Abstract
Papillomaviral DNA replicates as extrachromosomal plasmids in squamous epithelium. Viral DNA must segregate equitably into daughter cells to persist in dividing basal/parabasal cells. We have previously reported that the viral origin binding protein E2 of human papillomavirus types 11 (HPV-11), 16, and 18 colocalized with the mitotic spindles. In this study, we show the localization of the HPV-11 E2 protein to be dynamic. It colocalized with the mitotic spindles during prophase and metaphase. At anaphase, it began to migrate to the central spindle microtubules, where it remained through telophase and cytokinesis. It was additionally observed in the midbody at cytokinesis. A peptide spanning residues 285 to 308 in the carboxyl-terminal domain of HPV-11 E2 (E2C) is necessary and sufficient to confer localization on the mitotic spindles. This region is conserved in HPV-11, -16, and -18 and bovine papillomavirus type 4 (BPV-4) E2 and is also required for the respective E2C to colocalize with the mitotic spindles. The E2 protein of bovine papillomavirus type 1 is tethered to the mitotic chromosomes via the cellular protein Brd4. However, the HPV-11 E2 protein did not associate with Brd4 during mitosis. Lastly, a chimeric BPV-1 E2C containing the spindle localization domain from HPV-11 E2C gained the ability to localize to the mitotic spindles, whereas the reciprocal chimera lost the ability. We conclude that this region of HPV E2C is critical for localization with the mitotic apparatus, enabling the HPV DNA to sustain persistent infections.
Human papillomaviruses (HPVs) cause epithelial proliferative diseases, such as cutaneous and anogenital warts and laryngeal papillomas. Infections by the high-risk genotypes, notably HPV type 16 (HPV-16) and HPV-18, can progress over time to cancers at a low frequency, whereas the low-risk types, such as HPV-11 and HPV-6, rarely cause cancer (60). The productive phase of the infection takes place only in suprabasal cells undergoing terminal squamous differentiation. However, the initial viral infection is thought to occur in the basal cells of the squamous epithelium, where the viral DNA is maintained as extrachromosomal nuclear plasmids in low copy numbers over long periods of time (13). To persist in the basal cells, HPV must ensure equitable segregation of its DNA into daughter cells during cell division. For HPV, the E2 protein is responsible for DNA segregation (48). The viral E2 protein of approximately 42 kDa is also critical for the assembly of the preinitiation complex on the viral origin of replication (ori) (11, 29, 47, 54). It is the origin recognition protein, it binds as dimers to multiple E2 binding sites in the ori, and it recruits the viral E1 protein, which assembles into a dihexameric replicative DNA helicase (14, 19, 24, 31, 32a, 42, 43, 45, 46, 55). In turn, the E1 complex unwinds the ori and recruits the DNA polymerase α, the topoisomerase I, and the single-stranded DNA binding protein RPA to initiate replication (7, 15, 16, 33, 36, 39).
The E2 protein consists of three domains, an amino-terminal trans-acting domain (abbreviated hereafter as the N domain), a flexible hinge (H) domain, and a carboxyl-terminal (C) domain. Both the N domain of approximately 200 amino acids and the C domain of approximately 90 residues are relatively conserved in all papillomaviruses, while the H domain is variable in length and sequence. In HPV-11 E2, the H domain contains the nuclear localization sequence and also associates with nuclear matrix (59). The E2N domain interacts with the helicase domain of the E1 protein; E2 protein lacking the N domain does not support HPV DNA replication (1, 2, 12, 32, 34). The E2C domain is responsible for dimerization and binding to the palindromic consensus E2BS in the ori. The crystal structures of the dimeric C domains of several papillomaviruses have been elucidated (8, 20, 21). The dimer interface contains an eight-stranded β-barrel flanking two DNA binding α-helices, one from each monomer (see Fig. 2C).
FIG. 2.
Delineation of the mitotic-spindle localization domain in HPV-11 E2C. (A and B) Western blots of SDS-polyacrylamide gel electrophoresis of transfected COS7 cell lysates by using an antibody to GFP. (A) an SDS-4% to 12% polyacrylamide gradient gel. (B) An SDS-10% polyacrylamide gel. (C) Localization of selected GFP fusion peptides. Left column, GFP-E2C; middle column, β-tubulin (Cy3) with DAPI-stained chromosomal DNA (blue); right column, merged images. The cells were in prometaphase (a) or metaphase (b and c). (D) Crystal structure of the HPV-16 E2C dimer. The DNA binding helices are colored red, and the spindle association domain is white (adapted from Hegde and Androphy [22]).
Using fusion proteins to the enhanced green fluorescence protein (GFP), we have previously shown that the E2 proteins of HPV-11, -16 and -18 localize to the mitotic spindles (48). An epitope-tagged inducible HPV-11 E2 protein in a 293 cell line, 293(11-E2), exhibits similar properties. Moreover, in these cells, a plasmid containing the HPV-11 ori is also localized to mitotic-spindle fibers at metaphase, but only when the E2 protein is induced. Thus, this colocalization provides a mechanism for viral plasmid persistence in dividing basal and parabasal cells. Unlike HPV E2 proteins, the bovine papillomavirus type 1 (BPV-1) E2 protein associates with the mitotic chromosomes via its interactions with the cellular protein Brd4 to facilitate viral DNA segregation (25, 30, 44, 56). The region for this interaction has been localized to the N domain (4, 57). Association with the mitotic chromosomes as a mechanism for viral DNA segregation has previously been demonstrated for Epstein-Barr virus (EBV), which uses the virus-encoded nuclear antigen 1 protein, and for human herpesvirus 8, which uses the latency-associated nuclear antigen 1 protein (3, 28, 53).
For the HPV-11 E2 protein, the C and N domains each independently associate with the mitotic spindles (48). In this work, we show that the localization of HPV-11 E2 is dynamic through different phases during mitosis and that it does not associate with Brd4. In addition, we delineate the segment in the HPV-11 E2C domain critical for mitotic spindle localization to a segment spanning residues 285 to 308. This spindle localization motif is highly conserved in the C domain of the HPV-16, HPV-18, and BPV-4 E2 proteins. Deletion analyses demonstrated that this segment is necessary for their spindle localization as well. Reciprocal chimeric E2C proteins of HPV-11 and BPV-1 supported this conclusion. We discuss the implications of these findings.
MATERIALS AND METHODS
Cell cultures.
COS7 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Transfection was conducted by electroporation or lipofection (Invitrogen, Carlsbad, CA) as described previously (12). The 293(11-E2) tetracycline-inducible cells were maintained in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum in the presence of 1 μg/ml tetracycline to repress E2 expression (48). Induction was achieved by culturing the cells overnight in medium containing 0.1 μg/ml tetracycline.
Plasmid construction and mutagenesis.
pGFP-H11 E2C (spanning amino acid residues 285 to 367) was described previously (48). Plasmids expressing GFP fusions to E2C proteins of HPV-16 (pGFP-H16 E2C, residues 287 to 365), HPV-18 (pGFP-H18 E2C, residues 288 to 365), BPV-4 (pGFP-B4 E2C, residues 327 to 408), and BPV-1 (pGFP-B1 E2C, residues 327 to 410), as well as to deletion mutants of E2C proteins of these four viruses, pGFP-H11 E2C:285-308, pGFP-H11 E2C:285-318, pGFP-H11 E2C:285-328, pGFP-H11 E2C:285-338, pGFP-H11 E2C:285-348, pGFP-H11 E2C:285-358, pGFP-H11 E2C:295-367, pGFP-H16 E2C:296-365, pGFP-H18 E2C:297-365, and pGFP-B4 E2C:338-408, were each constructed by PCR amplification of the appropriate E2 fragments and placed in frame into the BglII and EcoRI sites of the pEGFP-C1 plasmid (Clontech, Mountain View, CA). pGFP-H11 E2H+:202-295, which extends HPV-11 E2H (residues 202 to 284) to include the first 11 residues of the C domain, was similarly prepared. The plasmids expressing the GFP fusions of the point mutations in HPV-11 E2HC (residues 285 to 367), namely, pGFP-H11 E2HC:V287A, pGFP-H11 E2HC:Q288A, pGPF-H11 E2HC:L289A, pGFP-H11 E2HC:Q290A, and pGFP-H11 E2HC:S293A, were constructed using PCR mutagenesis. pGFP-H11 E2HC:A7 contained alanine residues at positions 287 through 293. All of the above-mentioned clones, except pGFP-11H+:202-295, HPV-11E2C:285-308, and HPV-11E2C:295-367, contained an STR tag at the carboxyl terminus as previously described (48). Expression vectors of chimeric E2C were generated by PCR mutagenesis. For GFP fusion to HPV-11/BPV-1 E2C (pGFP-H11/B1 E2C), the forward primer incorporated nucleotides encoding amino acid residues 285 to 295 of HPV-11 E2, replacing the corresponding sequence of BPV-1 E2C. The reciprocal chimera, pGFP-B1/H11 E2C, was constructed using a forward primer that contained nucleotides encoding amino acid residues 327 to 337 of BPV-1 E2, followed by nucleotides encoding HPV-11 E2C. GFP-Brd4 was generated by PCR. The Brd4 coding region was amplified using a forward primer, GAATTCGTCTACCGAGAGCGGC, containing an EcoRI restriction site, and a reverse primer, GGATCCTCAAAAAAGATTTTCTTCAAATATTG, containing a BamHI restriction site. The PCR product was purified, digested with EcoRI and BamHI, and then inserted in frame into the pEGFP-C1 vector (Clontech, Mountain View, CA) at the same sites.
Western blots.
COS7 cells separately transfected with expression vectors of various pGFP-E2C fusion proteins were grown for 24 h and lysed with RIPA buffer (0.15 mM NaCl, 0.05 mM Tris-HCl, pH 7.2, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) containing protease inhibitors (Sigma, St. Louis, MO). Equal amounts of total proteins were separated on a 4 to 12% polyacrylamide-SDS gel (Fig. 2A) or by 10% SDS-polyacrylamide gel electrophoresis (see Fig. 2B, 3C, 4C, and 5B) and transferred to a nitrocellulose membrane by Western blotting. The nitrocellulose membranes were probed with a monoclonal antibody specific for GFP, followed by an anti-mouse immunoglobulin G antibody conjugated with peroxidase and developed with an enhanced chemiluminescence detection system (ECL; Amersham Pharmacia, Piscataway, NJ).
FIG. 3.
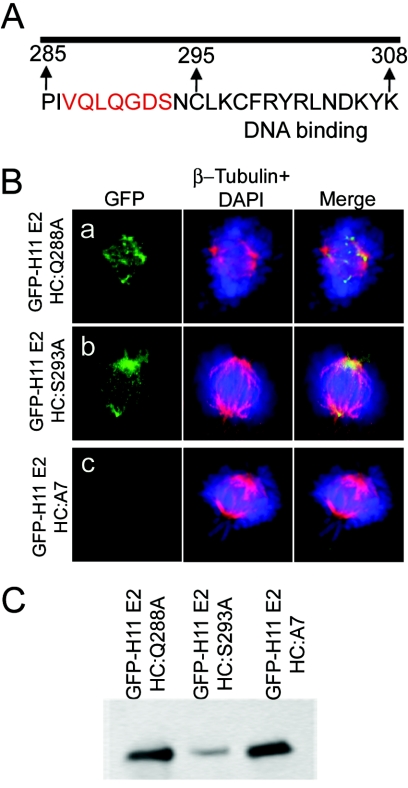
Site-directed mutagenic analysis of the spindle localization domain in HPV-11 E2C. (A) Sequence of and mutations in the amino-terminal region of E2C. In GFP-H11E2-HC:A7, residues 287 to 293 were replaced with seven alanine residues. In others, individual residues were separately mutated as described in Results. (B) Localization of representative mutated fusion proteins. Left column, GFP-E2C; middle column, β-tubulin (Cy3) with DAPI-stained chromosomal DNA (blue); right column, merged images. The cells were approaching metaphase. (C) A Western blot of transfected COS7 cell lysates with an antibody to GFP.
FIG. 4.
Conservation of the spindle localization domain in E2C proteins of HPV-11, HPV-18, HPV-16, and BPV-4, but not in that of BPV-1. (A) Sequence comparison of the amino-terminal portion of E2C implicated in colocalization with the mitotic spindles from papillomaviruses examined in this report. (B) Localization of GFP fused to the E2C domain of HPV-16, HPV-18, or BPV-4 (a, c, and e) and mutations with the putative spindle localization domain deleted (b, d, and f). Left column, GFP-E2C; middle column, β-tubulin (Cy3) with DAPI-stained chromosomal DNA (blue); right column, merged images. The cells were in prometaphase (b, c, and e) or approaching or in metaphase (a, d, and f). (C) Western blot of transfected COS7 cell lysates with an antibody to GFP.
FIG. 5.
Structure and properties of reciprocal chimeric HPV-11 and BPV-1 E2C fused to GFP. (A) Schematic diagram of GFP fusions of HPV-11 E2C, BPV-1 E2C, and the reciprocal chimeric H11/B1 E2C and B1/H11 E2C. (B) Western blot of transfected COS7 cell lysates using an antibody to GFP. (C) Localization of the fusion proteins in transfected cells. Left column, GFP-E2C; middle column, β-tubulin (Cy3) with DAPI-stained chromosomal DNA (blue); right column, merged images. The cells were in prometaphase (b), metaphase (c), or anaphase (a).
Fluorescence microscopy.
For preextracted slides, the cells on multichamber slides (LabTek, Campbell, CA) were permeabilized for 30 s at room temperature with a microtubule-stabilizing buffer {BRB80; 80 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], pH 6.8, 1 mM MgCl2, 1 mM EGTA} supplemented with 4 mM EGTA and 0.5% Triton X-100 to prevent degradation of the spindle fibers (51). The slides were then fixed in 4% paraformaldehyde for 10 min at room temperature. To stain for the mitotic spindles, the slides were incubated with a Cy3-conjugated primary antibody to β-tubulin (Sigma, St. Louis, MO) at 1:100 dilution for 1 h at 37°C. For immunofluorescence assays using the 293(11-E2) Tet-off cell line, the induced or uninduced cells were prepared as described above; following extraction and fixation, E2 was stained using a rabbit polyclonal anti-E2 antibody (1:100 dilution) (10) at 37°C for 1 h. After incubation, the slides were washed and incubated with a 1:750 dilution of anti-rabbit Alexa Fluor 488 (Molecular Probes, Portland, OR), and tubulin was stained as described above. Nuclei were stained using 4′,6-diamidino-2-phenylindole (DAPI) and mounted in Antifade (0.1 mM p-phenylenediamine in 100% glycerol; the pH was adjusted to 8.0 with a carbonate buffer containing 4 mM Na2CO3 and 46 mM NaHCO3). Alternatively, the FLAG-tagged E2 was detected with a monoclonal anti-FLAG M2 antibody (1:500) (Sigma, St. Louis, MO) by incubation at 37°C for 1 h. The slides were then washed, incubated with a 1:750 dilution of anti-mouse Alexa Fluor 488 (Molecular Probes, Portland, OR) at 37°C for 1 h, and then washed three times with phosphate-buffered saline plus 0.1% Tween 20 for 5 min each time. Mitotic spindles were stained with a Cy3-conjugated primary antibody to β-tubulin (1:10; Sigma) for 10 min. Nuclei were stained with DAPI and mounted in Antifade. For colocalization studies with GFP-Brd4, GFP-Brd4 was transfected into the 293(11-E2) cells. Expression of the E2 protein was induced by culturing the cells in tetracycline-free medium overnight. The cells were permeabilized and fixed in 4% paraformaldehyde with 0.5% Triton X-100. Mitotic spindles were stained with Cy3-conjugated primary antibody to β-tubulin as described above. Alternatively, the slides were incubated as described above with the E2 primary antibody, followed by a 1:1,000 dilution of tetramethyl rhodamine isothiocyanate-conjugated anti-rabbit antibody (Sigma, St. Louis, MO) for 1 h at 37°C. The slides were washed and mounted in Antifade after the nuclei were stained with DAPI. All images were acquired with an Olympus AX70 fluorescence microscope with a 100× objective lens and Speicher filters (Chroma, Rockingham, VT) and a Zeiss Axiocam digital camera (Thornwood, NY). Processing and assembly were accomplished with Photoshop (Adobe Systems, Mountain View, CA).
RESULTS
The localization of HPV-11 E2 is dynamic during mitosis and is independent of Brd4.
To determine the localization through mitosis of the epitope-tagged, tetracycline-regulatable HPV-11 E2 protein in the 293(11-E2) cell line, we extracted the uninduced or induced cells under conditions which stabilized the mitotic spindles. This procedure removed the E2 protein that was not attached to any stable subcellular structures. The remaining E2 was clearly and easily visualized by staining it with a polyclonal antibody (green fluor). The mitotic apparatus was visualized using antibody to β-tubulin (red fluor), and host chromosomes were stained with DAPI (blue). Uninduced cells yielded little or no E2 signal at any phase of the cell cycle (Fig. 1 Aa and data not shown). Upon induction, the localization of E2 was dynamic through the different phases of mitosis, but it remained colocalized with the mitotic-spindle fibers. During prophase, E2 was localized to the aster fibers that extended from the centrosomes (Fig. 1Ab). At metaphase, the E2 protein was seen to colocalize with mitotic spindles as described previously (Fig. 1Ac). During anaphase, the E2 protein remained localized to the mitotic-spindle fibers, which had started to pull the chromosomes toward the spindle poles. However, we also began to see an increased concentration of the E2 protein in the midplane of the cell, where the central spindle microtubules are located during progression to telophase (Fig. 1Ad). At late anaphase, telophase, and cytokinesis, the E2 protein was primarily localized to the central spindle microtubules (Fig. 1Ae and f). At cytokinesis, the E2 protein was additionally observed in the midbody in some of the cells (Fig. 1Af). The E2 protein expressed in the 293(11-E2) cell lines was tagged with the FLAG epitope at its amino terminus. To verify the observations made with the polyclonal E2 antibody, we repeated the experiment by using a monoclonal FLAG M2 antibody. The dynamic distribution patterns observed were essentially identical to those seen with the polyclonal E2 antibody (compare Fig. 1B to A). In the uninduced cells, no FLAG antibody reactivity was detected (Fig. 1Ba).
FIG. 1.
HPV-11 E2 localization is dynamic during mitosis and does not colocalize with Brd4. (A to C) Three images are presented for each cell, and each cell is denoted by a lowercase letter. (A and B) HPV-11 E2 localization is dynamic throughout the different phases of mitosis. Human 293(11-E2) cells that harbor FLAG-tagged HPV-11 E2 under the control of a tetracycline-regulated promoter were probed (A) with antibodies to HPV-11 E2 and β-tubulin (left column, E2 [Alexa488]; middle column, β-tubulin [Cy3] and DAPI-stained chromosomes [blue]; right column, merged images) and (B) with antibodies to the FLAG epitope and β-tubulin (left column, FLAG [Alexa488]; middle column, β-tubulin [Cy3] and DAPI-stained chromosomes [blue]; right column, merged images). (C) E2 does not associate with GFP-Brd4. 293(11-E2) cells were transfected with pEGFP-Brd4 and induced for HPV-11 E2 expression. The cells were stained with an antibody to β-tubulin (a) or an antibody to E2 (b). Both cells were in late anaphase. (Row a) Left panel, GFP-Brd4; middle panel, β-tubulin (Cy3) with DAPI-stained chromosomal DNA (blue); right panel, merged images. (Row b) Left panel, GFP-Brd4; middle panel, E2(Cy3); right panel, merged images with DAPI-stained chromosomal DNA (blue).
Brd4 is a cellular protein with bromodomains. It binds to acetylated histones and coats the chromosomes in a diffused manner throughout the cell cycle (17). In the presence of the BPV-1 E2 protein, Brd4 colocalizes with E2 and forms distinct foci on the mitotic chromosomes (4, 56). To examine the localization of the HPV-11 E2 protein relative to Brd4 through mitosis, we generated a GFP fusion construct of Brd4 and analyzed its localization pattern in transiently transfected human 293(11-E2) cells. The cells were then probed either with the HPV-11 E2 antibody or with the β-tubulin antibody. With or without prior induction of HPV-11 E2 protein expression, GFP-Brd4 diffusely coated the mitotic chromosomes as described previously (35), while the mitotic spindles formed normally. We observed no Brd4 focus formation on the chromosomes during prophase, metaphase, anaphase, telophase, and cytokinesis (Fig. 1Ca and data not shown). Figure 1Ca is a set of images of an induced anaphase cell in which the central spindle microtubules are clearly visible. As described above, the distribution of HPV-11 E2 protein was dynamic and remained localized to the spindle fibers (Fig. 1Cb and data not shown). In this anaphase cell, the E2 protein was observed to concentrate at the central spindle microtubules. No colocalization of E2 and Brd4 was observed. Thus, we conclude that during mitosis, HPV-11 E2 and Brd4 do not associate and do not influence each other's localization.
Residues 285 to 308 of HPV-11 E2 are necessary for localization to the mitotic spindles.
To localize the region of HPV-11 E2C (residues 285 to 348) responsible for its colocalization with the mitotic spindles, we generated a series of carboxyl-terminal truncations of GFP-H11E2C, yielding the following clones: GFP-H11 E2C:285-338, E2C:285-328, E2C:285-318, and E2C:285-308. Each expressed a fusion protein of the expected length in Western blots (Fig. 2A). The expression clones were each transfected into COS7 cells. The cells were extracted with microtubule-stabilizing buffer and stained for mitotic spindles using an anti-β-tubulin antibody as described above. Each of the truncated fusion proteins was seen on the mitotic apparatus, suggesting that the spindle localization domain was within residues 285 to 308 (Fig. 2Ca and data not shown). As inferred from the crystal structure of HPV-16 (Fig. 2D), residues 285 to 295 of HPV-11 E2 are expected to form a β-sheet, with residues 296 to 308 constituting the DNA binding helix. We reasoned that the DNA binding helix was not likely to be involved in spindle localization as well. Thus, the peptide spanning residues 285 to 295 was a logical candidate.
To test this hypothesis, we generated two additional fusion proteins. GFP-H11 E2C:295-367 had the first 11 residues of E2C deleted, and it no longer colocalized with the spindle fibers (Fig. 2Cb). HPV-11 E2H (spanning residues 201 to 284) did not colocalize with mitotic spindles (48). However, the extended hinge region (GFP-H11 E2H+:201-295) gained the ability to localize to the mitotic spindles (Fig. 2Cc). The expression of both fusion proteins was confirmed by Western blot analysis (Fig. 2B). Collectively, these results demonstrate that, for HPV-11 E2C, residues 285 to 308, and possibly as few as residues 285 to 295, are necessary and sufficient for localization to the mitotic spindles.
Mutational analysis of the HPV-11 E2C spindle localization domain.
We generated a number of single point mutations in HPV-11 E2HC (Q288A, L289A, Q290A, D292A, S293A, and N294A), as well as a clone containing seven alanines (HPV-11 E2HC:A7), in the spindle localization motif delineated above, each as a GFP fusion protein (Fig. 3A). We observed that none of the individual point mutations abrogated the ability of the fusion protein to colocalize with the mitotic spindles (Fig. 3Ba and b and data not shown). In contrast, HPV-11 E2HC:A7 no longer colocalized with the mitotic spindles, confirming the importance of the region (Fig. 3Bc). Western blot analyses demonstrated that the mutated proteins were synthesized in transfected cells (Fig. 2C).
HPV-16 E2C, HPV-18 E2C, and BPV-4 E2C colocalize with the mitotic spindles.
The E2 proteins of HPV-16 and the HPV-18 colocalize with the mitotic spindles (48). The spindle localization domain of HPV-11 E2C is highly conserved among these three viruses (Fig. 4A). To test if the conserved region in HPV-16 E2C and HPV-18 E2C also mediates this localization, we generated GFP fusion constructs of the E2C domains of these HPVs, as well as truncations that removed the segment spanning the putative mitotic-spindle localization domain. Only the fusions with the full-length E2C proteins, H16 E2C:287-365 and H18 E2C:288-365, colocalized with the mitotic spindles (Fig. 4B, rows a and c), whereas the truncated fusion proteins H16 E2C:296-365 and H18 E2C 297-365 did not (Fig. 4B, rows b and d). Western blotting showed that all four GFP-E2C fusion proteins were synthesized in the transfected cells (Fig. 4C).
Interestingly, the E2C domain of BPV-4 also contains a sequence very similar to the spindle localization domain of HPV E2C proteins (Fig. 4A). We were curious whether BPV-4 E2C also colocalizes with the mitotic apparatus. To test this possibility, we generated GFP fusion constructs of BPV-4 E2C (pGFP-B4 E2C, residues 327 to 408), as well as a truncation, BPV-4 E2C:336-408. The full-length E2C protein was able to colocalize with the mitotic spindles (Fig. 4B, row e), whereas the truncated form did not (row f). Western blotting showed that both proteins were expressed (Fig. 4C). These observations were corroborated by using an epitope-tagged BPV-4 E2C protein (data not shown). Collectively, these results support the notion that BPV-4 E2C colocalizes with the mitotic spindles and that GFP has no role in this localization.
A chimera of HPV-11 and BPV-1 E2C colocalizes with the mitotic spindles.
The N domain of BPV-1 E2 mediates the association with the mitotic chromosomes via the cellular protein Brd4, whereas the C domain is not involved (57). We constructed a GFP fusion to the E2C domain of BPV-1 (GFP-B1 E2C). Indeed, in the absence of extraction, it did not associate with either the mitotic spindles or the mitotic chromosomes but was diffusely distributed throughout the cell with a reduced intensity in areas occupied by the chromosomes, due to an excluded-volume effect (data no shown). Upon extraction, little GFP-B1 E2C remained in the cell (Fig. 5Ca). Sequence alignment indicated divergence in the HPV-11 E2C spindle localization domain compared to the corresponding region in BPV-1 E2C (Fig. 4A). To examine if this difference contributed to the distinct localization patterns of the HPV-11 and BPV-1 E2C proteins, we generated two chimeric E2C proteins. In the GFP-H11/B1 E2C chimera, the first 11 amino acids from BPV-1 E2C were replaced with the spindle localization domain of HPV-11 E2C. In the reciprocal chimera, GFP-B1/H11 E2C, the spindle localization domain of HPV-11 E2C was replaced with the corresponding region from BPV-1 E2C. Western blotting confirmed the synthesis of GFP-BPV-1 E2C, GFP-H11/B1 E2C, and GFP-B1/H11 E2C (Fig. 5B). The chimeric proteins were analyzed for the ability to colocalize with the mitotic spindles in transfected cells after extraction to remove any unattached GFP-E2C protein. Chimera GFP-H11/B1 E2C was observed to colocalize with the mitotic-spindle fibers (Fig. 5Cb), whereas the reciprocal chimera, GFP-B1/H11 E2C (Fig. 5Cc), exhibited no discernible patterns of localization, similar to GFP-BPV-1 E2C (Fig. 5Ca). These experiments verified our conclusion that residues 285 to 295 of HPV-11 E2C are critical for mitotic-spindle localization.
DISCUSSION
Throughout mitosis, the mitotic spindles constantly dissociate and reform as the mitotic chromosomes are pulled/pushed toward the two poles and the cell divides in two at the midplane. We have demonstrated that the distribution of the HPV ori binding protein E2, as revealed by either an antibody to E2 or an antibody to the FLAG epitope tag, is also dynamic and that its localization pattern changes as the cell progresses through mitosis, but always in association with the spindle fibers. During prophase, metaphase, and early anaphase, E2 colocalizes with mitotic spindles in close proximity to the mitotic chromosomes. Strikingly, however, E2 then relocalizes to the central spindle microtubules during late anaphase, telophase, and cytokinesis. It was additionally colocalized with the midbody during cytokinesis in some cells. Mass spectrometry of E2-containing complexes pulled down from the cell line harboring the inducible, tagged HPV-11 E2 protein (48) detected the presence of β-tubulin, as well as β-actin, a component of the midbody (our unpublished results). In addition, this dynamic E2 localization is reflected by the distribution of a self-tracking plasmid containing the HPV-11 origin in the presence, but not in the absence, of the E2 protein (R. J. Carter, T. R. Broker, and L. T. Chow, unpublished results).
It is intriguing that a very similar and dynamic localization during mitosis has been reported for human Orc6, one of the subunits of the cellular origin recognition complex, the counterpart of the HPV E2 protein. Chromatin-bound Orc complexes serve as sites for the assembly of the prereplication complexes (5). During early metaphase, the Orc6 protein localizes to the kinetochores, but at anaphase and telophase, as the chromosomes segregate, Orc6 relocalizes to the central spindle microtubules. Finally, during cytokinesis, the Orc6 protein can be seen in the midbody (41). The dynamic E2 localization during mitosis suggests that E2's apparent association with the spindle fibers might be mediated by one or more passenger proteins. Indeed, in vitro-translated E2 protein did not bind in vitro-translated β-tubulin (our unpublished observations). Passenger proteins are involved in coordinating events during mitosis and cytokinesis, regulating the movements of both the chromosomes and the mitotic apparatus. Several passenger proteins, including Aurora B (6) and Polo-like kinase (50), exhibit dynamic localization with some similarity to that observed for the E2 protein. Notably, Aurora B, which forms a complex with INCENP during mitosis, localizes to the kinetochores during prometaphase and then relocalizes to the central spindle fibers during anaphase and telophase (6). Interestingly, Aurora B plays a crucial role in regulating plasmid segregation for the EBV genome. EBV nuclear antigen 1 (EBNA-1) associates with the mitotic chromosomes in a process mediated by the human EBNA-1 binding protein 2 (hEBP2) (53). Silencing Aurora B caused hEPB2 to disassociate from the metaphase chromosomes, implicating this kinase in the segregation of EBV DNA, along with hEBP2.
By deletion and point mutations, the mitotic-spindle localization domain of HPV-11 E2C has been delineated to residues 285 to 308, and possibly as few as residues 285 to 295. The region as a whole is involved in mitotic-spindle association, because individual point mutations had no effect on this property. Furthermore, the domain is conserved, in both sequence and function, in the E2C peptides of HPV-16, HPV-18, and BPV-4 and is necessary to confer the ability to localize on the spindle apparatus. We propose that HPV-11, HPV-16, HPV-18, and BPV-4 may also use the abilities of the respective E2 proteins to associate with mitotic spindles as a means to maintain their extrachromosomal plasmids during cell division. Upon completion of mitosis, the viral plasmid DNA would presumably travel along the microtubules back into the nuclei of the daughter cells.
These patterns of localization of HPV E2 are different from that of BPV-1 E2, which is tethered to the mitotic chromosomes via an interaction with Brd4 (4, 56). Through chimeric E2C proteins, we showed that this spindle localization domain of HPV-11 E2C is sufficient to confer spindle localization on BPV-1 E2C, which by itself is diffusely distributed. We also investigated the localization patterns of HPV-11 E2 relative to GFP-Brd4 through different phases of mitosis. No colocalization of the FLAG-tagged HPV-11 E2 and GFP-Brd4 was observed throughout mitosis, and Brd4 remained associated exclusively with the chromosomes.
Intriguingly, HPV-16 and HPV-18 can induce carcinomas. We have previously suggested that, in addition to the oncogenic properties of the HPV E6 and E7 proteins, the association of the E2 protein with the mitotic spindles might play an important role in the carcinogenic conversion of the infected tissues (48). Oncogenic transformation by the high-risk HPV types results when the viral oncoproteins E6 and E7 are overexpressed in the dividing basal cells. High levels of E7 lead to extended periods of cellular proliferation by inactivating the pRB family of proteins, whereas overexpression of E6, which degrades the p53 tumor suppressor, leads to the survival of cells harboring chromosomal damage by abrogating various check points and apoptosis (60). Moreover, both E6 and E7 can independently induce chromosome instability (18). Intriguingly, BPV-4 can also induced carcinomas, but by a hit-and-run mechanism (9).
How might the E2 protein contribute to carcinogenesis? It is our opinion that, initially, both the E1 and E2 proteins might be expressed from intact copies of integrated HPV genomes. The repeated reinitiation of replication from the viral ori could generate unfinished replication bubbles susceptible to breakage. More importantly, the viral origin in integrated viral genomes may act as a virocentromere (48). During mitosis, the E2 protein would be able to bind to the viral origin as well as to the mitotic apparatus. If the virocentromere and the chromosomal centromere, on which the kinetochore assembles, are pulled to opposite poles during chromosome segregation, a break in the DNA might occur. Broken DNA ends are prime targets for recombination by nonhomologous end joining, thereby generating dicentric chromosomes and initiating breakage fusion bridge cycles (37). Breakage could also occur when the virocentromere-bound E2 relocalizes to the central spindle microtubules at telophase and cytokinesis, away from the rest of the chromosomes. Chromosome fragments could be lost when they fail to be included in the newly reformed nuclei of the daughter cells. Any of these events would result in genome instability and accelerate viral oncogenesis. Our hypothesis explains why, in patient lesions and in a cell line, HPV DNA integration is invariably and quickly accompanied by host chromosome instability (23, 38, 40).
Our hypothesis would also account for additional attributes consistently observed in HPV-induced cancers, cancer cell lines, and keratinocytes immortalized in vitro by HPVs. For instance, singly or tandemly integrated viral genomes are always disrupted in the E1 or the E2 gene, abolishing the expression of E2 or both genes (52). A predominant viral oncogene transcript that contained downstream host sequence and a polyadenlyation site was invariably detected in these cells (58). Such chimeric RNAs are thought to confer higher mRNA stability than the normal viral mRNA, leading to elevated levels of viral-oncoprotein expression and a growth advantage for the cell (26, 27). Indeed, in situ hybridization studies demonstrated that, in HPV-immortalized cells, cancers, and cervical-cancer cell lines, there is usually one transcription center, regardless of the copy number or number of integration sites. Moreover, in tandemly integrated viral genomes, the transcriptionally active copy was always located at the downstream integration site, which could not express an intact E2 protein. All the upstream copies, each containing an intact E2 gene, were silenced by DNA methylation (49). We suggest that E2 expression from integrated viral DNA has to cease in the cancer or immortalized cell which eventually emerges to avoid mitotic catastrophe induced by the association of E2 with the mitotic apparatus. However, it must also be noted that HPV-11 does not induce carcinomas in infected patients. This difference can be readily explained by the fact that the HPV-11 E6 and E7 proteins do not inactivate p53 and pRB as efficiently as those of the high-risk viruses, and in particular, E6 does not cause the degradation of p53. Thus, the low-risk HPV oncoproteins would not be able to overcome the various mitotic checkpoints or prevent apoptosis. The hypothesis that E2 contributes to the initial stage of HPV oncogenesis remains to be tested.
Acknowledgments
This research was supported by USPHS grants CA83679 and 107338 to L.T.C. and T.R.B. and CA103867 to C.-M.C.
We thank Keiko Ozato for Brd4 cDNA, Saveria Campo for the BPV-4 E2 clone, and Robert J. Carter for discussion.
REFERENCES
- 1.Abbate, E. A., J. M. Berger, and M. R. Botchan. 2004. The X-ray structure of the papillomavirus helicase in complex with its molecular matchmaker E2. Genes Dev. 18:1981-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin, A. A., S. Titolo, A. Pelletier, D. Fink, M. G. Cordingley, and J. Archambault. 2000. Identification of domains of the HPV11 E1 protein required for DNA replication in vitro. Virology 272:137-150. [DOI] [PubMed] [Google Scholar]
- 3.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 4.Baxter, M. K., M. G. McPhillips, K. Ozato, and A. A. McBride. 2005. The mitotic chromosome binding activity of the papillomavirus E2 protein correlates with interaction with the cellular chromosomal protein, Brd4. J. Virol. 79:4806-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell, S. P., and A. Dutta. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71:333-374. [DOI] [PubMed] [Google Scholar]
- 6.Bolanos-Garcia, V. M. 2005. Aurora kinases. Int. J. Biochem. Cell. Biol. 37:1572-1577. [DOI] [PubMed] [Google Scholar]
- 7.Bonne-Andrea, C., S. Santucci, P. Clertant, and F. Tillier. 1995. Bovine papillomavirus E1 protein binds specifically DNA polymerase alpha but not replication protein A. J. Virol. 69:2341-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bussiere, D. E., X. Kong, D. A. Egan, K. Walter, T. F. Holzman, F. Lindh, T. Robins, and V. L. Giranda. 1998. Structure of the E2 DNA-binding domain from human papillomavirus serotype 31 at 2.4 Å. Acta Crystallogr. D [DOI] [PubMed]
- 9.Campo, M. S. 1997. Bovine papillomavirus and cancer. Vet. J. 154:175-188. [DOI] [PubMed] [Google Scholar]
- 10.Chiang, C. M., T. R. Broker, and L. T. Chow. 1991. An E1M-E2C fusion protein encoded by human papillomavirus type 11 is a sequence-specific transcription repressor. J. Virol. 65:3317-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang, C. M., G. Dong, T. R. Broker, and L. T. Chow. 1992. Control of human papillomavirus type 11 origin of replication by the E2 family of transcription regulatory proteins. J. Virol. 66:5224-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang, C. M., M. Ustav, A. Stenlund, T. F. Ho, T. R. Broker, and L. T. Chow. 1992. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc. Natl. Acad. Sci. USA 89:5799-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow, L. T., and T. R. Broker. Human papillomavirus RNA transcription. In D. DiMaio and R. Garcea (ed.), The papillomaviruses, in press. Kluwer Academic Publishers, Amsterdam, The Netherlands.
- 14.Chow, L. T., and T. R. Broker. 2006. Mechanisms and regulation of papillomavirus DNA replication, p. 53-71. In M. S. Campo (ed.), Papillomavirus research: from natural history to vaccines and beyond. Caister Academic Press, Norwich, United Kingdom.
- 15.Clower, R. V., J. C. Fisk, and T. Melendy. 2006. Papillomavirus E1 protein binds to and stimulates human topoisomerase I. J. Virol. 80:1584-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conger, K. L., J. S. Liu, S. R. Kuo, L. T. Chow, and T. S. Wang. 1999. Human papillomavirus DNA replication. Interactions between the viral E1 protein and two subunits of human DNA polymerase alpha/primase. J. Biol. Chem. 274:2696-2705. [DOI] [PubMed] [Google Scholar]
- 17.Dey, A., F. Chitsaz, A. Abbasi, T. Misteli, and K. Ozato. 2003. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl. Acad. Sci. USA 100:8758-8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duensing, S., and K. Münger. 2003. Centrosomes, genomic instability, and cervical carcinogenesis. Crit. Rev. Eukaryot. Gene Expr. 13:9-23. [DOI] [PubMed] [Google Scholar]
- 19.Fouts, E. T., X. Yu, E. H. Egelman, and M. R. Botchan. 1999. Biochemical and electron microscopic image analysis of the hexameric E1 helicase. J. Biol. Chem. 274:4447-4458. [DOI] [PubMed] [Google Scholar]
- 20.Hegde, R. S. 2002. The papillomavirus E2 proteins: structure, function, and biology. Annu. Rev. Biophys. Biomol. Struct. 31:343-360. [DOI] [PubMed] [Google Scholar]
- 21.Hegde, R. S. 1995. Structure of the BPV-1 E2 DNA-binding domain bound to its DNA target. J. Nucl. Med. 36:25S-27S. [PubMed] [Google Scholar]
- 22.Hegde, R. S., and E. J. Androphy. 1998. Crystal structure of the E2 DNA-binding domain from human papillomavirus type 16: implications for its DNA binding-site selection mechanism. J. Mol. Biol. 284:1479-1489. [DOI] [PubMed] [Google Scholar]
- 23.Hopman, A. H., F. Smedts, W. Dignef, M. Ummelen, G. Sonke, M. Mravunac, G. P. Vooijs, E. J. Speel, and F. C. Ramaekers. 2004. Transition of high-grade cervical intraepithelial neoplasia to micro-invasive carcinoma is characterized by integration of HPV 16/18 and numerical chromosome abnormalities. J. Pathol. 202:23-33. [DOI] [PubMed] [Google Scholar]
- 24.Hughes, F. J., and M. A. Romanos. 1993. E1 protein of human papillomavirus is a DNA helicase/ATPase. Nucleic Acids Res. 21:5817-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilves, I., S. Kivi, and M. Ustav. 1999. Long-term episomal maintenance of bovine papillomavirus type 1 plasmids is determined by attachment to host chromosomes, which is mediated by the viral E2 protein and its binding sites. J. Virol. 73:4404-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon, S., B. L. Allen-Hoffmann, and P. F. Lambert. 1995. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J. Virol. 69:2989-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon, S., and P. F. Lambert. 1995. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc. Natl. Acad. Sci. USA 92:1654-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapoor, P., B. D. Lavoie, and L. Frappier. 2005. EBP2 plays a key role in Epstein-Barr virus mitotic segregation and is regulated by Aurora family kinases. Mol. Cell. Biol. 25:4934-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo, S.-R., J.-S. Liu, T. R. Broker, and L. T. Chow. 1994. Cell-free replication of the human papillomavirus DNA with homologous viral E1 and E2 proteins and human cell extracts. J. Biol. Chem. 269:24058-24065. [PubMed] [Google Scholar]
- 30.Lehman, C. W., and M. R. Botchan. 1998. Segregation of viral plasmids depends on tethering to chromosomes and is regulated by phosphorylation. Proc. Natl. Acad. Sci. USA 95:4338-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin, B. Y., A. M. Makhov, J. D. Griffith, T. R. Broker, and L. T. Chow. 2002. Chaperone proteins abrogate inhibition of the human papillomavirus (HPV) E1 replicative helicase by the HPV E2 protein. Mol. Cell. Biol. 22:6592-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, J.-S., S.-R. Kuo, T. R. Broker, and L. T. Chow. 1995. The functions of human papillomavirus type 11 E1, E2, and E2C proteins in cell-free DNA replication. J. Biol. Chem. 270:27283-27291. [DOI] [PubMed] [Google Scholar]
- 32a.Liu, J.-S., S.-R. Kuo, A. M. Makhov, D. M. Cyr, J. D. Griffith, T. R. Broker, and L. T. Chow. 1998. Human Hsp70 and Hsp40 chaperone proteins facilitate HPV-11 E1 protein binding to the origin and stimulate cell-free replication. J. Biol. Chem. 273:30704-30712. [DOI] [PubMed] [Google Scholar]
- 33.Loo, Y. M., and T. Melendy. 2004. Recruitment of replication protein A by the papillomavirus E1 protein and modulation by single-stranded DNA. J. Virol. 78:1605-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lusky, M., and E. Fontane. 1991. Formation of the complex of bovine papillomavirus E1 and E2 proteins is modulated by E2 phosphorylation and depends upon sequences within the carboxyl terminus of E1. Proc. Natl. Acad. Sci. USA 88:6363-6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruyama, T., A. Farina, A. Dey, J. Cheong, V. P. Bermudez, T. Tamura, S. Sciortino, J. Shuman, J. Hurwitz, and K. Ozato. 2002. A mammalian bromodomain protein, brd4, interacts with replication factor C and inhibits progression to S phase. Mol. Cell. Biol. 22:6509-6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masterson, P. J., M. A. Stanley, A. P. Lewis, and M. A. Romanos. 1998. A C-terminal helicase domain of the human papillomavirus E1 protein binds E2 and the DNA polymerase alpha-primase p68 subunit. J. Virol. 72:7407-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McClintock, B. 1951. Chromosome organization and genic expression. Cold Spring Harb. Symp. Quant. Biol. 16:13-47. [DOI] [PubMed] [Google Scholar]
- 38.Melsheimer, P., S. Vinokurova, N. Wentzensen, G. Bastert, and M. von Knebel Doeberitz. 2004. DNA aneuploidy and integration of human papillomavirus type 16 E6/E7 oncogenes in intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix uteri. Clin. Cancer Res. 10:3059-3063. [DOI] [PubMed] [Google Scholar]
- 39.Park, P., W. Copeland, L. Yang, T. Wang, M. R. Botchan, and I. J. Mohr. 1994. The cellular DNA polymerase alpha-primase is required for papillomavirus DNA replication and associates with the viral E1 helicase. Proc. Natl. Acad. Sci. USA 91:8700-8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pett, M. R., W. O. F. Alazawi, I. Roberts, S. Dowen, D. I. Smith, M. A. Stanley, and N. Coleman. 2004. Acquisition of high-level chromosomal instability is associated with integration of human papillomavirus type 16 in cervical keratinocytes. Cancer Res. 64:1359-1368. [DOI] [PubMed] [Google Scholar]
- 41.Prasanth, S. G., K. V. Prasanth, and B. Stillman. 2002. Orc6 involved in DNA replication, chromosome segregation, and cytokinesis. Science 297:1026-1031. [DOI] [PubMed] [Google Scholar]
- 42.Sedman, J., and A. Stenlund. 1998. The papillomavirus E1 protein forms a DNA-dependent hexameric complex with ATPase and DNA helicase activities. J. Virol. 72:6893-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo, Y. S., F. Muller, M. Lusky, and J. Hurwitz. 1993. Bovine papilloma virus (BPV)-encoded E1 protein contains multiple activities required for BPV DNA replication. Proc. Natl. Acad. Sci. USA 90:702-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skiadopoulos, M. H., and A. A. McBride. 1998. Bovine papillomavirus type 1 genomes and the E2 transactivator protein are closely associated with mitotic chromatin. J. Virol. 72:2079-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenlund, A. 2003. E1 initiator DNA binding specificity is unmasked by selective inhibition of non-specific DNA binding. EMBO J. 22:954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Titolo, S., A. Pelletier, A. M. Pulichino, K. Brault, E. Wardrop, P. W. White, M. G. Cordingley, and J. Archambault. 2000. Identification of domains of the human papillomavirus type 11 E1 helicase involved in oligomerization and binding to the viral origin. J. Virol. 74:7349-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ustav, M., and A. Stenlund. 1991. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 10:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Tine, B. A., L. D. Dao, S. Y. Wu, T. M. Sonbuchner, B. Y. Lin, N. Zou, C. M. Chiang, T. R. Broker, and L. T. Chow. 2004. Human papillomavirus (HPV) origin-binding protein associates with mitotic spindles to enable viral DNA partitioning. Proc. Natl. Acad. Sci. USA 101:4030-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Tine, B. A., J. C. Kappes, N. S. Banerjee, J. Knops, L. Lai, R. D. Steenbergen, C. L. Meijer, P. J. Snijders, P. Chatis, T. R. Broker, P. T. Moen, Jr., and L. T. Chow. 2004. Clonal selection for transcriptionally active viral oncogenes during progression to cancer. J. Virol. 78:11172-11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, Q., S. Xie, J. Chen, K. Fukasawa, U. Naik, F. Traganos, Z. Darzynkiewicz, M. Jhanwar-Uniyal, and W. Dai. 2002. Cell cycle arrest and apoptosis induced by human Polo-like kinase 3 is mediated through perturbation of microtubule integrity. Mol. Cell. Biol. 22:3450-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waters, J. C., T. J. Mitchison, C. L. Rieder, and E. D. Salmon. 1996. The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol. Biol. Cell 7:1547-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wentzensen, N., S. Vinokurova, and M. von Knebel Doeberitz. 2004. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 64:3878-3884. [DOI] [PubMed] [Google Scholar]
- 53.Wu, H., D. F. Ceccarelli, and L. Frappier. 2000. The DNA segregation mechanism of Epstein-Barr virus nuclear antigen 1. EMBO Rep. 1:140-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, L., R. Li, I. J. Mohr, R. Clark, and M. R. Botchan. 1991. Activation of BPV-1 replication in vitro by the transcription factor E2. Nature 353:628-632. [DOI] [PubMed] [Google Scholar]
- 55.Yang, L., I. Mohr, E. Fouts, D. A. Lim, M. Nohaile, and M. Botchan. 1993. The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc. Natl. Acad. Sci. USA 90:5086-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.You, J., J. L. Croyle, A. Nishimura, K. Ozato, and P. M. Howley. 2004. Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell 117:349-360. [DOI] [PubMed] [Google Scholar]
- 57.Zheng, P. S., J. Brokaw, and A. A. McBride. 2005. Conditional mutations in the mitotic chromosome binding function of the bovine papillomavirus type 1 E2 protein. J. Virol. 79:1500-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziegert, C., N. Wentzensen, S. Vinokurova, F. Kisseljov, J. Einenkel, M. Hoeckel, and M. von Knebel Doeberitz. 2003. A comprehensive analysis of HPV integration loci in anogenital lesions combining transcript and genome-based amplification techniques. Oncogene 22:3977-3984. [DOI] [PubMed] [Google Scholar]
- 59.Zou, N., B. Y. Lin, F. Duan, K. Y. Lee, G. Jin, R. Guan, G. Yao, E. J. Lefkowitz, T. R. Broker, and L. T. Chow. 2000. The hinge of the human papillomavirus type 11 E2 protein contains major determinants for nuclear localization and nuclear matrix association. J. Virol. 74:3761-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2:342-350. [DOI] [PubMed] [Google Scholar]