Abstract
Recombinant vesicular stomatitis virus (rVSV) vectors offer an attractive approach for the induction of robust cellular and humoral immune responses directed against human pathogen target antigens. We evaluated rVSV vectors expressing full-length glycoprotein D (gD) from herpes simplex virus type 2 (HSV-2) in mice and guinea pigs for immunogenicity and protective efficacy against genital challenge with wild-type HSV-2. Robust Th1-polarized anti-gD immune responses were demonstrated in the murine model as measured by induction of gD-specific cytotoxic T lymphocytes and increased gamma interferon expression. The isotype makeup of the serum anti-gD immunoglobulin G (IgG) response was consistent with the presence of a Th1-CD4+ anti-gD response, characterized by a high IgG2a/IgG1 IgG subclass ratio. Functional anti-HSV-2 neutralizing serum antibody responses were readily demonstrated in both guinea pigs and mice that had been immunized with rVSV-gD vaccines. Furthermore, guinea pigs and mice were prophylactically protected from genital challenge with high doses of wild-type HSV-2. In addition, guinea pigs were highly protected against the establishment of latent infection as evidenced by low or absent HSV-2 genome copies in dorsal root ganglia after virus challenge. In summary, rVSV-gD vectors were successfully used to elicit potent anti-gD Th1-like cellular and humoral immune responses that were protective against HSV-2 disease in guinea pigs and mice.
Herpes simplex virus type 2 (HSV-2) infections remain a serious public health problem worldwide. HSV-2 genital lesions are not only painful and disfiguring but also facilitate the transmission of human immunodeficiency virus (HIV) (7). The seroprevalence in the United States has increased by 30% between 1976 and 1994, and roughly one of every five people over the age of 12 in the United States is infected with HSV-2 (15). Individuals latently infected with HSV-2 remain infected for life and can exhibit asymptomatic viral shedding. It is therefore believed that, without intervention, such as the development of prophylactic and/or therapeutic HSV-2 vaccines, HSV-2 prevalence will continue to rise in the future.
Small experimental animal vaginal challenge models in mice and guinea pigs have been used for preclinical evaluation of a number of HSV-2 vaccine strategies, including subunit vaccines (gB and/or gD with or without interleukin-12 [IL-12]), plasmid HSV DNA vaccines (gD and/or gB with or without cytokine DNA (IL-2, IL-4, IL-10, IL-12, IL-15, or IL-18), attenuated HSV-2 vaccines (TK−, BlacZ, dl5-29, RAV 9395, ICP10ΔPK, or AD472), and virus-vectored HSV-2 vaccines (adenovirus, varicella-zoster virus, or vaccinia virus) (1, 9, 12, 17, 21, 22, 34, 39, 40, 45, 60, 62, 66). Various degrees of success have been achieved in these preclinical studies, but limited success has carried over to the clinical setting, where the experience with HSV-2 subunit vaccines has had mixed results (10). Nonetheless, an adjuvanted gD subunit approach has achieved some success in early clinical trials and is currently under phase III assessment (64).
Live recombinant vectors expressing pertinent HSV-2 target genes can be divided into vectors capable of replication and those that are limited to a single cycle of infection. One of the major advantages associated with the use of nonreplicating vectors is increased safety. However, this inability to replicate may reduce total recombinant antigen expression, resulting in reduced immunogenicity. For success, replicating viral vectors require a balance between safety and immunogenicity, both of which are dependent on the level of viral replication and antigen expression.
Vesicular stomatitis virus (VSV) is an enveloped, negative-strand RNA virus of the Rhabdoviridae family. In nature, VSV is transmitted by insects and infects livestock, causing a self-limiting disease that is marked by vesicular lesions of the mouth and teats. VSV rarely infects humans but, when infection does occur, it can result in disease ranging from asymptomatic infection to mild flu-like illness (51). Since the development of a system for recovery of recombinant VSV (rVSV) from plasmid DNA, rVSV vectors have been assessed in animal models as vaccine vectors for numerous pathogens, including influenza virus, human immunodeficiency virus, respiratory syncytial virus, hepatitis C virus, measles virus, Ebola virus, Lassa cottontail rabbit papillomavirus, fever virus, Marburg virus, and severe acute respiratory syndrome virus (5, 16, 18, 19, 25-28, 47, 49, 56). Depending on the foreign antigen expressed, rVSV vectors can induce potent humoral and cellular immune responses that are protective in many animal models of infection. Specifically, rVSV vectors expressing HIV-Env and SIV-Gag were highly protective in the rhesus macaque SHIV 89.6P challenge model (13). More recently, rVSV vectors pseudotyped with G proteins from the Ebola and Marburg viruses protected nonhuman primates from lethal challenge with these viruses (26).
We describe here the use of recombinant VSV vectors expressing HSV-2 gD as a genital HSV-2 vaccine. The anti-gD immune responses elicited by these rVSV-gD vectors were evaluated in immunized mice and guinea pigs, and genital challenge models were used to measure vaccine efficacy. Immunization with rVSV-gD induced potent and protective immunity in both murine and guinea pig models.
MATERIALS AND METHODS
Animals.
Seven-week-old female BALB/c mice were obtained from Taconic Laboratories (Germantown, NY). Female Dunkin-Hartley guinea pigs weighing approximately 250 to 350 g were obtained from Charles River Laboratories. All animal care and procedures conformed to Institutional Animal Care and Use Committee guidelines. Mice were housed in microisolator cages (five animals/cage), and guinea pigs were kept in shoebox style cages (two animals/box) and were permitted to feed and drink ad libitum.
Viruses and cells.
Recombinant VSV vectors expressing gD (rVSV-gD) were derived from the prototypic rVSV vectors described by Rose and coworkers (33, 49, 57, 58). Briefly, to create rVSVI-gD, a PCR product spanning the complete HSV-2 gD (strain 12) open reading frame was cloned into an XhoI/NheI expression cassette between the G and L genes of genomic cDNA from the Indiana (I) serotype of VSV (Fig. 1A). Infectious virus was recovered from the resulting rVSVI-gD cDNA after transfection into BHK cells, along with a mixture of plasmids expressing the VSV N, P, and L proteins under the control of the bacteriophage T7 RNA polymerase transcription promoter. Confluent overnight BHK cell monolayers in six-well dishes were transfected with 2 to 4 μg of plasmid containing the full-length genomic cDNA, 1.0 μg of N plasmid, 0.5 μg of P plasmid, and 0.15 μg of L plasmid using CaPO4. To provide a source of T7 RNA polymerase, MVA-T7-GK16 was added at a multiplicity of infection (MOI) of 3 to 4 PFU/cell, along with cytosine arabinoside, at a final concentration of 20 μg/ml to prevent replication of MVA-T7 (32). Cells were incubated at 32°C, 3% CO2 for 3 h, followed by a 2-h heat shock at 43°C and 3% CO2 (43). Cells were incubated at 32°C in 3% CO2 overnight. Transfection medium was replaced with 2 ml of fresh growth medium containing 20 μg of cytosine arabinoside/ml, and cells were then incubated at 37°C in 5% CO2 for 48 to 72 h. Transfected cells were scraped into suspension, gently pipetted, and transferred to confluent Vero cell monolayers. The next day, cocultures were supplemented with 1 ml of fresh growth medium and incubated for 3 to 5 days, during which time a viral cytopathic effect became apparent. Rescued virus was triple plaque purified and further amplified for animal studies. Expression of intact gD was verified by Western blot.
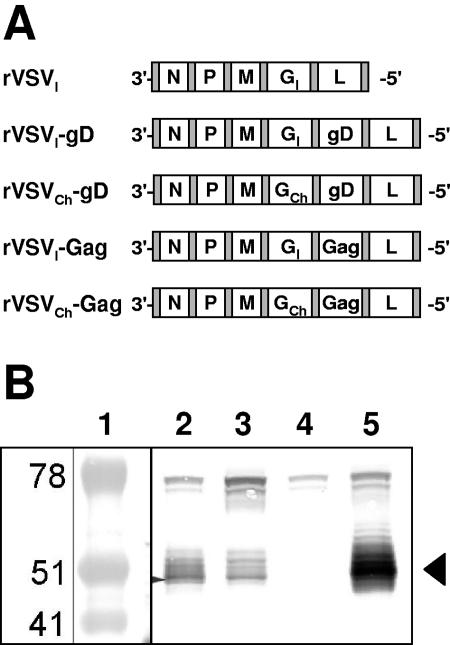
FIG. 1.
Genomes and protein expression of rVSV-gD vectors. (A) The genomes of the rVSV vectors created for the present study are diagrammed in a 3′-to-5′ orientation of the negative-stranded viral RNA. (B) Vero cells were infected at an MOI of 5 with rVSV. At 24 h postinfection, cell lysates were prepared and electophoresed in a 5 to 20% sodium dodecyl sulfate-polyacrylamide gel. Proteins were electroblotted on to a nylon membrane and probed with DL6 monoclonal antibody against HSV-2 gD. Lane 1, molecular weight standard; lane 2, rVSVI-gD; lane 3, rVSVCh-gD; lane 4, rVSVI; lane 5, HSV-2 gD standard (position is indicated by arrow).
The rVSVCh-gD vector was generated by swapping the G gene of rVSVI-gD with the G gene from the related vesiculovirus Chandipura virus (Ch) (53). The G switch was made by restriction digestion of pVSV(GCh)XN-1 with MluI and XhoI and agarose gel electrophoresis purification of a 1.6-kb fragment containing the GCh protein open reading frame. The GCh protein fragment was ligated with pVSVI-gD2 DNA previously digested with MluI and XhoI and treated with alkaline phosphatase. STBL2 cells transformed with the ligation mixture were screened for clonal selection, and one clone was expanded as a maxiprep. The resultant plasmid vector pVSVI[GCh]-gD2 was used to rescue pseudotyped virus as described above.
The 186 or MS strains of HSV-2, amplified and titrated in Vero cells, were used as the challenge viruses for mice or guinea pigs, respectively.
Vaccination protocol.
BALB/c mice were primed either intranasally or intramuscularly with the indicated dose of rVSVCh-gD and boosted 3 to 4 weeks later by the same route with the corresponding rVSVI-gD. Negative controls consisted of naive mice or mice immunized with rVSV vectors expressing an irrelevant HIV Gag antigen: rVSVCh-Gag followed by rVSVI-Gag. Intramuscular immunizations were given by injection into the calf muscle of anesthetized mice in 50 μl of phosphate-buffered saline (PBS). Intranasal immunizations were given by dropwise instillation into the nares of anesthetized mice by a micropipettor in 10 μl of PBS (5 μl per naris). Serum samples and spleen cells were harvested from mice (n = 5) 3 to 4 weeks after the prime or boost to test for humoral and cellular immune responses. Protective efficacy was determined by resistance to an intravaginal challenge with HSV-2 in groups of 10 mice as described below at 3 to 4 weeks after either priming or boosting.
Groups of 10 Dunkin-Hartley guinea pigs were vaccinated by intranasal instillation of rVSV in PBS. The 100-μl dose was distributed equally to each naris of the anesthetized animal by using a micropipettor. Priming and boosting immunizations were performed 3 weeks apart, with the boost occurring 3 weeks before challenge with HSV-2. The VSVI-gD was administered first for the primary immunization, and VSVCh-gD was used for the booster immunization.
Murine vaginal challenge model.
Five days prior to the virus challenge, all mice received 2.0 mg of DEPO-PROVERA (Upjohn, Kalamazoo, MI) subcutaneously in the scruff of the neck to synchronize their estrus cycles and to increase their susceptibility to HSV-2 vaginal infection (44). Prior to infection, mice were anesthetized, and their vaginas were swabbed with sterile PBS-soaked Dacron polyester tip applicators (Puritan, Guilford, ME) to remove the associated mucous. Mice were subsequently inoculated intravaginally with 100 50% lethal doses (LD50) of wild-type HSV-2 strain 186 (1 LD50 = 250 PFU, empirically determined for this specific stock of virus). Virus was instilled into the vaginal vault by using a micropipettor (10 μl/dose in PBS), and anesthetized mice were placed into dorsal recumbency until they recovered from anesthesia. Naive mice served as negative controls. The mice were monitored daily for 4 weeks for symptoms of viral infection and mortality using the following scale: 0, no signs of disease; 1, vaginal erythema; 2, vaginal erythema and edema; 3, vaginal herpetic lesions; 4, unilateral paralysis or severe genital ulceration with hair loss from genital and surrounding tissue; and 5, bilateral paralysis or death.
Guinea pig vaginal challenge model.
Guinea pigs were challenged with HSV-2 strain MS by intravaginal instillation of 100 μl of PBS containing 2 × 105 PFU of virus. The vaginal vault was cleansed by swabbing with saline-dipped swabs, followed by a dry swab prior to inoculation. The virus inoculum was delivered slowly using a 1-ml syringe tipped with a 1-cm length of narrow Teflon tubing. Instillation was repeated to ensure infection rates of 90 to 100%. Vaginas were swabbed on day 2, and virus titers were assessed by reverse transcription-PCR of swab samples. Lesion scores were determined by using a modification of the scoring system reported by Stanberry et al. (63). Briefly, disease severity was scored by counting discrete lesions; for animals with more then 15 discrete lesions, assessment of disease severity was based on the area covered by confluent lesions. The area covered by confluent lesions (as a percentage of the whole genital area) was converted back to individual lesion numbers based on the extrapolation that confluent lesions covering the entire genital area would be equivalent to a score of 30. This resulted in scores that were proportional to disease severity and thus more appropriate for statistical analysis.
ELISA for gD-specific immunoglobulin.
Murine gD-specific antibody responses were quantified by standard enzyme-linked immunosorbent assay (ELISA) as previously described (70). Briefly, 96-well plates were coated with 20 ng of purified gD/well, washed three times, and then blocked with PBS plus 1% bovine serum albumin. Serial twofold dilutions of mouse sera in 0.05 M Tris-buffered saline were added to duplicate wells, followed by incubation for 1 h. Bound gD-specific antibodies were detected with biotinylated goat anti-mouse immunoglobulin G1 (IgG1) or IgG2a or with goat anti-guinea pig IgG, followed by avidin-horseradish peroxidase (Sigma, St. Louis, MO) and ABTS substrate (Kirkgaard and Perry Laboratories, Gaithersburg, MD). The intensity of the resulting color was measured at 405 nm, and the endpoint titer was defined as the reciprocal of the serum dilution that resulted with an absorbance (optical density at 405 nm [OD405]) value that was equal to the mean absorbance value of control naive sera plus two standard deviations. The geometric mean titer ± the standard error for each group was calculated by using Origin and Excel software.
HSV-2 neutralization titers (ELVIS assay).
Individual mouse or guinea pig sera were evaluated for HSV neutralizing antibody titer by using a colorimetric assay with the ELVIS HSV cell line (Diagnostic Hybrids, Athens, OH) as described previously (9). Briefly, threefold diluted test sera were incubated with 4 × 104 PFU of HSV-2 in the presence of 10% (vol/vol) guinea pig plasma for 1 h at 37°C. Aliquots were overlaid onto confluent ELVIS HSV cell monolayers in 96-well microtiter plates. After an overnight incubation, the culture fluid was aspirated, and the cells were overlaid with medium containing detergent and frozen at −70°C. Upon thawing, medium containing a β-d-galactopyranoside substrate was added, the microtiter plates were incubated at 37°C, and the OD570 was determined. The neutralization titer was defined as the reciprocal of the serum dilution added that decreased the OD570 obtained using positive virus controls by 50%. The geometric mean and standard error of the geometric mean of titers for each group were calculated.
VSV neutralization assay.
Test sera were diluted 1:50 in PBS, and serial twofold dilutions were carried out in duplicate in U-bottom 96-well plates in 50 μl of PBS. rVSVI or rVSVCh was diluted in Dulbecco modified Eagle medium, and 100 PFU in 50 μl per well was added to the plates. After a 1-h incubation at 37°C, 2 × 103 log phase BHK cells in Dulbecco modified Eagle medium plus 10% fetal bovine serum were added to all wells. Plates were incubated for 3 to 4 days or until “no virus” control wells showed yellow color associated with cell overgrowth, while “no serum” control wells retained the original red color of the medium. The neutralization titer was defined as the reciprocal of the highest dilution that exhibited complete inhibition of the VSV cytopathic effect.
Th1/Th2 cytokine detection by cytometric bead array analysis.
Pooled spleen cells (108) from five mice per group had red blood cells lysed with ACK lysis buffer (BioWhittaker, Walkerville, MD) and were restimulated in vitro in 40 ml of T-cell medium in a T-75 T-flask with 108 PFU of HSV-2 (strain 186) that was UV inactivated with 100 mJ of UV light (UV Stratalinker; Stratagene, La Jolla, CA). After 3 days of restimulation, supernatant fluids were frozen and stored at −20°C for later analysis. The Th1/Th2 cytokine content was determined by BD Pharmingen's (San Diego, CA) mouse Th1/Th2 cytokine cytometric bead array as described in the manufacturer's protocol.
CTL assay.
Pooled spleen cells were restimulated with UV-inactivated HSV-2 as described above. After 5 days, live effector cells were isolated on Lympholyte-M gradients (Cedarlane, Hornby, Ontario, Canada) and assessed for cytolytic activity against HSV-2-infected (MOI = 10, 4 h) A20 B-cell lymphoma target cells (American Type Culture Collection) in a 3-h europium (Eu3+) release assay (65). Uninfected A20 cells were used as targets for background lysis. Target cells were labeled with Eu3+ (Sigma), and Eu3+ release was detected by time resolved fluorescence on a Victor2 Multilabel Counter (Perkin-Elmer, Gaithersburg, MD). The mean percent lysis was calculated from the average of triplicates based on the formula: percent lysis = [(experimental release − spontaneous release)/(maximal release-spontaneous release)] × 100. The percent specific lysis was determined by subtracting the percent lysis of uninfected targets from the percent lysis of infected targets for each group.
Intracellular cytokine staining protocol.
Pooled murine splenocyte suspensions were restimulated in vitro for 3 days as described above. Cell surface staining was accomplished with fluorescein isothiocyanate-conjugated and biotinylated antibodies (59). Briefly, cells were stained with fluorescein isothiocyanate-conjugated rat anti-mouse CD4 and PerCP-conjugated rat anti-mouse CD8a (Pharmingen, San Jose, CA) monoclonal antibodies. After cell surface staining, cells were washed with PBS, fixed with 4% paraformaldehyde (Cytofix; Pharmingen, San Jose, CA) for 30 min, and permeabilized with 0.1% saponin (Sigma Chemical Co.). After permeabilization, cells were incubated with phycoerythrin-conjugated rat anti-mouse gamma interferon (IFN-γ) monoclonal antibody (Pharmingen) for 20 min. Isotype-matched immunoglobulins were used as negative controls. Flow cytometry analyses were immediately performed with a FACScalibur (Becton Dickinson).
Real-time PCR measurement of HSV-2 DNA.
Vaginal swabs were placed into 1 ml of cell culture medium and frozen at −70°C. Detection of HSV-2 DNA in swab samples by real-time PCR was performed as described previously (4). Purified viral HSV-2 DNA was used as standard to obtain an estimate of the number of genomic copies of virus per ml of sample (limit of detection = 50 copies).
Estimation of HSV-2 DNA copy numbers in guinea pig dorsal root ganglia.
Sacral dorsal root ganglia (six to eight per animal) were dissected at the termination of the experiment and weighed, and the viral DNA was extracted by using a QIAamp DNA minikit (QIAGEN). Real-time PCR was performed on extracted DNA samples. A standard curve was constructed for each experiment by using purified plasmid containing HSV-2 gD gene sequences. The data were normalized by using probes specific for guinea pig lactalbumin DNA in order to correct for variable amounts of neural material in the dissected ganglia.
Statistical analysis.
Where indicated Student t test (two tailed) was used to determine statistical differences between test and control groups.
RESULTS
Construction and expression of rVSV-gD.
Recombinant VSV vectors expressing HSV-2 gD (rVSVI-gD) were constructed by inserting the gene for gD into the fifth position of the VSVI genome (Fig. 1A). A strong neutralizing antibody response to VSV can be generated against the G protein of VSV after a single immunization and can preclude the ability to immunize multiple times with the same rVSV vector (30). Therefore, a second VSV vector expressing gD was made by replacing the gene for the VSVI G protein from rVSVI-gD with the G protein from Chandipura virus, creating rVSVCh-gD. Similar rVSV vectors expressing HIV Gag in the fifth position (rVSVI-Gag and rVSVCh-Gag) were used as control rVSV vectors expressing an irrelevant antigen. Expression of authentic HSV-2 gD protein by rVSVI-gD and rVSVCh-gD was demonstrated by Western blotting (Fig. 1B).
Humoral and cellular immune responses in rVSV-gD vaccinated mice.
Immunization of BALB/c mice with gD subunit induces potent antibody responses but weak cellular responses. Specifically, in BALB/c mice there is no CD8+ T-cell response to gD subunit, but the combined CD4+ T-cell response and gD-specific antibody response is protective in vaginal challenge models. Coadministration of IL-12 with subunit gD induces a potent Th1 response with high anti-gD IgG2a titers and a CD4+ CTL response, and this redirection of the response to a Th1-like response enhances protection from a vaginal challenge (9).
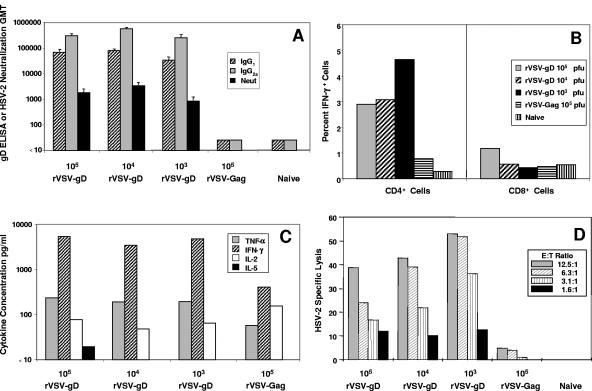
Both cellular and humoral immune responses were assessed in mice immunized with ascending doses of rVSV-gD. Mice primed and boosted intranasally with rVSVCh-gD and rVSVI-gD, respectively, responded with robust Th1 polarized anti-gD cellular and humoral immune responses (Fig. 2). Comparable levels of serum anti-gD IgG2a ELISA titers and functional anti-HSV-2 neutralizing antibody titers were detected at all doses of rVSV-gD (Fig. 2A). Anti-gD titers for IgG2a were very high and well above the IgG1 titers, suggesting that the presentation of gD to the immune system by rVSV vectors induced a Th1-like humoral response since gD subunit immunization leads to a more Th2-like profile of high IgG1 titers and low to undetectable IgG2a titers (9). Anti-HSV-2 neutralization titers were also very high (geometric mean titer, 900 to 1,200) and comparatively much higher than we have seen previously with neat subunit vaccine formulations of gD, which generate neutralization titers of less than 200 (9). Similarly, strong Th1-polarized cellular anti-gD responses, as measured by determining the levels of IFN-γ and tumor necrosis factor alpha (TNF-α), were detected in spleen cell populations harvested from rVSV-gD-immunized mice (Fig. 2B and C). IFN-γ and TNF-α secretion from restimulated spleen cells were high, whereas the expression of IL-5 or IL-4 (not shown) was low to undetectable. Internal cytokine staining determined that the expression of IFN-γ was restricted to the CD4+ population, which was consistent with our previous results using immunization of mice with subunit gD formulated with murine IL-12 (9). Previously, we have only been able to induce a moderate CD4+ CTL response to gD in mice when subunit gD is coadministered with IL-12. We assessed the cytolytic activity of restimulated spleen cells from rVSV-gD-vaccinated mice against HSV-2-infected A20 B cells that express major histocompatibility complex class II and can be lysed by CD4+ CTLs. Functional anti-gD CTL responses were detected in mice at all doses of rVSV-gD (Fig. 2D). Overall, strong gD-specific responses were seen in mice regardless of the immunizing dose of rVSV-gD used (from 103 to 105 PFU per mouse), which most likely reflects the ability of rVSV to replicate in vivo after intranasal inoculation. Surprisingly, there was a trend toward an inverse dose response in the CTL response (Fig. 2D) and to a much lesser extent internal IFN-γ staining (Fig. 2B). This may also reflect somewhat that the intranasal route of inoculation with this rVSV backbone can cause some weight loss and illness in the animals. Moreover, similar dose responses (or the lack thereof) have been seen with intranasal inoculation of mice with similar rVSV vectors expressing other unrelated foreign antigens, including HIV-gag (unpublished observations), influenza virus-hemagglutinin (49), and bovine viral diarrhea virus-E2 (20).
FIG. 2.
Humoral and cellular anti-gD responses elicited after prime-boosting with rVSV-gD. Mice were primed intranasally with the indicated dose in PFU of rVSVCh-gD or rVSVCh-Gag at week 0, boosted intranasally with the same dose of rVSVI-gD or rVSVI-Gag at week 4, and euthanized to collect sera and spleen cells at week 8. (A) Individual serum samples were tested for presence of IgG1 and IgG2a anti-gD antibodies by ELISA and for anti-HSV-2 neutralizing antibodies. Spleen cells were pooled per treatment group and were restimulated in vitro with UV-inactivated HSV-2 to evaluate the cellular immune responses. (B) Three-day antigen-restimulated pooled spleen cell populations were evaluated by fluorescence-activated cell sorting for internal IFN-γ expression. (C) Culture supernatants obtained from 3-day restimulated pooled spleen cells were analyzed by cytokine bead array testing for the presence of TNF-α, IFN-γ, IL-2, IL-4, and IL-5. Concentrations of IL-4 were below background and are not shown. (D) Five-day antigen-restimulated pooled spleen cell populations were examined for their ability to recognize and lyse HSV-2-infected and mock-infected target cells.
Primary and recall responses in rVSV-gD-vaccinated mice.
Since rVSV vectors are replication-competent viruses, we wanted to determine the ability of rVSV-gD to induce immunity after a single immunization compared to a prime-boost regimen. Furthermore, rVSV vectors have been shown to be immunogenic in mice after intranasal or intraperitoneal immunization (46). We wanted to assess the ability of rVSV-gD to prime responses after the more common clinical route of intramuscular immunization. Therefore, mice were immunized with rVSVCh-gD by either intranasal or intramuscular administration, and 3 weeks later groups of 5 mice were harvested for the evaluation of cellular and humoral immunity, and groups of 10 mice were vaginally challenged with a lethal dose of HSV-2. A second cohort of mice was boosted with rVSVI-gD at week 3 and 3 weeks later were either sacrificed to measure immune responses or challenged as described above with HSV-2.
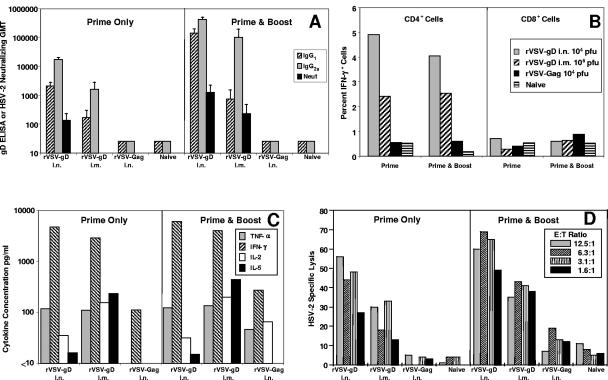
The humoral anti-gD responses elicited after a single intranasal immunization were superior to those measured after a single intramuscular immunization (Fig. 3A). Serum samples from the intranasal group had anti-gD ELISA IgG1 and IgG2a responses that were 10-fold higher than the corresponding intramuscular group. Furthermore, functional anti-HSV-2 neutralizing antibodies were only detected in the sera collected from the intranasally immunized group. After booster immunization, all anti-gD ELISA responses increased, but the humoral responses obtained from the intranasal group remained superior to those of the intramuscular group. However, significant neutralizing antibody titers, although still 10-fold lower than the intranasal group, were readily detectable in the intramuscularly immunized group, and there was a strong IgG2a/IgG1 ratio indicative of a Th1 polarized response in both the intranasal and the intramuscular groups.
FIG. 3.
Humoral and cellular anti-gD responses elicited after one or two immunizations by either intranasal or intramuscular administration of rVSV-gD. Mice were primed with rVSVCh-gD or the control, rVSVCh-Gag, via the intranasal (104 PFU per dose) or the intramuscular (5 × 105 PFU per dose) route at week 0. Three weeks later mice were either euthanized (Prime Only) to collect serum and spleen samples or were boosted with the corresponding rVSVI vector via the intranasal (104 PFU per dose) or intramuscular (106 PFU dose) route. The boosted mice were euthanized, and serum and spleen samples were collected at week 6 (Prime & Boost). (A) Individual serum samples were tested for presence of IgG1 and IgG2a anti-gD antibodies by ELISA and for anti-HSV-2 neutralizing antibodies. Spleen cells were pooled per treatment group and were restimulated in vitro with UV-inactivated HSV-2 to evaluate the cellular immune responses with respect to cytokine expression and functional lytic activity. (B) Three-day antigen restimulated pooled spleen cell populations from each treatment group were stained for CD4+ and CD8+ and evaluated by fluorescence-activated cell sorting for internal cytokine expression of IFN-γ. (C) The culture supernatants obtained from 3-day restimulated pooled spleen cells were analyzed by cytokine bead array testing for the presence of TNF-α, IFN-γ, IL-2, IL-4, and IL-5. Concentrations of IL-4 were below background and are not shown. (D) Five-day antigen-restimulated pooled spleen cell populations were examined for their ability to recognize and lyse HSV-2-infected and mock-infected target cells.
In contrast to what was seen with the humoral response, strong cellular responses were detected after a single intranasal or intramuscular immunization. Cytokine responses peaked after a single immunization and only returned back to post-prime levels after boosting (Fig. 3B and C). Interestingly, moderate IL-5 secretion was seen in restimulated spleen cells from the intramuscularly immunized mice but not in the intranasally immunized mice. As shown previously, the T-cell response was limited to the CD4+ spleen cell fraction (Fig. 3B). The gD-specific CTL response was relatively strong after a single intranasal or intramuscular immunization but was significantly enhanced after boosting, resulting again in some of the strongest gD-specific CTL responses we have detected in BALB/c mice (Fig. 3D). Overall, both humoral and cellular responses were stronger after intranasal immunization, although responses after priming and boosting intramuscularly were good.
Efficacy of rVSV-gD vaccination in mice against HSV-2 vaginal challenge.
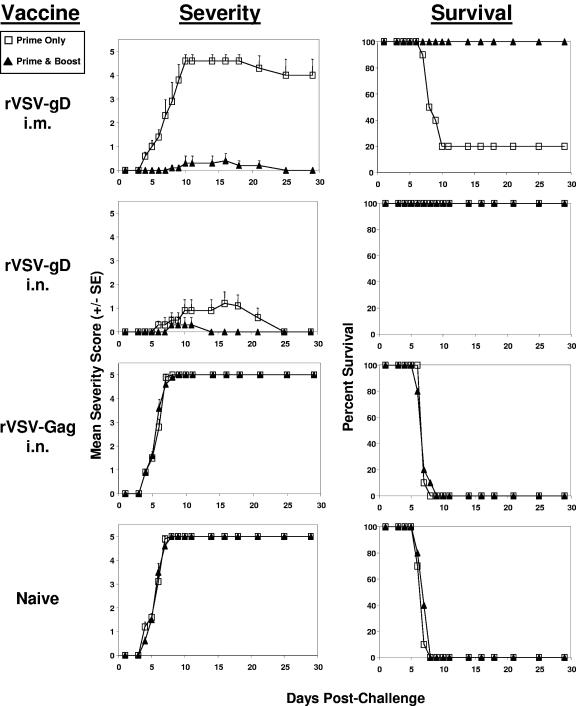
To determine the efficacy of the level of immunity detected after one or two immunizations, inoculated or control mice were challenged by intravaginal instillation with 100 LD50 of HSV-2 (Fig. 4). Naive and rVSV-Gag-immunized negative control mice were all dead by 9 days after virus challenge. A single intramuscular immunization with rVSVCh-gD failed to protect mice from HSV-2 infection and subsequent mortality, whereas a single intranasal rVSVCh-gD immunization offered significant protection against HSV-2-induced morbidity and mortality. The lack of anti-HSV-2 neutralization titers in the mice immunized a single time intramuscularly most likely explains the lack of efficacy in this group. Two intramuscular immunizations with rVSV-gD, however, did offer protection against disease and death. Although a single intranasal administration was shown to be quite protective with only modest disease severity and no mortality, two intranasal immunizations appeared to be superior because the observed disease severity was even further reduced from that measured after a single immunization (Fig. 4).
FIG. 4.
Immunization with rVSV-gD protects mice from a lethal intravaginal challenge with HSV-2. Mice were challenged with 100 LD50 of HSV-2 strain 186 after one immunization (Prime Only, open squares) or two immunizations (Prime & Boost, solid triangles) by either intranasal or intramuscular administration with rVSV-gD or the control rVSV-Gag vector as indicated. Naive mice served as unimmunized age-matched controls. Mice were immunized as indicated in Fig. 2 and challenged with HSV-2 3 weeks after either the primary or the booster immunization. The mice were monitored daily for 4 weeks for disease severity using the following scale: 0, no signs of disease; 1, vaginal erythema; 2, vaginal erythema and edema; 3, vaginal herpetic lesions; 4, unilateral paralysis or severe genital ulceration with hair loss from genital and surrounding tissue; and 5, bilateral paralysis or death. The mean of the severity scores for the 10 mice per group plus the standard error of the mean are shown (Severity), along with the percent survival for each group (Survival).
Humoral responses in rVSV-gD-vaccinated guinea pigs.
The guinea pig HSV-2 model is attractive since, unlike mice, guinea pigs do establish a latent infection with recurrences that somewhat approximates HSV-2 disease in humans (63). Unlike in mice, immunological reagents are limited for guinea pigs—especially for the assessment of T-cell responses—so serum responses, disease severity, and the establishment of latent infection were monitored after rVSV-gD immunization and vaginal challenge with HSV-2.
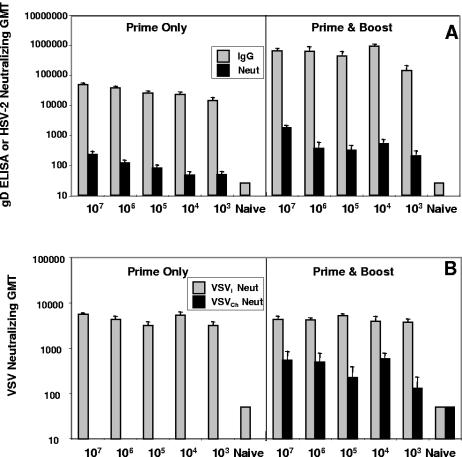
Guinea pigs were immunized with two intranasal instillations of rVSV-gD at doses ranging from 103 PFU to 107 PFU. The priming dose used rVSVI-gD, while the boost consisted of an equivalent dose of rVSVCh-gD. Animal weights were monitored throughout the immunization period and, in contrast to mice, we observed no significant weight loss in guinea pigs due to the administration of rVSV-gD compared to unimmunized controls (data not shown). Sera were collected 3 weeks after each immunization and examined for gD-specific IgG and neutralizing activity directed at HSV-2 (Fig. 5A). As was seen in the murine model, comparable anti-gD IgG titers were observed regardless of the dose. This suggests that some threshold dose was required to initiate an infection and that all of the doses tested eventually reach a plateau that was responsible for evoking comparable serological responses. A very similar pattern was also observed when evaluating the guinea pig anti-HSV-2 neutralizing serum responses as relatively high anti-HSV-2 neutralization titers were observed regardless of the dose. Booster immunization appeared to augment both anti-gD IgG titers and anti-HSV-2 neutralization titers by approximately 1 log10. Tenfold or stronger anti-VSVI neutralizing antibody titers were elicited after immunization with the priming vector compared to the boosting rVSVCh (Fig. 5B), suggesting that cross-protective immunity against internal VSV proteins may be restricting the rVSV replication after booster immunization.
FIG. 5.
Guinea pig serum responses to rVSV-gD immunization. Guinea pigs were primed intranasally with indicated doses of rVSVI-gD and boosted intranasally with the same doses of rVSVCh-gD at week 3. Sera were collected at weeks 3 and 6. (A) Individual serum samples were tested for presence of IgG anti-gD antibodies by ELISA and for anti-HSV-2 neutralizing antibodies (A) and neutralizing antibodies against VSVI and VSVCh (B).
Efficacy of rVSV-gD vaccination in guinea pigs against HSV-2 vaginal challenge.
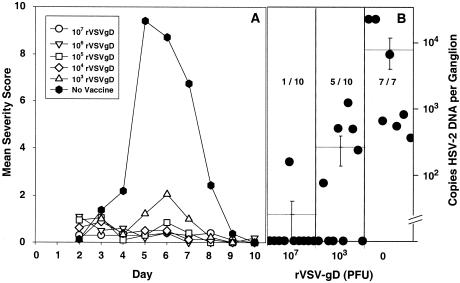
To determine the efficacy of the level of immunity detected after rVSV-gD immunization, inoculated or control guinea pigs were challenged by intravaginal instillation of HSV-2. After intravaginal challenge with HSV-2, all of the vaccinated groups exhibited a high degree of protection from development of genital lesions (Fig. 6A). Doses between 107 and 104 PFU gave complete protection, whereas animals immunized with 103 PFU showed a slight increase in lesion scores on days 5 to 7. All control unvaccinated animals tested positive for HSV-2 in vaginal swabs at day 2 and developed disease.
FIG. 6.
Immunization with rVSV-gD protects guinea pigs against intravaginal challenge with HSV-2. Groups of 10 guinea pigs were immunized by two intranasal instillations of rVSV-gD vectors on days −42 (rVSVI-gD) and −21 (rVSVCh-gD). On day 0 animals were challenged by intravaginal instillation of 2 × 105 PFU of HSV-2. Disease severity was scored on days 2 through 10. On day 24 dorsal root ganglia were dissected and analyzed for HSV-2 genome copies by reverse transcription-PCR. (A) Mean lesion severity score for groups receiving 107 and 103 PFU of rVSV-gD vector or no vaccine. (B) Copies of HSV-2 DNA per ganglion for the individual animals in the groups indicated below the chart. The horizontal lines indicate the mean, and the error bars represent the standard error of the mean. Analysis of the data using the Student t test showed that the P values for the differences between the control group and the vaccinated group were 0.032 and 0.037, respectively, for the 107 and 103 PFU dose groups. The fractions in the figure denote the proportions of animals that were PCR positive. Three animals in the unvaccinated group did not survive the challenge and had to be euthanized between days 11 and 24.
A highly efficacious prophylactic vaccine for HSV-2 should prevent the establishment of a latent infection that is the source of HSV-2 genital reactivation. Therefore, the ability of the rVSV-gD vaccine to prevent the accumulation of viral genomes in guinea pig dorsal root ganglia was evaluated. At the lowest dose tested (103 PFU), only 50% of the guinea pigs had ganglia that were HSV-2 positive and the positive ganglia had low HSV-2 genome copy numbers (Fig. 6B). At the highest dose tested (107 PFU), only 1 of 10 guinea pigs had ganglia that were HSV-2 positive, and these ganglia had only about 20 copies compared to up to 1,000 copies in unimmunized control animals.
DISCUSSION
An effective HSV-2 vaccine will most likely need to elicit effective Th1 cell-mediated immune responses, as well as neutralizing antibodies. One type of vaccine technology that holds promise for achieving this goal is the use of attenuated replication-competent viral vectors. Here we describe the immunogenicity and efficacy of replication-competent rVSV vectors expressing HSV-2 gD in both murine and guinea pig models. In mice, rVSV-gD induced potent humoral and cellular immune responses that were Th1 polarized and protective in the vaginal challenge model. In guinea pigs, rVSV-gD induced strong humoral responses that were also protective in the vaginal challenge model.
The rVSV-vectored technology offers a convenient mechanism for the production of replication-competent vectored vaccines to express key viral antigens in an appropriate manner to elicit robust immune responses (33, 49, 50). Other researchers have demonstrated that even a single administration of rVSV vectors was capable of providing protective immune responses to influenza virus, severe acute respiratory syndrome virus, or cottontail rabbit papillomavirus challenges in animal models (16, 28, 49, 50). Moreover, single immunizations of nonhuman primates with rVSV vectors pseudotyped with the glycoprotein from Ebola or Marburg virus conferred protection against challenge with the wild-type viruses (26). With the murine HSV-2 challenge model we found that a single intranasal immunization with rVSV-gD was protective, whereas intramuscular immunization required boosting in order to protect against a vaginal HSV-2 challenge. In general, with our murine studies we found that the intranasal route was superior to the intramuscular route in terms of eliciting both humoral and cellular responses. It is possible that VSV replicates better in nasal tissue than in muscle, and this may account for the need to boost after intramuscular priming. VSV is more virulent in mice after intranasal inoculation than after intramuscular inoculation since VSV will cause severe weight loss and even death after intranasal inoculation but not after intramuscular inoculation. VSV is not as virulent in primates (and presumably humans) after intranasal inoculation as it is in mice (52). Although this should show VSV vectors to be safer in primates than in mice, there is the possibility that reduced immunogenicity will be observed as these vectors are tested in humans.
Boosting is possible within the rVSV system by circumventing the neutralizing antibody response to the VSV G protein through the use of rVSV glycoprotein exchange vectors that exchange out the Indiana VSV G protein with a G protein from a different VSV serotype (NJ) or from the related Chandipura virus (53). Boosting in this manner was used to show efficacious protection from a lethal SHIV challenge in rhesus macaques immunized with rVSV vectors expressing HIV-Env and SIV-Gag (52). We were able to boost responses in mice in this manner, especially by the intramuscular route, which only achieved full efficacy after boosting.
Here, we have demonstrated that either intranasal or intramuscular priming with rVSVCh-gD and boosting with rVSVI-gD prototypic recombinants were highly effective at generating strong Th1 anti-gD humoral and cellular immune responses that conferred protective immunity against a lethal HSV-2 challenge in mice. To our knowledge there has been no formal direct demonstration of the ability of rVSV vectors to elicit CD4+ T-cell responses directed to the inserted recombinant protein other than the demonstration of antibody responses, which presumably used CD4+ T-cell help. Here, we directly have shown that rVSV-gD can activate splenic CD4+ T cells to respond with the production of Th1 cytokines (IFN-γ and TNF-α) and the development of cytolytic activity. The HSV-2 gD antigen has been demonstrated to elicit cytotoxic T cells that belong to the CD4+ subset in both humans (69) and mice (9). The ability of rVSV vectors to produce Th1 CD4+ effector cells directed against the inserted recombinant antigen correlates well with the natural immune responses generated against VSV infections (6, 35).
Other investigators have assessed HSV vaccines based on viral or bacterial vectors expressing gD. Specifically, the HSV gD gene or epitope gene segments of gD have been expressed by adenovirus, adeno-associated virus, vaccinia virus, modified vaccinia virus Ankara, varicella-zoster virus, and Salmonella enterica serovar Typhimurium vectors, some of which were tested in mice and/or guinea pigs for immunogenicity and protection (8, 21, 29, 36-38, 41, 66, 67, 72). Similar to our observations, serum neutralizing anti-gD antibodies were measured after administration of recombinant gD vaccines expressed by adenovirus, Salmonella serovar Typhimurium, modified vaccinia virus Ankara, and varicella-zoster virus vectors (8, 21, 41, 72). Likewise, cellular responses such as delayed-type hypersensitivity, lymphoproliferative IFN-γ expression, and CTL activity were shown to be induced after vaccination with recombinant gD vaccines expressed by vaccinia virus and modified vaccinia virus Ankara (37, 38, 41, 66, 67). In agreement with our findings, many of the observed cellular responses, such as the CTL lytic activity were shown to be mediated by CD4+ cells (37, 38, 67). Although many of these vectored gD vaccines have shown promise preclinically, rVSV has several advantages over these approaches other than its robust ability to induce immune responses, including the following: (i) there is little to no preexisting immunity to VSV in human populations; (ii) although not a human pathogen, VSV can replicate in human cells and tissues; (iii) whereas VSV can cause neurovirulence in rodents, there has been no confirmed cases of neurological involvement in people that have become infected either by laboratory accident or proximity to livestock; and (iv) the VSV genome can easily be manipulated genetically to create further attenuations if required (14, 18, 48, 51).
While we detected both gD-specific CD4+ cellular responses and gD-specific neutralizing humoral responses in mice after immunization with rVSV-gD, it is most likely the neutralizing antibodies play the major role in protection from the vaginal challenge with 100 LD50 of wild-type HSV-2, although the CD4+ immune response may augment this protection. We have examined a number of recombinant vaccines expressing other HSV-2 antigens (both glycoproteins and internal proteins), where some induce HSV-2 neutralizing antibodies and some do not. We have observed that protection from the 100 LD50 HSV-2 challenge only occurs when HSV-2 serum neutralizing antibodies are detectable (data not shown). Furthermore, we have previously demonstrated that gD subunit immunization (without adjuvants) elicits neutralizing antibodies without the induction of a detectable CD4+ CTL response and still protects mice from HSV-2 vaginal challenge (9). In addition, the ability of anti-gD antibodies to protect mice in the absence of a cellular response was demonstrated by a number of passive transfer studies with monoclonal anti-gD antibodies (2, 23, 55, 68, 71). Moreover, it was shown that the Th1-like IgG2a monoclonal anti-gD antibody was superior to the IgG1 monoclonal anti-gD antibody with regard to the relative efficacy of the passive protection conferred (24). Indeed, although the murine challenge model relies on the presence of neutralizing antibodies for protection, protection from disease in humans is presumed to require HSV-specific cellular responses as well, so it was noteworthy to see the induction of strong CD4+ T-cell responses after VSV gD vaccination in this model.
As far back as the 1920s, guinea pigs have occasionally been used as a convenient permissive small animal model to study VSV infections by either intradermal injection of the metatarsal pads (11) or intracranial injection or intranasal instillation (54). In a manner similar to that observed in young mice, intracranial administration of low doses of VSV produces fatal encephalitis in young (8 to 12 days old) guinea pigs, but nasal instillation of VSV does not induce fatal encephalitis in young guinea pigs in contrast to young mice (54). Intranasal administration to older guinea pigs results with some local replication in the nasal mucosa but with no viral spread to the brain (54). Intranasal, intramuscular, and intradermal administration of VSV to adult animals results in the induction of protective immune responses directed against VSV that are readily observed on subsequent rechallenge (42, 54, 61). Multiple intramuscular immunizations produce sensitized lymphocyte populations recovered from the peritoneal cavity and peripheral blood that demonstrates lymphoproliferative and migration inhibition responses to VSV antigens (31). It is understandable that, with the lack of immunological reagents available to characterize immune responses, the guinea pig model would not be a primary animal model for evaluating rVSV vectors. The advantage that HSV-2-induced primary and recurrent disease in the guinea pig vaginal infection model closely mimics the course of the clinical genital infection (63) has made this model an attractive one to evaluate. In guinea pigs, we found that priming with VSVI-gD followed by boosting with rVSVCh-gD also provided a high degree of protection against HSV-2 challenge. Guinea pigs tolerated intranasal doses as high as 107 PFU very well with little or no weight loss. Doses as low as 104 PFU were capable of protecting most guinea pigs against intravaginal HSV-2 challenge. Guinea pigs were also effectively protected against the establishment of a latent infection, as evidenced by the absence or sharp reduction of the accumulation of HSV-2 genome copies in dorsal root ganglia. The ability of rVSV gD vectored immunization to protect guinea pigs from a vaginal challenge was in agreement with the findings of other investigators using vaccinia virus or varicella-zoster recombinants expressing the gD antigen (3, 21, 66).
In conclusion, we demonstrated that rVSV vectors expressing HSV-2 gD can elicit robust and protective Th1-polarized MHC class II-restricted CD4+ T-cell responses and HSV-2 neutralizing antibody responses in mice. Furthermore, rVSV-gD induced robust and protective immune response in guinea pigs. Therefore, rVSV-gD shows promise as the basis for genital herpes vaccines that could be enhanced by the addition of other antigens from HSV-2. Although the results obtained with these VSV recombinants are quite promising, we are currently designing new VSV recombinants that have been further modified to increase the attenuation of the vector without compromising the ability of the vector to express the inserted recombinant antigen in infected cells in case the current vectors are deemed not suitable to proceed to assessment in humans.
REFERENCES
- 1.Aurelian, L., H. Kokuba, and C. C. Smith. 1999. Vaccine potential of a herpes simplex virus type 2 mutant deleted in the PK domain of the large subunit of ribonucleotide reductase (ICP10). Vaccine 17:1951-1963. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran, N., S. Bacchetti, and W. E. Rawls. 1982. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect. Immun. 37:1132-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein, D. I. 2000. Effect of route of vaccination with vaccinia virus expressing HSV-2 glycoprotein D on protection from genital HSV-2 infection. Vaccine 18:1351-1358. [DOI] [PubMed] [Google Scholar]
- 4.Blakeney, S., J. Kowalski, D. Tummolo, J. DeStefano, D. Cooper, M. Guo, S. Gangolli, D. Long, T. Zamb, R. J. Natuk, and R. J. Visalli. 2005. Herpes simplex virus type 2 UL24 gene is a virulence determinant in murine and guinea pig disease models. J. Virol. 79:10498-10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buonocore, L., K. J. Blight, C. M. Rice, and J. K. Rose. 2002. Characterization of vesicular stomatitis virus recombinants that express and incorporate high levels of hepatitis C virus glycoproteins. J. Virol. 76:6865-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, B. N., B. S. Huneycutt, C. P. Gapud, R. J. Arceci, and C. S. Reiss. 1993. Lymphokine expression profile of resting and stimulated CD4+ CTL clones specific for the glycoprotein of vesicular stomatitis virus. Cell. Immunol. 146:147-156. [DOI] [PubMed] [Google Scholar]
- 7.Celum, C., R. Levine, M. Weaver, and A. Wald. 2004. Genital herpes and human immunodeficiency virus: double trouble. Bull. W. H. O. 82:447-453. [PMC free article] [PubMed] [Google Scholar]
- 8.Chabalgoity, J. A., C. M. Khan, A. A. Nash, and C. E. Hormaeche. 1996. A Salmonella typhimurium htrA live vaccine expressing multiple copies of a peptide comprising amino acids 8-23 of herpes simplex virus glycoprotein D as a genetic fusion to tetanus toxin fragment C protects mice from herpes simplex virus infection. Mol. Microbiol. 19:791-801. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, D., M. W. Pride, M. Guo, M. Cutler, J. C. Mester, F. Nasar, J. She, V. Souza, L. York, E. Mishkin, J. Eldridge, and R. J. Natuk. 2004. Interleukin-12 redirects murine immune responses to soluble or aluminum phosphate adsorbed HSV-2 glycoprotein D toward Th1 and CD4+ CTL responses. Vaccine 23:236-246. [DOI] [PubMed] [Google Scholar]
- 10.Corey, L., A. G. Langenberg, R. Ashley, R. E. Sekulovich, A. E. Izu, J. M. Douglas, Jr., H. H. Handsfield, T. Warren, L. Marr, S. Tyring, R. DiCarlo, A. A. Adimora, P. Leone, C. L. Dekker, R. L. Burke, W. P. Leong, S. E. Straus, et al. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331-340. [DOI] [PubMed] [Google Scholar]
- 11.Cotton, W. E. 1926. Vesicular stomatitis in its relation to the diagnosis of foot-and-mouth disease. J. Am. Vet. Med. Assoc. 69:313-332. [Google Scholar]
- 12.Da Costa, X. J., N. Bourne, L. R. Stanberry, and D. M. Knipe. 1997. Construction and characterization of a replication-defective herpes simplex virus 2 ICP8 mutant strain and its use in immunization studies in a guinea pig model of genital disease. Virology 232:1-12. [DOI] [PubMed] [Google Scholar]
- 13.Egan, M. A., S. Y. Chong, N. F. Rose, S. Megati, K. J. Lopez, E. B. Schadeck, J. E. Johnson, A. Masood, P. Piacente, R. E. Druilhet, P. W. Barras, D. L. Hasselschwert, P. Reilly, E. M. Mishkin, D. C. Montefiori, M. G. Lewis, D. K. Clarke, R. M. Hendry, P. A. Marx, J. H. Eldridge, S. A. Udem, Z. R. Israel, and J. K. Rose. 2004. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res. Hum. Retrovir. 20:989-1004. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan, E. B., J. M. Zamparo, L. A. Ball, L. L. Rodriguez, and G. W. Wertz. 2001. Rearrangement of the genes of vesicular stomatitis virus eliminates clinical disease in the natural host: new strategy for vaccine development. J. Virol. 75:6107-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 16.Fukushi, S., T. Mizutani, M. Saijo, S. Matsuyama, N. Miyajima, F. Taguchi, S. Itamura, I. Kurane, and S. Morikawa. 2005. Vesicular stomatitis virus pseudotyped with severe acute respiratory syndrome coronavirus spike protein. J. Gen. Virol. 86:2269-2274. [DOI] [PubMed] [Google Scholar]
- 17.Gallichan, W. S., and K. L. Rosenthal. 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 177:1155-1161. [DOI] [PubMed] [Google Scholar]
- 18.Garbutt, M., R. Liebscher, V. Wahl-Jensen, S. Jones, P. Moller, R. Wagner, V. Volchkov, H. D. Klenk, H. Feldmann, and U. Stroher. 2004. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J. Virol. 78:5458-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisbert, T. W., S. Jones, E. A. Fritz, A. C. Shurtleff, J. B. Geisbert, R. Liebscher, A. Grolla, U. Stroher, L. Fernando, K. M. Daddario, M. C. Guttieri, B. R. Mothe, T. Larsen, L. E. Hensley, P. B. Jahrling, and H. Feldmann. 2005. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2:e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grigera, P. R., M. P. Marzocca, A. V. Capozzo, L. Buonocore, R. O. Donis, and J. K. Rose. 2000. Presence of bovine viral diarrhea virus (BVDV) E2 glycoprotein in VSV recombinant particles and induction of neutralizing BVDV antibodies in mice. Virus Res. 69:3-15. [DOI] [PubMed] [Google Scholar]
- 21.Heineman, T. C., L. Pesnicak, M. A. Ali, T. Krogmann, N. Krudwig, and J. I. Cohen. 2004. Varicella-zoster virus expressing HSV-2 glycoproteins B and D induces protection against HSV-2 challenge. Vaccine 22:2558-2565. [DOI] [PubMed] [Google Scholar]
- 22.Hoshino, Y., S. K. Dalai, K. Wang, L. Pesnicak, T. Y. Lau, D. M. Knipe, J. I. Cohen, and S. E. Straus. 2005. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J. Virol. 79:410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue, Y., Y. Ohashi, H. Watanabe, and R. Manabe. 1992. Protective effects of anti-glycoprotein D monoclonal antibodies in murine herpetic keratitis. Curr. Eye Res. 11:53-60. [DOI] [PubMed] [Google Scholar]
- 24.Ishizaka, S. T., P. Piacente, J. Silva, and E. M. Mishkin. 1995. IgG subtype is correlated with efficiency of passive protection and effector function of anti-herpes simplex virus glycoprotein D monoclonal antibodies. J. Infect. Dis. 172:1108-1111. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, J. E., M. J. Schnell, L. Buonocore, and J. K. Rose. 1997. Specific targeting to CD4+ cells of recombinant vesicular stomatitis viruses encoding human immunodeficiency virus envelope proteins. J. Virol. 71:5060-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, S. M., H. Feldmann, U. Stroher, J. B. Geisbert, L. Fernando, A. Grolla, H. D. Klenk, N. J. Sullivan, V. E. Volchkov, E. A. Fritz, K. M. Daddario, L. E. Hensley, P. B. Jahrling, and T. W. Geisbert. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 11:786-790. [DOI] [PubMed] [Google Scholar]
- 27.Kahn, J. S., M. J. Schnell, L. Buonocore, and J. K. Rose. 1999. Recombinant vesicular stomatitis virus expressing respiratory syncytial virus (RSV) glycoproteins: RSV fusion protein can mediate infection and cell fusion. Virology 254:81-91. [DOI] [PubMed] [Google Scholar]
- 28.Kapadia, S. U., J. K. Rose, E. Lamirande, L. Vogel, K. Subbarao, and A. Roberts. 2005. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. [DOI] [PMC free article] [PubMed]
- 29.Karem, K. L., J. Bowen, N. Kuklin, and B. T. Rouse. 1997. Protective immunity against herpes simplex virus (HSV) type 1 following oral administration of recombinant Salmonella typhimurium vaccine strains expressing HSV antigens. J. Gen. Virol. 78(Pt. 2):427-434. [DOI] [PubMed] [Google Scholar]
- 30.Kelley, J. M., S. U. Emerson, and R. R. Wagner. 1972. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J. Virol. 10:1231-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koszinowski, U., and G. Bandlow. 1975. Induction and in Vitro demonstration of cellular immunity to DNA and RNA viruses in guinea-pigs. Clin. Exp. Immunol. 20:143-154. [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacs, G. R., C. L. Parks, N. Vasilakis, and S. A. Udem. 2003. Enhanced genetic rescue of negative-strand RNA viruses: use of an MVA-T7 RNA polymerase vector and DNA replication inhibitors. J. Virol. Methods 111:29-36. [DOI] [PubMed] [Google Scholar]
- 33.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA 92:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long, D., T. J. Madara, M. Ponce de Leon, G. H. Cohen, P. C. Montgomery, and R. J. Eisenberg. 1984. Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect. Immun. 43:761-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maloy, K. J., C. Burkhart, T. M. Junt, B. Odermatt, A. Oxenius, L. Piali, R. M. Zinkernagel, and H. Hengartner. 2000. CD4+ T-cell subsets during virus infection. Protective capacity depends on effector cytokine secretion and on migratory capability. J. Exp. Med. 191:2159-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manning, W. C., X. Paliard, S. Zhou, M. Pat Bland, A. Y. Lee, K. Hong, C. M. Walker, J. A. Escobedo, and V. Dwarki. 1997. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. J. Virol. 71:7960-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin, S., B. Moss, P. W. Berman, L. A. Laskey, and B. T. Rouse. 1987. Mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: cytotoxic T cells. J. Virol. 61:726-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin, S., and B. T. Rouse. 1987. The mechanisms of antiviral immunity induced by a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: clearance of local infection. J. Immunol. 138:3431-3437. [PubMed] [Google Scholar]
- 39.McClements, W. L., M. E. Armstrong, R. D. Keys, and M. A. Liu. 1996. Immunization with DNA vaccines encoding glycoprotein D or glycoprotein B, alone or in combination, induces protective immunity in animal models of herpes simplex virus-2 disease. Proc. Natl. Acad. Sci. USA 93:11414-11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDermott, M. R., J. R. Smiley, P. Leslie, J. Brais, H. E. Rudzroga, and J. Bienenstock. 1984. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J. Virol. 51:747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meseda, C. A., K. L. Elkins, M. J. Merchlinsky, and J. P. Weir. 2002. Prime-boost immunization with DNA and modified vaccinia virus ankara vectors expressing herpes simplex virus-2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA vaccine alone. J. Infect. Dis. 186:1065-1073. [DOI] [PubMed] [Google Scholar]
- 42.Olitsky, P. K., J. Traum, and H. W. Schoening. 1926. Comparative studies on vesicular stomatitis and foot-and-mouth disease. J. Am. Vet. Med. Assoc. 79:147-167. [Google Scholar]
- 43.Parks, C. L., R. A. Lerch, P. Walpita, M. S. Sidhu, and S. A. Udem. 1999. Enhanced measles virus cDNA rescue and gene expression after heat shock. J. Virol. 73:3560-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parr, M. B., L. Kepple, M. R. McDermott, M. D. Drew, J. J. Bozzola, and E. L. Parr. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Investig. 70:369-380. [PubMed] [Google Scholar]
- 45.Prichard, M. N., R. Kaiwar, W. T. Jackman, D. C. Quenelle, D. J. Collins, E. R. Kern, G. M. Kemble, and R. R. Spaete. 2005. Evaluation of AD472, a live attenuated recombinant herpes simplex virus type 2 vaccine in guinea pigs. Vaccine 23:5424-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Publicover, J., E. Ramsburg, and J. K. Rose. 2004. Characterization of nonpathogenic, live, viral vaccine vectors inducing potent cellular immune responses. J. Virol. 78:9317-9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reuter, J. D., B. E. Vivas-Gonzalez, D. Gomez, J. H. Wilson, J. L. Brandsma, H. L. Greenstone, J. K. Rose, and A. Roberts. 2002. Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease. J. Virol. 76:8900-8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73:3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts, A., J. D. Reuter, J. H. Wilson, S. Baldwin, and J. K. Rose. 2004. Complete protection from papillomavirus challenge after a single vaccination with a vesicular stomatitis virus vector expressing high levels of L1 protein. J. Virol. 78:3196-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1244. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 52.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 53.Rose, N. F., A. Roberts, L. Buonocore, and J. K. Rose. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 74:10903-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabin, A. B., and P. K. Olitsky. 1938. Influence of host factors on neuroinvasiveness of vesicular stomatitis virus. IV. Variations in neuroinvasiveness in different species. J. Exp. Med. 67:229-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanna, P. P., A. De Logu, R. A. Williamson, Y. L. Hom, S. E. Straus, F. E. Bloom, and D. R. Burton. 1996. Protection of nude mice by passive immunization with a type-common human recombinant monoclonal antibody against HSV. Virology 215:101-106. [DOI] [PubMed] [Google Scholar]
- 56.Schlereth, B., J. K. Rose, L. Buonocore, V. ter Meulen, and S. Niewiesk. 2000. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J. Virol. 74:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnell, M. J., L. Buonocore, E. Kretzschmar, E. Johnson, and J. K. Rose. 1996. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. USA 93:11359-11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schnell, M. J., L. Buonocore, M. A. Whitt, and J. K. Rose. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 70:2318-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.She, J., M. C. Ruzek, P. Velupillai, I. de Aos, B. Wang, D. A. Harn, J. Sancho, C. A. Biron, and C. Terhorst. 1999. Generation of antigen-specific cytotoxic T lymphocytes and regulation of cytokine production takes place in the absence of CD3ζ. Int. Immunol. 11:845-857. [DOI] [PubMed] [Google Scholar]
- 60.Sin, J. I., J. J. Kim, J. D. Boyer, R. B. Ciccarelli, T. J. Higgins, and D. B. Weiner. 1999. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J. Virol. 73:501-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skinner, H. H. 1957. The virus of vesicular stomatitis in small experimental hosts. II. Guinea pigs and rabbits. J. Comp. Pathol. 67:87-105. [DOI] [PubMed] [Google Scholar]
- 62.Spector, F. C., E. R. Kern, J. Palmer, R. Kaiwar, T. A. Cha, P. Brown, and R. R. Spaete. 1998. Evaluation of a live attenuated recombinant virus RAV 9395 as a herpes simplex virus type 2 vaccine in guinea pigs. J. Infect. Dis. 177:1143-1154. [DOI] [PubMed] [Google Scholar]
- 63.Stanberry, L. R., E. R. Kern, J. T. Richards, T. M. Abbott, and J. C. Overall, Jr. 1982. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J. Infect. Dis. 146:397-404. [DOI] [PubMed] [Google Scholar]
- 64.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin. 2002. Glycoprotein-d-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 65.Velders, M. P., S. McElhiney, M. C. Cassetti, G. L. Eiben, T. Higgins, G. R. Kovacs, A. G. Elmishad, W. M. Kast, and L. R. Smith. 2001. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 61:7861-7867. [PubMed] [Google Scholar]
- 66.Wachsman, M., L. Aurelian, C. C. Smith, B. R. Lipinskas, M. E. Perkus, and E. Paoletti. 1987. Protection of guinea pigs from primary and recurrent herpes simplex virus (HSV) type 2 cutaneous disease with vaccinia virus recombinants expressing HSV glycoprotein D. J. Infect. Dis. 155:1188-1197. [DOI] [PubMed] [Google Scholar]
- 67.Wachsman, M., J. H. Luo, L. Aurelian, and E. Paoletti. 1992. Protection from herpes simplex virus type 2 is associated with T cells involved in delayed type hypersensitivity that recognize glycosylation-related epitopes on glycoprotein D. Vaccine 10:447-454. [DOI] [PubMed] [Google Scholar]
- 68.Whaley, K. J., L. Zeitlin, R. A. Barratt, T. E. Hoen, and R. A. Cone. 1994. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J. Infect. Dis. 169:647-649. [DOI] [PubMed] [Google Scholar]
- 69.Yasukawa, M., H. Ohminami, Y. Yakushijin, J. Arai, A. Hasegawa, Y. Ishida, and S. Fujita. 1999. Fas-independent cytotoxicity mediated by human CD4+ CTL directed against herpes simplex virus-infected cells. J. Immunol. 162:6100-6106. [PubMed] [Google Scholar]
- 70.York, L. J., D. P. Giorgio, and E. M. Mishkin. 1995. Immunomodulatory effects of HSV2 glycoprotein D in HSV1-infected mice: implications for immunotherapy of recurrent HSV infection. Vaccine 13:1706-1712. [DOI] [PubMed] [Google Scholar]
- 71.Zeitlin, L., K. J. Whaley, P. P. Sanna, T. R. Moench, R. Bastidas, A. De Logu, R. A. Williamson, D. R. Burton, and R. A. Cone. 1996. Topically applied human recombinant monoclonal IgG1 antibody and its Fab and F(ab′)2 fragments protect mice from vaginal transmission of HSV-2. Virology 225:213-215. [DOI] [PubMed] [Google Scholar]
- 72.Zheng, B., F. L. Graham, D. C. Johnson, T. Hanke, M. R. McDermott, and L. Prevec. 1993. Immunogenicity in mice of tandem repeats of an epitope from herpes simplex gD protein when expressed by recombinant adenovirus vectors. Vaccine 11:1191-1198. [DOI] [PubMed] [Google Scholar]