viral survival, as evidenced by the existence of viruses lacking anti-IFN proteins, which can be sensitive to cellular antiviral responses. One such strategy may be for viruses to preferentially propagate in cells resistant to IFN action. Alternatively, viruses may suppress the IFN response indirectly, by manipulating cellular signaling pathways that inhibit the IFN antiviral mechanism.
Viral infection plays a key role in the activation of alpha interferon (IFN-α)- and IFN-β-responsive promoters (40, 53). Once secreted, IFN-α and IFN-β, binding to their cognate receptors, activate the Jak-1 and Tyk-2 kinases, which leads to the phosphorylation of STAT-1 and STAT-2 proteins (14, 54). Phosphorylated STAT-1 and STAT-2 associate in a complex with IFN regulatory factor 9, to form IFN-stimulated gene factor 3, which in turn binds to IFN-stimulated response elements to activate gene transcription (52, 55). Among the IFN-inducible genes, RNA-activated protein kinase (PKR), 2′,5′-oligoadenylate synthetases, and Mx proteins have been particularly well studied with respect to their antiviral activities. PKR is an RNA-dependent kinase, which autophosphorylates following binding to double-stranded RNA (dsRNA) generated during viral infection (64). Activated PKR can directly phosphorylate selected cellular proteins, such as the α subunit of eukaryotic translation initiation factor 2 (eIF2α) (51), which leads to inhibition of viral protein synthesis. Similar to PKR, 2′,5′-oligoadenylate synthetase is also activated by dsRNA and generates 2′,5′-oligoadenylates (2-5A) (32, 57). The 2-5A molecules, in turn, bind and activate the latent RNase, RNase L, resulting in RNA degradation and inhibition of virus replication. The IFN-induced Mx proteins, first identified as anti-influenza virus proteins (27), are members of the superfamily of dynamin-like guanosine triphosphatases (GTPases) (26). The antiviral mechanism of Mx proteins is poorly understood. Most experimental evidence to date supports a model in which either viral nucleocapsid transport or viral RNA synthesis is blocked by Mx proteins, depending on their localization (21, 47, 63).
Ras is a membrane-bound GTP that functions as a molecular switch to transduce a wide range of signals from the cell membrane to the nucleus (56). Activation of Ras stimulates a broad range of cellular signaling pathways, resulting in regulation of a variety of cellular functions (11). Ras interacts with and activates one of its downstream elements, the serine/threonine protein kinase Raf, in a GTP-dependent manner, to regulate cell cycle progression, transformation, differentiation or apoptosis. The family of Raf protein kinases consists of A-Raf, B-Raf, and Raf-1. Activated Raf proteins phosphorylate and activate MEK-1 and -2, which then phosphorylate and activate extracellular signal-regulated kinase 1 (ERK-1) and ERK-2. Phosphorylated ERKs can affect gene expression directly by phosphorylating transcription factors such as Ets, Elk, and c-Myc as well as indirectly through other substrates such as p90-RSK (ribosomal S6 kinase) (15, 38). Several viruses are known to activate the Ras/Raf/MEK pathway during infection. Coxsackie virus infection activates Raf/MEK/ERK pathway by inducing cleavage of p21GTPase-activating protein (RasGAP) (28). Treatment of cells with U0126, a MEK inhibitor, significantly inhibits Coxsackie viral protein synthesis and progeny production (39). Borna disease virus also activates the Raf/MEK/ERK signaling cascade so that it can spread to neighboring cells in cell culture (49). Activation of ERK is also necessary for efficient infection by respiratory syncytial virus and visna virus (6, 35).
Several cellular mechanisms negatively regulate the IFN-induced antiviral state. Ras signaling has been suggested as one possible negative regulator of the IFN pathway. Introduction of viral oncogene (v-Ras) to BALB/c-3T3 cells blocks expression of major histocompatibility complex class I induced by IFN (45). Inhibition of PKR by activated Ras was initially reported by Mundschau and Faller (42). A recent study has shown that activation of K-Ras inhibits another component of the IFN response, specifically gamma-activated site-mediated expression of type II IFN (IFN-γ)-stimulated genes (33). Downregulation (dephosphorylation) of PKR has also been observed in Ras-transformed cells during infection by oncolytic viruses (9, 16, 59), suggesting that suppression of PKR by Ras signaling could be a common requirement for viral oncolysis. Although several studies have indicated that Ras downregulates cellular antiviral pathways, further systematic studies are required to determine exactly which downstream elements are responsible for the downregulation and how this takes place. In this study, we demonstrate that activation of Ras signaling pathways interrupts antiviral responses induced by IFN and further identify the downstream elements responsible for downregulation of the IFN response.
MATERIALS AND METHODS
Cells and viruses.
Murine fibroblast cell lines, NIH 3T3 and L929 cells, were obtained from the American Type Culture Collection. All cell lines used in this study were maintained in high-glucose Dulbecco's modified Eagle's medium (Invitrogen, Burlington, Ontario, Canada) with 10% fetal bovine serum (Cansera, Etobicoke, Ontario, Canada). Activated Ras and Ras effector mutant constructs in the pBABE retroviral vector were generously provided by P. W. Lee (Dalhousie University, Halifax, Canada) (43). The retroviral vectors containing the mutant genes were transfected into Bosc23 packaging cells (provided by P. W. Lee) by using Superfect (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Supernatants containing retroviruses were harvested at 48 h after transfection, filtered, and stored at −70°C. NIH 3T3 cells infected with retrovirus were selected with 2 μg of puromycin/ml for 2 weeks. Vesicular stomatitis virus (VSV) (Indiana strain, provided by J. C. Bell, University of Ottawa, Ottawa, Canada) (58) was amplified and titers determined using L929 cells. Vaccinia viruses (wild type [WR] and mutant virus lacking a gene encoding E3L [delE3L]) were provided by B. L. Jacobs (Arizona State University) (7).
Virus infection.
Cells were plated in 6- or 24-well plates and pretreated with or without recombinant mouse IFN-α (500 U/ml) (Sigma, St. Louis, MO) for 16 h. The cells were then infected with VSV or vaccinia virus at a multiplicity of infection (MOI) of 1 PFU. For drug treatment, cells were incubated with either of two MEK inhibitors, U0126 or PD98059 (Calbiochem, La Jolla, CA) for the duration of the experiments. At specified time points after infection, the supernatant was harvested for a progeny virus assay. The viral concentrations of supernatants from the triplicate wells were determined by plaque assay as described before (24). For Western blot analysis, cells were washed in phosphate-buffered saline (PBS) and lysed in PBS containing 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 10 μg/ml aprotinin, 100 μg/ml phenylmethylsulfonyl fluoride, and 1% phosphatase inhibitor cocktail (Sigma). Protein samples were cleared of debris by centrifugation. For reverse transcription (RT)-PCR, cells were washed in PBS and harvested in Trizol (Invitrogen).
RNAi.
Two oligonucleotides corresponding to nucleotide sequences of H-Ras, MEK1, or MEK2 were synthesized by Invitrogen (H-Ras, 5′-CCACUAUAGAGGAUUCCUACCGGAA-3′ [positions 289 to 314] and 5′-CCUGUGUGUGUUUGCCAUAACAAC-3′ [positions 422 to 446]; MEK1, 5′-AGUUCUGAAGAAAGCUGGAAGAAUU-3′ [positions 499 to 524] and 5′-UCAGCGGGCAGCUAAUUGACUCUAU-3′ [positions 672 to 697]; MEK2, 5′-GAGCUCAAGGACGACGACUUUGAGA-3′ [positions 413 to 438] and 5′-GAUCGACUCCAUGGCCAACUCGUUU [positions 875 to 900]). Negative control RNA interference (RNAi) comprised of a random nucleotide sequence was used as a control for nonspecific effects due to transfection of duplex RNA. Cells were grown to 40 to 50% confluence in 24-well plates, washed twice with PBS, and then incubated with a transfection mixture containing Dulbecco's modified Eagle's medium without antibiotics, 10% fetal bovine serum, RNAi Lipofectamine (20 μg/ml), and oligonucleotides (final concentration range, 10 to 50 pM). The transfection was repeated after 24 h for greater suppression of target genes. To confirm the efficacy of the RNAi system on silencing the target genes, we tested for a decrease either in phosphorylation of downstream elements by Western blot analysis or in target mRNA by RT-PCR using the following primers, as previously described (23): MEK1, 5′-TGCTGAGTTGCAGGCTCTTT-3′ and 5′-CTGGAGTCTCTCAGGCGACA-3′; MEK2, 5′-AGGCAAACCTGGTGGACCT-3′ and 5′-GAAGGCGTGGTTCATCAGC-3′; GAPDH, 5′-GGGTGGAGCCAAACGGGTCA-3′ and 5′-GGAGTTGCTGTTGAAGGTCGCA-3′.
Western blot analysis.
Protein samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose membrane (Bio-Rad, Mississauga, Ontario, Canada). The membrane was blocked with 5% skim milk in TBS (20 mM Tris and 137 mM NaCl [pH 7.3]) containing 0.1% Tween 20 and then incubated with the following primary antibodies: anti-VSV-G (Alpha Diagnostic, San Antonio, TX), anti-vaccinia virus (ViroStat, Portland, ME), anti-actin (Sigma), anti-phospho-ERK-1/2, anti-total ERK-1/2 (Calbiochem), anti-phospho-eIF2α (Biosource, Camarillo, CA), and anti-PKR (provided by P. W. Lee). After washing, the membrane was incubated with peroxidase-conjugated goat anti-mouse immunoglobulin G or anti-rabbit immunoglobulin G (Santa Cruz, CA), and specific bands were detected by enhanced chemiluminescence (Amersham, Baie d'Urfe, Quebec, Canada) as described previously (25). Quantification of the intensity of bands corresponding to phospho-ERK and total ERK was performed by using Kodak Molecular Imaging Software (Eastman Kodak Company, N.Y.).
Statistical analysis.
Statistical analyses were conducted with Student's t test.
RESULTS
Activation of Ras interferes with antiviral responses induced by IFN-α.
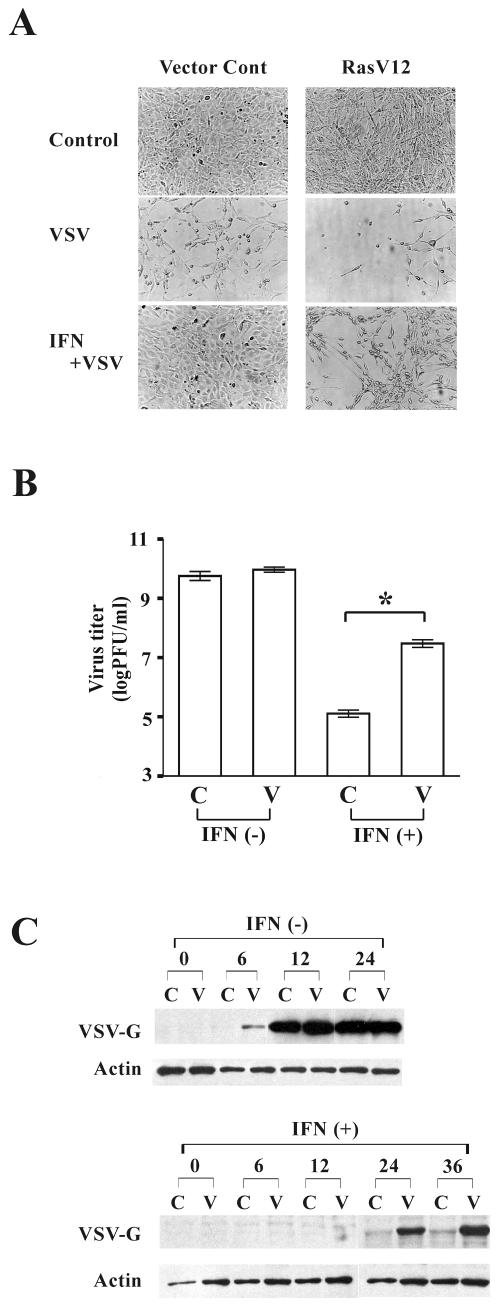
To test whether activated Ras signaling suppresses a protective IFN-α response and thus promotes viral replication in cells treated with IFN, H-Ras-transformed cells (RasV12) and vector control cells were generated by transformation of NIH 3T3 cells with an active mutant Ras gene or an empty vector. Eighty percent confluent vector control and RasV12 cultures were treated with or without IFN-α (500 unit/ml) for 16 h and then infected with an IFN-sensitive virus, VSV, at an MOI of 1. Following VSV infection, both vector control and RasV12 cells clearly exhibited cytopathic effects in the absence of IFN (Fig. 1A). In the presence of IFN, no morphological change was detected in VSV-infected control cells. In contrast, RasV12 cells were susceptible to VSV infection despite the addition of IFN. These results were confirmed by analysis of progeny virus production (Fig. 1B) and by Western blot analysis using an antibody against VSV-G protein (Fig. 1C). While there was no significant difference in production of progeny virus between vector control and RasV12 in the absence of IFN at 24 h after infection, more progeny virus (300 times) was produced in RasV12 culture than vector control culture following infection in the presence of IFN (P < 0.01). It should be noted that IFN treatment showed some inhibition on VSV replication in RasV12 cells even though it was to a much lesser degree than that in vector control cells. Compared to RasV12 not treated with IFN, accumulation of VSV-G protein was delayed and progeny virus production was reduced in RasV12 treated with IFN.
FIG. 1.
Activation of Ras interrupts IFN-induced antiviral responses. Vector control (Cont) cells (C) and RasV12 cells (V) were incubated with or without IFN (500 U/ml) for 16 h and then challenged with VSV (MOI = 1). Cytopathic effects (A) and progeny virus production (B) in vector control cells and RasV12 cells at 24 h after VSV infection are shown. *, P < 0.01. (C) Viral protein synthesis in vector control cells and RasV12 cells in the absence or presence of IFN at 0, 6, 12, 24, and 36 h after VSV infection, as determined by Western blot analysis for VSV-G and β-actin.
To further demonstrate the interaction of the Ras signaling pathways and the IFN pathway, we silenced the active mutant Ras gene in RasV12 cells by using Stealth RNAi to H-Ras. A possible problem of using small interfering RNA/RNAi in this particular study was the nonspecific activation of IFN/PKR responses by the introduced dsRNA. To circumvent this problem, we used Stealth RNAi, since this novel system is designed to eliminate nonspecific activation of the IFN pathway. We confirmed this by Western blot analysis, where no increased expression of PKR or phosphorylation of eIF2α was observed in Ras RNAi or control RNAi-treated RasV12 cells compared to mock-treated cells (Fig. 2A). Phosphorylation levels of the downstream element of Ras, ERK-1/2, was decreased in cells treated with Ras RNAi (10 to 50 pmol), confirming that the RNAi silences the target Ras gene in RasV12 cells. After 24 h of treatment with RNAi, the cells were treated with IFN-α for 16 h and then challenged with VSV. Viral protein synthesis was clearly blocked in vector control cells and RasV12 cells treated with the Ras RNAi, while VSV infected RasV12 control and RasV12 cells treated with negative control RNAi in the presence of IFN. Thus, IFN-induced antiviral effects in RasV12 cells were restored by suppression of Ras by RNAi targeted to the active mutant Ras gene (Fig. 2B). In contrast, the Ras silencing did not alter cell susceptibility to VSV infection in the absence of IFN. Taken together, these results suggest clearly that activation of Ras signaling pathways interrupts IFN-induced antiviral responses.
FIG. 2.
IFN-induced antiviral responses are restored in RasV12 cells by knockdown of active mutant Ras gene using RNAi. RNA oligonucleotides to Ras (RasRNAi) or random sequence (NGRNAi) were transfected to RasV12 cells twice with a 24-h interval. At 24 h after the second RNAi treatment, the cells were incubated with IFN (500 U/ml) for 16 h followed by infection of VSV (MOI = 1). (A) Expression of PKR and phosphorylation of eIF2α in RasV12 cells at 24 h after the RNAi treatment. (B) Viral protein synthesis in RasV12 cells with RNAi-mediated depletion of active mutant Ras gene at 24 h after VSV infection in the presence (+) or absence (−) of IFN. Western blot analysis for PKR, phosphorylated eIF2α (p-eIF2α), VSV-G, phosphorylated ERK (p-ERK), and total ERK (t-ERK). p-ERK/t-ERK, ratio of intensity of phosphorylated ERK and total ERK corresponding to the value of RasV12 control.
Involvement of Ras/Raf/MEK pathway in downregulation of antiviral responses induced by IFN.
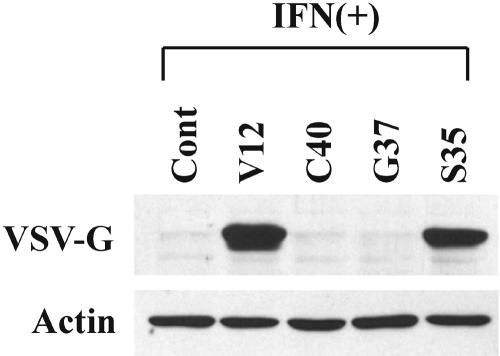
Among the wide range of cellular signaling pathways stimulated by activation of Ras, three effectors of Ras are well characterized: phosphatidylinositol 3-kinase (PI3-kinase), Ral guanine nucleotide exchange factors (RalGEFs), and Raf kinase. Point mutations within the effector domain of Ras generates mutants which impair the activation of specific downstream elements (11, 62). For example, the V12C40 mutant shows selective affinity for PI3-kinase, but not for RalGEFs or Raf kinase; the V12G37 signals to RalGEFs, but not PI3-kinase or Raf kinase; the V12S35 only selects for Raf kinase without stimulating the other two pathways. To identify whether one of these effectors suppresses the IFN response, Ras effector mutant cells were established by infecting NIH 3T3 cells with retrovirus containing three Ras effector domain mutants (V12C40, V12G37, and V12S35), RasV12 (activated Ras), or empty vector control. The Ras effector mutant cells were infected with VSV in the presence of IFN (Fig. 3). Although VSV infection was clearly blocked in vector control cells and two Ras effector mutants, namely V12C40 cells (PI3 kinase) and V12G37 cells (RalGEFs), IFN failed to protect RasV12 cells (Ras) and V12S35 cells (Raf kinase) from virus infection. This result indicates that one branch of Ras, the Ras/Raf pathway, plays a critical role in regulating IFN-stimulated antiviral responses to virus infection.
FIG. 3.
Raf is the responsible element downstream of Ras that mediates suppression of the IFN antiviral response. Ras effector mutants (vector control [Cont], RasV12 [Ras, V12], RasC40 [PI3-kinase, C40], RasG37 [RalGEFs, G37], and RasS35 [Raf, S35]) were incubated with IFN-α (500 U/ml) for 16 h and then challenged with VSV (MOI = 1). Virus infection at 24 h after VSV infection as determined by Western blot analysis for VSV-G and β-actin is shown.
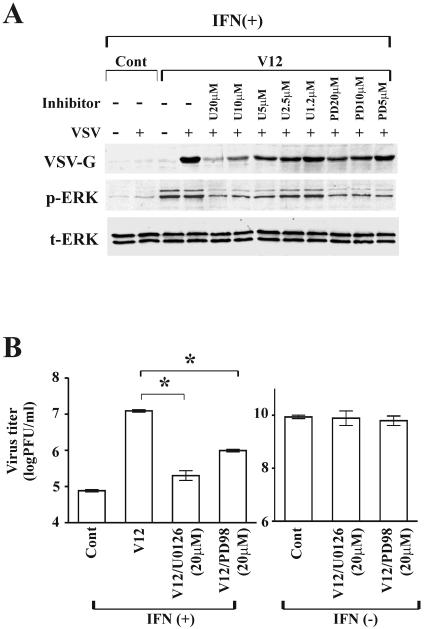
Next, we sought to identify the downstream elements of the Ras/Raf pathway involved in interrupting the IFN-induced antiviral response by testing the effects of MEK inhibitors, U0126 and PD98059, on VSV replication in Ras-activated cells (RasV12). As shown Fig. 4A, treatment with both MEK inhibitors clearly decreased phosphorylation of the downstream elements (ERK-1/2) in a dose-dependent manner. When pretreated with U0126, the IFN response was almost completely restored in RasV12 cells; in the presence of U0126, IFN protected RasV12 cells to a similar extent as seen in vector control cells. Partial restoration of the IFN response was also observed for PD98059. These results were also evident by quantitative assay measuring progeny virus production (Fig. 4B). At 24 h postinfection in the presence of IFN, progeny virus of VSV-infected RasV12 cells was significantly decreased by MEK inhibitor treatment (P < 0.01). The inhibitory effect of U0126 on progeny virus production was four times greater than that of PD98059. Importantly, U0126 and PD98059 did not inhibit VSV progeny virus production in the absence of IFN, suggesting that the protective effect induced by the MEK inhibitors is dependent on antiviral responses induced by IFN but not on VSV replication directly. This agrees with a previous report by Balachandran and Barber, which showed that VSV replication is not directly affected by PD98059 (3).
FIG. 4.
IFN-induced antiviral responses are restored in RasV12 cells treated with a MEK inhibitor. Vector control cells (Cont) and RasV12 cells (V12) were treated with or without a MEK inhibitor (U0126 [U] or PD98059 [PD]) in the presence (+) or absence (−) of IFN (500 U/ml) for 16 h and then challenged with VSV. (A) Viral protein synthesis in RasV12 cells treated with a MEK inhibitor at 24 h after VSV infection as determined by Western blot analysis for VSV-G, phosphorylated ERK (p-ERK), and total ERK (t-ERK); (B) progeny virus production at 24 h after infection. *, P < 0.01.
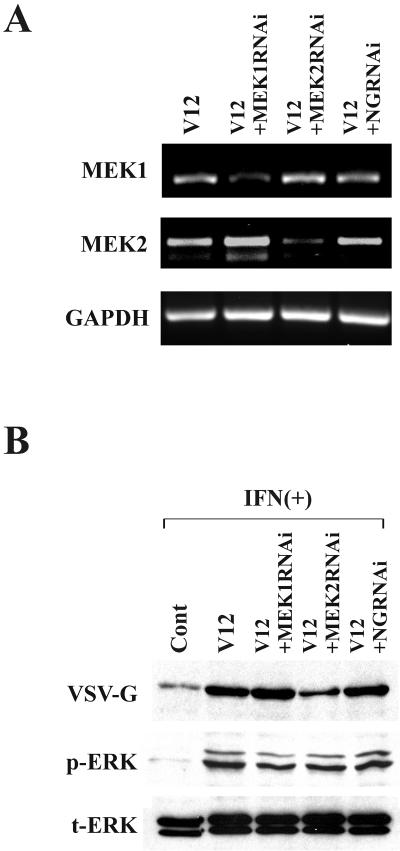
The differential efficacy of the two MEK inhibitors in restoration of IFN-induced antiviral responses may be due to the fact that PD98059 more selectively inhibits MEK1 activity than MEK2, compared to U0126 which inhibits both MEKs to a similar extent (2, 17). Therefore, we hypothesized that MEK2, rather than MEK1, plays a critical role in the interruption of the IFN pathways. To test this, we suppressed the expression levels of MEK1 and MEK2 in RasV12 cells using RNAi. We confirmed that the expression of MEK1 or MEK2 mRNA in RasV12 cells was reduced at 24 h after RNAi transfection by RT-PCR (Fig. 5A). RasV12 cells were treated with RNAi and incubated with IFN for 16 h and then challenged with VSV. Western blot analysis using VSV-G antibody showed that the infection was inhibited in RasV12 cells treated with MEK2 RNAi, whereas infection was unaffected by MEK1 knockdown or negative control RNAi (Fig. 5B). The phosphorylation levels of ERK-1 and -2 were partially inhibited by RNAi treatment of either MEK1 or MEK2. These results suggest that MEK2, rather than MEK1, is the element downstream of Ras/Raf that is responsible for suppression of the IFN response.
FIG. 5.
MEK2, but not MEK1, is responsible for interruption of IFN-induced antiviral responses. RNA oligonucleotides to MEK1 (MEK1RNAi), MEK2 (MEK2RNAi), or random sequence (NGRNAi) were transfected to RasV12 cells twice with a 24-h interval. At 24 h after the second RNAi treatment, the cells were incubated with IFN (500 U/ml) for 16 h followed by infection of VSV (MOI = 1). (A) Knockdown of MEK1 and MEK2 mRNA expression by RNAi. Total cellular RNA was extracted at 24 h after the RNAi treatment and subjected to RT-PCR for MEK1, MEK2, and GAPDH. (B) Viral protein synthesis in vector control cells (Cont) and RasV12 cells (V12) with RNAi-mediated depletion of MEK1 or MEK2 at 24 h after VSV infection as determined by Western blot analysis for VSV-G, phosphorylated ERK (p-ERK), and total ERK-1/2 (t-ERK) at 24 h after VSV infection.
Altogether, using Ras effector domain mutants, chemical inhibitors, and RNAi, we suggest that the Ras/Raf/MEK2 pathway plays a critical role in interrupting IFN-induced antiviral responses.
Negative regulation of IFN responses by the Ras/Raf/MEK pathway during infection by an IFN-sensitive vaccinia virus.
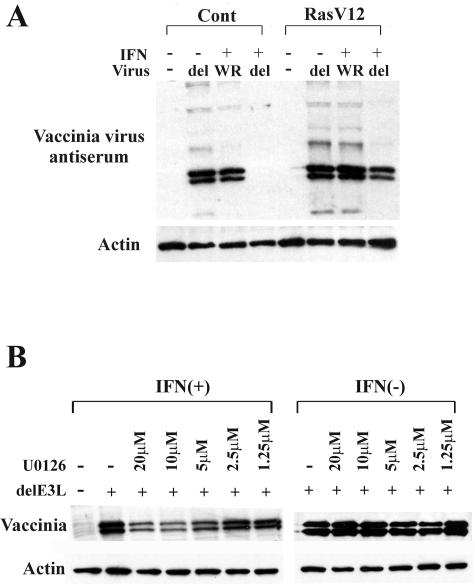
To examine whether resistance to IFN in cells with an activated Ras/Raf/MEK pathway occurs during infection by other IFN-sensitive viruses, vaccinia viruses (wild type [WR] and mutant vaccinia virus lacking a gene encoding an anti-IFN protein [delE3L]) were used. E3L is a member of the dsRNA-binding proteins, which prevents the activation of PKR and 2-5A synthetases (13). While mutant delE3L virus is known to be highly sensitive to IFN and exhibits a restricted host range, replication of wild-type vaccinia virus is not affected by the activation of PKR and 2-5A/RNase L (10). As expected, wild-type vaccinia virus, which is somewhat resistant to IFN action, was capable of infecting both vector control cells and RasV12 cells, regardless of the addition of IFN (Fig. 6A). In contrast, the IFN-sensitive mutant virus did not replicate in vector control cells but could replicate in RasV12 cells in the presence of IFN. To further examine whether infection of the IFN-sensitive vaccinia virus in the presence of IFN is dependent on activity of the MEK, an MEK inhibitor, U0126, was used. We found that delE3L virus infection of V12 cells was decreased in the presence of IFN and U0126 (Fig. 6B). U0126 did not affect virus replication in the absence of IFN, indicating that replication inhibition was not through a direct effect by U0126 but was rather achieved through the restoration of the IFN-induced antiviral response. Thus, the Ras/Raf/MEK pathway is also involved in interrupting the IFN responses in cells infected with the IFN-sensitive vaccinia virus. Since similar results were obtained in VSV experiments, it seems that the interaction of the IFN pathway and the Ras/Raf/MEK pathway may be a common event during infection by IFN-sensitive viruses.
FIG. 6.
Ras/Raf/MEK pathway suppresses antiviral responses induced by IFN during infection of another IFN-sensitive virus. (A) Viral protein synthesis in vector control cells (Cont) and RasV12 cells at 48 h after vaccinia virus infection. Vector control cells and RasV12 cells were incubated with (+) or without (−) IFN (500 U/ml) for 16 h and then challenged with wild-type vaccinia virus (WR) or mutant delE3L vaccinia virus (del) at an MOI of 1. (B) Viral protein synthesis in RasV12 cells treated with a MEK inhibitor U0126 in the presence (+) or absence (−) of IFN (500 U/ml) at 48 h after mutant delE3L vaccinia virus infection as determined by Western blot analysis with vaccinia virus antiserum and β-actin antibody.
The PKR/eIF2α antiviral pathway is not targeted by the Ras/Raf/MEK pathway.
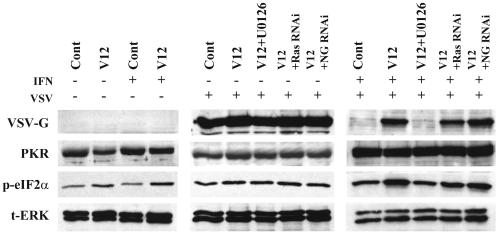
Downregulation (dephosphorylation) of PKR is often observed in Ras-transformed cells during infection by oncolytic viruses, suggesting that the Ras-PKR connection may be a common requirement for Ras-dependent viral oncolysis (9, 16, 59). Next, a set of experiments were performed to examine whether activation of the PKR/eIF2α pathway induced by IFN is regulated by the Ras/Raf/MEK pathway. Vector control cells and RasV12 cells were treated with either U0126, RNAi to Ras, or RNAi to random sequences and then treated with or without IFN for 16 h, followed by infection of VSV. At 12 (in the absence of IFN) and 24 (in the presence of IFN) h after infection, cell lysates were examined by Western blot analysis for VSV-G, PKR, phosphorylated eIF2α, or total ERK-1/2. In the absence of IFN, there was no difference in VSV infection, PKR expression, and eIF2α phosphorylation among the cells with or without activation of the Ras/Raf/MEK pathway (Fig. 7). In the presence of IFN, VSV replicated in RasV12 cells, but not in vector control cells, and viral protein synthesis was suppressed in RasV12 cells treated with U0126 or Ras RNAi, but not negative control RNAi. Activation of the Ras/Raf/MEK pathway did not affect induction of PKR. Interestingly, we found that eIF2α was phosphorylated to a greater extent in RasV12 cells than vector control cells or RasV12 cells with inhibition of the Ras/Raf/MEK pathway. In brief, phosphorylation of eIF2α positively correlated with the degree of infection. These results indicate that the PKR/eIF2α pathway is not inhibited by the Ras/Raf/MEK pathway.
FIG. 7.
The PKR/eIF2a pathway is not the target of Ras/Raf/MEK pathway. Vector control cells (Cont), RasV12 cells (V12), RasV12 cells treated with U0126 (V12+U0126), and RasV12 cells treated with RNAi to Ras or random sequence (NG) were incubated in the absence (−) or presence (+) of IFN (500 U/ml) for 16 h and then challenged with VSV (MOI = 1). Western blot analysis for VSV-G, PKR, phosphorylated eIF2α (p-eIF2α), and total ERK (t-ERK) at 12 h (in the absence of IFN) and 24 h (in the presence of IFN) after VSV infection is shown.
DISCUSSION
The IFN system is the primary line of host defense that targets replication steps common to the propagation of many different viruses. However, this antiviral system becomes ineffective when viruses take advantage of cellular negative regulators of the IFN response. In this study, we have found that activation of the Ras/Raf/MEK pathway downregulates IFN-induced antiviral responses. Since Ras/Raf/MEK signaling regulates cell proliferation, differentiation, and cell death in diverse cell types, activation of this pathway varies depending on cell type and cellular conditions. Thus, the activation status of the Ras/Raf/MEK pathway may be one defining factor of cellular sensitivity to IFN action, which underlies host susceptibilities and organ tropisms in vivo. In addition, viruses have evolved to modulate host cellular signaling pathways to promote viral replication. Cell signal modulation during virus infection is mainly a consequence of the binding of virus to its cellular receptor, cross talk between viral and cellular proteins, and stress caused by the infection. As several viruses are known to activate the Ras/Raf/MEK pathway early during infection (6, 28, 35, 41, 48, 49, 50), it is possible that the activation of this pathway may be a strategy used by these viruses to evade the host IFN system.
There is a significant body of literature implicating Ras activation in viral infection and replication (4, 9, 12, 16, 59). Reovirus was the first virus reported to specifically kill cancer cells harboring constitutively activated Ras (59). Following the discovery of reovirus oncolysis, it has been suggested that other viruses, such as wild-type herpes simplex virus (HSV) (16), VSV (4), influenza virus (delNS1 strain) (9), and adenovirus (VAI mutant) (12) were found to similarly flourish in cells with deregulated Ras signaling. However, it has been reported that two oncolytic viruses exploit different elements downstream of Ras for their replication: reovirus (Ras/RalGEF/p38MAPK) (43) and wild-type HSV (Ras/Raf/MEK) (16). The basis of viral oncolysis by these viruses is the connection between Ras activation and the PKR/eIF2α response. Therefore, the interruption of IFN-induced antiviral responses by the Ras/Raf/MEK pathway demonstrated in this study, which does not involve the PKR/eIF2α pathway, may not be of primary importance in their particular oncolytic capability. In addition, reovirus and HSV are somewhat insensitive to the antiviral status induced by IFN since they both harbor viral proteins to evade the IFN system (19, 22, 29). In contrast, other oncolytic viruses are either inherently sensitive to IFN (VSV) or are missing essential components of their anti-IFN defense due to mutation (influenza and adenovirus mutants). Thus, Ras/Raf/MEK suppression of IFN pathways may be a highly relevant and essential common mechanism for infection and oncolysis by these viruses. Recent studies indicate that VSV oncolysis relies on two independent mechanisms: first, deregulation of PKR and its downstream targets, and second, interruption of IFN signaling pathways (4, 5, 44). Deregulation of PKR signaling has been shown recently, where it was found that eIF2B-mediated nucleotide exchange downstream of PKR/eIF2α was aberrant in transformed cells compared to primary cells (3). Mechanism for the interruption of IFN signaling, however, remained unclear. The present study suggests that the other arm of VSV's oncolytic mechanism, namely interruption of IFN signaling, depends on Ras/Raf/MEK. It is of interest to examine whether the same mechanism applies to other cell lines, especially human cancer cell lines.
In our current study, we have found that activation of MEK2, rather than MEK1, is responsible for the suppression of the IFN-induced antiviral responses. MEK1 and MEK2 are nearly identical at the amino acid level (34, 66). Despite their similarity, functional differences between MEK1 and MEK2 have been reported. MEK1−/− mice die in the early gestation period due to a placental vascularization defect, while MEK2 is dispensable for growth and development (8, 18). Ussar and Voss suggested that the balance of MEK1 and MEK2 activities plays a critical role in regulating cell cycle progression (61). The MEK1 signal promotes proliferation via cyclin D-CDK4/6 activation, whereas activation of MEK2 leads to p21cip1-mediated growth arrest. MEK1 and MEK2 are known to equally phosphorylate downstream elements ERK-1 and -2 (34). This agrees with our result which shows that knockdown of either MEK1 or MEK2 induced only partial inhibition of phosphorylation of ERKs (Fig. 5B). This result suggests that it is unlikely that the ERKs are the downstream elements of MEK2 responsible for the interruption of antiviral responses induced by IFN. Further investigation is required to identify the responsible downstream elements of MEK2.
Which elements of the IFN response are targeted by MEK2 remains unknown. One of the possibilities is that MEK2 counteracts the antiviral effectors activated by IFN and virus infection, such as PKR/eIF2α, 2-5A/RNase L, and Mx. Indeed, it has been reported that Ras suppression of PKR underlies one mechanism of tumor lysis by oncolytic viruses (9, 16, 59). However, in our study, we did not observe downregulation of the PKR/eIF2α pathway in Ras-activated cells (RasV12) infected with VSV in the presence of IFN compared to the vector control cells, suggesting that PKR/eIF2α is not targeted by the Ras/Raf/MEK pathway in our experimental system. Yet, it is possible that the Ras/Raf/MEK pathway interferes with other antiviral effectors, such as 2-5A/RNase L and Mx. Another possible mechanism of MEK2 action on the IFN response may involve interruption of Jak-STAT signaling/IFN-stimulated response element-mediated transcription. IFN-α is an important therapeutic cytokine that exerts antitumor activity in a variety of tumor cells (20). However, therapeutic effects become limited when tumor cells become insensitive to the IFN treatment (31, 60). Lack of activation of the Jak-STAT pathway has been reported as a possible cause of cancer cell resistance to IFN (36, 46, 65). In addition, a recent study has shown that activation of K-Ras inhibits another component of the IFN response, specifically gamma-activated site-mediated expression of IFN-γ-stimulated genes (33). Therefore, the Ras/Raf/MEK pathway may inhibit activation of the Jak-STAT pathway upon IFN stimulation which would suppress expression of IFN-inducible genes. To answer this, we will further investigate how the Ras/Raf/MEK pathway counteracts the IFN pathway by conducting a step-by-step analysis of IFN receptor expression, activation of the JAK-STAT pathway, expression of IFN-inducible genes, and modification of antiviral effectors in Ras-transformed cells.
The MEK inhibitor U0126 by itself did not inhibit VSV or IFN-sensitive vaccinia virus replication, which suggests that U0126 does not have any direct effects on their replication cycles. This result indicates clearly that the Ras/Raf/MEK pathway modulates IFN-induced antiviral responses but not VSV replication directly. This agrees with a previous report which showed that differential susceptibility between primary and transformed cells in the absence of IFN is not dependent on the MEK-ERK pathway (3). However, several viruses have been reported to be sensitive to U0126 treatment (1, 30, 39, 49, 50). For example, inhibition of MEK by U0126 during influenza virus infection resulted in nuclear retention of viral ribonucleoprotein complexes and reduction of viral progeny production (50). The spread of Borna disease virus from cell to cell was inhibited in the presence of U0126 (49). Although these experiments were performed with naive cells without treatment of exogenous IFN, U0126 may have enhanced the effect of endogenous IFN produced during the course of infection.
In conclusion, Ras interrupts the IFN-induced antiviral response through activation of Raf/MEK2. This study provides new insights into how the cellular signaling environment affects virus infection through modification of the IFN system. Viruses often directly manipulate cellular signaling pathways; therefore, this study may illuminate potential signaling elements modulated by viruses with the ultimate goal of suppressing the IFN-induced antiviral response. Strengthening the IFN response by suppressing cellular negative regulators may be a new strategy for the development of novel antiviral drugs.
Acknowledgments
This work was supported by grants (to K.H.) from the Canadian Institutes for Health Research (CIHR), Breast Cancer Society of Canada, and Newfoundland Cancer Treatment and Research Foundation. K.H. is a recipient of a New Investigator Award from CIHR.
We thank Patrick Lee, Bertram Jacobs, and John Bell for generous gifts of constructs, cell lines, and viruses.
REFERENCES
- 1.Akula, S. M., P. W. Ford, A. G. Whitman, K. E. Hamden, J. G. Shelton, and J. A. McCubrey. 2004. Raf promotes human herpesvirus-8 (HHV-8/KSHV) infection. Oncogene 23:5227-5241. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 3.Balachandran, S., and G. N. Barber. 2004. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 5:51-65. [DOI] [PubMed] [Google Scholar]
- 4.Balachandran, S., M. Porosnicu, and G. N. Barber. 2001. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J. Virol. 75:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. [DOI] [PubMed] [Google Scholar]
- 6.Barber, S. A., L. Bruett, B. R. Douglass, D. S. Herbst, M. C. Zink, and J. E. Clements. 2002. Visna virus-induced activation of MAPK is required for virus replication and correlates with virus-induced neuropathology. J. Virol. 76:817-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beattie, E., K. L. Denzler, J. Tartaglia, M. E. Perkus, E. Paoletti, and B. L. Jacobs. 1995. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J. Virol. 69:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belanger, L. F., S. Roy, M. Tremblay, B. Brott, A. M. Steff, W. Mourad, P. Hugo, R. Erikson, and J. Charron. 2003. Mek2 is dispensable for mouse growth and development. Mol. Cell. Biol. 23:4778-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmann, M., I. Romirer, M. Sachet, R. Fleischhacker, A. Garcia-Sastre, P. Palese, K. Wolff, H. Pehamberger, R. Jakesz, and T. Muster. 2001. A genetically engineered influenza A virus with ras-dependent oncolytic properties. Cancer Res. 61:8188-8193. [PubMed] [Google Scholar]
- 10.Brandt, T. A., and B. L. Jacobs. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell, S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 12.Cascallo, M., G. Capella, A. Mazo, and R. Alemany. 2003. Ras-dependent oncolysis with an adenovirus VAI mutant. Cancer Res. 63:5544-5550. [PubMed] [Google Scholar]
- 13.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darnell, J. E., Jr., I. M. Kerr, and G. R. Stark. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415-1421. [DOI] [PubMed] [Google Scholar]
- 15.Davie, J. R., and V. A. Spencer. 2001. Signal transduction pathways and the modification of chromatin structure. Prog. Nucleic Acid Res. Mol. Biol. 65:299-340. [DOI] [PubMed] [Google Scholar]
- 16.Farassati, F., A. D. Yang, and P. W. Lee. 2001. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat. Cell Biol. 3:745-750. [DOI] [PubMed] [Google Scholar]
- 17.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273:18623-18632. [DOI] [PubMed] [Google Scholar]
- 18.Giroux, S., M. Tremblay, D. Bernard, J. F. Cardin-Girard, S. Aubry, L. Larouche, S. Rousseau, J. Huot, J. Landry, L. Jeannotte, and J. Charron. 1999. Embryonic death of Mek1-deficient mice reveals a role for this kinase in angiogenesis in the labyrinthine region of the placenta. Curr. Biol. 9:369-372. [DOI] [PubMed] [Google Scholar]
- 19.Grandvaux, N., B. R. tenOever, M. J. Servant, and J. Hiscott. 2002. The interferon antiviral response: from viral invasion to evasion. Curr. Opin. Infect. Dis. 15:259-267. [DOI] [PubMed] [Google Scholar]
- 20.Gutterman, J. U. 1994. Cytokine therapeutics: lessons from interferon alpha. Proc. Natl. Acad. Sci. USA 91:1198-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haller, O., M. Frese, and G. Kochs. 1998. Mx proteins: mediators of innate resistance to RNA viruses. Rev. Sci. Tech. 17:220-230. [DOI] [PubMed] [Google Scholar]
- 22.He, B., M. Gross, and B. Roizman. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirasawa, K., H. S. Jun, K. Maeda, Y. Kawaguchi, S. Itagaki, T. Mikami, H. S. Baek, K. Doi, and J. W. Yoon. 1997. Possible role of macrophage-derived soluble mediators in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J. Virol. 71:4024-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirasawa, K., A. Kim, H. S. Han, J. Han, H. S. Jun, and J. W. Yoon. 2003. Effect of p38 mitogen-activated protein kinase on the replication of encephalomyocarditis virus. J. Virol. 77:5649-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirasawa, K., S. G. Nishikawa, K. L. Norman, T. Alain, A. Kossakowska, and P. W. K. Lee. 2002. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 62:1696-1701. [PubMed] [Google Scholar]
- 26.Horisberger, M. A. 1992. Interferon-induced human protein MxA is a GTPase which binds transiently to cellular proteins. J. Virol. 66:4705-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horisberger, M. A., P. Staeheli, and O. Haller. 1983. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc. Natl. Acad. Sci. USA 80:1910-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber, M., K. A. Watson, H. C. Selinka, C. M. Carthy, K. Klingel, B. M. McManus, and R. Kandolf. 1999. Cleavage of RasGAP and phosphorylation of mitogen-activated protein kinase in the course of coxsackievirus B3 replication. J. Virol. 73:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imani, F., and B. L. Jacobs. 1988. Inhibitory activity for the interferon-induced protein kinase is associated with the reovirus serotype 1 sigma 3 protein. Proc. Natl. Acad. Sci. USA 85:7887-7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, R. A., X. L. Ma, A. D. Yurochko, and E. S. Huang. 2001. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J. Gen. Virol. 82:493-497. [DOI] [PubMed] [Google Scholar]
- 31.Kantarjian, H. M., S. O'Brien, P. Anderlini, and M. Talpaz. 1996. Treatment of myelogenous leukemia: current status and investigational options. Blood 87:3069-3081. [PubMed] [Google Scholar]
- 32.Kerr, I. M. 1987. The 2-5A system: a personal view. J. Interferon Res. 7:505-510. [DOI] [PubMed] [Google Scholar]
- 33.Klampfer, L., J. Huang, G. Corner, J. Mariadason, D. Arango, T. Sasazuki, S. Shirasawa, and L. Augenlicht. 2003. Oncogenic Ki-ras inhibits the expression of interferon-responsive genes through inhibition of STAT1 and STAT2 expression. J. Biol. Chem. 278:46278-46287. [DOI] [PubMed] [Google Scholar]
- 34.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351(Pt 2):289-305. [PMC free article] [PubMed] [Google Scholar]
- 35.Kong, X., H. San Juan, A. Behera, M. E. Peeples, J. Wu, R. F. Lockey, and S. S. Mohapatra. 2004. ERK-1/2 activity is required for efficient RSV infection. FEBS Lett. 559:33-38. [DOI] [PubMed] [Google Scholar]
- 36.Landolfo, S., A. Guarini, L. Riera, M. Gariglio, G. Gribaudo, A. Cignetti, I. Cordone, E. Montefusco, F. Mandelli, and R. Foa. 2000. Chronic myeloid leukemia cells resistant to interferon-alpha lack STAT1 expression. Hematol. J. 1:7-14. [DOI] [PubMed] [Google Scholar]
- 37.Levy, D. E., and A. Garcia-Sastre. 2001. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 12:143-156. [DOI] [PubMed] [Google Scholar]
- 38.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49-139. [DOI] [PubMed] [Google Scholar]
- 39.Luo, H., B. Yanagawa, J. Zhang, Z. Luo, M. Zhang, M. Esfandiarei, C. Carthy, J. E. Wilson, D. Yang, and B. M. McManus. 2002. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 76:3365-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mamane, Y., C. Heylbroeck, P. Genin, M. Algarte, M. J. Servant, C. LePage, C. DeLuca, H. Kwon, R. Lin, and J. Hiscott. 1999. Interferon regulatory factors: the next generation. Gene 237:1-14. [DOI] [PubMed] [Google Scholar]
- 41.Mischiati, C., F. Pironi, D. Milani, M. Giacca, P. Mirandola, S. Capitani, and G. Zauli. 1999. Extracellular HIV-1 Tat protein differentially activates the JNK and ERK/MAPK pathways in CD4 T cells. AIDS 13:1637-1645. [DOI] [PubMed] [Google Scholar]
- 42.Mundschau, L. J., and D. V. Faller. 1992. Oncogenic ras induces an inhibitor of double-stranded RNA-dependent eukaryotic initiation factor 2 alpha-kinase activation. J. Biol. Chem. 267:23092-23098. [PubMed] [Google Scholar]
- 43.Norman, K. L., K. Hirasawa, A. D. Yang, M. A. Shields, and P. W. Lee. 2004. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc. Natl. Acad. Sci. USA 101:11099-11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obuchi, M., M. Fernandez, and G. N. Barber. 2003. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 77:8843-8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Offermann, M. K., and D. V. Faller. 1990. Effect of cellular density and viral oncogenes on the major histocompatibility complex class I antigen response to gamma-interferon in BALB-c/3T3 cells. Cancer Res. 50:601-605. [PubMed] [Google Scholar]
- 46.Pansky, A., P. Hildebrand, E. Fasler-Kan, L. Baselgia, S. Ketterer, C. Beglinger, and M. H. Heim. 2000. Defective Jak-STAT signal transduction pathway in melanoma cells resistant to growth inhibition by interferon-alpha. Int. J. Cancer 85:720-725. [DOI] [PubMed] [Google Scholar]
- 47.Pavlovic, J., A. Schroder, A. Blank, F. Pitossi, and P. Staeheli. 1993. Mx proteins: GTPases involved in the interferon-induced antiviral state. Ciba Found. Symp. 176:233-243. [DOI] [PubMed] [Google Scholar]
- 48.Perkins, D., E. F. Pereira, M. Gober, P. J. Yarowsky, and L. Aurelian. 2002. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 76:1435-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Planz, O., S. Pleschka, and S. Ludwig. 2001. MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells. J. Virol. 75:4871-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pleschka, S., T. Wolff, C. Ehrhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 51.Samuel, C. E. 1979. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc. Natl. Acad. Sci. USA 76:600-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schafer, S. L., R. Lin, P. A. Moore, J. Hiscott, and P. M. Pitha. 1998. Regulation of type I interferon gene expression by interferon regulatory factor-3. J. Biol. Chem. 273:2714-2720. [DOI] [PubMed] [Google Scholar]
- 54.Schindler, C. 1999. Cytokines and JAK-STAT signaling. Exp. Cell Res. 253:7-14. [DOI] [PubMed] [Google Scholar]
- 55.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 56.Shields, J. M., K. Pruitt, A. McFall, A. Shaub, and C. J. Der. 2000. Understanding Ras: ‘it ain't over ′til it's over’. Trends Cell Biol. 10:147-154. [DOI] [PubMed] [Google Scholar]
- 57.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 58.Stojdl, D. F., B. Lichty, S. Knowles, R. Marius, H. Atkins, N. Sonenberg, and J. C. Bell. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821-825. [DOI] [PubMed] [Google Scholar]
- 59.Strong, J. E., M. C. Coffey, D. Tang, P. Sabinin, and P. W. Lee. 1998. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 17:3351-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talpaz, M., H. Kantarjian, R. Kurzrock, J. M. Trujillo, and J. U. Gutterman. 1991. Interferon-alpha produces sustained cytogenetic responses in chronic myelogenous leukemia. Philadelphia chromosome-positive patients. Ann. Intern. Med. 114:532-538. [DOI] [PubMed] [Google Scholar]
- 61.Ussar, S., and T. Voss. 2004. MEK1 and MEK2, different regulators of the G1/S transition. J. Biol. Chem. 279:43861-43869. [DOI] [PubMed] [Google Scholar]
- 62.Webb, C. P., L. Van Aelst, M. H. Wigler, and G. F. Woude. 1998. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc. Natl. Acad. Sci. USA 95:8773-8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weber, F., O. Haller, and G. Kochs. 2000. MxA GTPase blocks reporter gene expression of reconstituted Thogoto virus ribonucleoprotein complexes. J. Virol. 74:560-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams, B. R. 1999. PKR; a sentinel kinase for cellular stress. Oncogene 18:6112-6120. [DOI] [PubMed] [Google Scholar]
- 65.Wong, L. H., K. G. Krauer, I. Hatzinisiriou, M. J. Estcourt, P. Hersey, N. D. Tam, S. Edmondson, R. J. Devenish, and S. J. Ralph. 1997. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J. Biol. Chem. 272:28779-28785. [DOI] [PubMed] [Google Scholar]
- 66.Zheng, C. F., and K. L. Guan. 1993. Cloning and characterization of two distinct human extracellular signal-regulated kinase activator kinases, MEK1 and MEK2. J. Biol. Chem. 268:11435-11439. [PubMed] [Google Scholar]