Abstract
The mechanism by which respiratory syncytial virus (RSV) suppresses T-cell proliferation to itself and other antigens is poorly understood. We used monocyte-derived dendritic cells (MDDC) and CD4 T cells and measured [3H]thymidine incorporation to determine the factors responsible for RSV-induced T-cell suppression. These two cell types were sufficient for RSV-induced suppression of T-cell proliferation in response to cytomegalovirus or Staphylococcus enterotoxin B. Suppressive activity was transferable with supernatants from RSV-infected MDDC and was not due to transfer of live virus or RSV F (fusion) protein. Supernatants from RSV-infected MDDC, but not MDDC exposed to UV-killed RSV or mock conditions, contained alpha interferon (IFN-α; median, 43 pg/ml) and IFN-λ (approximately 1 to 20 ng/ml). Neutralization of IFN-α with monoclonal antibody (MAb) against one of its receptor chains, IFNAR2, or of IFN-λ with MAb against either of its receptor chains, IFN-λR1 (interleukin 28R [IL-28R]) or IL-10R2, had a modest effect. In contrast, blocking the two receptors together markedly reduced or completely blocked the RSV-induced suppression of CD4 T-cell proliferation. Defining the mechanism of RSV-induced suppression may guide vaccine design and provide insight into previously uncharacterized human T-cell responses and activities of interferons.
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family of negative-strand RNA viruses that includes parainfluenza virus 1 (PIV1), PIV3, mumps, and measles, as well as newly recognized pathogens such as metapneumovirus, Nipa virus, and Hendra virus. RSV is often the first pathogen encountered by infants and accounts for about 24% of hospitalizations for pediatric respiratory tract disease. RSV is the primary cause of infantile pneumonia and bronchiolitis, the latter being an obstructive disease characterized by wheezing and hypoxia due to necrosis of the respiratory epithelium and airway plugging (9, 14, 22, 33). Severe infantile RSV-induced lower respiratory infections (LRI) may herald the onset of allergic diseases and/or asthma later in life (57). While RSV-induced LRI beyond age 3 are uncommon, RSV is a known trigger for wheezing in older asthmatics, and frequent upper respiratory infections in children and adults are common. Finally, RSV is a significant cause of morbidity in the elderly and mortality in bone marrow transplant recipients (22).
Among Paramyxoviridae family members, RSV, PIV3, and measles are known to suppress T-cell responses to secondary antigenic stimuli (43, 45, 50, 55). Clinical immunosuppression is a common complication of measles, and secondary bacterial and viral infections contribute to its high morbidity and mortality (6). The mechanism by which measles suppresses T-cell responses is not clear, but altered cytokine expression and impaired antigen presentation have been implicated (for reviews, see references 35 and 50). PIV3 is also immunosuppressive, perhaps a consequence of increased expression of interleulin-10 (IL-10) (54, 55).
Suppression of in vitro T-cell responses by human RSV (43, 46) and bovine RSV has been described (26, 51, 52). Unlike measles, however, it is not immediately obvious that suppression of T-cell responses in vitro is clinically relevant. It is quite possible that this immunosuppressive activity contributes to the phenomena of repeat infections with RSV, despite a limited number of subtypes and a genome that is not prone to mutation, as is, for example, influenza. In addition, secondary infection with Pasteurella haemolytica is not rare in bovine RSV-infected sheep and goats (10), and Streptococcus pneumoniae may complicate human RSV LRI more commonly than has been appreciated (30). Furthermore, immunosuppression may be mechanistically linked to a bias toward responses dominated by Th2 cytokines such as IL-4 and IL-13 induced by early infection with RSV in animal models (15). This effect may in turn contribute to subsequent episodes of wheezing in childhood with repeat exposure to this virus and may also cause predisposition to the development of asthma and allergic diseases.
The mechanism by which RSV suppresses T-cell responses is not well understood. Contact-dependent mechanisms (49), IL-1 receptor antagonist (IL-1RA) (32, 46), and alpha interferon (IFN-α) (42) have each been implicated but inconclusively so. The implication that IFN-α is immunosuppressive is somewhat paradoxical, since it is well documented that the nonstructural genes of RSV, NS1 and NS2, also decrease expression of the cellular responses to type I IFN (IFN-α and -β) (47, 48, 58).
To date, T-cell responses to RSV have been studied using peripheral blood mononuclear cells, which is a heterogeneous population. This may have impeded efforts to define the mechanisms of RSV-induced immunosuppression. Therefore, we first designed a simplified experimental system using only monocyte-derived dendritic cells (MDDC) and autologous CD4 T cells and then focused on identifying soluble factors expressed by MDDC in response to RSV that suppress T-cell proliferation to the superantigen Staphylococcus enterotoxin B (SEB).
MATERIALS AND METHODS
Cell culture.
Human MDDC were generated from elutriated monocytes obtained from healthy adult donors (<65 years of age) at the National Institutes of Health (NIH) Clinical Center blood bank. The monocytes were cultured at 3 × 105/ml in RPMI medium with 1% nonessential amino acids and 1% sodium pyruvate (Biosource, Rockville, MD), 10% fetal bovine serum (HyClone, Logan, UT), 0.05 mM β-mercaptoethanol (Sigma, St. Louis, MO), 20 μg/ml gentamicin (Invitrogen, Grand Island, NY), 800 U/ml recombinant human granulocyte-macrophage colony-stimulating factor (Leukine; Immunex, Seattle, WA), and 500 U/ml recombinant human IL-4 (rhIL-4; BD Pharmingen, San Diego, CA) for 7 days.
Elutriated lymphocytes were purified with Ficoll-Hypaque density centrifugation (Sigma) and frozen in liquid nitrogen. After thawing and overnight incubation, CD4 T cells were purified from the lymphocytes by positive selection with a magnetic column and beads (AutoMACS; Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol, and purity was verified by flow cytometry. The Institutional Review Boards of the NIH and at the U.S. Food and Drug Administration approved the study.
Monoclonal antibodies and recombinant cytokines.
Mouse anti-IFN-α/β receptor chain 2 (IFNAR2, CD118) monoclonal antibody (MAb) was purchased from PBL laboratory (Piscataway, NJ). Anti-IL-10R2 MAb and mouse immunoglobulin G2a (IgG2a) and IgG1 isotype controls were obtained from R & D systems (Minneapolis, MN). The humanized MAbs palivizumab and omalizumab were obtained from Medimmune (Gaithersburg, MD) and Genentech (San Francisco, CA), respectively. Anti-IL28R was generated as previously described (19).
Viral infection of MDDC.
PIV3 was propagated at 32°C in LLC-MK2 cells or Vero cells, as previously described (23). The Udorn strain of influenza A virus (A/Udorn/72) was propagated in HEp-2 cells in the presence of 7.5 μg of trypsin/ml as previously described (62). The wild-type A2 strain of RSV and recombinant RSV expressing green fluorescent protein (rgRSV) (24) were grown in HEp-2 cells (14). Wild-type RSV was further purified by centrifugation through a sucrose gradient to eliminate cytokine contaminants (58). Virus concentrations were determined by plaque assay (28). RSV was inactivated with UV light (UV-RSV) in a Stratalinker UV cross-linker for 10 min (0.12 J/cm2; Stratagene, La Jolla, CA). Complete inactivation of RSV was confirmed by plaque assay. Cytomegalovirus (CMV) grade 2 antigen was purchased from Microbix Biosystems (Toronto, Ontario).
MDDC (1 × 106/ml) were exposed to virus for 4 h at 37°C and, when stated, washed extensively in culture medium to remove any nonadherent virus prior to coculture with lymphocytes or CD4 T cells. MDDC supernatants were collected after overnight culture of infected and washed MDDC. Mock-infected MDDC were exposed to sucrose solutions of a concentration equal to those in the viral stocks as measured by the invertase-hexokinase-G6PD assay (Sigma). Infection of MDDC with rgRSV was verified with an Olympus BX41 (Tokyo, Japan) fluorescent microscope and digitally photographed with the Spot RT Color system (Sterling Heights, MI).
Cell stimulation and in vitro proliferation assay.
Virus-exposed MDDC were cultured with lymphocytes at a ratio of 1:10 or with CD4 T cells at a ratio adjusted according to the percentage of CD4 T cells in lymphocytes of that donor, which was usually between 1:3 and 1:5. CD4 T cells were stimulated with Staphylococcus enterotoxin B (1 μg/ml; Sigma). Unless otherwise stated, the cells were pulsed with [3H]thymidine (0.5 μCi/well; MP Biomedicals, Inc., Irvine, CA) on day 4 and harvested on day 5 for measurement of cell lysate-associated tritium by β-scintillation counting (Perkin-Elmer, Boston, MA). All proliferation experiments were performed in triplicate and reported as counts per minute.
To test MDDC supernatants for antiproliferative activity, CD4 T cells (0.6 × 105 to 1 × 105/well) were incubated in 96-well plates with SEB for 3 days in the presence of 100 μl tissue culture medium/well, of which a maximum of 84 μl was supernatant from an overnight culture of virus or mock-infected MDDC. The cells were then pulsed with [3H]thymidine and harvested for β-scintillation the next day. To test dependence on IFN-α and IFN-λ, MAb to IFNAR2, IL-10R2, and IL-28R were used at concentrations of 15, 10, and 20 μg/ml, respectively. CD4 T cells were incubated with MAb in half the final volume of medium for 1 h at 37°C before addition to the MDDC supernatant and SEB.
Transwell cultures.
Virus-exposed MDDC were placed in the top chamber of 24-well Transwell culture plates (1.4 × 105 cells/well in 100 μl; Costar, Cambridge, MA). SEB (1 μg/ml) and CD4 T cells (4 × 105 to 7 × 105) treated with MAb were incubated in 600 μl of media in the lower chamber. After 4 days, the CD4 T cells were transferred to 96-well plates (0.6 × 105 to 1 × 105/well) for measurement of proliferation using [3H]thymidine.
Enzyme-linked immunosorbent assay (ELISA) for RSV proteins.
Rabbit anti-RSV polyclonal Abs were generated by immunizing a rabbit with UV-inactivated sucrose gradient-purified virus at a dose equivalent to 6.0 log10 50% tissue culture infective doses given subcutaneously four times at 3-week intervals and then terminally bled 1 month after the last dose. Rabbit anti-RSV antisera had a 50% plaque reduction neutralization titer of 1:11,000 when tested against the A2 strain of RSV (not shown).
For detection of RSV proteins, 96-well plates were coated at 4°C overnight with either MDDC supernatants or RSV standards (RPMI medium containing serial twofold dilutions of live RSV). The sample, standard dilutions, and control wells were washed three times with phosphate-buffered saline-0.05% Tween 20 solution and blocked with 1% bovine serum albumin in phosphate-buffered saline-0.05% Tween 20 solution prior to incubation with rabbit polyclonal anti-RSV. Horseradish peroxidase-conjugated goat anti-rabbit IgG and 3,3′,5,5′-tetramethylbenzidin (KPL, Inc., Gaithersburg, MD) were used to reveal RSV-derived proteins. Concentrations of RSV proteins in MDDC supernatants are expressed in equivalents relative to the amount of protein in the standard containing 1 × 106/ml of live RSV.
Measurements of cytokines.
ELISA kits for IFN-α and IFN-β were purchased from PBL Laboratories (Piscataway, NJ). All other cytokines were detected with Luminex multiplex bead assay (Linco Research, St. Charles, MO), except IL-1α, for which the bead assay was purchased from Biosource (Camarillo, CA). The data were analyzed using the MasterPlex QT quantitation software (MiraiBio, Alameda, CA).
Quantitative reverse transcription-PCR (RT-PCR) for IFN-λ.
Total cellular RNA of virus-infected or mock-infected MDDC was isolated using RNeasy (QIAGEN, Valencia, CA) and reverse transcribed to cDNA with Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Primers from SuperArray (Frederick, MD) specific for IFN-λ1 and β-actin were used for PCR amplification. The cDNA was mixed with Brilliant SYBR green quantitative PCR (qPCR) master mix (Stratagene, La Jolla, CA), and qPCR was performed using the Mx3005P system (Stratagene). IFN-λ1 mRNA quantification was normalized using β-actin.
Biological assays for IFN-λ activity.
Signal transduction by electrophoretic mobility shift assay (EMSA) was used to detect activation of STAT1 by IFN-λ in the HT-29 cell line transfected with the IL-28 receptor complex. The effect of IFN-λ on proliferation of BWLICR2 cells (stable transfectants with hIL-28R) was measured with the hexominidase-based chromogenic viability assay as previously described (19, 27, 29).
Statistical analysis.
Differences were analyzed for statistical significance with the Wilcoxon signed rank test using JMP software (version 5; SAS Institute, Inc., Cary, NC).
RESULTS
RSV infects MDDC.
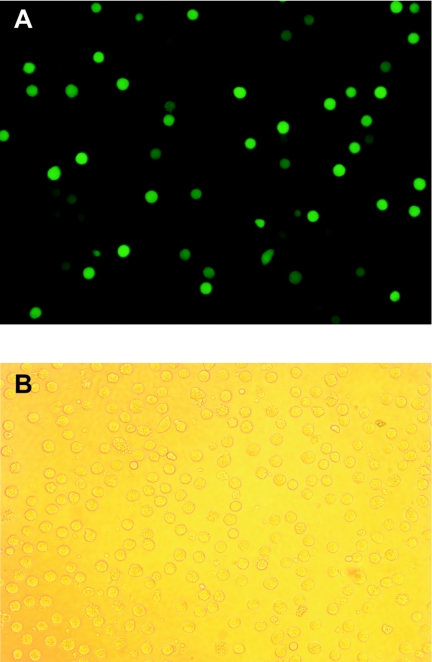
To determine whether RSV actively infects MDDC, we exposed MDDC overnight to rgRSV. Figure 1 shows that approximately 10 to 15% of the MDDC express green fluorescent protein, suggesting that RSV is actively transcribing and probably replicating in those cells. By contrast, CD4 T cells exposed to rgRSV did not fluoresce (data not shown). To determine the amounts of virus in the supernatant after overnight incubation with MDDC, we performed plaque assays using supernatants generated from unwashed RSV-exposed MDDC. To our surprise, only 1.9% of infectious PFU were in the supernatants after overnight incubation. In contrast, 25% of infectious PFU was recovered when RSV was incubated overnight in medium alone (not shown). Furthermore, analysis of MDDC supernatants by ELISA demonstrates that the concentration of RSV proteins after overnight incubation (whether from live or dead virus) was 38% ± 10% (mean ± standard deviation [SD]) of the dose to which the MDDC were exposed. The RSV infectious PFU and proteins found in the MDDC supernatants may be either newly replicated or due to virus that had been added to the MDDC but had not adsorbed onto the cells. Either way, it is clear that RSV replicates poorly in MDDC for the first 12 to 18 h of exposure.
FIG. 1.
RSV infects MDDC. MDDC (1 × 106/ml) were exposed overnight to rgRSV (multiplicity of infection of 3) and washed twice before being examined by fluorescent microscopy. The fluorescent (A) and bright field (B) images of the same microscopic field were photographed. The figure is representative of the results of three experiments.
RSV inhibits proliferation in response to either SEB or CMV antigens.
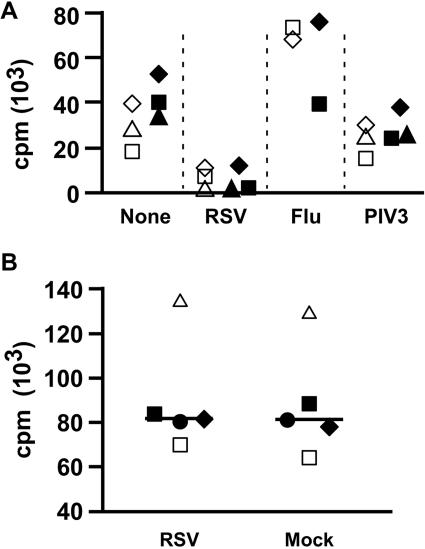
To determine factors responsible for RSV-induced immunosuppression, we first sought to design a simplified, reproducible experimental system. We used MDDC as antigen-presenting cells and to measure proliferation of autologous T cells in response to SEB as the primary readout for responses and suppression. Figure 2A shows that RSV and not influenza virus inhibits proliferation of T cells in response to SEB. Exposure of MDDC to PIV3, another paramyxovirus, modestly inhibited T-cell proliferation as previously reported (54, 55). To simplify the experimental system, we mixed purified CD4 T cells (97 to 99% pure, not shown) with RSV-exposed MDDC and found suppression to be essentially the same (Fig. 2A). The addition of RSV directly to CD4 T cells (in the absence of MDDC), however, did not suppress SEB-induced proliferation (Fig. 2B). Thus, RSV-induced immunosuppression could readily be demonstrated in a system in which MDDC were treated with RSV and mixed with superantigen and T cells (either CD4 or CD8 T cells).
FIG. 2.
RSV induces MDDC-mediated suppression of T-cell proliferation. (A) CD4 T cells together with MDDC are sufficient to demonstrate RSV-induced immunosuppression. MDDC were exposed to RSV (multiplicity of infection of 2.5), influenza, or PIV3 (multiplicity of infection of 3) or mock infected for 4 h before the addition of either SEB-stimulated autologous lymphocytes or purified CD4 T cells. The cells were pulsed with [3H]thymidine on day 4 and harvested on day 5. Each symbol shape is one of three donors. Open symbols indicate proliferation of T cells in a population of total lymphocytes, and filled symbols represent purified CD4 T cells. (B) RSV does not directly inhibit CD4 T-cell proliferation. RSV (final density, 1 × 106 PFU/ml) or the same volume of sucrose buffer was added to SEB-stimulated CD4 T cells and SEB for 3 days prior to pulsing with [3H]thymidine. The bar indicates the median of data from five donors, each represented by a different symbol.
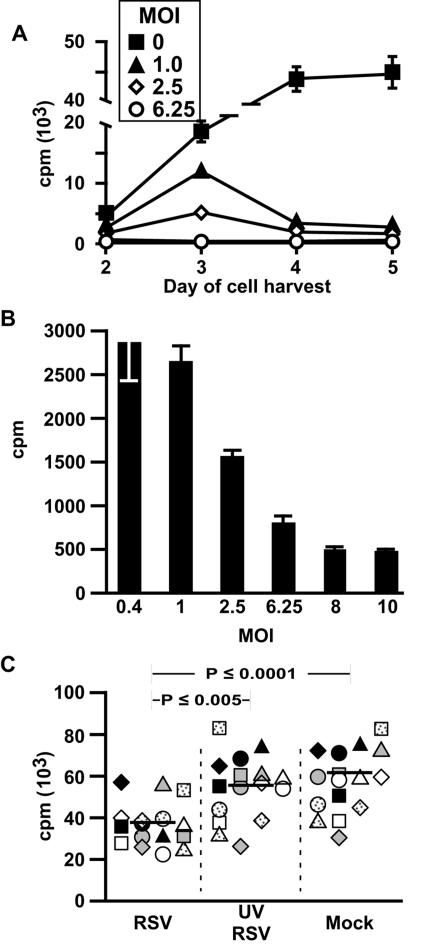
Since lymphocytes were harvested on day 5 after exposure to MDCC, we sought to determine whether the immunosuppressive effects could be observed earlier and to verify the appropriate experimental time course. Figure 3A demonstrates that T-cell proliferation in response to CMV antigens is suppressed as early as day 3, in a dose-dependent manner. However, maximal suppression was observed on day 5 (Fig. 3A and B). Figure 3C demonstrates reproducibility of experiments in which only CD4 T cells and MDDC were used and the necessity of live RSV for maximal immunosuppression. For these, and subsequent experiments, the MDDC were washed 4 h after incubation with RSV to minimize any effect of RSV carryover. Consistent with the dose response shown in Fig. 3A, immunosuppression was diminished when exposure of MDDC to live RSV was limited to 4 h.
FIG. 3.
RSV-induced immunosuppression is dependent on time, dose, and live virus. (A and B) Time and dose dependency of RSV-induced immunosuppression of CMV antigen-stimulated proliferation. MDDC were exposed to CMV antigen (vol/vol = 1:100) and RSV (multiplicity of infection [MOI] of 0, 0.4, 1, 2.5, 6.25, 8, and 10) for 4 h before incubation with autologous lymphocytes. Cells were pulsed with [3H]thymidine at different time points for proliferation assay. The results, expressed as mean cpm ± SD, are from one representative experiment of three. (A) Only MOI 0, 1, 2.5, and 6.25 are shown for clarity. (B) Proliferation on day 5 of each experimental condition showing dose dependency at that time point. (C) Only live RSV induces immunosuppression. MDDC were incubated with live RSV or UV-RSV, each at an MOI of 1, or an equal volume of sucrose buffer for 4 h (mock), washed, and incubated with SEB-stimulated autologous purified CD4 T cells for 4 days before harvest on day 5 for [3H]thymidine uptake assay. The bar indicates the median of data from 15 donors.
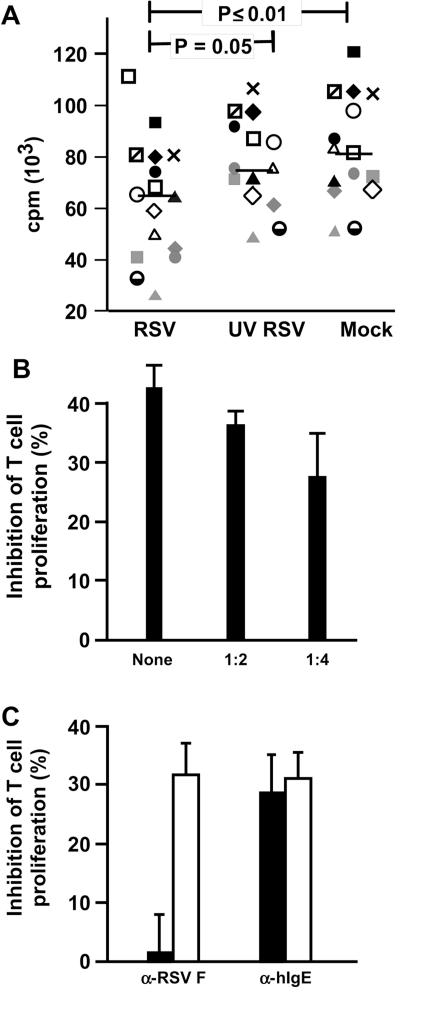
Supernatants from RSV-exposed MDDC transfers immunosuppression.
To determine whether soluble factors might be responsible for RSV-induced immunosuppression, we exposed MDDC to RSV, UV-RSV, or sucrose buffer (mock) for 4 h, after which time the cells were washed and incubated overnight before harvesting supernatant. Figures 4A and B show that only supernatants from MDDC exposed to live RSV suppress proliferation of CD4 T cells in response to SEB and that the suppressive effect is dose dependent. To confirm that RSV carryover is not responsible for this immunosuppression, we first incubated virus with humanized MAb (1 mg/ml) against either RSV F (fusion) protein (palivizumab), or human IgE (omalizumab) as a control to demonstrate the former's neutralization capacity. As expected, supernatants from MDDC exposed to RSV that was neutralized by palivizumab did not suppress CD4 T-cell proliferation (Fig. 4C). In contrast, when added to supernatant that was harvested from RSV-exposed MDDC, palivizumab did not reverse immunosuppression (Fig. 4C), just as live RSV added to supernatants from mock-infected MDDC did not immunosuppress (Fig. 2B). Thus, the immunosuppressive activity transferred in supernatants from RSV-exposed MDDC is not due to RSV itself and is more likely due to one or more soluble factors that are MDDC derived.
FIG. 4.
Supernatants from RSV-exposed MDDC transfer immunosuppressive activity. (A) Supernatants from RSV, but not UV-RSV or mock-exposed MDDC, inhibit SEB-stimulated CD4 T-cell proliferation. MDDC were exposed to RSV or UV-RSV or mock exposed for 4 h and washed. Supernatants were harvested after overnight incubation and incubated with SEB-stimulated CD4 T cells for 3 days prior to pulsing with [3H]thymidine for 24 h. The bar indicates the median of data from 15 donors. (B) Inhibitory effect of RSV-exposed MDDC supernatants decreases with dilution. Means and SD of results from one representative experiment of three are shown. (C) RSV or RSV F proteins in the supernatants are not responsible for immunosuppression of CD4 T cells. RSV or supernatants from RSV- or mock-exposed MDDC were incubated with palivizumab or omalizumab (1-mg/ml final concentration) for 1 h at 37°C before addition to MDDC or SEB-stimulated CD4 T cells, respectively. The data, expressed as mean counts per minute ± SD, are cumulative from three donors. Filled bars, MAb added directly to RSV prior to addition of virus to MDDC; open bars, MAb added to MDDC supernatants prior to mixing with CD4 T cells; a-RSV F, anti-RSV F protein; a-hIgE, anti-hIgE.
Analysis of cytokines in MDDC supernatants.
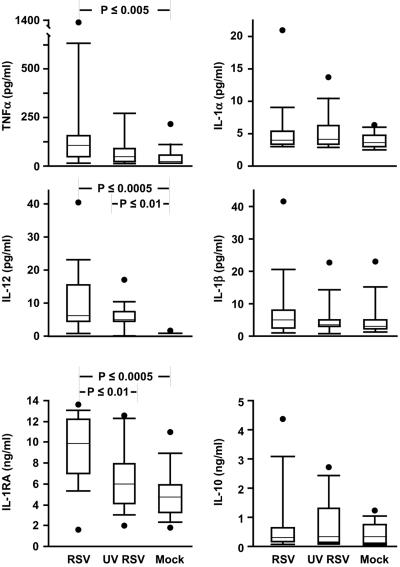
To determine which soluble factors might be responsible for immunosuppression, we measured levels of a number of cytokines using multiplex bead assays. Figure 5 shows that there were no differences in supernatant derived from RSV-, UV-RSV-, or mock-exposed MDDC in concentrations of IL-1α or IL-1β. Consistent with previous reports (32, 46), IL-1RA levels were high in RSV supernatants and low in UV-RSV and mock-infected supernatants. Since we found very little IL-1α or -1β, with no differences between the conditions and since the only known activity of IL-1RA is to block binding of IL-1α/β to IL-1R, we did not explore further an immunosuppressive role for IL-1RA.
FIG. 5.
RSV induces secretion of tumor necrosis factor alpha, IL-12, and IL-1RA, but not IL-1α, IL-1β, or IL-10, from MDDC. Data from 14 donors are shown. The upper and lower error bars indicate the 90th and 10th percentiles, respectively; the borders of each box indicate the 75th and 25th percentiles; the bar within the box indicates the median, and the filled circles show the highest (and in the case of IL-1RA, the lowest) data points of each set.
There were no differences in IL-10, and in all three conditions,transforming growth factor β1 (TGF-β1) was either very low or undetectable (not shown). Because IL-10 and TGF-β1 are known to suppress T-cell proliferation, we tested whether addition of rIL-10 (25 ng/ml) or TGF-β1 (10 ng/ml) would suppress proliferation in our system and found that they did not (not shown). Similarly, when we removed suppressor CD4 T cells, which secrete IL-10 and TGF-β1, by magnetic bead selection (i.e., CD25+, CD4+ lymphocytes), we saw no differences in SEB-induced proliferation (not shown).
Tumor necrosis factor alpha levels were highest in the supernatants from RSV-infected MDDC, a difference that was significant only when compared to mock-infected MDDC supernatants. IL-12 was found at high levels in both RSV and UV-RSV supernatant but not in supernatant from the mock-infected MDDC. Exogenous IL-12 (2 ng/ml) or IL-2 (20 U/ml) and anti-IL-12 (2 μg/ml) also had no effect in our system (not shown).
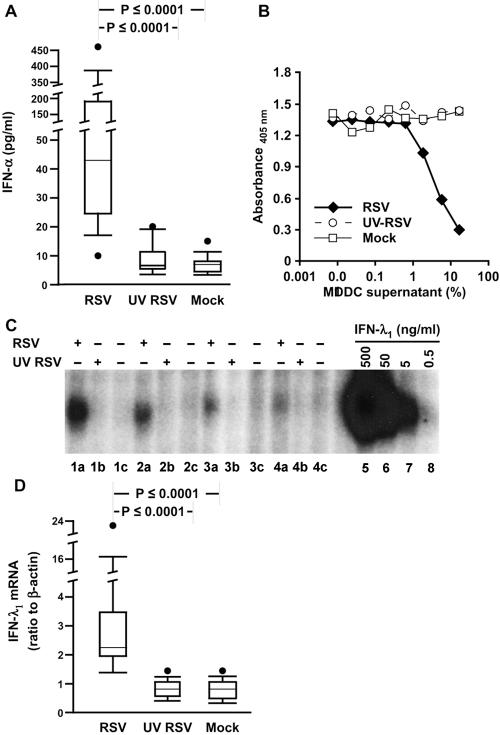
IFN-α has been previously reported to be solely responsible for RSV-induced immunosuppression (42). We therefore measured IFN-α using an ELISA that detects 12 of the 14 isoforms and found high concentrations uniquely in the RSV-exposed MDDC supernatants (Fig. 6A). By contrast, IFN-β was undetectable in any of the MDDC supernatants (data not shown).
FIG. 6.
RSV induces secretion of IFN-α and IFN-λ from MDDC supernatants. (A) IFN-α protein is expressed in response to live RSV. Data from 15 donors are shown. (B and C) IFN-λ activity is expressed in response to live RSV. (B) Supernatants from RSV-infected MDDC inhibit proliferation of BWLICR2 cells. The parent line BW5147 was unaffected by any of the supernatants (not shown). The figure shown is representative of data from five donors. (C) HT-29 cells transfected with the IL-28 receptor complex were exposed to MDDC supernatants, harvested, and analyzed by EMSA for activation of STAT1 in response to supernatants from MDDC that were exposed to RSV (lanes a), UV RSV (lanes b), or mock infection (lanes c). Lanes 5 to 8 are nuclear extracts from transfected HT-29 cells treated with rhIFN-λ1 at 500, 50, 5, and 0.5 ng/ml, respectively. +, present; −, absent. (D) IFN-λ1 mRNA is expressed in response to live RSV. RNA harvested from MDDC was analyzed for expression of the gene for IFN-λ1 by qRT-PCR and shown as a ratio to expression of the gene for β-actin. Data from 16 donors are shown.
Type III interferons, which include IFN-λ1, 2, and 3 (also known as IL-29, -28A, and -28B, respectively), are expressed by macrophages at low levels in response to RSV (58). Currently, there are no commercially available MAbs for measurement of IFN-λ by ELISA, so we used two biological assays. The first assay system measures activation of signal transducer and activator of transcription 1 (STAT1) induced by IFN-λ in a hamster cell line that is stably transfected with a cDNA encoding a chimeric receptor composed of the extracellular ligand-binding domain of IFN-λR1 (IL-28R) and the intracellular domain of the IFN-γR1 chain (27). The second assay system measures IFN-λ activity by quantifying the suppression of cell division using a chromogenic viability assay of a human lymphoma cell line that was engineered to express the full-length IFN-λR1 chain (19). Figures 6B and C demonstrate that only supernatants from RSV-exposed MDDC contained appreciable levels of IFN-λ, The concentration of type I IFN in the supernatants is estimated to be between 1 to 5 ng/ml and 5 to 20 ng/ml, based on the specific activity of rIFN-λ in the EMSA and proliferation assays, respectively. Expression of IFN-λ by RSV-infected MDDC was confirmed by qRT-PCR (Fig. 6D).
IFN-α and IFN-λ contribute to the inhibitory effect of RSV-exposed MDDC supernatants.
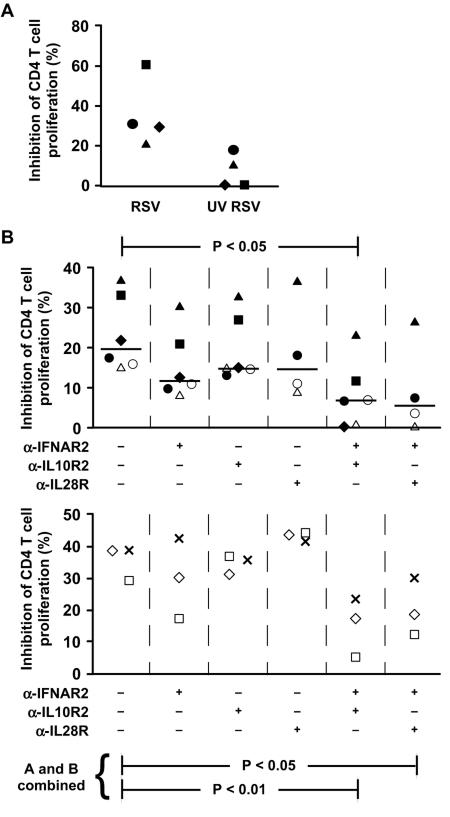
We then asked whether IFN-α and IFN-λ mediate RSV-induced immunosuppression by treating the CD4 T cells with neutralizing MAb to IFNAR2 (CD118), one of the two chains of IFN-α/β receptor, and MAbs to both of the members of the IFN-λ receptor complex, IL-10R2 and IL-28R. Two experimental systems were used: transfer of supernatants and Transwell cultures by placing MDDC in the top chamber and the CD4 T cells in the bottom chamber for the first 3 days of incubation. RSV-induced immunosuppression in the Transwell system is shown in Fig. 7A. While each of the MAbs alone may have a modest effect, the combination of either anti-IFNAR2 with anti-IL10R2 or with anti-IL28R substantially abrogated suppression after transfer of supernatants and in the Transwell system (Fig. 7B, top and bottom, respectively).
FIG. 7.
IFN-α and IFN-λ contribute to the inhibitory effect of RSV-exposed MDDC supernatants. (A) RSV-induced immunosuppression can be demonstrated with the Transwell system. MDDC were exposed to mock, live, or UV-inactivated RSV for 4 h, washed, and placed in the top chamber of Transwell plates (pore size, 0.4 μm). The bottom chamber contained autologous purified CD4 T cells stimulated with SEB (1 μg/ml). Each symbol represents a different donor. (B) Neutralizing MAbs to IFN-α and IFN-λ receptors together substantially reverse the inhibitory effect of RSV-exposed MDDC supernatant (top) or in the Transwell system (5-μm pore size, bottom). Each symbol represents a different donor. Since an n of 6 is required for the Wilcoxon signed-rank test, the data from the top and bottom plots were pooled for additional statistical analysis. λ-IFNAR2, anti-IFNAR2; λ-IL10R2, anti-IL10R2; λ-IL28R, anti-IL28R; +, present; −, absent.
DISCUSSION
We explored the mechanism of RSV-induced immunosuppression by first simplifying the experimental system to MDDC and autologous CD4 T cells, thus eliminating interactions with CD8 T cells, B cells, and NK cells. Our approach was similar to that of Bartz and colleagues (5), who used cord blood CD4 T cells, which are all naive, and cord blood CD34+ cells to derive dendritic cells (DC). In that report, treatment of DC with RSV suppressed IFN-γ secretion but not proliferation in response to superantigen. Preliminarily, we have seen no difference in suppression by RSV-infected MDDC of sorted naive versus memory CD4 T cells (B. Chi and R. L. Rabin, unpublished data), but the difference in the source of DC, age of donor, or both may easily account for the difference in functional suppression between the two reports.
Since RSV had no direct effect on CD4 T cells (Fig. 2B), we could concentrate on MDDC as a source of suppressive activity, and since suppressive activity could be transferred with supernatants from MDDC (Fig. 4A), we turned our attention toward soluble factors secreted by them. And since suppression of T-cell proliferation is induced only with infection of MDDC by live RSV (Fig. 3C and 4A), we ruled out the possibility that live RSV directly suppresses CD4 T-cell proliferation by demonstrating, first, that CD4 T-cell suppression was unaffected by addition of palivizumab to supernatants from RSV-infected MDDC (Fig. 4C) and, second, that direct addition of live (Fig. 2B) or UV-killed RSV (not shown) to CD4 T cells did not suppress proliferation in response to SEB. Furthermore, consistent with a previous report using a similar experimental system (5), live RSV, and RSV-derived protein levels in harvested supernatants actually diminished. However, there are some obvious limitations to these experiments that must be acknowledged. First, it is not known if palivizumab blocks virus or F binding to cells or if palivizumab neutralization is mediated at a postbinding step. Because palivizumab specifically interacts with a single epitope within the F1 region, it would not be expected to block virus attachment mediated by the F2 portion of the fusion glycoprotein or inhibit interactions with the G glycoprotein. Second, these experiments do not rule out the possibility that RSV-secreted G glycoprotein may play a role in T-cell suppression. It is apparent, however, that type I and III IFN-mediated suppression is distinct from inhibition of NF-κB mediated by RSV-soluble G glycoprotein, the latter of which is not dependent on live virus (1, 41). Taken together, it appeared unlikely that RSV virions or proteins directly suppress T cells in our experiments; thus, we focused on MDDC-derived soluble factors.
One such factor that has been held responsible for RSV-induced immunosuppression is IL-1RA (32, 46). We found high levels of IL-1RA in the MDDC supernatants but very little IL-1α and IL-1β, suggesting that IL-1RA probably has no role in this context. Similarly, TGF-β1 and IL-10 are immunosuppressive cytokines that were ruled out because TGF-β1 was undetectable in the MDDC supernatants (not shown) and IL-10 levels were similar in all experimental conditions. Furthermore, neither addition of rhTGF-β1 or rhIL-10 to control MDDC supernatants nor exclusion of suppressor CD4 T cells (CD4+, CD25+ lymphocytes), an important source of TGF-β1 and IL-10 blocked RSV-induced suppression (not shown). IL-12 enhances T-cell proliferation (61) and was induced by RSV and UV-RSV. However, neither neutralization with anti-IL-12 MAb nor supplementation with rhIL-12 blocked RSV-induced immunosuppression (not shown). Similarly, IFN-γ, classically a product of T and NK cells, can be also expressed by DC (60) and may be immunosuppressive (12, 37). Consistent with a previous report (39), neutralizing MAb to IFN-γ enhanced, rather than diminished, suppression of proliferation (data not shown).
IFN-α levels were elevated in supernatants of MDDC exposed to live RSV (Fig. 6A). Similarly, RSV induces expression of IFN-λ in monocyte-derived macrophages (58) and MDDC (Fig. 6B, C, and D). IFN-λ1, IFN-λ2, and IFN-λ3 comprise the newly described type III IFN, which may be expressed by a variety of cells, including plasmacytoid DC, MDDC, and macrophages in response to a variety of stimuli, including viruses (13). The receptor for the type III IFN consists of the ligand-binding chain, IL-28R, and the accessory receptor chain, IL-10R2 (27, 53). IL-28R is unique to the type III IFN, but IL-10R2 also dimerizes with IL-10R1, IL-22R, and IL-26R to signal in response to their respective cytokines (for a review, see reference 18). In contrast to these three IL-10 family members, STAT1 and STAT2 primarily transduce signals through the IFN-λR complex, as they do for the IFN-α/β and -γ receptors (16, 38).
To determine whether IFN-α and IFN-λ contribute to RSV-induced immunosuppression, we used MAb to one of the two chains of IFN-α/β receptor, IFNAR2 (CD118), and to the two chains of the IFN-λR complex, IL-10R2 and IL-28R, to block receptor binding. In contrast to a previous report that IFN-α is solely responsible for RSV immunosuppression (42), anti-IFNAR2 only modestly diminished immunosuppression (Fig. 7). Similarly, anti-IL10R2 or anti-IL-28R was relatively ineffective. But when anti-IFNAR2 was combined with either anti-IL-10R2 or anti-IL-28R, the level of suppression was substantially decreased, thus demonstrating synergism between the two IFN. Since type I and III IFN both signal through STAT1 and STAT2, the two IFN might synergize by surpassing a threshold of signaling that neither could achieve alone. On the other hand, the two IFN may qualitatively signal such that only when present together are unique pathways activated or blocked. To our knowledge, this is the first report that type III IFN affect T-cell function.
The dependence of RSV-induced immunosuppression on type I and III IFN is paradoxical considering that (i) IFN are expressed in response to a variety of stimuli, including many other viruses that are not associated with T-cell suppression; (ii) plasmacytoid DC may express 100-fold as much IFN-α as MDDC and yet are critical to the ultimate antiviral adaptive response (56); and (iii) type I IFN prevent activation-induced death of murine T cells in vitro after stimulation with superantigen in vivo (31). The emerging story, however, is that the effects of type I IFN on CD4 T cells are dependent on the context in which they are exposed. For example, Dondi and colleagues showed that, in the context of TcR stimulation, exogenous IFN-α is antiapoptotic early and proapoptotic later (17). Most relevant to our report, entry into the cell cycle was delayed when TcR stimulation followed IFN-α exposure (17), apparently due to sequestration by the type I IFN receptor complex of CD45, Lck, and ZAP-70 (40), all of which are necessary to initiate, but not to sustain, cellular responses to stimulation through the TcR (11). Similarly, high concentrations of IFN-α cause STAT2 to associate with STAT6 and enhance signaling through the IL-4 receptor for the first 6 h of exposure; thereafter, IFN-α inhibits IL-4 responses (20). Finally, IFN-α may activate STAT4 (36), which while insufficient to induce Th1 differentiation (7), may enhance it when induced by IL-12. It is likely that the contextual complexity of T-cell responses to IFN-α is only increased by the addition of type III IFN into the equation. The combination of type I and type III IFN could favor signaling through pathways that preferentially activate antiproliferative effects of IFN rather than those pathways that mediate the antiviral effects.
RSV has a number of strategies to minimize its vulnerability to IFN-α. The products of the NS1 and NS2 genes inhibit the upstream expression of IFN-α (8, 25, 47, 58) by suppressing activation and nuclear translocation of interferon regulatory factor 3 (8, 59) and block downstream responses by decreasing STAT2 levels (2, 44). Since cellular responses to IFN-α are complex and dependent upon context, it is reasonable to suspect that RSV may have achieved the fine balance of impairing antiviral properties of IFN-α while its suppressive activity may persist. Proliferation is not likely the only impaired T-cell response. For example, since IL-4 attenuates CD8 T-cell cytolytic function (3, 4), cytolysis may be further impaired when IFN-α enhances signaling in response to IL-4 (20). This is but one potential mechanism by which RSV may deviate T-cell responses in ways that are highly relevant to infection with RSV and its sequelae, including childhood wheezing and asthma.
Conversely, suppression of T-cell responses during RSV infection may actually offer some benefit to the host. Our model system uses MDDC and autologous CD4 T cells derived from healthy, young adult donors, a population in which RSV lower respiratory tract disease is rare (22). Since the host inflammatory response contributes significantly to lower respiratory disease (34), diminished or absent suppressive activity in infants 2 to 6 months of age (22) and the infirm elderly (21) may account for their susceptibility to RSV pneumonia.
Acknowledgments
We thank Yeqiang Li for excellent technical support.
This work was supported by CBER intramural funds and a collaborative grant from the Vaccine Research Center, NIAID. S.V.K. is supported by Public Health Services grant RO1 AI51139 from the National Institute of Allergy and Infectious Diseases and by American Heart Association grant AHA 0245131N.
The views expressed in this report are the personal opinions of the authors and are not the official opinion of the U.S. Food and Drug Administration, the National Institutes of Health, or the Department of Health and Human Services.
REFERENCES
- 1.Arnold, R., B. Konig, H. Werchau, and W. Konig. 2004. Respiratory syncytial virus deficient in soluble G protein induced an increased proinflammatory response in human lung epithelial cells. Virology 330:384-397. [DOI] [PubMed] [Google Scholar]
- 2.Atreya, P. L., and S. Kulkarni. 1999. Respiratory syncytial virus strain A2 is resistant to the antiviral effects of type I interferons and human MxA. Virology 261:227-241. [DOI] [PubMed] [Google Scholar]
- 3.Aung, S., and B. S. Graham. 2000. IL-4 diminishes perforin-mediated and increases Fas ligand-mediated cytotoxicity In vivo. J. Immunol. 164:3487-3493. [DOI] [PubMed] [Google Scholar]
- 4.Aung, S., J. A. Rutigliano, and B. S. Graham. 2001. Alternative mechanisms of respiratory syncytial virus clearance in perforin knockout mice lead to enhanced disease. J. Virol. 75:9918-9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartz, H., O. Turkel, S. Hoffjan, T. Rothoeft, A. Gonschorek, and U. Schauer. 2003. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells. Immunology 109:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckford, A. P., R. O. Kaschula, and C. Stephen. 1985. Factors associated with fatal cases of measles. A retrospective autopsy study. S. Afr. Med. J. 68:858-863. [PubMed] [Google Scholar]
- 7.Berenson, L. S., N. Ota, and K. M. Murphy. 2004. Issues in T-helper 1 development-resolved and unresolved. Immunol. Rev. 202:157-174. [DOI] [PubMed] [Google Scholar]
- 8.Bossert, B., S. Marozin, and K. K. Conzelmann. 2003. Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3. J. Virol. 77:8661-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breese Hall, C. 1998. Respiratory syncytial virus, p. 2084-3011. In R. D. Feigin and J. D. Cherry (ed.), Textbook of pediatric infectious diseases, 4th ed. Saunders, Philadelphia, Pa.
- 10.Brogden, K. A., H. D. Lehmkuhl, and R. C. Cutlip. 1998. Pasteurella haemolytica complicated respiratory infections in sheep and goats. Vet. Res. 29:233-254. [PubMed] [Google Scholar]
- 11.Chan, A. C., D. M. Desai, and A. Weiss. 1994. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu. Rev. Immunol. 12:555-592. [DOI] [PubMed] [Google Scholar]
- 12.Chin, Y. E., M. Kitagawa, W. C. Su, Z. H. You, Y. Iwamoto, and X. Y. Fu. 1996. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science 272:719-722. [DOI] [PubMed] [Google Scholar]
- 13.Coccia, E. M., M. Severa, E. Giacomini, D. Monneron, M. E. Remoli, I. Julkunen, M. Cella, R. Lande, and G. Uze. 2004. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 34:796-805. [DOI] [PubMed] [Google Scholar]
- 14.Collins, P., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1443-1485. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 15.Culley, F. J., J. Pollott, and P. J. Openshaw. 2002. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 196:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decker, T., S. Stockinger, M. Karaghiosoff, M. Muller, and P. Kovarik. 2002. IFNs and STATs in innate immunity to microorganisms. J. Clin. Investig. 109:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dondi, E., G. Roue, V. J. Yuste, S. A. Susin, and S. Pellegrini. 2004. A dual role of IFN-alpha in the balance between proliferation and death of human CD4+ T lymphocytes during primary response. J. Immunol. 173:3740-3747. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly, R. P., F. Sheikh, S. V. Kotenko, and H. Dickensheets. 2004. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J. Leukoc. Biol. 76:314-321. [DOI] [PubMed] [Google Scholar]
- 19.Dumoutier, L., A. Tounsi, T. Michiels, C. Sommereyns, S. V. Kotenko, and J. C. Renauld. 2004. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J. Biol. Chem. 279:32269-32274. [DOI] [PubMed] [Google Scholar]
- 20.Eriksen, K. W., V. H. Sommer, A. Woetmann, A. B. Rasmussen, C. Brender, A. Svejgaard, S. Skov, C. Geisler, and N. Odum. 2004. Bi-phasic effect of interferon (IFN)-alpha: IFN-alpha up- and down-regulates interleukin-4 signaling in human T cells. J. Biol. Chem. 279:169-176. [DOI] [PubMed] [Google Scholar]
- 21.Falsey, A. R., P. A. Hennessey, M. A. Formica, C. Cox, and E. E. Walsh. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749-1759. [DOI] [PubMed] [Google Scholar]
- 22.Hall, C. B. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344:1917-1928. [DOI] [PubMed] [Google Scholar]
- 23.Hall, S. L., A. Stokes, E. L. Tierney, W. T. London, R. B. Belshe, F. C. Newman, and B. R. Murphy. 1992. Cold-passaged human parainfluenza type 3 viruses contain ts and non-ts mutations leading to attenuation in rhesus monkeys. Virus Res. 22:173-184. [DOI] [PubMed] [Google Scholar]
- 24.Hallak, L. K., D. Spillmann, P. L. Collins, and M. E. Peeples. 2000. Glycosaminoglycan sulfation requirements for respiratory syncytial virus infection. J. Virol. 74:10508-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hengel, H., U. H. Koszinowski, and K. K. Conzelmann. 2005. Viruses know it all: new insights into IFN networks. Trends Immunol. 26:396-401. [DOI] [PubMed] [Google Scholar]
- 26.Keles, I., A. K. Sharma, Z. Woldehiwet, and R. D. Murray. 1999. The effects of bovine respiratory syncytial on normal ovine lymphocyte responses to mitogens or antigens in vitro. Comp. Immunol. Microbiol. Infect. Dis. 22:1-13. [DOI] [PubMed] [Google Scholar]
- 27.Kotenko, S. V., G. Gallagher, V. V. Baurin, A. Lewis-Antes, M. Shen, N. K. Shah, J. A. Langer, F. Sheikh, H. Dickensheets, and R. P. Donnelly. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69-77. [DOI] [PubMed] [Google Scholar]
- 28.Krempl, C., B. R. Murphy, and P. L. Collins. 2002. Recombinant respiratory syncytial virus with the G and F genes shifted to the promoter-proximal positions. J. Virol. 76:11931-11942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landegren, U. 1984. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J. Immunol. Methods 67:379-388. [DOI] [PubMed] [Google Scholar]
- 30.Madhi, S. A., and K. P. Klugman. 2004. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 10:811-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrack, P., J. Kappler, and T. Mitchell. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarthy, D. O., F. M. Domurat, J. E. Nichols, and N. J. Roberts, Jr. 1989. Interleukin-1 inhibitor production by human mononuclear leukocytes and leukocyte subpopulations exposed to respiratory syncytial virus: analysis and comparison with the response to influenza virus. J. Leukoc. Biol. 46:189-198. [DOI] [PubMed] [Google Scholar]
- 33.McIntosh, K. 2004. Respiratory syncytial virus, p. 1076-1079. In R. E. Behrman, R. M. Kleigman, and H. B. Jenson (ed.), Nelson textbook of pediatrics, 17th ed. Saunders, Philadelphia, Pa.
- 34.McNamara, P. S., and R. L. Smyth. 2002. The pathogenesis of respiratory syncytial virus disease in childhood. Br. Med. Bull. 61:13-28. [DOI] [PubMed] [Google Scholar]
- 35.Moss, W. J., M. O. Ota, and D. E. Griffin. 2004. Measles: immune suppression and immune responses. Int. J. Biochem. Cell. Biol. 36:1380-1385. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen, K. B., W. T. Watford, R. Salomon, S. R. Hofmann, G. C. Pien, A. Morinobu, M. Gadina, J. J. O'Shea, and C. A. Biron. 2002. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 297:2063-2066. [DOI] [PubMed] [Google Scholar]
- 37.Novelli, F., M. M. D'Elios, P. Bernabei, L. Ozmen, L. Rigamonti, F. Almerigogna, G. Forni, and G. Del Prete. 1997. Expression and role in apoptosis of the alpha- and beta-chains of the IFN-gamma receptor on human Th1 and Th2 clones. J. Immunol. 159:206-213. [PubMed] [Google Scholar]
- 38.O'Shea, J. J., M. Gadina, and R. D. Schreiber. 2002. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 109(Suppl.):S121-S131. [DOI] [PubMed] [Google Scholar]
- 39.Paton, A. W., and P. N. Goldwater. 1990. Respiratory syncytial virus modulation of adult and neonatal lymphocyte mitogenic responses and the role of interferon-gamma. Microb. Pathog. 9:235-241. [DOI] [PubMed] [Google Scholar]
- 40.Petricoin, E. F., III, S. Ito, B. L. Williams, S. Audet, L. F. Stancato, A. Gamero, K. Clouse, P. Grimley, A. Weiss, J. Beeler, D. S. Finbloom, E. W. Shores, R. Abraham, and A. C. Larner. 1997. Antiproliferative action of interferon-alpha requires components of T-cell-receptor signalling. Nature 390:629-632. [DOI] [PubMed] [Google Scholar]
- 41.Polack, F. P., P. M. Irusta, S. J. Hoffman, M. P. Schiatti, G. A. Melendi, M. F. Delgado, F. R. Laham, B. Thumar, R. M. Hendry, J. A. Melero, R. A. Karron, P. L. Collins, and S. R. Kleeberger. 2005. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc. Natl. Acad. Sci. USA 102:8996-9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preston, F. M., P. L. Beier, and J. H. Pope. 1995. Identification of the respiratory syncytial virus-induced immunosuppressive factor produced by human peripheral blood mononuclear cells in vitro as interferon-alpha. J. Infect. Dis. 172:919-926. [DOI] [PubMed] [Google Scholar]
- 43.Preston, F. M., P. L. Beier, and J. H. Pope. 1992. Infectious respiratory syncytial virus (RSV) effectively inhibits the proliferative T cell response to inactivated RSV in vitro. J. Infect. Dis. 165:819-825. [DOI] [PubMed] [Google Scholar]
- 44.Ramaswamy, M., L. Shi, M. M. Monick, G. W. Hunninghake, and D. C. Look. 2004. Specific inhibition of type I interferon signal transduction by respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 30:893-900. [DOI] [PubMed] [Google Scholar]
- 45.Roberts, N. J., Jr. 1982. Different effects of influenza virus, respiratory syncytial virus, and Sendai virus on human lymphocytes and macrophages. Infect. Immun. 35:1142-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts, N. J., Jr., A. H. Prill, and T. N. Mann. 1986. Interleukin 1 and interleukin 1 inhibitor production by human macrophages exposed to influenza virus or respiratory syncytial virus. Respiratory syncytial virus is a potent inducer of inhibitor activity. J. Exp. Med. 163:511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlender, J., B. Bossert, U. Buchholz, and K. K. Conzelmann. 2000. Bovine respiratory syncytial virus nonstructural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J. Virol. 74:8234-8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K. K. Conzelmann. 2005. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlender, J., G. Walliser, J. Fricke, and K. K. Conzelmann. 2002. Respiratory syncytial virus fusion protein mediates inhibition of mitogen-induced T-cell proliferation by contact. J. Virol. 76:1163-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider-Schaulies, S., K. Bieback, E. Avota, I. Klagge, and V. ter Meulen. 2002. Regulation of gene expression in lymphocytes and antigen-presenting cells by measles virus: consequences for immunomodulation. J. Mol. Med. 80:73-85. [DOI] [PubMed] [Google Scholar]
- 51.Sharma, R., and Z. Woldehiwet. 1991. Depression of lymphocyte responses to phytohaemagglutinin in lambs experimentally infected with bovine respiratory syncytial virus. Res. Vet. Sci. 50:152-156. [DOI] [PubMed] [Google Scholar]
- 52.Sharma, R., and Z. Woldehiwet. 1990. Increased susceptibility to Pasteurella haemolytica in lambs infected with bovine respiratory syncytial virus. J. Comp. Pathol. 103:411-420. [DOI] [PubMed] [Google Scholar]
- 53.Sheppard, P., W. Kindsvogel, W. Xu, K. Henderson, S. Schlutsmeyer, T. E. Whitmore, R. Kuestner, U. Garrigues, C. Birks, J. Roraback, C. Ostrander, D. Dong, J. Shin, S. Presnell, B. Fox, B. Haldeman, E. Cooper, D. Taft, T. Gilbert, F. J. Grant, M. Tackett, W. Krivan, G. McKnight, C. Clegg, D. Foster, and K. M. Klucher. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63-68. [DOI] [PubMed] [Google Scholar]
- 54.Sieg, S., C. King, Y. Huang, and D. Kaplan. 1996. The role of interleukin-10 in the inhibition of T-cell proliferation and apoptosis mediated by parainfluenza virus type 3. J. Virol. 70:4845-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sieg, S., C. Muro-Cacho, S. Robertson, Y. Huang, and D. Kaplan. 1994. Infection and immunoregulation of T lymphocytes by parainfluenza virus type 3. Proc. Natl. Acad. Sci. USA 91:6293-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 57.Sigurs, N., R. Bjarnason, F. Sigurbergsson, and B. Kjellman. 2000. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 161:1501-1507. [DOI] [PubMed] [Google Scholar]
- 58.Spann, K. M., K. C. Tran, B. Chi, R. L. Rabin, and P. L. Collins. 2004. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages. J. Virol. 78:4363-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spann, K. M., K. C. Tran, and P. L. Collins. 2005. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J. Virol. 79:5353-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamaguchi, N., Y. Fujimori, Y. Fujibayashi, I. Kasumoto, H. Okamura, K. Nakanishi, and H. Hara. 2005. Interferon-gamma production by human cord blood monocyte-derived dendritic cells. Ann. Hematol. 84:423-428. [DOI] [PubMed] [Google Scholar]
- 61.Zeh, H. J., III, S. Hurd, W. J. Storkus, and M. T. Lotze. 1993. Interleukin-12 promotes the proliferation and cytolytic maturation of immune effectors: implications for the immunotherapy of cancer. J. Immunother. 14:155-161. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, L., M. E. Peeples, R. C. Boucher, P. L. Collins, and R. J. Pickles. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 76:5654-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]