Abstract
Open reading frames (ORFs) 21, 29, 62, 63, and 66 of varicella-zoster virus (VZV) are transcribed during latency in human ganglia. ORF 63 is the most frequently expressed gene, and ORF 62 encodes a transcriptional activator. The mechanisms regulating the expression of these genes are not well understood, although analyses of other alphaherpesviruses indicate a role for chromatin in virus gene regulation during latent infection. Using chromatin immunoprecipitation (ChIP) assays to analyze the euchromatic state of ORFs 62 and 63 compared to the centromere from human chromosome 4 (heterochromatic) and the human glyceraldehyde-3-phosphate dehydrogenase promoter (euchromatic), we show that the promoters of ORFs 62 and 63 are associated with the histone protein H3K9(Ac) and thus maintained in a euchromatic state during latency. Conversely, the promoters of ORF 36 (thymidine kinase) and ORF 14 (glycoprotein C), genes expressed during lytic but not latent infection, were not enriched in the fraction of latently infected ganglia that bound to anti-H3K9(Ac) antibody. A ChIP assay using productively infected MeWo cells revealed that VZV ORFs 62, 63, 36, and 14 are all euchromatic. Together, these data indicate that the expression of the two latency-related VZV genes, ORFs 62 and 63, is regulated epigenetically through chromatin structure.
Varicella-zoster virus (VZV) is an exclusively human neurotropic alphaherpesvirus. Primary infection almost always produces chickenpox (varicella), after which the virus becomes latent in cranial nerve, dorsal root, and autonomic ganglia along the entire neuraxis. Virus reactivation, usually many decades later, results in shingles (zoster). While a recent randomized, double-blind, placebo-controlled trial of >38,000 adults demonstrated that VZV vaccination significantly decreased the incidence of and morbidity from zoster (23), a better understanding of the physical state of the virus during latency in human ganglia is necessary to understand the mechanisms by which VZV latency is maintained and the virus reactivates.
To date, sequence analysis has verified the transcription of 5 of the approximately 70 known VZV open reading frames (ORFs 21, 29, 62, 63, and 66) in latently infected human ganglia (4, 5). Nevertheless, the mechanism by which these five genes are regulated during latency is unknown. Analyses of other human herpesviruses have revealed that virus gene regulation is associated with chromatin. Promoter regions regulating both Epstein-Barr virus (EBV) and herpes simplex virus type 1 (HSV-1) latently transcribed genes are associated with posttranslationally modified histone proteins, indicative of a euchromatic, transcriptionally permissive state (16, 18, 19, 27).
The basic unit of chromatin is the nucleosome, which is composed of a histone protein core entwined with a coil of DNA. Gene transcription, replication, and DNA repair result from modifications of histone core proteins, usually by methylation, phosphorylation, and acetylation (25, 30). For example, acetylation at histone protein H3 residues lysine 9 and lysine 14 in the histone core results in a euchromatic configuration of nucleosomes (12, 29). To determine whether VZV DNA is associated with chromatin in latently infected human ganglia, we studied four virus genes, two of which (VZV genes 62 and 63) are frequently transcribed in latently infected human ganglia (4, 6, 14) and two of which (VZV genes 36 and 14) are not transcribed during latency (11). A chromatin immunoprecipitation (ChIP) assay was used to determine the association of the histone protein H3 modified by acetylation at lysine 9 [H3K9(Ac)] with the promoters regulating VZV gene 14, 36, 62, and 63 transcription in latently infected ganglia compared to that in cells in tissue culture productively infected with VZV.
MATERIALS AND METHODS
Virus and cells.
The VZV Duman strain was used to infect MeWo cells propagated in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, Calif.) supplemented with 9% fetal bovine serum, and VZV DNA was extracted from isolated nucleocapsids (6).
Preparation of VZV-infected MeWo cells.
VZV-infected MeWo cells in 100-mm2 dishes were washed at 3 days postinfection with 1.0 ml of ice-cold phosphate-buffered saline (PBS) containing protease inhibitors (1× Complete Mini, used according to the manufacturer's instructions; Roche, Penzberg, Germany) (PBS-PI). After the removal of PBS-PI and the addition of 1.0 ml of fresh PBS-PI, cells were scraped into 15-ml conical tubes and brought to a final volume of 2.0 ml. DNA-protein complexes were cross-linked by the addition of 43.2 μl formaldehyde, vortexed for 10 s, and rocked horizontally on an orbital shaker for 10 min at room temperature. Glycine was added to a final concentration of 0.128 M, followed by vortexing and rocking on an orbital shaker for 5 min at room temperature. After centrifugation at 5,000 rpm for 5 min at 4°C, the cell pellet was washed and resuspended in 1 ml PBS-PI by vortexing. The pellet was recentrifuged at 5,000 rpm at 4°C for 5 min, washed once in PBS-PI, resuspended in 500 μl of sodium dodecyl sulfate lysis buffer (Upstate Biotech, Charlottesville, Va.), and incubated for 10 min on ice. Lysates were transferred to sterile 5-ml polypropylene culture tubes, brought to 1 ml with ChIP dilution buffer (Upstate), and placed in an ice-salt slurry at −7°C. Samples were sonicated (Sonifer W140D cell disruptor; Heat Systems-Ultrasonics, Farmingdale, NY) three times for 30 s each time at a setting of 3, with 90-s cooling intervals.
Preparation of human tissue.
Trigeminal ganglia (TG) were removed within 24 h of death from the subjects listed in Table 1. No subject had any signs of recent herpesvirus reactivation, and the patients were not immunocompromised before death. Each TG was washed twice in modified Eagle's medium and ground separately with a mortar and pestle in 750 μl of ice-cold PBS-PI. The homogenate was removed and, after the addition of 750 μl of ice-cold PBS-PI to the remaining tissue, further homogenized. Both 750-μl homogenates were combined, followed by the addition of 430 μl PBS-PI and 43.2 μl formaldehyde to yield a final concentration of 0.6% formaldehyde. The samples were vortexed for 10 s and rocked on an orbital shaker for 10 min at room temperature. The remaining preparation procedures were identical to those described above for MeWo cells.
TABLE 1.
Clinical information for subjects in this study
| Subject | Age (yr) | Sex | Cause of death |
|---|---|---|---|
| 1 | 78 | Female | Pneumonia |
| 2 | 85 | Female | Hypoxia |
| 3 | 42 | Male | Aneurysm-hemorrhage |
DNA fragment size determination.
After sonication, 30 μl of each sample (ganglia or tissue culture cells) was removed to ensure that chromatin was sheared to 0.5- to 1.0-kb fragments. Cross-linking of the samples was reversed by incubation with NaCl (final concentration, 0.2 M) at 65°C for 4 h followed by incubation with RNase (final concentration, 40 μg/ml) for 30 min at 37°C, and the samples were phenol-chloroform extracted, ethanol precipitated, washed in 70% cold ethanol, and resolved in a 1.6% agarose gel.
Chromatin preclearing.
After confirmation of chromatin size, the left and right TG samples were combined and centrifuged at 13,000 rpm at 4°C for 10 min. The supernatant was collected, diluted 1:10 with ChIP dilution buffer (Upstate), and incubated with a 50% (wt/vol) salmon sperm DNA-protein A agarose slurry (Upstate) (240 μl per TG pair) for 2 h at 4°C with shaking. Samples were centrifuged, and the supernatant was divided into 950-μl fractions.
Chromatin immunoprecipitation.
TG or cell samples were incubated with antibodies (3 μl) directed against the H3K9(Ac) protein (Upstate) or with control human immunoglobulin G (IgG; Vector, Burlingame, CA) overnight at 4°C with shaking in the dark. After further inoculation with a 50% (wt/vol) salmon sperm DNA-protein A agarose slurry (30 μl) at 4°C for 90 min with shaking, the antibody complexes were pelleted at 7,000 rpm for 30 s.
Supernatants (300 μl) from the human IgG controls were removed (unbound fractions). The pellets precipitated with H3 antibody (bound fractions) were washed according to the manufacturer's instructions (ChIP assay kit; Upstate Biotech), and the complexes were eluted from beads with preheated (65°C) elution buffer (0.1% sodium dodecyl sulfate, 0.1 M NaHCO3). Cross-linking was reversed by incubation with NaCl (final concentration, 0.2 M) for 4 h at 65°C. Samples were also treated with RNase (40 μg/ml) for 30 min at 37°C. Protein in the sample was degraded by incubation with EDTA (final concentration, 0.01 M), Tris, pH 6.5 (final concentration, 0.02 M), and proteinase K (40 μg/ml) at 65°C for 4 h.
PCR.
DNA was purified using a QIAquick PCR purification kit (QIAGEN, Germany). Equivalent volumes of the bound and unbound fractions were analyzed by PCR. Figure 1 shows the complete 125-kbp VZV genome and identifies the positions of VZV ORFs 14, 36, 62, and 63. Both a putative early gene (ORF 36) that encodes thymidine kinase and a late gene (ORF 14) encoding glycoprotein C were chosen. The promoter regions for the selected ORFs studied herein are also shown (Fig. 1D and E). Table 2 lists the sequences of VZV and cell primers used. For PCR, samples were initially incubated for 1 min at 95°C, followed by 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72 for 1 min, with a final cycle of 94°C for 30 s, 55°C for 30 s, and 72°C for 7 min. The amplified material was resolved in 8.0% acrylamide gels, visualized with SYBR green at a concentration recommended by the supplier's (Invitrogen) instructions, and imaged on a Storm imager (Molecular Dynamics, Fairfield, Conn.).
FIG. 1.
Schematic representation of VZV genome and locations of oligonucleotide primers. (A) The VZV genome consists of unique long (UL) and unique short (US) segments, each bracketed by repeat sequences. The UL is bracketed by terminal repeat (TRL) and internal repeat (IRL) sequences; US is also bracketed by terminal repeat sequences (TRS) and internal repeat sequences (TRS/IRS). (B) ORFs 14 and 36 are located within the UL and are transcribed in opposite directions. ORFs 62 and 63 map within IRS and also within TRS (ORFs 70 and 71, respectively). (C) Details of the VZV ORFs showing the locations of the ORF start sites and the origin of DNA replication (ori) between ORFs 62 and 63. (D) Further details of VZV ORFs 14, 36, 62, and 63, including TATA sites, minor transcriptional start sites, poly(A) sequences, and locations of untranslated regions (UTR). The major transcriptional start site of each ORF is referenced as +1. (E) Locations of oligonucleotide primers.
TABLE 2.
PCR primers used in this study
| Target | Primer name | Primer sequence | 5′ Location (chromosome [human] or position [VZV]) |
|---|---|---|---|
| Human chromosome | GAPdH 1 | ATC AGG TCT GGT GCC CTC TTC | 9 |
| GAPdH 2 | CTA ATG GTC CTA GTT AAA ACT | 9 | |
| GAPdH 3 | GGA TGC CTG TGT TGG GAC CC | 9 | |
| GAPdH 4 | CGT CTG CCC CAG TTT ATG AGC | 9 | |
| GAPdH 5 | CCC AAC TTT CCC GCC TCT C | 9 | |
| GAPdH 6 | CAG CCG CCT GGT TCA ACT G | 9 | |
| Chr 4 sat 3 | CTG CAC TAC CTG AAG AGG AC | 4 | |
| Chr 4 sat 4 | GAT GGT TCA ACA CTC TTA CA | 4 | |
| VZV | p62 3 | CGA TGT AGT GAT TGG ACG AGA CTC G | 109,310 |
| p62 4 | CAT GTC TGG TAT CCC TTG TTA TG | 109,451 | |
| p63 1 | GCT TTT TAA AAT CGA TTT GAC G | 110,335 | |
| p63 2 | CGA GAC CTT CGA TTG GGT TGC C | 110,486 | |
| VZV pTK 1 | GAG TAC ATC GTA AAA ACG AGT G | 64,679 | |
| VZV pTK 2 | CTT CGG CGG CGG TTG TTT TTC C | 64,897 | |
| VZV pgC 1 | CAG TCG ATA ATT GTA TAC ACG | 21,056 | |
| VZV pgC 2 | GGT ACA TAT GTA TCG TAA TCG AG | 21,275 |
RESULTS
Primer sensitivities.
To determine sensitivities of amplification for the cell controls, i.e., glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the centromere, and for VZV genes ORF 14, 36, 62, and 63, cell- and virus-specific primers were assayed by PCR. For cell targets, normal human brain (NHB) DNA was serially diluted 10-fold from 3.0 × 104 to 3.0 × 100 genome copies (Fig. 2A). Both primer sets amplified products to equal levels of sensitivity (3.0 × 100 genome copies). Control reactions with no DNA did not yield any product. PCRs of serially diluted (105 to 101) copies of VZV ORFs 62 and 63 revealed equivalent sensitivities, which detected products to the 103 dilution; similarly, VZV ORFs 14 and 36 were detected at 103 viral genome copies, although the faint ORF 14 band for 103 copies of VZV DNA in the original photograph did not reproduce clearly (Fig. 2B). Additionally, the ORF 62 and 63 PCR-amplified products were more intense than those obtained with the ORF 14 and 36 primers, most likely because the latter ORFs are single-copy genes, in contrast to ORFs 62 and 63, which are diploid.
FIG. 2.
Sensitivities of PCR primers. (A) NHB DNA was extracted, quantitated, diluted from 3 × 104 to 3 × 100 cell genome equivalents (based on the presence of 3 × 109 bp in one cell), and amplified with PCR primers specific for GAPDH (primer set 5/6) or the chromosome 4 centromere. Both cellular control PCR primer sets detected 30 genome equivalents of NHB DNA. (B) DNA was extracted from isolated VZV nucleocapsids, quantitated, diluted (105 to 101 virus DNA copies), and amplified with primers specific for VZV ORFs 62, 63, 36, and 14. A VZV-specific PCR product (103 copies) was detected with every primer set.
Chromatin profile during acute infection in tissue culture.
Initially, ChIP experiments were used to determine whether the promoters of VZV genes 14, 36, 62, and 63, as well as those for two regions of the cellular genome, were associated with H3K9(Ac) in infected MeWo cells (Fig. 3). At the height of a VZV-induced cytopathic effect, all four VZV gene promoters as well as the euchromatic control GAPDH promoter, but not the heterochromatic control centromeric region, were bound by H3K9(Ac). PCR analysis of the sample that did not bind the H3K9(Ac) antibody revealed the presence of both cellular controls and all four VZV ORFs (Fig. 3B).
FIG. 3.
ChIP assay of VZV-infected MeWo cells (2 ×107) harvested 3 days after infection. (A) DNA within the immunocomplex formed by antibody directed against H3K9(Ac) was extracted and PCR amplified in duplicate with cell control- and VZV-specific primer sets. Centromere-specific primers did not yield a detectable product (<103 cell genome equivalents). The GAPDH (primer set 1/2) and VZV-specific primer sets yielded PCR products. (B) DNA was extracted from supernatants of samples immunoprecipitated with a human anti-IgG control antibody. All six targets were amplified when unbound DNA was used as the PCR template.
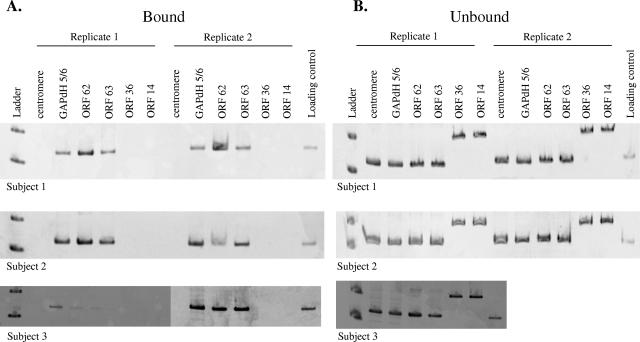
Chromatin profile in latently infected human TG.
Three pairs of human TG obtained at autopsy (Table 1 lists the clinical details) were analyzed for their association with H3K9(Ac) (Fig. 4). ChIP analysis with antibody directed against H3K9(Ac) detected the promoter of cellular GAPDH and the promoters of VZV ORFs 62 and 63, but not the promoters of ORFs 14 and 36 or the centromeric cellular control. PCR analysis of the supernatants from ganglionic samples incubated with a nonhistone protein antibody (anti-human IgG) revealed the presence of both cellular controls and all four VZV ORFs. The apparent size difference in PCR products obtained by amplification of centromeric DNA (Fig. 3B and 4B) occurred because cellular DNA was amplified from a transformed human malignant melanoma cell line for Fig. 3B, in contrast to cellular DNA amplified from human ganglia removed at autopsy for Fig. 4B.
FIG. 4.
ChIP assay of latently infected human trigeminal ganglia. Both trigeminal ganglia were removed from three subjects at autopsy and processed for ChIP analysis as described in Materials and Methods. (A) DNA contained within the immunocomplex formed by antibody directed against H3K9(Ac) was extracted and PCR amplified in duplicate with cell control- and VZV-specific primer sets. Centromere-specific primers and primers specific for VZV ORFs 36 and 14 did not yield detectable products (<103 cell genome equivalents and <103 VZV DNA copies, respectively). GAPDH primer set 5/6 and primers specific for VZV ORFs 62 and 63 yielded PCR products. (B) DNA was extracted from the supernatants of samples immunoprecipitated with a human anti-IgG control antibody. All six targets were amplified when this unbound DNA was used as the PCR template.
DISCUSSION
The present data demonstrate that the promoters for the latently transcribed VZV genes 62 and 63 are maintained in a euchromatic configuration by their association with the histone protein H3 acetylated at lysine 9. We also show that the promoters for VZV ORFs 14 and 36 are not immunoprecipitated with antibody directed against H3K9(Ac), indicating that they are silent during latent infection, consistent with earlier studies reporting the absence of the respective transcripts in latently infected ganglia (3, 7). Similarly, early studies by Deshmane and Fraser (8) indicating an association between histones and the HSV-1 genome in latently infected mouse ganglia were confirmed by the demonstration that the promoter regulating the transcription of the HSV-1 latency-associated transcript (LAT) was associated with H3K9(Ac) and K14(Ac) (18, 19).
In VZV-infected MeWo cells, the promoters for ORFs 14, 36, 62, and 63 were associated with histone H3K9(Ac), in accord with the previous detection of these transcripts in diverse productively infected cells (6, 13, 15, 17, 24). In HSV-1-infected cells in tissue culture, virus genes of all three kinetic classes (immediate-early, early, and late) were associated with H3K9(Ac) as well as with H3K14(Ac) (16).
Epigenetic regulation of virus gene transcription through chromatin structure has also been demonstrated for cells productively and latently infected with EBV. Specifically, in type I latently infected B cells, the promoters regulating EBNA-2 and LMP-1 transcription are associated with acetylated and methylated H3 and H4 proteins, resulting in transcriptional repression (1, 9, 10, 21, 22, 26, 27). Demethylation of the EBNA-2 and LMP-1 promoters activates transcription and mediates a switch from type I to type III EBV latency (1, 30).
Our results demonstrate that in ganglia latently infected with VZV, promoter regions regulating the transcription of two latently expressed VZV genes are associated with H3K9(Ac). Conversely, the promoters regulating the transcription of two VZV genes expressed solely during productive infection were not associated with H3K9(Ac). Therefore, the promoters for VZV genes 14 and 36 may be associated with a form of the H3 protein that leads to repression of gene transcription. Such an H3 modification is methylation at lysine 9 [H3K9(Me)] (20). We are currently investigating the association of H3K9(Me) with VZV ORF 14 and 36 promoter regions by a quantitative ChIP analysis that incorporates real-time PCR analysis of immunoprecipitated DNA. Associations of the VZV gene 14 and 36 promoters with H3K9(Me) would maintain those DNA regions in a heterochromatic state and keep them inaccessible for transactivation by VZV IE62. VZV IE62 is required for activation of the VZV gene 14 and 36 promoters (13). Removal of the methyl group on H3K9 by cellular demethylase, followed by K9 acetylation, has been shown to initiate a transition from type I to type III latency in EBV (1, 30). Thus, histone H3 modification, methylation, or acetylation may be a key regulator in the transcription of alphaherpesvirus genes leading to the establishment and maintenance of virus latency in human ganglia. Supporting this notion are recent reports comparing histone H3 modifications on HSV-1 gene promoters during latent infection and after virus reactivation in mouse models (2, 28). A greater association of heterochromatic H3K9(Me) with HSV-1 lytic gene promoters was found during latency than during virus reactivation. In addition, the HSV-1 LAT promoter and 5′ exon/enhancer region were shown to undergo a transition from euchromatic to heterochromatic H3 modification concomitant with an increase in euchromatic H3K9 hyperacetylation of the HSV-1 ICP4 promoter during reactivation. Taken together, these findings suggest that the HSV-1 LAT locus, associated with euchromatic H3K9(Ac) and H3K4(Me), is actively transcribed and functions, in part, to maintain the HSV-1 ICP4 promoter in a heterochromatic H3K9(Me) state. Upon reactivation, the LAT promoter/enhancer becomes progressively heterochromatic, whereas the HSV-1 ICP4 promoter becomes progressively euchromatic. Interestingly, VZV lacks a definable LAT locus, and VZV ORF 62 transcripts have been detected in latently infected human ganglia (4). Thus, a basic difference in the transcriptional patterns of HSV-1 ICP4 and VZV ORF 62 during latency is the posttranslational modification of histone protein H3, controlled in part by LAT, a gene present in HSV-1 but not found in VZV.
In summary, our study describes the first detection of a modified histone H3 protein associated with promoters for VZV genes that are transcribed in latently infected human ganglia and provides a rationale for further study of epigenetic gene regulation by alphaherpesviruses during latent infection.
Acknowledgments
This work was supported in part by Public Health Service grants AG 06127 and NS 32623 from the National Institutes of Health (NIH). Lee Gary is supported by Public Health Service training grant NS 07321 from the NIH.
We thank Mark Burgoon for the anti-human IgG antibody, Marina Hoffman for editorial assistance, and Cathy Allen for manuscript preparation.
REFERENCES
- 1.Ambinder, R. F., K. D. Robertson, and Q. Tao. 1999. DNA methylation and the Epstein-Barr virus. Semin. Cancer Biol. 9:369-375. [DOI] [PubMed] [Google Scholar]
- 2.Amelio, A. L., P. K. McAnany, and D. C. Bloom. 2006. A chromatin insulator-like element in the herpes simplex virus type 1 latency-associated transcript region binds CCCTC-binding factor and displays enhancer-blocking and silencing activities. J. Virol. 80:2358-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohrs, R. J., R. Mahalingam, A. N. Dueland, W. Wolf, M. Wellish, and D. H. Gilden. 1992. Restricted transcription of varicella-zoster virus in latently infected human trigeminal and thoracic ganglia. J. Infect. Dis. 166:S24-S29. [DOI] [PubMed] [Google Scholar]
- 4.Cohrs, R. J., M. Barbour, and D. H. Gilden. 1996. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J. Virol. 70:2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohrs, R. J., D. H. Gilden, P. R. Kinchington, E. Grinfeld, and P. G. Kennedy. 2003. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J. Virol. 77:6660-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohrs, R. J., M. P. Hurley, and D. H. Gilden. 2003. Array analysis of viral gene transcription during lytic infection of cells in tissue culture with varicella-zoster virus. J. Virol. 77:11718-11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croen, K. D., J. M. Ostrove, L. J. Dragovic, and S. E. Straus. 1988. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc. Natl. Acad. Sci. USA 85:9773-9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshmane, S. L., and N. W. Fraser. 1989. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J. Virol. 63:943-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernberg, I., K. Falk, J. Minarovits, P. Busson, T. Tursz, M. G. Masucci, and G. Klein. 1989. The role of methylation in the phenotype-dependent modulation of Epstein-Barr nuclear antigen 2 and latent membrane protein genes in cells latently infected with Epstein-Barr virus. J. Gen. Virol. 70:2989-3002. [DOI] [PubMed] [Google Scholar]
- 10.Falk, K. I., L. Szekely, A. Aleman, and I. Ernberg. 1998. Specific methylation patterns in two control regions of Epstein-Barr virus latency: the LMP-1-coding upstream regulatory region and an origin of DNA replication (oriP). J. Virol. 72:2969-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinfeld, E., C. Sadzot-Delvaux, and P. G. Kennedy. 2004. Varicella-zoster virus proteins encoded by open reading frames 14 and 67 are both dispensable for the establishment of latency in a rat model. Virology 323:85-90. [DOI] [PubMed] [Google Scholar]
- 12.Hansen, J. C., C. Tse, and A. P. Wolffe. 1998. Structure and function of the core histone N-termini: more than meets the eye. Biochemistry 37:17637-17641. [DOI] [PubMed] [Google Scholar]
- 13.Inchauspe, G., S. Nagpal, and J. M. Ostrove. 1989. Mapping of two varicella-zoster virus-encoded genes that activate the expression of viral early and late genes. Virology 173:700-709. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy, P. G., E. Grinfeld, and J. E. Bell. 2000. Varicella-zoster virus gene expression in latently infected and explanted human ganglia. J. Virol. 74:11893-11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy, P. G., E. Grinfeld, M. Craigon, K. Vierlinger, D. Roy, T. Forster, and P. Ghazal. 2005. Transcriptomal analysis of varicella-zoster virus infection using long oligonucleotide-based microarrays. J. Gen. Virol. 86:2673-2684. [DOI] [PubMed] [Google Scholar]
- 16.Kent, J. R., P. Y. Zeng, D. Atanasiu, J. Gardner, N. W. Fraser, and S. L. Berger. 2004. During lytic infection herpes simplex virus type 1 is associated with histones bearing modifications that correlate with active transcription. J. Virol. 78:10178-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinchington, P. R., P. Ling, M. Pensiero, B. Moss, W. T. Ruyechan, and J. Hay. 1990. The glycoprotein products of varicella-zoster virus gene 14 and their defective accumulation in a vaccine strain (Oka). J. Virol. 64:4540-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubat, N. J., A. L. Amelio, N. V. Giordani, and D. C. Bloom. 2004. The herpes simplex virus type 1 latency-associated transcript (LAT) enhancer/rcr is hyperacetylated during latency independently of LAT transcription. J. Virol. 78:12508-12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubat, N. J., R. K. Tran, P. McAnany, and D. C. Bloom. 2004. Specific histone tail modification and not DNA methylation is a determinant of herpes simplex virus type 1 latent gene expression. J. Virol. 78:1139-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehnertz, B., Y. Ueda, A. A. H. A. Derijck, U. Braunschweig, L. Perez-Burgos, S. Kubicek, T. Chen, E. Li, T. Jenuwein, and A. H. F. M. Peters. 2003. Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr. Biol. 13:1192-1200. [DOI] [PubMed] [Google Scholar]
- 21.Li, H., and J. Minarovits. 2003. Host cell-dependent expression of latent Epstein-Barr virus genomes: regulation by DNA methylation. Adv. Cancer Res. 89:133-156. [DOI] [PubMed] [Google Scholar]
- 22.Minarovits, J., L. F. Hu, S. Minarovits-Kormuta, G. Klein, and I. Ernberg. 1994. Sequence-specific methylation inhibits the activity of the Epstein-Barr virus LMP 1 and BCR2 enhancer-promoter regions. Virology 200:661-667. [DOI] [PubMed] [Google Scholar]
- 23.Oxman, M. N., M. J. Levin, G. R. Johnson, K. E. Schmader, S. E. Straus, L. D. Gelb, R. D. Arbeit, M. S. Simberkoff, A. A. Gershon, L. E. Davis, A. Weinberg, K. D. Boardman, H. M. Williams, J. H. Y. Zhang, P. N. Peduzzi, C. E. Beisel, V. A. Morrison, J. C. Guatelli, P. A. Brooks, C. A. Kauffman, C. T. Pachucki, K. M. Neuzil, R. F. Betts, P. F. Wright, M. R. Griffin, P. Brunell, N. E. Soto, A. R. Marques, S. K. Keay, R. P. Goodman, D. J. Cotton, J. W. Gnann, J. Loutit, M. Holodniy, W. A. Keitel, G. E. Crawford, S. S. Yeh, Z. Lobo, J. F. Toney, R. N. Greenberg, P. M. Keller, R. Harbecke, A. R. Hayward, M. R. Irwin, T. C. Kyriakides, C. Y. Chan, I. S. F. Chan, W. W. B. Wang, P. W. Annunziato, J. L. Silber, and the Shingles Prevention Study Group. 2005. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 352:2271-2284. [DOI] [PubMed] [Google Scholar]
- 24.Perera, L. P., J. D. Mosca, W. T. Ruyechan, and J. Hay. 1992. Regulation of varicella-zoster virus gene expression in human T lymphocytes. J. Virol. 66:5298-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 26.Tierney, R. J., H. E. Kirby, J. K. Nagra, J. Desmond, A. I. Bell, and A. B. Rickinson. 2000. Methylation of transcription factor binding sites in the Epstein-Barr virus latent cycle promoter Wp coincides with promoter down-regulation during virus-induced B-cell transformation. J. Virol. 74:10468-10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, L., S. R. Grossman, and E. Kieff. 2000. Epstein-Barr virus nuclear protein 2 interacts with p300, CBP, and PCAF histone acetyltransferases in activation of the LMP1 promoter. Proc. Natl. Acad. Sci. USA 97:430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, Q. Y., C. Zhou, K. E. Johnson, R. C. Colgrove, D. M. Coen, and D. M. Knipe. 2005. Herpesviral latency-associated transcript gene promotes assembly of heterochromatin on viral lytic-gene promoters in latent infection. Proc. Natl. Acad. Sci. USA 102:16055-16059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolffe, A. P. 1998. Packaging principle: how DNA methylation and histone acetylation control the transcriptional activity of chromatin. J. Exp. Zool. 282:239-244. [PubMed] [Google Scholar]
- 30.Zhang, Y., and D. Reinberg. 2001. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 15:2343-2360. [DOI] [PubMed] [Google Scholar]