Abstract
Here, we present the genome sequence, with analysis, of a poxvirus infecting Nile crocodiles (Crocodylus niloticus) (crocodilepox virus; CRV). The genome is 190,054 bp (62% G+C) and predicted to contain 173 genes encoding proteins of 53 to 1,941 amino acids. The central genomic region contains genes conserved and generally colinear with those of other chordopoxviruses (ChPVs). CRV is distinct, as the terminal 33-kbp (left) and 13-kbp (right) genomic regions are largely CRV specific, containing 48 unique genes which lack similarity to other poxvirus genes. Notably, CRV also contains 14 unique genes which disrupt ChPV gene colinearity within the central genomic region, including 7 genes encoding GyrB-like ATPase domains similar to those in cellular type IIA DNA topoisomerases, suggestive of novel ATP-dependent functions. The presence of 10 CRV proteins with similarity to components of cellular multisubunit E3 ubiquitin-protein ligase complexes, including 9 proteins containing F-box motifs and F-box-associated regions and a homologue of cellular anaphase-promoting complex subunit 11 (Apc11), suggests that modification of host ubiquitination pathways may be significant for CRV-host cell interaction. CRV encodes a novel complement of proteins potentially involved in DNA replication, including a NAD+-dependent DNA ligase and a protein with similarity to both vaccinia virus F16L and prokaryotic serine site-specific resolvase-invertases. CRV lacks genes encoding proteins for nucleotide metabolism. CRV shares notable genomic similarities with molluscum contagiosum virus, including genes found only in these two viruses. Phylogenetic analysis indicates that CRV is quite distinct from other ChPVs, representing a new genus within the subfamily Chordopoxvirinae, and it lacks recognizable homologues of most ChPV genes involved in virulence and host range, including those involving interferon response, intracellular signaling, and host immune response modulation. These data reveal the unique nature of CRV and suggest mechanisms of virus-reptile host interaction.
Crocodile poxviruses (CRV) and caiman poxviruses are uncharacterized and unclassified members of the subfamily Chordopoxvirinae in the family Poxviridae that infect host species of the order Crocodylia worldwide (55, 56, 59, 84, 88, 90, 97). Similarly, uncharacterized poxviruses infecting other reptiles, including Hermann's tortoise, wild flap-necked chameleon, and lizards, have been described (60, 87, 117). Morphologically, CRV and caiman poxvirus virions are brick shaped with rounded corners and have particle dimensions and a dumbbell-shaped central core with lateral bodies similar to orthopoxvirus virions; however, they also exhibit a regular, crisscross surface structure pattern characteristic of parapoxvirus virions (42, 59, 88, 90, 97).
CRV and caiman poxviruses are significant pathogens which cause economic losses on crocodile farms in Southeast Asia, Australia, and southern Africa and on caiman farms in South America, largely by affecting hatchlings and smaller yearlings (55, 56, 59, 90). Poxvirus infection reported by a Brazilian farm affected 15% of 5- to 9-month-old Brazilian caimans, while adults remained asymptomatic (97). Poxvirus infections have been associated with 3.4% of all skin lesions in Australian farmed crocodiles (Crocodylus porosus and Crocodylus johnstoni) (16). In a CRV outbreak of 9-month-old Nile crocodiles (Crocodylus niloticus), 40% presented with clinical disease (52). On a Zambian farm of 4,000 hatchling to 5-year-old animals, 300 yearlings were affected and exhibited nodular skin lesions and complications resulting in 27% mortality (88). CRV infections in Nile crocodiles currently occur on farms in Zimbabwe (D. Wallace, personal communication). Although infections with CRV have not yet been reported in the wild, the prevalence of these viruses in diverse crocodilian populations worldwide and their ability to cause significant disease suggest that crocodilians are natural hosts.
CRV causes a disease that varies from a nonfatal dermatitis with complete recovery to more severe disease characterized by ophthalmia, rhinitis resulting in asphyxia, and debilitating illness with stunting and high mortality (52, 56, 88). In one CRV disease outbreak (52), yellow or brownish mucocutaneous lesions that varied from flat spots of 2 to 3 mm in diameter to nodules of 8 mm, and occasionally shallow ulcers, were common on the sides of the mouth, eyelids, nostrils, ventral aspects of the neck, sides of the body, belly, and limbs and were less common in the oral cavity and on other external parts of the body. Histopathologically, skin lesions exhibited large focal areas of severe hyperkeratosis and parakeratosis and cells containing intracytoplasmic inclusions.
Although complete genomic sequences have been determined for representative, and often multiple, viruses from classified chordopoxvirus (ChPV) genera infecting mammalian or avian hosts (Capripoxvirus [124, 125], Leporipoxvirus [17, 128], Molluscipoxvirus [106], Orthopoxvirus [5, 7, 24, 44, 49, 76, 108, 109], Parapoxvirus [30], Suipoxvirus [4], Yatapoxvirus [14, 68], and Avipoxvirus [3, 123]), the CRV genome has not been characterized. Reptiles, considered to be the most immediate ancestor of birds and mammals, are poikilothermic animals with immune systems that remain relatively unstudied (13, 32). Analysis of the CRV genome may reveal genes that suggest novel mechanisms of poxvirus host range and virus-reptile host interaction. Here, we describe the complete genome sequence, with analysis, of CRV, identifying both its divergent nature relative to characterized ChPVs and novel genes likely affecting aspects of CRV replication and virus-host interactions.
MATERIALS AND METHODS
Virus isolation and purification.
Lesion material was obtained from a 6-month-old Nile crocodile (Crocodylus niloticus) during a disease outbreak on the Ume crocodile farm at Lake Kariba in Zimbabwe. The animal presented with generalized skin lesions (5 mm in diameter and 1 to 2 mm high) on the belly and sides of the tail. The lesion material was ground with mortar and pestle in McIlvains's hypotonic buffer and clarified by low-speed centrifugation (450 × g for 5 min) in 1% Triton X-100 and 2.6 μl β-mercaptoethanol/ml. Semipurified virus was obtained by centrifugation (19,000 × g, 60 min) through 36% and 20% sucrose cushions.
DNA isolation, cloning, and sequencing.
Semipurified virions were disrupted with 1% sodium dodecyl sulfate and 100 μg/ml proteinase K as described by others (77), and DNA was extracted and precipitated using standard protocols (102). Random DNA fragments were obtained by incomplete enzymatic digestion with AciI and TaqI endonucleases (New England BioLabs, Beverly, MA), and DNA fragments larger than 1.0 kbp were cloned and used in dideoxy sequencing reactions as previously described (3). Reaction products were resolved by using an ABI PRISM 3730xl automated DNA sequencer (Applied Biosystems, Foster City, CA). Sequence data were assembled with the Phrap and CAP3 software programs (http://www.phrap.org) (54), and sequence gaps were closed as described previously (3). Final DNA consensus sequences represented, on average, 10-fold redundancy at each base position, with a Consed estimated error rate of less than 0.01 error per 10 kbp (45).
Genome analysis.
Genome DNA composition, structure, repeats, and restriction enzyme patterns were analyzed as previously described (2) using the GCG v.10 software package and EMBOSS programs (31, 99). The concatemer resolution motif was identified using alignments of known poxvirus resolution motifs (80) and predicted secondary structure (78) using the computer programs CMBUILD and CMSEARCH (35). Briefly, known concatemer resolution motifs (80) were used to conduct a FASTA (89) search against the GenBank database, and all identified motifs were aligned with the program Pileup (31) to obtain a consensus. A secondary stem loop structure for the consensus was predicted using the program FoldRNA (31). A hidden Markov model was constructed from the alignment and was used to search a complete viral database that included CRV genomic sequences using HMMSEARCH and CMSEARCH (34). Once CRV concatemer sequences and score distribution values were obtained, a covariance model from predicted secondary structures of multiple alignment, CRV, and consensus sequences was built (35). Only terminally located concatemer resolution sequence-like motifs were identified for other poxviruses, indicating the specificity of the prediction.
Open reading frames (ORFs) longer than 30 codons were evaluated for coding potential as previously described (3) and by using Framefinder (http://www.ebi.ac.uk/∼guy/estate/). ORFs 30 to 60 codons in length with coding potential and 408 ORFs greater than 60 codons were subjected to similarity searches as previously described, with additional searches against UniGene, Pfam, TIGRFAMs, and SMART databases (2, 3, 39). Here, 173 ORFs were annotated as potential genes and numbered from left to right. Given the predicted nature of all CRV genes and gene products, ORF names were used throughout the text to indicate both the predicted gene and its putative protein product. Multiple genomic and protein alignments were done using Dialign (81), Dialign-T (118), Clustal (121), MUSCLE (36), and/or Kalign (67) programs. Phylogenetic analysis was performed using PHYLO_WIN, TREE-PUZZLE, IQPNNI, PHYLIP, PHYML, and MRBAYES software packages, and ProtTest was used to select evolutionary models (1, 38, 40, 50, 57, 104, 126). Additional analyses were conducted for alignments in which poorly aligned regions were removed with Gblocks (21). Supertree analysis of neighbor-joining, maximum parsimony, and maximum likelihood trees from individually aligned protein homologues was performed using Puzzling (http://bioinf.may.ie/software/puzzling/index.html). F-box motif searches were done with hidden Markov models using the program HMMER (34) and publicly available matrixes (SCOP v1.69 [46] and Pfam PF00646).
Nucleotide sequence accession number.
The CRV genome has been deposited in GenBank under accession number DQ356948.
RESULTS AND DISCUSSION
Organization of the CRV genome.
The genome of Nile crocodile poxvirus (CRV) is 190,054 bp and contains an overall nucleotide composition of 62% C+G. Notably, an island of lower G+C content (50% over 5,864 bp) encoding a novel gene family of unknown function (CRV033, CRV034, and CRV035) is located between nucleotide positions 27796 and 33659. As terminal hairpin loops were not sequenced, the left-most nucleotide of the assembled genome was arbitrarily designated base 1. A putative poxvirus concatemer resolution motif was identified only at positions 50 to 68 from each terminus, suggesting that the assembled sequence contains all but the most terminal 50 to 100 bp of the CRV genome (Fig. 1). The CRV genome contains a large unique coding region bounded by two identical inverted terminal repeat (ITR) regions of 1,754 bp. ITRs contain three 113-bp tandem repeats that share approximately 92% nucleotide identity with each other and are located between positions 719 and 1057 within the left ITR and between positions 188998 and 189336 within the right ITR. No additional repeats were detected in terminal genomic regions.
FIG. 1.
Alignment of the putative CRV concatemer resolution sequence region with those of other poxviruses. Genomic positions are indicated on the right; the putative concatemer resolution sequence is underlined. GenBank accession numbers are as follows: rabbit fibroma virus, AF170722; myxoma virus, AF170726; Yaba-like disease virus, YDI293568; sheeppox virus, M28823; fowlpox virus, AJ581527; swinepox virus, AF410153; vaccinia virus, AY243312; cowpox virus, AF482758.
One hundred-seventy three ORFs were annotated here as potential genes, encoding proteins of 53 to 1,941 amino acids in length and representing a coding density of 94%. The central genomic region (CRV036 to CRV158) contains conserved poxvirus genes involved in basic replicative mechanisms (RNA transcription and modification and DNA replication), in virion structure, and in assembly of intracellular mature, intracellular enveloped, and extracellular enveloped virions (IMV, IEV, and EEV, respectively) (Table 1) (82). Terminal genomic regions (CRV001 to CRV035 and CRV159 to CRV173) largely contain genes of unknown function, including one gene duplicated in the ITRs and 12 genes that comprise two novel gene families. Sixty-two CRV genes lack recognizable homologues in other poxviruses. Of these, 33 are located in the left-terminal region, 15 are located in the right-terminal region, and 14 are present in regions that disrupt colinearity within the core of conserved ChPV genes (Table 1). Notably, a majority of the CRV genes present in six gene families were novel, as only seven of 29 resembled genes found in other poxviruses. Forty-four of these unique CRV genes are completely novel, with predicted products lacking similarity to all known proteins or protein domains.
TABLE 1.
CRV ORFs
| ORF | Position (length [aa])a | Best matchb
|
Predicted structure and/or function | ORF homologuef
|
||||
|---|---|---|---|---|---|---|---|---|
| Accession no.c | Species | Scored | % Id.e | MOCV | VACV | |||
| CRV001 | 396-629 (78) | |||||||
| CRV002 | 3453-1174 (760) | |||||||
| CRV003 | 4562-3576 (329) | |||||||
| CRV004 | 6189-4738 (484) | |||||||
| CRV005 | 7034-6309 (242) | |||||||
| CRV006 | 7414-7211 (68) | |||||||
| CRV007 | 7679-7479 (67) | |||||||
| CRV008 | 9316-7823 (498) | |||||||
| CRV009 | 9935-9369 (189) | AF428140 | Homo sapiens | 99 | 32 | F-box domain protein | ||
| CRV010 | 10793-9966 (276) | AE003835 | Drosophila melanogaster | 98 | 38 | F-box domain protein | ||
| CRV011 | 11511-10717 (265) | BC012385 | Homo sapiens | 113 | 44 | F-box domain protein | ||
| CRV012 | 12101-11514 (196) | BC012385 | Homo sapiens | 98 | 51 | F-box domain protein | ||
| CRV013 | 12860-12186 (225) | BC012385 | Homo sapiens | 104 | 39 | F-box domain protein | ||
| CRV014 | 13491-12904 (196) | AY007380 | Oryza sativa | 107 | 40 | F-box domain protein | ||
| CRV015 | 14185-13568 (206) | AE002690 | Drosophila melanogaster | 118 | 38 | F-box domain protein | ||
| CRV016 | 15613-14282 (444) | |||||||
| CRV017 | 16212-15703 (170) | |||||||
| CRV018 | 16815-16300 (172) | |||||||
| CRV019 | 17395-16910 (162) | |||||||
| CRV020 | 17881-17477 (135) | |||||||
| CRV021 | 19113-17800 (438) | L22174 | Gallid herpesvirus 2 | 177 | 38 | SORF2 domain protein | ||
| CRV022 | 20308-19403 (302) | |||||||
| CRV023 | 21309-20395 (305) | |||||||
| CRV024 | 21975-22205 (77) | |||||||
| CRV025 | 22584-22216 (123) | |||||||
| CRV026 | 23363-22611 (251) | AC114983 | Oryza sativa | 122 | 36 | F-box domain protein | ||
| CRV027 | 24125-23382 (248) | |||||||
| CRV028 | 25327-24380 (316) | |||||||
| CRV029 | 25892-25515 (126) | |||||||
| CRV030 | 26679-25993 (229) | 312 | 35 | J-domain protein | MC013L | |||
| CRV031 | 26857-26660 (66) | |||||||
| CRV032 | 27712-26963 (250) | |||||||
| CRV033 | 29408-27981 (476) | |||||||
| CRV034 | 31619-29556 (688) | |||||||
| CRV035 | 33591-31948 (548) | |||||||
| CRV036 | 34288-33650 (213) | 407 | 46 | MC016L | F9L | |||
| CRV037 | 35662-34292 (457) | 1122 | 50 | Ser/Thr protein kinase | MC017L | F10L | ||
| CRV038 | 37738-35675 (688) | 384 | 28 | IEV protein | MC019L | F12L | ||
| CRV039 | 38905-37793 (371) | 684 | 44 | Palmitylated EEV envelope lipase | MC021L | F13L | ||
| CRV040 | 44775-39052 (1908) | L22579 | Variola virus | 1237 | 30 | VARV B22R-like protein | MC035R | |
| CRV041 | 50525-44904 (1874) | 1179 | 28 | VARV B22R-like protein | MC035R | |||
| CRV042 | 56371-58035 (555) | AY070554 | Drosophila melanogaster | 102 | 28 | RING-like motif protein | ||
| CRV043 | 56486-50664 (1941) | 1318 | 32 | VARV B22R-like protein | MC035R | |||
| CRV044 | 58267-58025 (81) | |||||||
| CRV045 | 58348-60159 (604) | AY532606 | Rock bream iridovirus | 114 | 29 | RING-like motif protein | ||
| CRV046 | 61179-60535 (215) | AY318871 | Canarypox virus | 246 | 35 | MC025L | F15L | |
| CRV047 | 61457-61215 (81) | AY340984 | Squirrel parapoxvirus | 115 | 30 | Apc11-like protein | MC026L | |
| CRV048 | 63339-61411 (643) | |||||||
| CRV049 | 64738-63293 (482) | |||||||
| CRV050 | 65456-64734 (241) | 109 | 24 | MC028L | ||||
| CRV051 | 66163-65477 (229) | AF426834 | Staphylococcus epidermidis Tn552 | 99 | 30 | Site-specific recombinase-like protein | MC029L | F16L |
| CRV052 | 67293-66214 (360) | AY653733 | Acanthamoeba polyphaga mimivirus | 126 | 31 | NAD+-dependent DNA ligase | ||
| CRV053 | 67365-67715 (117) | AX754989 | Orf virus | 144 | 34 | DNA-binding virion core protein | MC030R | F17R |
| CRV054 | 69126-67708 (473) | AF198100 | Fowlpox virus | 940 | 41 | Poly(A) polymerase large subunit | MC031L | E1L |
| CRV055 | 71484-69154 (777) | 338 | 28 | E2L/O1L-like protein | MC032L | E2L | ||
| CRV056 | 72065-73762 (566) | 1295 | 43 | MC037R | E6R | |||
| CRV057 | 72066-71503 (188) | AY318871 | Canarypox virus | 567 | 58 | RNA polymerase subunit RP030 | MC034L | E4L |
| CRV058 | 73770-76151 (794) | AF170726 | Myxoma virus | 674 | 48 | Virion core protein | MC038R | E8R |
| CRV059 | 79159-76196 (988) | 2554 | 51 | DNA polymerase | MC039L | E9L | ||
| CRV060 | 79194-79484 (97) | AJ581527 | Fowlpox virus | 281 | 48 | IMV redox protein | MC040R | E10R |
| CRV061 | 79851-79468 (128) | 151 | 33 | Virion core protein | MC041L | E11L | ||
| CRV062 | 82021-79841 (727) | 560 | 29 | E2L/OIL-like protein | MC042L | O1L | ||
| CRV063 | 84329-82035 (765) | 186 | 27 | E2L/OIL-like protein | MC042L | O1L | ||
| CRV064 | 86716-84452 (755) | X94355 | Cowpox virus | 112 | 23 | E2L/OIL-like protein | MC042L | E2L |
| CRV065 | 87777-86851 (309) | AY318871 | Canarypox virus | 856 | 54 | DNA-binding virion core protein | MC044L | I1L |
| CRV066 | 87983-87789 (65) | AY386265 | Bovine papular stomatitis virus | 120 | 50 | MC045L | I2L | |
| CRV067 | 88709-87990 (240) | AF438165 | Camelpox virus | 176 | 28 | DNA-binding phosphoprotein | MC046L | I3L |
| CRV068 | 88962-88726 (79) | P18521 | Fowlpox virus | 152 | 35 | IMV membrane protein | MC047L | I5L |
| CRV069 | 90129-88966 (388) | E48563 | Fowlpox virus | 571 | 32 | Telomere binding protein | MC048L | I6L |
| CRV070 | 91421-90129 (431) | 1064 | 50 | Virion core proteinase | MC049L | I7L | ||
| CRV071 | 91427-93448 (674) | 1685 | 51 | RNA helicase NPH-II | MC050R | I8R | ||
| CRV072 | 95364-93466 (633) | 1322 | 47 | Metalloprotease | MC056L | G1L | ||
| CRV073 | 95681-95364 (106) | 97 | 37 | MC057L | G3L | |||
| CRV074 | 95692-96390 (233) | 276 | 37 | Transcriptional elongation factor | MC058R | G2R | ||
| CRV075 | 96686-96339 (116) | AF198100 | Fowlpox virus | 131 | 24 | Glutaredoxin 2 | MC059L | G4L |
| CRV076 | 96689-98053 (455) | 546 | 36 | Virion core protein | MC060R | G5R | ||
| CRV077 | 98034-98222 (63) | AF198100 | Fowlpox virus | 236 | 68 | RNA polymerase subunit RPO7 | MC061R | G5.5R |
| CRV078 | 98237-98818 (194) | 351 | 48 | MC062R | G6R | |||
| CRV079 | 99883-98852 (344) | AF170726 | Myxoma virus | 378 | 31 | Virion core protein | MC065L | G7L |
| CRV080 | 99914-100687 (258) | AF170722 | Rabbit fibroma virus | 754 | 55 | Late transcription factor VLTF-1 | MC067R | G8R |
| CRV081 | 100703-101686 (328) | P15909 | Fowlpox virus | 614 | 37 | Myristylated protein | MC068R | G9R |
| CRV082 | 101690-102442 (251) | AF198100 | Fowlpox virus | 727 | 56 | Myristylated IMV envelope protein | MC069R | L1R |
| CRV083 | 102510-102776 (89) | AF482758 | Cowpox virus | 105 | 36 | MC070R | L2R | |
| CRV084 | 103047-104528 (494) | P35886 | Streptomyces coelicolor | 136 | 28 | GyrB-like ATPase domain protein | ||
| CRV085 | 104673-106115 (481) | P30182 | Arabidopsis thaliana | 77 | 24 | GyrB-like ATPase domain protein | ||
| CRV086 | 106180-107355 (392) | AB110283 | Trichophyton verrucosum | 110 | 28 | GyrB-like ATPase domain protein | ||
| CRV087 | 107445-108695 (417) | AB049145 | Pichia guilliermondii | 123 | 23 | GyrB-like ATPase domain protein | ||
| CRV088 | 108890-110134 (415) | AE010515 | Fusobacterium nucleatum | 127 | 28 | GyrB-like ATPase domain protein | ||
| CRV089 | 110183-111709 (509) | P30182 | Arabidopsis thaliana | 91 | 28 | GyrB-like ATPase domain protein | ||
| CRV090 | 111854-114904 (1017) | CNS07EGB | Encephalitozoon cuniculi | 250 | 23 | GyrB-like ATPase domain protein | ||
| CRV091 | 114900-115190 (97) | 60 | 39 | MC071R | ||||
| CRV092 | 116070-115165 (302) | AY318871 | Canarypox virus | 612 | 42 | MC072L | L3L | |
| CRV093 | 116097-116831 (245) | AY318871 | Canarypox virus | 410 | 36 | DNA-binding virion core protein | MC073R | L4R |
| CRV094 | 116827-117216 (130) | AY386265 | Bovine papular stomatitis virus | 207 | 40 | IMV membrane protein | MC074R | L5R |
| CRV095 | 117182-117649 (156) | 257 | 44 | IMV membrane protein | MC075R | J1R | ||
| CRV096 | 117667-118548 (294) | 820 | 56 | Poly(A) polymerase small subunit | MC076R | J3R | ||
| CRV097 | 118585-119151 (189) | 510 | 49 | RNA polymerase subunit RPO22 | MC077R | J4R | ||
| CRV098 | 119536-119141 (132) | M17418 | Fowlpox virus | 359 | 45 | MC078L | J5L | |
| CRV099 | 119586-123452 (1289) | 4673 | 67 | RNA polymerase subunit RPO147 | MC079R | J6R | ||
| CRV100 | 123495-123719 (75) | 69 | 31 | MC081R | ||||
| CRV101 | 124106-124804 (233) | 547 | 55 | IMV membrane protein | MC083R | H2R | ||
| CRV102 | 124206-123706 (167) | P33064 | Variola virus | 357 | 44 | Tyr/Ser protein phosphatase | MC082L | H1L |
| CRV103 | 125614-124781 (278) | AY318871 | Canarypox virus | 241 | 24 | IMV envelope protein | MC084L | H3L |
| CRV104 | 128017-125618 (800) | AF380138 | Monkeypox virus | 1951 | 47 | RNA-polymerase-associated protein | MC085L | H4L |
| CRV105 | 128136-128504 (123) | AY386264 | Orf virus | 88 | 32 | Late transcription factor VLTF-4 | MC086R | H5R |
| CRV106 | 128504-129475 (324) | AF198100 | Fowlpox virus | 881 | 52 | DNA topoisomerase IB | MC087R | H6R |
| CRV107 | 129438-129863 (142) | 123 | 34 | MC088R | H7R | |||
| CRV108 | 130203-129847 (119) | 69 | 25 | MC089L | ||||
| CRV109 | 130219-132762 (848) | 2241 | 56 | mRNA capping enzyme large subunit | MC090R | D1R | ||
| CRV110 | 133117-133992 (292) | 178 | 32 | Virion core protein | MC092R | D3R | ||
| CRV111 | 133118-132765 (118) | 147 | 42 | Virion core protein | MC091L | D2L | ||
| CRV112 | 133928-134602 (225) | AY386263 | Orf virus | 634 | 49 | Uracil DNA glycosylase | MC093R | D4R |
| CRV113 | 134666-137008 (781) | AY318871 | Canarypox virus | 2022 | 49 | NTPase, DNA replication | MC094R | D5R |
| CRV114 | 137008-138906 (633) | 2442 | 73 | Early transcription factor small subunit | MC095R | D6R | ||
| CRV115 | 138896-139390 (165) | 443 | 52 | RNA polymerase subunit RPO18 | MC097R | D7R | ||
| CRV116 | 139425-140120 (232) | AY318871 | Canarypox virus | 429 | 43 | MutT motif | MC098R | D9R |
| CRV117 | 140080-140829 (250) | 239 | 31 | MutT motif | MC099R | D10R | ||
| CRV118 | 142709-140832 (626) | S42251 | Fowlpox virus | 1813 | 53 | NPH-1, transcription termination | MC100R | D11L |
| CRV119 | 143578-142712 (289) | 829 | 54 | mRNA capping enzyme small subunit | MC101L | D12L | ||
| CRV120 | 145205-143574 (544) | 1503 | 52 | Rifampin resistance protein | MC102L | D13L | ||
| CRV121 | 145665-145228 (146) | AY318871 | Canarypox virus | 306 | 41 | Late transcription factor VLTF-2 | MC103L | A1L |
| CRV122 | 146386-145712 (225) | AY318871 | Canarypox virus | 832 | 68 | Late transcription factor VLTF-3 | MC104L | A2L |
| CRV123 | 146592-146386 (69) | AF198100 | Fowlpox virus | 115 | 36 | Virion redox protein | MC105L | A2.5L |
| CRV124 | 148623-146599 (675) | 1617 | 49 | Virion core protein P4b | MC106L | A3L | ||
| CRV125 | 148942-148673 (90) | Virion core protein | MC107L | A4L | ||||
| CRV126 | 148982-149455 (158) | 405 | 54 | RNA polymerase subunit RPO19 | MC108R | A5R | ||
| CRV127 | 151081-149492 (530) | 692 | 36 | MC109L | A6L | |||
| CRV128 | 153193-151097 (699) | AF198100 | Fowlpox virus | 2202 | 58 | Early transcription factor large subunit | MC110L | A7L |
| CRV129 | 153237-154112 (292) | AF170726 | Myxoma virus | 479 | 39 | Intermediate transcription factor VITF-3 | MC111R | A8R |
| CRV130 | 154313-154092 (74) | 195 | 52 | IMV membrane protein | MC112L | A9L | ||
| CRV131 | 157057-154334 (908) | 1525 | 37 | Virion core protein P4a | MC113L | A10L | ||
| CRV132 | 157072-158016 (315) | 545 | 40 | Nonstructural protein | MC114R | A11R | ||
| CRV133 | 158505-158086 (140) | 216 | 41 | Virion core protein | MC115L | A12L | ||
| CRV134 | 159194-158814 (127) | 42 | 47 | MC116R | ||||
| CRV135 | 159439-159239 (67) | 95 | 31 | IMV membrane protein | MC117L | A13L | ||
| CRV136 | 159730-159443 (96) | 177 | 41 | IMV membrane protein | MC118L | A14L | ||
| CRV137 | 159909-159751 (53) | 127 | 47 | IMV membrane protein | MC119L | A14.5 | ||
| CRV138 | 160193-159909 (95) | AY386263 | Orf virus | 59 | 48 | Virion core protein | MC120L | A15L |
| CRV139 | 161277-160114 (388) | 823 | 48 | Myristylated membrane protein | MC121L | A16L | ||
| CRV140 | 161823-161299 (175) | P68592 | Vaccinia virus | 163 | 31 | IMV membrane protein | MC122L | A17L |
| CRV141 | 161828-163204 (459) | AF198100 | Fowlpox virus | 1140 | 49 | DNA helicase, transcription elongation | MC123R | A18R |
| CRV142 | 163451-163188 (88) | AF198100 | Fowlpox virus | 123 | 36 | MC124L | A19L | |
| CRV143 | 163765-165096 (444) | 243 | 27 | DNA polymerase processivity factor | MC126R | A20R | ||
| CRV144 | 163766-163455 (104) | 226 | 47 | IMV membrane protein | MC125L | A21L | ||
| CRV145 | 165044-165523 (160) | 244 | 41 | Holliday junction resolvase | MC127R | A22R | ||
| CRV146 | 165531-166679 (383) | 882 | 49 | Intermediate transcription factor VITF-3 | MC128R | A23R | ||
| CRV147 | 166707-170153 (1149) | 4399 | 71 | RNA polymerase subunit RPO132 | MC129R | A24R | ||
| CRV148 | 171565-170228 (446) | AF198100 | Fowlpox virus | 137 | 22 | IMV A type inclusion-like protein P4c | MC133L | A27L |
| CRV149 | 171985-171569 (139) | 391 | 51 | IMV membrane protein | MC134L | A28L | ||
| CRV150 | 172897-171989 (303) | 541 | 39 | RNA polymerase subunit RPO35 | MC135L | A29L | ||
| CRV151 | 173075-172863 (71) | 99 | 36 | Virion core protein | MC136L | A30L | ||
| CRV152 | 173175-173507 (111) | AF198100 | Fowlpox virus | 182 | 29 | MC138R | A31R | |
| CRV153 | 173519-173875 (119) | 74 | 25 | MC139R | ||||
| CRV154 | 174596-173850 (249) | 622 | 49 | ATPase, DNA packaging | MC140L | A32L | ||
| CRV155 | 174649-175326 (226) | |||||||
| CRV156 | 175209-175661 (151) | |||||||
| CRV157 | 175674-176054 (127) | |||||||
| CRV158 | 176306-176791 (162) | AF380138 | Monkeypox virus | 98 | 23 | EEV envelope protein | MC143R | A34R |
| CRV159 | 176835-177158 (108) | |||||||
| CRV160 | 177231-177980 (250) | |||||||
| CRV161 | 177970-178368 (133) | |||||||
| CRV162 | 178454-178741 (96) | |||||||
| CRV163 | 178795-180225 (477) | |||||||
| CRV164 | 180365-180697 (111) | |||||||
| CRV165 | 180979-181680 (234) | KDEL motif | ||||||
| CRV166 | 181750-182316 (189) | |||||||
| CRV167 | 182779-183597 (273) | AF176524 | Mus musculus | 111 | 51 | F-box domain protein | ||
| CRV168 | 184231-186165 (645) | |||||||
| CRV169 | 186333-186863 (177) | |||||||
| CRV170 | 187094-187690 (199) | |||||||
| CRV171 | 187753-188211 (153) | |||||||
| CRV172 | 188256-189398 (381) | |||||||
| CRV173 | 189659-189426 (78) | |||||||
aa, amino acids.
Best-matching protein sequence from Blast2 analysis of nonredundant protein database.
GenBank or SwissProt database accession number.
Blast2 score. Scores in boldface type indicate best matches to MOCV homologues, and italics indicate scores generated in searches against a MOCV database.
% Id., percent amino acid identity in local match. Values in boldface type are matches against MOCV homologues.
Notable CRV genes. (i) CRV ubiquitin ligase-related genes.
CRV encodes multiple proteins which may have functions associated with ubiquitin ligase (E3) enzyme components of the ubiquitin proteolytic system, a conserved system which selectively targets proteins for degradation to affect many critical cellular functions (51, 93). E3 enzymes function to recruit ubiquitin conjugating (E2) enzymes to specific ubiquitination substrates. The multitude of cellular E3 enzymes identified, including both multisubunit complexes and single subunit enzymes, reflects the array of cellular processes controlled through the ubiquitin proteolytic system. CRV encodes 10 proteins with features similar to components of Skp1-Cul1-F box (SCF1) and anaphase-promoting complex/cyclosome (APC/C) E3 ligase complexes, including 9 proteins containing F-box motifs and F-box-associated regions and 1 homologue of APC/C subunit 11 (Apc11). CRV also encodes two novel proteins containing motifs similar to C3HC4 RING fingers characteristic of single-subunit E3 enzymes.
Nine genes (CRV009 to CRV015, CRV026, and CRV167) comprise the largest CRV gene family. Their predicted proteins contain a 44-amino-acid F-box motif and conserved residues at the amino terminus (Table 1; Fig. 2) (65). In addition, these proteins contain an adjacent region resembling the F-box-associated region present in a subset of eukaryotic F-box-containing proteins (58). Seven CRV F-box proteins (CRV009 to CRV015), ranging in size from 189 to 276 amino acids, are located in tandem in the left-terminal genomic region. However, they share only limited amino acid identity outside the F-box domain (26 to 32%). Notably, CRV167 lacks any similarity to other family members outside the amino-terminal F-box region.
FIG. 2.
Alignment of CRV and cellular F-box proteins. The highlighted, boldface, and capital letters indicate residues similar to a previously described 234 F-box consensus (65). Residues in boldface type are present in >40% of this previously described data set, those highlighted are present in 20 to 40%, and those in capital letters are present in greater than 10%. Amino acid positions are indicated on the right. F-box protein sequence names correspond to the following GenBank accession numbers: rat, Q7TSL3; mouse, Q9QZM8; and human, Q96EF6.
F-box proteins are critical components of the conserved SCF E3 complex, in which any one of a large number of interchangeable F-box proteins serve as substrate-specificity/recognition subunits (20, 27, 91). Substrate specificity is dictated by the bipartite structure of the F-box protein, with the conserved F-box motif binding to Skp1 adapter protein in the core complex and a divergent protein-protein interaction motif selectively binding the cognate substrate to be recruited for ubiquitination. Sequence diversity among the many currently identified F-box proteins suggests that SCF-dependent proteolysis affects numerous substrates and cellular pathways; indeed, F-box proteins have roles ranging from nutrient sensing in yeast to developmental and cell cycle pathways in plants and animals and, recently, regulation of the inhibitor of apoptosis protein (IAP1) in Drosophila spp. (20, 27, 105). CRV F-box proteins may have a similar role in ubiquitination, with variability among CRV proteins conceivably resulting in multiple target substrates affecting multiple cellular pathways.
Given the presence of F-box-like proteins in CRV, ORFs from other ChPVs were examined for similar motifs. Notably, a shorter (32-amino-acid), carboxyl-terminal F-box-like domain is present in many of the ankyrin repeat proteins encoded by multigene families in mammalian and avian ChPVs (data not shown) (114). Known cellular E3 substrate-receptor subunits contain a range of E3 core binding and substrate interaction motifs, including carboxyl-terminal core binding motifs and ankyrin repeat substrate interaction motifs (10, 71, 91). Vaccinia virus (VACV) K1L ankyrin repeat protein is able to prevent degradation of IκB-α, an NF-κB inhibitor normally ubiquitinated during induction of proinflammatory responses, implicating poxvirus-encoded ankyrin repeats in ubiquitin-mediated events (110). Although the functional significance of a shorter, poxviral F-box-like domain is unclear, its presence in a family of proteins containing a diverse array of ankyrin repeats suggests that these ChPV proteins may encode F-box and substrate interaction functions and provide novel substrate specificities to endogenous E3 ligase complexes. In support of this hypothesis, the myxoma virus ankyrin repeat protein and virulence factor M-T5 was recently shown to contain a carboxyl-terminal F-box-like motif, to interact with the cellular E3 ligase subunit cul-1, to promote ubiquitination of the cul-1 substrate p27, and to affect viral inhibition of cell-cycle arrest and cell death (61). Interestingly, CRV lacks homologues of ChPV ankyrin repeat proteins and also of ChPV gene family proteins containing both BTB and kelch-like motifs, motifs known to act as E3 core binding and substrate recognition motifs, respectively, in cellular substrate receptor subunits (91). Conceivably, CRV F-box-containing proteins could provide E3 complex-related host-range functions analogous to those of ankyrin repeat and kelch-like proteins in other ChPVs.
CRV047 (81 amino acids) is homologous to molluscum contagiosum virus (MOCV) MC026L, parapox virus 014, and squirrelpox virus 026L and is comprised largely of a modified RING finger motif similar to the RING-H2 motif of cellular APC/C subunit 11 (Apc11) and ring box E3 subunits from divergent species (93). The RING-H2 domain of cellular Apc11 proteins (84 to 132 amino acids) interacts directly with E2 enzyme to recruit it to the APC and allow ubiquitination of protein substrates (43, 120), and it is essential for the minimum E3 ligase activity observed for Saccharomyces cerevisiae Apc11 (69). A similar role for CRV047 in E3 ubiquitin ligase complex formation or function, conceivably directing E3 activity to unique cellular substrates and affecting ubiquitin-mediated CRV-host interaction, is possible.
CRV042 and CRV045 contain carboxyl-terminal motifs similar to C3HC4-type RING finger motifs characteristic of a diverse array of single subunit E3 ubiquitin ligases, including ectromelia virus p28, a host range factor whose C3HC4 motif is required for E3 activity and viral virulence (53, 85, 92, 107). CRV042 and CRV045 contain all C and additional non-C residues present in C3HC4 RING motifs; however, they lack the conserved H residue critical for binding one of two zinc ions coordinated by the C3HC4 RING motif (data not shown) (11). Outside the RING-like domain, CRV042 and CRV045 share similarity with each other (22% amino acid identity over 410 amino acids) but lack similarity to other C3HC4 RING finger proteins. Although other novel RING-like motifs lacking the H residue have been shown to coordinate single zinc ions and contain intrinsic E3 activity, the function of RING-like motifs in CRV042 and CRV045 is unclear (19).
The presence of multiple CRV genes with the potential to affect E3 activities suggests that this is an effective mechanism through which CRV, like other viruses, may modulate protein degradation for the manipulation of host responses to infection. E3 ubiquitin ligases are involved in the regulation of many aspects of innate and adaptive immune responses, including activation of NF-κB and antigen-receptor-mediated signaling pathways, proteasome-dependent processing of antigenic peptides in antigen-presenting cells, activation of T lymphocytes, induction of T-cell tolerance, and Toll-like receptor signaling intensity and duration, and they also affect virion budding during certain viral infections (25, 72). F-box pathways are also exploited by human immunodeficiency virus to rid infected T cells of the CD4 membrane receptor, thereby reducing superinfection and exposure of infected cells to immune surveillance (75). Binding of herpes simplex virus 1 replication initiator protein UL9 to the E3 component neural F-box 42-kDa protein (NFB42) leads to a significant decrease in UL9 and is a specific interaction potentially involved in the switch of herpes simplex virus 1 from active replication to neuronal latency (37). Human papillomavirus E6 protein recruits cellular E3 for the degradation of tumor suppressor protein p53 (103). Viral F-box proteins include those encoded by atadeno- and nanoviruses, which have been reported to manipulate SCF complexes (8, 12). Thus, the large number of CRV-encoded proteins likely to affect ubiquitination suggests that CRV has potential for manipulating the ubiquitin proteolytic system through a diverse range of ubiquitin-mediated degradation pathways. This may be of particular relevance for virus-reptile host interactions.
(ii) GyrB-like ATPase domain gene family.
CRV084, CRV085, CRV086, CRV087, CRV088, CRV089, and CRV090 comprise a family of genes which encode proteins sharing 26 to 42% amino acid identity with each other and similarity to the amino-terminal GyrB-like ATPase domain of type II DNA topoisomerases (topo II). CRV GyrB-like ATPase domain ORFs are tandemly arranged in a central genomic location that disrupts colinearity with other ChPVs (Table 1). Eukaryotic topo II holoenzymes are homodimers of polypeptides that contain an amino-terminal ATPase domain similar to that of the bacterial DNA gyrase subunit GyrB, a centrally located cleavage and religation (CR) domain, and a carboxyl-terminal tail involved in nuclear localization and interaction with other proteins (22). The GyrB ATPase domain is an ATP binding module that forms a fold common with several enzymes now grouped into the GHKL family, which includes the gyrase class of topoisomerases, heat shock protein 90 (Hsp90), histidine kinases, and MutL DNA mismatch repair proteins, and which likely confers a common ATP-dependent mechanism to functionally distinct enzymes (33). All CRV GyrB-like ATPase domains contain sequences similar to the topo II-like GyrB ATPase domain, including Bergerat fold motifs I and III, suggestive of an ATP binding and hydrolysis function (Fig. 3 and data not shown) (9). Notably, only CRV090 contains sequences reminiscent of a CR domain, making it the longest (1,017 amino acids) and most topo II-like member of the CRV family. CRV090 shares, over its length, 23% amino acid identity to topo II of fungi and Paramecium bursaria chlorella virus 1. However, the CRV090 CR-like domain is divergent within the putative metal binding Toprim (topoisomerase-primase) region conserved in other topo II, including the PROSITE topo II signature (PS00177), and it lacks a Y residue homologous to the topo II CR catalytic site (data not shown) (26). All CRV GyrB-like ATPase domain proteins lack a recognizable topo II-like carboxyl-terminal tail. Thus, all CRV GyrB-like ATPase domain proteins appear deficient in domains or residues that would predict metal binding and DNA cleavage and reunion activity of fully functional topo II. Notably, CRV also encodes a homologue of the type IB DNA topoisomerase conserved in other poxviruses (CRV106), likely providing similar transcription-related functions in CRV (28). Features of CRV GyrB ATPase domain proteins suggest that they may have energy-dependent functions potentially involving novel virus-host interactions.
FIG. 3.
Amino acid alignment of CRV GyrB-like ATPase domain proteins and cellular topoisomerase II. Highlighted are residues identical to those in CRV genes. Underlined are Bergerat fold motifs I (where E involves hydrolysis and N binds Mg+) and III. h, hydrophobic; x, any residue; Talaromyces, Talaromyces flavus (fungi) (GenBank accession number AB078356); Microspora, Encephalitozoon cuniculi (AL590444); Nicotiana, Nicotiana tabacum (AY169238); Drosophila, Drosophila melanogaster (P15348). Numbers on the right indicate amino acid positions.
(iii) Additional CRV gene families.
CRV contains gene families with homologues in other ChPVs, including those similar to variola virus (VARV) B22R and those similar to VACV E2L and O1L. CRV040, CRV041, and CRV043 are similar to VARV B22R, making CRV and avipoxviruses the only ChPVs to encode multiple full-length homologues. B22R-like genes are the largest known poxvirus genes, encoding proteins of unknown function but predicted to contain transmembrane domains. CRV B22R-like genes occupy genomic locations distinct from mammalian ChPV homologues (Table 1), with encoded proteins sharing less than 32% amino acid identity with full-length ChPV homologues. Notably, CRV B22R-like genes are located in a region that also contains a B22R-like gene in avipoxviruses, viruses which contain six B22R-like genes (data not shown). CRV055, CRV062, CRV063, and CRV064 represent a gene family characterized by similarity to two ChPV genes of unknown function (homologues of VACV E2L and O1L) (106). Notably, CRV028, CRV033, CRV034, and CRV035 are members of a previously undescribed, highly variable gene family whose products share only 26 to 30% amino acid identity and lack similarity to known proteins (Table 1).
(iv) Other notable CRV genes.
CRV contains four genes of particular interest, including two (CRV052 and CRV021) that have homologues identified thus far only for viruses infecting nonmammalian hosts, one (CRV030) shared uniquely with MOCV, and one (CRV051) that suggests a novel function for homologues in other mammalian poxviruses.
CRV052 is similar to NAD+-dependent DNA ligases encoded by mimivirus, entomopoxviruses, insect iridovirus, and bacteria (approximately 26% amino acid identity) (Table 1). CRV052 (360 amino acids) contains a modified NAD+ ligase signature (PROSITE PS01056), including the active site motif (KXDG, position 192 to 195) and AMP binding residue. It is, however, considerably smaller than other viral (520 to 636 amino acids) and bacterial (approximately 630 to 840 amino acids) ligases. CRV052 contains nucleotidyl transferase motifs I, III, and IV, with motif IV exhibiting a D/E substitution not predicted to affect coordination of catalytic metal ions. CRV052 lacks, however, discernible motifs IIIa and V, including a K residue likely important for ligation, and the zinc finger motif present in many bacterial ligases. An additional 150 amino acids present in the amino terminus of CRV052 may compensate for its lack of motif V. CRV052 may be a functional, albeit divergent, NAD+-dependent ligase similar to those in entomopoxviruses; if so, it would be the first ChPV NAD+ ligase described (73, 115). Alternatively, CRV052 may function as a novel nucleotidyl transferase (W. Cao, personal communication). CRV, like MOCV, parapoxviruses, and yatapoxviruses, does not contain a homologue of VACV A50R, a nonessential ATP-dependent DNA ligase that affects viral sensitivity to genotoxic agents and viral virulence in vivo and is present in other poxvirus genera (64).
CRV021 contains a 160-amino-acid domain similar (31 to 37% amino acid identity) to those encoded by several unrelated viruses infecting birds (avian poxvirus, herpesvirus, and adenovirus isolates), vertebrate iridoviruses, and in cDNA sequences expressed in vertebrate species (Table 1 and data not shown). CRV021 also contains a carboxyl-terminal domain absent in other related ORFs, suggesting that additional functional domains are present. The CRV021-like protein (SORF2) encoded by Marek's disease virus, a tumorogenic alphaherpesvirus of domestic fowl, has been previously characterized as similar to the FPV250 protein of fowlpox virus and is highly similar (73% amino acid identity) to a predicted protein encoded in chicken cDNA and genomic sequences (data not shown) (15). Notably, the nonessential Marek's disease virus SORF2 protein interacts with chicken growth hormone, a cellular protein associated with resistance to Marek's disease (70). The presence of this CRV021-like domain in different viral and eukaryotic genes suggests that CRV021 may be involved in a conserved cellular function, possibly affecting aspects of viral host range.
CRV030 contains a J domain similar to those of the eukaryotic family of 40-kDa heat shock proteins (Hsp40) and bacterial DNA-J proteins. Notably, the only poxvirus homologue of CRV030 MOCV is MC013L, a protein shown to interfere with nuclear steroid receptor-mediated transcription (23). While CRV030 is most similar to cellular proteins at the 60-amino-acid amino-terminal J domain, including the highly conserved HPD tripeptide, the carboxyl-terminal domain (positions 118 to 209) also shares similarity with the less conserved carboxyl-terminal domain of Hsp40/DNA-J homologues (24% amino acid identity with positions 250 to 346 of the human DnaJ-like subfamily A protein; GenBank accession number AL162590). CRV030 lacks both G/F-rich and putative zinc-finger domains adjacent to the J domain found in many Hsp40/DNA-J homologues; however, cellular J-domain proteins lacking these domains are also common and potentially provide substrate specificity to Hsp70 recruitment (63).
J-domain proteins are cochaperones to the ubiquitous Hsp70/DNA-K class of protein chaperones, proteins that mediate ATP-dependent protein folding and assembly. By recruiting Hsp70 partners and accelerating the ATP hydrolysis coupled with Hsp70 substrate binding and release, J-domain proteins play important regulatory roles in the chaperone system and affect multiple cellular processes (63). J-domain proteins are also encoded by or affected by viruses. Three J-domain proteins encoded by mimivirus have been speculated to have chaperone functions and to interact with other mimivirus proteins predicted to affect protein folding (98). Polyomavirus T antigens, proteins with multiple functions in viral replication, virus-host cell interaction, and cellular transformation, contain amino-terminal J domains that possess Hsp40-like activities and mediate interaction with cellular proteins, including members of the retinoblastoma tumor suppressor family and protein phosphatase 2A (63, 119). Cellular J-domain proteins have been shown to function in pestivirus polyprotein processing (100). Influenza virus infection results in Hsp40/chaperone-mediated inhibition of P58IPK, a protein which inhibits the interferon (IFN)-induced protein PKR, a key regulator of cellular antiviral responses (79). CRV030L may function as a virally encoded cochaperone, affecting chaperone-mediated processes in infected cells and mediating virus-cell interactions. Notably, MC013L has been shown to interact in vitro directly with, and to inhibit the transcriptional effects of, vitamin D and glucocorticoid nuclear receptors, proteins that complex with cellular chaperones to affect their intracellular concentration or ligand affinity (23, 74, 95). While the MC013L J domain could conceivably interfere with normal steroid receptor-chaperone complexes, mutation of the MC013L HPD motif important for J-domain/Hsp70 interaction failed to abrogate MC013L-mediated transcriptional inhibition (23). Although the functional specifics of CRV030L relative to MC013L are unclear, the lack of homologues in other poxviruses suggests that their functions may affect mechanisms of virus-host interaction common to, and perhaps of particular relevance for, these two viruses.
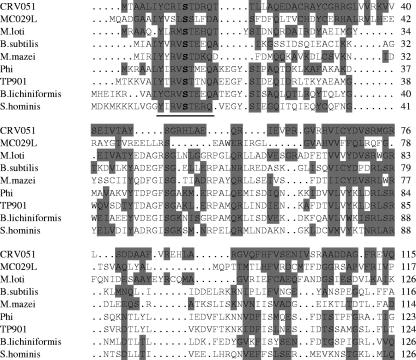
CRV051 shares similarity with both bacterial transposon resolvases and poxvirus homologues of VACV F16L, a protein of unknown function. Transposon resolvases are proteins of 180 to 200 amino acids containing an amino-terminal catalytic and dimerization domain, with a conserved S active-site residue involved in transient covalent binding to DNA, and a carboxyl-terminal helix-turn-helix DNA binding motif (113). CRV051 shares similarity with the amino-terminal domain of Staphylococcus site-specific serine recombinase-resolvases, including the recognized site-specific recombinase motif and potential active site residue at position 9 (PROSITE PS00397) (Fig. 4), and it contains a portion of the carboxyl-terminal region similar to recombinase helix-turn-helix regions (position 170 to 188, data not shown). CRV051 also shares 24% amino acid identify and a similar genomic location with MC029L, the MOCV homologue of VACV F16L (Table 1). Notably, poxvirus F16L homologues share very limited similarity with recombinases; however, all contain amino-terminal S residues which, conceivably, include a recombinase active site (data not shown). This unexpected similarity of CRV051 to both bacterial recombinases and poxvirus F16L homologues suggests that F16L homologues may function as site-specific recombinases.
FIG. 4.
Multiple sequence alignment CRV051, MC029L (GenBank accession number U60315) and site-specific recombinase/integrase amino-terminal regions. Highlighted are amino acids similar to CRV051. M. loti, Mesorhizobium loti (AP003017); B. subtilis, Bacillus subtilis (P17867); M. mazei, Methanosarcina mazei (AE013525); Phi, bacteriophage phi-FC1 (AF124258); TP901, Lactococcus lactis bacteriophage TP901-1 (X85213); B. lichiniformis, Bacillus lichiniformis (AX930120); S. hominis, Staphylococus hominis (AB063171). Underlined letters are PROSITE motif residues (PS00397). The putative active site residue S is indicated by boldface type. Numbers on the right indicate amino acid positions.
Site-specific recombination of two DNA molecules results in cointegration or excision of a DNA fragment, and it has been hypothesized as a mechanism utilized during the replication of linear genomes or for viral gene acquisition (66, 122). CRV contains homologues of VACV H6R DNA topoisomerase I (CRV106) and A22R Holliday junction resolvase (CRV145), genes involved in the resolution of Holliday junction replicative intermediates (telomere resolution) and in genomic recombination (41). It has been suggested that an additional, more highly specialized viral enzyme(s) may be involved in telomere resolution (66). Conceivably, CRV051 and other F16L homologues may function in telomere resolution and/or gene acquisition. Notably, the absence of F16L homologues in avipoxviruses suggests that this gene is highly divergent in avipoxviruses or, alternatively, nonessential for viral replication.
Comparison of CRV with other ChPVs.
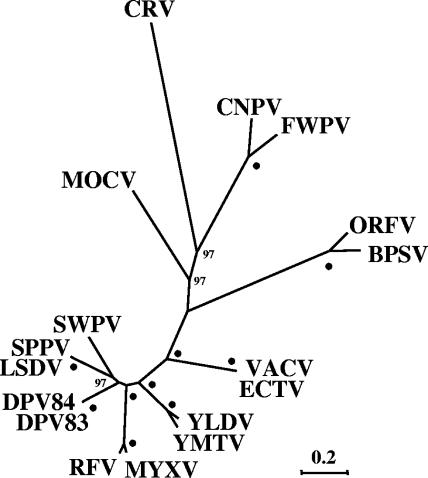
Genome analysis indicated that CRV is a highly divergent ChPV, containing unique sequence features in both a ChPV-like central genomic region and in completely novel terminal genomic regions. Between positions 33650 and 176791 (the region containing homologues of VACV F9L to A34R), CRV contained the 90 genes present in all known ChPVs, with the most conserved genes involved in DNA replication and RNA biosynthesis (Table 1) (48). Despite maintaining this minimal ChPV gene complement, phylogenetic analyses of these proteins revealed relatively large genetic distances between CRV and viruses of other ChPV genera, indicating that CRV represents a new poxvirus genus (Fig. 5).
FIG. 5.
Phylogenetic analysis of CRV proteins. Eighty-three conserved proteins between CRV036 and CRV147 were concatenated and aligned with similar data sets from other ChPVs using MUSCLE. The unrooted tree for 32,633 aligned characters was generated using maximum likelihood with WAG correction for multiple substitutions, four-category discrete gamma model, estimation for invariant residues, and 100 bootstrap replicates as implemented in Phyml. Bootstrap values greater than 70 are indicated at appropriate nodes, and dots indicate values of 100. Homologous protein sequences from the following viruses and accession numbers were compared: bovine papular stomatitis virus (BPSV; GenBank accession number AY386265); canarypox virus (CNPV; AY318871); ectromelia virus (ECTV; AF012825); deerpox virus W-848-83 (DPV83; AY689436); deerpox virus W-1170-84 (DPV84; AY689437); fowlpox virus (FWPV; AF198100); lumpy skin disease virus (LSDV; AF325528); molluscum contagiosum virus (MOCV; U60315); myxoma virus (MYXV; AF170726); orf virus (ORFV; AY386264); rabbit (Shope) fibroma virus (SFV; AF170722); sheeppox virus (SPPV; AY077833); swinepox virus (SWPV; AF410153); VACV, M35027; Yaba-like disease virus (YLDV; AJ293568); Yaba monkey tumor virus (YMTV; AY386371). Scale indicates estimated changes per residue. Similar topologies were obtained using an alignment (22,055 characters) in which poorly aligned regions were trimmed with Gblocks; using additional maximum likelihood analyses of the MUSCLE alignment as implemented in PHYLIP, TREE-PUZZLE, IQPNNI, and MRBAYES; using similar analyses on alignments generated with with Dialign-T and Kalign; using Phyml results for supertree analysis of multiple concatenated datasets and for supertree analysis of individual proteins aligned with Kalign; or by conducting similar analyses on a data set including only one virus per genus or major viral group (10 taxa).
Divergence between CRV and ChPVs in the central conserved regions included notable differences in amino acid identity and length of encoded proteins. CRV proteins sharing a low level of amino acid identity with ChPV homologues (less than 34% to VACV homologues) included putative virion core (CRV061, CRV079, and CRV110), IMV envelope (CRV103, CRV135, and CRV140), IEV (CRV038), and EEV (CRV158) proteins; glutaredoxin (CRV075); proteins of unknown function (CRV083 and CRV107); and, notably, several proteins with putative functions in DNA replication or gene expression (CRV051, CRV067, CRV105, CRV117, CRV143). CRV125 (70 amino acids) shares limited similarity with the amino-terminal regions of the VACV A4L homologues, virion core proteins that exhibit a relatively high degree of variability in sequence identity and length among other ChPVs (148 to 421 amino acids).
A repetitive, proline-rich amino-terminal extension makes CRV058 significantly larger (794 amino acids) than ChPV homologues (VACV E8R, 273 amino acids); however, an alternative start codon (M526) at genomic position 75345 would yield a ChPV-like protein (270 amino acids). CRV105, the homologue of VACV late transcription factor 4 (VLTF-4) is smaller in size (123 amino acids) than ChPV homologues (170 to 228 amino acids), lacking an equivalent amino-terminal domain. Similarly, CRV133, the homologue of VACV A12L virion core protein, is smaller (140 amino acids) than its ChPV homologues (161 to 260 amino acids), lacking a central domain of low amino acid complexity (data not shown). These differences between CRV proteins and ChPV homologues exemplify the divergent nature of CRV and potentially reflect specific adaptation to the reptile host.
Novel gene arrangements at discrete loci within the central genomic region also reflect the divergent nature of CRV. ChPV-like gene colinearity is disrupted at several loci in the CRV central genomic region, including the insertion of seven GyrB-like ATPase domain genes (CRV084 to CRV090) at the VACV L2R/L3L locus and two novel E2L/O1L-like genes (CRV063 and CRV064) at the VACV O1L locus (Table 1). In addition, CRV contains a single gene (CRV148) encoding a homologue of the multiple A-type inclusion proteins present at the homologous locus in other ChPVs, including MOCV, parapoxviruses, and avipoxviruses (3, 30, 106).
Several features within or adjacent to the CRV central genomic region are similar to other relatively divergent ChPVs, including the avipoxviruses, parapoxviruses, and MOCV to which CRV proteins were most similar in pairwise searches (Table 1). CRV, like avipoxviruses, lacks homologues of the VACV E3L interferon resistance protein and A33R EEV glycoprotein required for efficient actin tail-mediated cell-to-cell spread of VACV (101), and CRV contains three VARV B22R-like homologues (CRV040, CRV041, CRV043) encoded at a locus also containing a B22R-like gene in avipoxviruses (3). The CRV genome shares certain features previously noted as common between MOCV and parapoxviruses, including a G+C-rich nucleotide composition and lack of genes present in most other ChPVs (30). Notably, several CRV genes, although generally small in size and interspersed within the central region, were similar to homologues found only in MOCV (CRV030, CRV050, CRV091, CRV100, CRV108, CRV134, and CRV153), suggesting a monophyletic relationship between the two viruses. Although neighbor joining and parsimony-based phylogenetic analyses of proteins from central conserved regions indeed suggested CRV/MOCV monophyly (data not shown), more robust maximum likelihood analyses indicated that CRV and MOCV are not monophyletic relative to other ChPVs (Fig. 5). Thus, despite the potentially synaptomorphic nature of uniquely shared gene orthologues, the phylogenetic relevance of other features shared between CRV and other ChPVs is unclear, leaving CRV distinct within the subfamily Chordopoxvirinae.
Consistent with the relatively divergent nature of the CRV central genomic region, proteins encoded by CRV genes located within the left 33-kbp and right 13-kbp terminal genomic regions are largely of unknown function and lacked similarity to known poxvirus proteins (Table 1). CRV lacks ChPV gene families and ChPV homologues involved in viral virulence, host range, modulation of innate and adaptive immune responses, and modulation of apoptotic responses. CRV lacks recognizable homologues of MC053L and MC054L interleukin 18 (IL-18) binding proteins, MOCV MC148R chemokine receptor antagonist, MC159L and MC160L vFLIP-like inhibitors of apoptosis, and MC002L, MC161R, and MC162R SLAM/CD150-like proteins (83, 111, 129). CRV also lacks homologues of VACV C7L host range protein, K3L PKR/IFN response antagonist, N1L, A46R, and A52R inhibitors of intracellular signaling, A38L CD47-like protein, A39R semaphorin-like protein, A41L virulence protein, A44L β-hydroxysteroid dehydrogenase, A45R superoxide dismutase, B5R EEV protein, B7R virulence protein, B8R gamma interferon receptor, B16R IL-1 receptor, and B19R alpha/beta interferon receptor (6, 86, 112, 116). Absent are homologues of VACV F1L and myxoma virus M004 and M011R antiapoptotic proteins, myxoma virus α-2,3-sialyltransferase, and ChPV chemokine binding proteins and receptors, complement binding proteins, and tumor necrosis factor receptor-like proteins (6, 17, 82, 127).
CRV is the first poxvirus of a reptile to be studied, and the differences in the genome of CRV relative to other ChPVs, especially the absence of recognizable poxvirus virulence and host range genes, are notable. Although understanding of the reptile immune system and its response to viral infection is limited, it is known that reptiles produce immune cell populations morphologically similar to those found in avian and mammalian species, and they are thought to be capable of mounting inflammatory, humoral, cellular, and cell-mediated immune responses comparable to those present in higher vertebrates, albeit affected by seasonal and thermoregulatory changes (13, 18). Conservation of vertebrate innate and adaptive immune responses, including interferon, IL-1, tumor necrosis factor-like receptor, complement, and Toll-like receptor-mediated responses, that are interdicted by poxviruses of birds and mammals suggest that such mechanisms are also likely manipulated by CRV (13, 32, 62, 94). Thus, the complete lack of genes predicted to involve manipulation of host immune responses in CRV is striking. As is the case with other poxviruses encoding novel proteins affecting host immune and apoptotic responses (29, 47, 96, 127), it is likely that many of the novel genes present in CRV encode proteins capable of affecting similar host responses.
ADDENDUM IN PROOF
Since the completion of the analyses presented here, similar observations regarding F-box-like motifs in poxvirus ankyrin repeat proteins have been reported (A. A. Mercer, S. B. Fleming, and N. Ueda, Virus Genes 31:127-133, 2005).
Acknowledgments
We thank Chris Foggin of the Department of Veterinary Services, Zimbabwe, for supplying viral material and information on recent outbreaks in Zimbabwe; James M. Berger of the University of California, Berkeley, for topoisomerase II analysis; Weiguo Cao of Clemson University, South Carolina, for comments on NAD+-dependent DNA ligase; and Kristin Zaffuto and Adrienne Lakowitz for providing excellent technical assistance.
REFERENCES
- 1.Abascal, F., R. Zardoya, and D. Posada. 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21:2104-2105. [DOI] [PubMed] [Google Scholar]
- 2.Afonso, C. L., E. R. Tulman, Z. Lu, E. Oma, G. F. Kutish, and D. L. Rock. 1999. The genome of Melanoplus sanguinipes entomopoxvirus. J. Virol. 73:533-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, G. F. Kutish, and D. L. Rock. 2000. The genome of fowlpox virus. J. Virol. 74:3815-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, F. A. Osorio, C. Balinsky, G. F. Kutish, and D. L. Rock. 2002. The genome of swinepox virus. J. Virol. 76:783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, N. T. Sandybaev, U. Z. Kerembekova, V. L. Zaitsev, G. F. Kutish, and D. L. Rock. 2002. The genome of camelpox virus. Virology 295:1-9. [DOI] [PubMed] [Google Scholar]
- 6.Alcami, A. 2003. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 3:36-50. [DOI] [PubMed] [Google Scholar]
- 7.Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. [DOI] [PubMed] [Google Scholar]
- 8.Aronson, M. N., A. D. Meyer, J. Gyorgyey, L. Katul, H. J. Vetten, B. Gronenborn, and T. Timchenko. 2000. Clink, a nanovirus-encoded protein, binds both pRB and SKP1. J. Virol. 74:2967-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergerat, A., B. de Massy, D. Gadelle, P. C. Varoutas, A. Nicolas, and P. Forterre. 1997. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature 386:414-417. [DOI] [PubMed] [Google Scholar]
- 10.Boengler, K., F. Pipp, B. Fernandez, A. Richter, W. Schaper, and E. Deindl. 2003. The ankyrin repeat containing SOCS box protein 5: a novel protein associated with arteriogenesis. Biochem. Biophys. Res. Commun. 302:17-22. [DOI] [PubMed] [Google Scholar]
- 11.Borden, K. L., and P. S. Freemont. 1996. The RING finger domain: a recent example of a sequence-structure family. Curr. Opin. Struct. Biol. 6:395-401. [DOI] [PubMed] [Google Scholar]
- 12.Both, G. W. 2002. Identification of a unique family of F-box proteins in atadenoviruses. Virology 304:425-433. [DOI] [PubMed] [Google Scholar]
- 13.Brown, D. R. 2002. Mycoplasmosis and immunity of fish and reptiles. Front. Biosci. 7:d1338-1346. [DOI] [PubMed] [Google Scholar]
- 14.Brunetti, C. R., H. Amano, Y. Ueda, J. Qin, T. Miyamura, T. Suzuki, X. Li, J. W. Barrett, and G. McFadden. 2003. Complete genomic sequence and comparative analysis of the tumorigenic poxvirus Yaba monkey tumor virus. J. Virol. 77:13335-13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunovskis, P., and L. F. Velicer. 1995. The Marek's disease virus (MDV) unique short region: alphaherpesvirus-homologous, fowlpox virus-homologous, and MDV-specific genes. Virology 206:324-338. [DOI] [PubMed] [Google Scholar]
- 16.Buenviaje, G. N., P. W. Ladds, and Y. Martin. 1998. Pathology of skin diseases in crocodiles. Aust. Vet. J. 76:357-363. [DOI] [PubMed] [Google Scholar]
- 17.Cameron, C., S. Hota-Mitchell, L. Chen, J. Barrett, J. X. Cao, C. Macaulay, D. Willer, D. Evans, and G. McFadden. 1999. The complete DNA sequence of myxoma virus. Virology 264:298-318. [DOI] [PubMed] [Google Scholar]
- 18.Campbell, T. W. 1996. Clinical pathology, p. 248-257. In D. R. Mader (ed.), Reptile medicine and surgery. W.B. Saunders Company, Philadelphia, Pa.
- 19.Capili, A. D., E. L. Edghill, K. Wu, and K. L. Borden. 2004. Structure of the C-terminal RING finger from a RING-IBR-RING/TRIAD motif reveals a novel zinc-binding domain distinct from a RING. J. Mol. Biol. 340:1117-1129. [DOI] [PubMed] [Google Scholar]
- 20.Cardozo, T., and M. Pagano. 2004. The SCF ubiquitin ligase: insights into a molecular machine. Nat. Rev. Mol. Cell Biol. 5:739-751. [DOI] [PubMed] [Google Scholar]
- 21.Castresana, J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17:540-552. [DOI] [PubMed] [Google Scholar]
- 22.Champoux, J. J. 2001. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem. 70:369-413. [DOI] [PubMed] [Google Scholar]
- 23.Chen, N., T. Baudino, P. N. MacDonald, M. Green, W. L. Kelley, J. W. Burnett, and R. M. Buller. 2000. Selective inhibition of nuclear steroid receptor function by a protein from a human tumorigenic poxvirus. Virology 274:17-25. [DOI] [PubMed] [Google Scholar]
- 24.Chen, N., M. I. Danila, Z. Feng, R. M. Buller, C. Wang, X. Han, E. J. Lefkowitz, and C. Upton. 2003. The genomic sequence of ectromelia virus, the causative agent of mousepox. Virology 317:165-186. [DOI] [PubMed] [Google Scholar]
- 25.Chuang, T. H., and R. J. Ulevitch. 2004. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat. Immunol. 5:495-502. [DOI] [PubMed] [Google Scholar]
- 26.Corbett, K. D., and J. M. Berger. 2004. Structure, molecular mechanisms, and evolutionary relationships in DNA topoisomerases. Annu. Rev. Biophys. Biomol. Struct. 33:95-118. [DOI] [PubMed] [Google Scholar]
- 27.Craig, K. L., and M. Tyers. 1999. The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction. Prog. Biophys. Mol. Biol. 72:299-328. [DOI] [PubMed] [Google Scholar]
- 28.Da Fonseca, F., and B. Moss. 2003. Poxvirus DNA topoisomerase knockout mutant exhibits decreased infectivity associated with reduced early transcription. Proc. Natl. Acad. Sci. USA 100:11291-11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deane, D., C. J. McInnes, A. Percival, A. Wood, J. Thomson, A. Lear, J. Gilray, S. Fleming, A. Mercer, and D. Haig. 2000. Orf virus encodes a novel secreted protein inhibitor of granulocyte-macrophage colony-stimulating factor and interleukin-2. J. Virol. 74:1313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delhon, G., E. R. Tulman, C. L. Afonso, Z. Lu, A. de la Concha-Bermejillo, H. D. Lehmkuhl, M. E. Piccone, G. F. Kutish, and D. L. Rock. 2004. Genomes of the parapoxviruses ORF virus and bovine papular stomatitis virus. J. Virol. 78:168-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Pasquier, L., and M. Flajnik. 1999. Origin and evolution of the vertebrate immune system, p. 605-650. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven, New York, N.Y.
- 33.Dutta, R., and M. Inouye. 2000. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem. Sci. 25:24-28. [DOI] [PubMed] [Google Scholar]
- 34.Eddy, S. R. 1996. Hidden Markov models. Curr. Opin. Struct. Biol. 6:361-365. [DOI] [PubMed] [Google Scholar]
- 35.Eddy, S. R., G. Mitchison, and R. Durbin. 1995. Maximum discrimination hidden Markov models of sequence consensus. J. Comput. Biol. 2:9-23. [DOI] [PubMed] [Google Scholar]
- 36.Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eom, C. Y., W. D. Heo, M. L. Craske, T. Meyer, and I. R. Lehman. 2004. The neural F-box protein NFB42 mediates the nuclear export of the herpes simplex virus type 1 replication initiator protein (UL9 protein) after viral infection. Proc. Natl. Acad. Sci. USA 101:4036-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felsenstein, J. 1989. PHYLIP: phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 39.Galperin, M. Y. 2005. The molecular biology database collection: 2005 update. Nucleic Acids Res. 33:D5-D24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galtier, N., M. Gouy, and C. Gautier. 1996. SEAVIEW and PHYLO WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biol. Sci. 12:543-554. [DOI] [PubMed] [Google Scholar]
- 41.Garcia, A. D., L. Aravind, E. V. Koonin, and B. Moss. 2000. Bacterial-type DNA Holliday junction resolvases in eukaryotic viruses. Proc. Natl. Acad. Sci. USA 97:8926-8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerdes, G. H. 1991. Morphology of poxviruses from reptiles. Vet. Rec. 128:452. [DOI] [PubMed] [Google Scholar]
- 43.Gmachl, M., C. Gieffers, A. V. Podtelejnikov, M. Mann, and J. M. Peters. 2000. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc. Natl. Acad. Sci. USA 97:8973-8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goebel, S. J., G. P. Johnson, M. E. Perkus, S. W. Davis, J. P. Winslow, and E. Paoletti. 1990. The complete DNA sequence of vaccinia virus. Virology 179:247-266. [DOI] [PubMed] [Google Scholar]
- 45.Gordon, D., C. Abajian, and P. Green. 1998. Consed: a graphical tool for sequence finishing. Genome Res. 8:192-202. [DOI] [PubMed] [Google Scholar]
- 46.Gough, J., K. Karplus, R. Hughey, and C. Chothia. 2001. Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J. Mol. Biol. 313:903-919. [DOI] [PubMed] [Google Scholar]
- 47.Graham, K. A., A. S. Lalani, J. L. Macen, T. L. Ness, M. Barry, L.-Y. Liu, A. Lucas, I. Clark-Lewis, R. W. Moyer, and G. McFadden. 1997. The T1/35kDa family of poxvirus-secreted proteins bind chemokines and modulate leukocyte influx into virus-infected tissues. Virology 229:12-24. [DOI] [PubMed] [Google Scholar]
- 48.Gubser, C., S. Hue, P. Kellam, and G. L. Smith. 2004. Poxvirus genomes: a phylogenetic analysis. J. Gen. Virol. 85:105-117. [DOI] [PubMed] [Google Scholar]
- 49.Gubser, C., and G. L. Smith. 2002. The sequence of camelpox virus shows it is most closely related to variola virus, the cause of smallpox. J. Gen. Virol. 83:855-872. [DOI] [PubMed] [Google Scholar]
- 50.Guindon, S., and O. Gascuel. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696-704. [DOI] [PubMed] [Google Scholar]
- 51.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 52.Horner, R. F. 1988. Poxvirus in farmed Nile crocodiles. Vet. Rec. 122:459-462. [DOI] [PubMed] [Google Scholar]
- 53.Huang, J., Q. Huang, X. Zhou, M. M. Shen, A. Yen, S. X. Yu, G. Dong, K. Qu, P. Huang, E. M. Anderson, S. Daniel-Issakani, R. M. Buller, D. G. Payan, and H. H. Lu. 2004. The poxvirus p28 virulence factor is an E3 ubiquitin ligase. J. Biol. Chem. 279:54110-54116. [DOI] [PubMed] [Google Scholar]
- 54.Huang, X., and A. Madan. 1999. CAP3: a DNA sequence assembly program. Genome Res. 9:868-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huchzermeyer, F. W. 2002. Diseases of farmed crocodiles and ostriches. Rev. Sci. Tech. 21:265-276. [DOI] [PubMed] [Google Scholar]
- 56.Huchzermeyer, F. W., K. D. Huchzermeyer, and J. F. Putterill. 1991. Observations on a field outbreak of pox virus infection in young Nile crocodiles (Crocodylus niloticus). J. S. Afr. Vet. Assoc. 62:27-29. [PubMed] [Google Scholar]
- 57.Huelsenbeck, J. P., and F. Ronquist. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754-755. [DOI] [PubMed] [Google Scholar]
- 58.Ilyin, G. P., A. L. Serandour, C. Pigeon, M. Rialland, D. Glaise, and C. Guguen-Guillouzo. 2002. A new subfamily of structurally related human F-box proteins. Gene 296:11-20. [DOI] [PubMed] [Google Scholar]
- 59.Jacobson, E. R., J. A. Popp, R. P. Shields, and J. M. Gaskin. 1979. Poxlike skin lesions in captive caimans. J. Am. Vet. Med. Assoc. 175:937-940. [PubMed] [Google Scholar]
- 60.Jacobson, E. R., and S. R. Telford. 1990. Chlamydial and poxvirus infections of circulating monocytes of a flap-necked chameleon (Chamaeleo dilepis). J. Wildl. Dis. 26:572-577. [DOI] [PubMed] [Google Scholar]
- 61.Johnston, J. B., G. Wang, J. W. Barrett, S. H. Nazarian, K. Colwill, M. Moran, and G. McFadden. 2005. Myxoma virus M-T5 protects infected cells from the stress of cell cycle arrest through its interaction with host cell cullin-1. J. Virol. 79:10750-10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaiser, P., L. Rothwell, S. Avery, and S. Balu. 2004. Evolution of the interleukins. Dev. Comp. Immunol. 28:375-394. [DOI] [PubMed] [Google Scholar]
- 63.Kelley, W. L. 1998. The J-domain family and the recruitment of chaperone power. Trends Biochem. Sci. 23:222-227. [DOI] [PubMed] [Google Scholar]
- 64.Kerr, S. M., L. H. Johnston, M. Odell, S. A. Duncan, K. M. Law, and G. L. Smith. 1991. Vaccinia DNA ligase complements Saccharomyces cerevisiae cdc9, localizes in cytoplasmic factories and affects virulence and virus sensitivity to DNA damaging agents. EMBO J. 10:4343-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kipreos, E. T., and M. Pagano. 2000. The F-box protein family. Genome Biol. 1:REVIEWS3002.1-3002.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kobryn, K., and G. Chaconas. 2001. The circle is broken: telomere resolution in linear replicons. Curr. Opin. Microbiol. 4:558-564. [DOI] [PubMed] [Google Scholar]
- 67.Lassmann, T., and E. L. L. Sonnhammer. 2005. Kalign—an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics. 6:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee, H. J., K. Essani, G. L. Smith, F. Jeanmougin, and D. G. Higgins. 2001. The genome sequence of Yaba-like disease virus, a yatapoxvirus. Virology 281:170-192. [DOI] [PubMed] [Google Scholar]
- 69.Leverson, J. D., C. A. Joazeiro, A. M. Page, H. Huang, P. Hieter, and T. Hunter. 2000. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol. Biol. Cell 11:2315-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu, H. C., H. J. Kung, J. E. Fulton, R. W. Morgan, and H. H. Cheng. 2001. Growth hormone interacts with the Marek's disease virus SORF2 protein and is associated with disease resistance in chicken. Proc. Natl. Acad. Sci. USA 98:9203-9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu, Y., J. Li, F. Zhang, W. Qin, G. Yao, X. He, P. Xue, C. Ge, D. Wan, and J. Gu. 2003. Molecular cloning and characterization of the human ASB-8 gene encoding a novel member of ankyrin repeat and SOCS box containing protein family. Biochem. Biophys. Res. Commun. 300:972-979. [DOI] [PubMed] [Google Scholar]
- 72.Liu, Y. C. 2004. Ubiquitin ligases and the immune response. Annu. Rev. Immunol. 22:81-127. [DOI] [PubMed] [Google Scholar]
- 73.Lu, J., J. Tong, H. Feng, J. Huang, C. L. Afonso, D. L. Rock, F. Barany, and W. Cao. 2004. Unique ligation properties of eukaryotic NAD+-dependent DNA ligase from Melanoplus sanguinipes entomopoxvirus. Biochim. Biophys. Acta 1701:37-48. [DOI] [PubMed] [Google Scholar]
- 74.Lutz, W., K. Kohno, and R. Kumar. 2001. The role of heat shock protein 70 in vitamin D receptor function. Biochem. Biophys. Res. Commun. 282:1211-1219. [DOI] [PubMed] [Google Scholar]
- 75.Margottin, F., S. P. Bour, H. Durand, L. Selig, S. Benichou, V. Richard, D. Thomas, K. Strebel, and R. Benarous. 1998. A novel human WD protein, h-βTrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1:565-574. [DOI] [PubMed] [Google Scholar]
- 76.Massung, R. F., L.-I. Liu, J. Qi, J. C. Knight, T. E. Yuran, A. R. Kerlavage, J. M. Parsons, J. C. Venter, and J. J. Esposito. 1994. Analysis of the complete genome of smallpox variola major virus strain Bangladesh—1975. Virology 201:215-240. [DOI] [PubMed] [Google Scholar]
- 77.Massung, R. F., and R. W. Moyer. 1991. The molecular biology of swinepox virus. I. A characterization of the viral DNA. Virology 180:347-354. [DOI] [PubMed] [Google Scholar]
- 78.Mathews, D. H., J. Sabina, M. Zuker, and D. H. Turners. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911-940. [DOI] [PubMed] [Google Scholar]
- 79.Melville, M. W., W. J. Hansen, B. C. Freeman, W. J. Welch, and M. G. Katze. 1997. The molecular chaperone hsp40 regulates the activity of P58IPK, the cellular inhibitor of PKR. Proc. Natl. Acad. Sci. USA 94:97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merchlinsky, M. 1990. Mutational analysis of the resolution sequence of vaccinia virus DNA: essential sequence consists of two separate AT-rich regions highly conserved among poxviruses. J. Virol. 64:5029-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morgenstern, B., K. Frech, A. Dress, and T. Werner. 1998. DIALIGN: finding local similarities by multiple sequence alignment. Bioinformatics 14:290-294. [DOI] [PubMed] [Google Scholar]
- 82.Moss, B. 2001. Poxviridae: The viruses and their replication, p. 2849-2883. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 83.Moss, B., J. L. Shisler, Y. Xiang, and T. G. Senkevich. 2000. Immune-defense molecules of Molluscum contagiosum virus, a human poxvirus. Trends Microbiol. 8:473-477. [DOI] [PubMed] [Google Scholar]
- 84.Moyer, R. W., B. Arif, D. N. Black, D. B. Boyle, R. M. Buller, K. R. Dumbell, J. J. Esposito, G. McFadden, B. Moss, A. A. Mercer, S. Ropp, D. N. Tripathy, and C. Upton. 2000. Family Poxviridae, p. 137-157. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Academic Press, New York, N.Y.
- 85.Nerenberg, B. T., J. Taylor, E. Bartee, K. Gouveia, M. Barry, and K. Fruh. 2005. The poxviral RING protein p28 is a ubiquitin ligase that targets ubiquitin to viral replication factories. J. Virol. 79:597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ng, A., D. C. Tscharke, P. C. Reading, and G. L. Smith. 2001. The vaccinia virus A41L protein is a soluble 30 kDa glycoprotein that affects virus virulence. J. Gen. Virol. 82:2095-2105. [DOI] [PubMed] [Google Scholar]
- 87.Oros, J., J. L. Rodriguez, S. Deniz, L. Fernandez, and A. Fernandez. 1998. Cutaneous poxvirus-like infection in a captive Hermann's tortoise (Testudo hermanni). Vet. Rec. 143:508-509. [DOI] [PubMed] [Google Scholar]
- 88.Pandey, G. S., N. Inoue, K. Ohshima, K. Okada, Y. Chihaya, and Y. Fujimoto. 1990. Poxvirus infection in Nile crocodiles (Crocodylus niloticus). Res. Vet. Sci. 49:171-176. [PubMed] [Google Scholar]
- 89.Pearson, W. R. 1990. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 183:63-98. [DOI] [PubMed] [Google Scholar]
- 90.Penrith, M. L., J. W. Nesbit, and F. W. Huchzermeyer. 1991. Pox virus infection in captive juvenile caimans (Caiman crocodilus fuscus) in South Africa. J. S. Afr. Vet. Assoc. 62:137-139. [PubMed] [Google Scholar]
- 91.Petroski, M. D., and R. J. Deshaies. 2005. Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6:9-20. [DOI] [PubMed] [Google Scholar]
- 92.Pickart, C. M. 2001. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70:503-533. [DOI] [PubMed] [Google Scholar]
- 93.Pickart, C. M., and M. J. Eddins. 2004. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta 1695:55-72. [DOI] [PubMed] [Google Scholar]
- 94.Plouffe, D. A., P. C. Hanington, J. G. Walsh, E. C. Wilson, and M. Belosevic. 2005. Comparison of select innate immune mechanisms of fish and mammals. Xenotransplantation 12:266-277. [DOI] [PubMed] [Google Scholar]
- 95.Pratt, W. B., and D. O. Toft. 1997. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 18:306-360. [DOI] [PubMed] [Google Scholar]
- 96.Puehler, F., H. Schwarz, B. Waidner, J. Kalinowski, B. Kaspers, S. Bereswill, and P. Staeheli. 2003. An interferon-gamma-binding protein of novel structure encoded by the fowlpox virus. J. Biol. Chem. 278:6905-6911. [DOI] [PubMed] [Google Scholar]
- 97.Ramos, M. C., S. D. Coutinho, E. R. Matushima, and I. L. Sinhorini. 2002. Poxvirus dermatitis outbreak in farmed Brazilian caimans (Caiman crocodilus yacare). Aust. Vet. J. 80:371-372. [DOI] [PubMed] [Google Scholar]
- 98.Raoult, D., S. Audic, C. Robert, C. Abergel, P. Renesto, H. Ogata, B. La Scola, M. Suzan, and J. M. Claverie. 2004. The 1.2-megabase genome sequence of Mimivirus. Science 306:1344-1350. [DOI] [PubMed] [Google Scholar]
- 99.Rice, P., I. Longden, and A. Bleasby. 2000. EMBOSS: the European molecular biology open software suite. Trends Genet. 16:276-277. [DOI] [PubMed] [Google Scholar]
- 100.Rinck, G., C. Birghan, T. Harada, G. Meyers, H. J. Thiel, and N. Tautz. 2001. A cellular J-domain protein modulates polyprotein processing and cytopathogenicity of a pestivirus. J. Virol. 75:9470-9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roper, R. L., E. J. Wolffe, A. Weisberg, and B. Moss. 1998. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J. Virol. 72:4192-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 103.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 104.Schmidt, H. A., K. Strimmer, M. Vingron, and A. von Haeseler. 2002. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18:502-504. [DOI] [PubMed] [Google Scholar]
- 105.Schreader, B. A., Y. Wang, and J. R. Nambu. 2003. Drosophila morgue and the intersection between protein ubiquitination and programmed cell death. Apoptosis 8:129-139. [DOI] [PubMed] [Google Scholar]
- 106.Senkevich, T. G., E. V. Koonin, J. J. Bugert, G. Darai, and B. Moss. 1997. The genome of Molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology 233:19-42. [DOI] [PubMed] [Google Scholar]
- 107.Senkevich, T. G., E. V. Koonin, and R. M. L. Buller. 1994. A poxvirus protein with a RING zinc finger motif is of crucial importance for virulence. Virology 198:118-128. [DOI] [PubMed] [Google Scholar]
- 108.Shchelkunov, S. N., A. V. Totmenin, V. N. Loparev, P. F. Safronov, V. V. Gutorov, V. E. Chizhikov, J. C. Knight, J. M. Parsons, R. F. Massung, and J. J. Esposito. 2000. Alastrim smallpox variola minor virus genome DNA sequences. Virology 266:361-386. [DOI] [PubMed] [Google Scholar]
- 109.Shchelkunov, S. N., A. V. Totmenin, P. F. Safronov, M. V. Mikheev, V. V. Gutorov, O. I. Ryazankina, N. A. Petrov, I. V. Babkin, E. A. Uvarova, L. S. Sandakhchiev, J. R. Sisler, J. J. Esposito, I. K. Damon, P. B. Jahrling, and B. Moss. 2002. Analysis of the monkeypox virus genome. Virology 297:172-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shisler, J. L., and X. L. Jin. 2004. The vaccinia virus K1L gene product inhibits host NF-κB activation by preventing IκBα degradation. J. Virol. 78:3553-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sidorenko, S. P., and E. A. Clark. 2003. The dual-function CD150 receptor subfamily: the viral attraction. Nat. Immunol. 4:19-24. [DOI] [PubMed] [Google Scholar]
- 112.Smith, G. L., Y. S. Chan, and S. T. Howard. 1991. Nucleotide sequence of 42 kbp of vaccinia virus strain WR from near the right inverted terminal repeat. J. Gen. Virol. 72:1349-1376. [DOI] [PubMed] [Google Scholar]
- 113.Smith, M. C., and H. M. Thorpe. 2002. Diversity in the serine recombinases. Mol. Microbiol. 44:299-307. [DOI] [PubMed] [Google Scholar]
- 114.Sonnhammer, E. L. L., S. R. Eddy, E. Birney, A. Bateman, and R. Durbin. 1998. Pfam: multiple sequence alignments and HMM-profiles of protein domains. Nucleic Acids Res. 26:320-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sriskanda, V., R. W. Moyer, and S. Shuman. 2001. NAD+-dependent DNA ligase encoded by a eukaryotic virus. J. Biol. Chem. 276:36100-36109. [DOI] [PubMed] [Google Scholar]
- 116.Stack, J., I. R. Haga, M. Schroder, N. W. Bartlett, G. Maloney, P. C. Reading, K. A. Fitzgerald, G. L. Smith, and A. G. Bowie. 2005. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J. Exp. Med. 201:1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stauber, E., and R. Gogolewski. 1990. Poxvirus dermatitis in a tegu lizard (Tubinambis teguixin). J. Zoo Wildl. Med. 21:228-230. [Google Scholar]
- 118.Subramanian, A. R., J. Weyer-Menkhoff, M. Kaufmann, and B. Morgenstern. 2005. DIALIGN-T: an improved algorithm for segment-based multiple sequence alignment. BMC Bioinformatics 6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sullivan, C. S., and J. M. Pipas. 2002. T antigens of simian virus 40: molecular chaperones for viral replication and tumorigenesis. Microbiol. Mol. Biol. Rev. 66:179-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang, Z., B. Li, R. Bharadwaj, H. Zhu, E. Ozkan, K. Hakala, J. Deisenhofer, and H. Yu. 2001. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol. Biol. Cell 12:3839-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tourand, Y., K. Kobryn, and G. Chaconas. 2003. Sequence-specific recognition but position-dependent cleavage of two distinct telomeres by the Borrelia burgdorferi telomere resolvase, ResT. Mol. Microbiol. 48:901-911. [DOI] [PubMed] [Google Scholar]
- 123.Tulman, E. R., C. L. Afonso, Z. Lu, L. Zsak, G. F. Kutish, and D. L. Rock. 2004. The genome of canarypox virus. J. Virol. 78:353-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tulman, E. R., C. L. Afonso, Z. Lu, L. Zsak, G. F. Kutish, and D. L. Rock. 2001. Genome of lumpy skin disease virus. J. Virol. 75:7122-7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tulman, E. R., C. L. Afonso, Z. Lu, L. Zsak, J. H. Sur, N. T. Sandybaev, U. Z. Kerembekova, V. L. Zaitsev, G. F. Kutish, and D. L. Rock. 2002. The genomes of sheeppox and goatpox viruses. J. Virol. 76:6054-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vinh, L. S., and A. Von Haeseler. 2004. IQPNNI: moving fast through tree space and stopping in time. Mol. Biol. Evol. 21:1565-1571. [DOI] [PubMed] [Google Scholar]
- 127.Wasilenko, S. T., T. L. Stewart, A. F. Meyers, and M. Barry. 2003. Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc. Natl. Acad. Sci. USA 100:14345-14350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Willer, D. O., G. McFadden, and D. H. Evans. 1999. The complete genome sequence of shope (rabbit) fibroma virus. Virology 264:319-343. [DOI] [PubMed] [Google Scholar]
- 129.Xiang, Y., and B. Moss. 1999. IL-18 binding and inhibition of interferon gamma induction by human poxvirus-encoded proteins. Proc. Natl. Acad. Sci. USA 96:11537-11542. [DOI] [PMC free article] [PubMed] [Google Scholar]