Abstract
Interactions between the oncogenic retrovirus human T-cell leukemia virus type 1 (HTLV-1) and dendritic cells (DCs) are poorly characterized. We show here that monocyte-derived DCs form syncytia and are infected upon coculture with HTLV-1-infected lymphocytes. We examined the role of DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN), a C-type lectin expressed in DCs, in HTLV-1-induced syncytium formation. DC-SIGN is known to bind with high affinity to various viral envelope glycoproteins, including human immunodeficiency virus (HIV) and hepatitis C virus, as well as to the cellular receptors ICAM-2 and ICAM-3. After cocultivating DCs and HTLV-1-infected cells, we found that anti-DC-SIGN monoclonal antibodies (MAbs) were able to decrease the number and size of HTLV-1-induced syncytia. Moreover, expression of the lectin in epithelial-cell lines dramatically enhanced the ability to fuse with HTLV-1-positive cells. Interestingly, in contrast to the envelope (Env) glycoproteins of HIV and other viruses, that of HTLV-1 does not bind directly to DC-SIGN. The facilitating role of the lectin in HTLV-1 syncytium formation is mediated by its interaction with ICAM-2 and ICAM-3, as demonstrated by use of MAbs directed against these adhesion molecules. Altogether, our results indicate that DC-SIGN facilitates HTLV-1 infection and fusion of DCs through an ICAM-dependent mechanism.
Dendritic cells (DCs), which are antigen-presenting cells (APCs), have been shown to play a crucial role in generating and maintaining antiviral immunity. They act within the barriers first exposed to infectious agents, such as the epithelial (e.g., skin) and mucosal (e.g., respiratory, genital, and digestive tract) surfaces in which they are located (21). In fact, although DCs and macrophages represent only approximately 2% of the cells in peripheral blood (4), they are present in peripheral tissues and mucosal membranes in larger numbers than CD4+ and CD8+ T cells. DCs express molecules used by viruses to invade host cells. These molecules include the DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN) molecule CD209. DC-SIGN is a mannose-specific C-type lectin (9) specific to dendritic cells and macrophage subsets. It has been shown to bind the envelope glycoproteins (Env) of several viruses, including human immunodeficiency virus (HIV) (41), hepatitis C virus (23), dengue virus (34), Ebola virus (42), Marburg virus, and severe acute respiratory syndrome coronavirus (31). It has been demonstrated that its binding to some of these viruses enhances the spread of viral infection within the host, as shown for HIV transmission through breast milk macrophages (41). Such binding might also be involved in promoting exogenous major histocompatibility complex class I (MHC-I)-restricted antigen presentation (32).
Human T-cell leukemia virus type 1 (HTLV-1) is a human retrovirus associated with severe clinical manifestations, including adult T-cell leukemia/lymphoma (ATL) (47) and tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) (10, 37), as well as with other inflammatory disorders. The pathogenesis of HTLV-1-associated diseases is still poorly understood. A number of viral and host factors, such as the proviral load and the immune response (HLA haplotype), are involved in disease progression (3). Besides heparan sulfate molecules (38), Glut-1, a glucose transporter at the cell membrane, has been shown to interact with the HTLV-1 envelope glycoprotein (27). The route and age at occurrence of the primary viral infection contribute to the course of disease linked to HTLV-1 infection. Whereas primary viral infection through blood transfusion and sexual transmission have been correlated with TSP/HAM, ATL has been associated with HTLV-1 transmission via prolonged breastfeeding; most persons who develop ATL were infected at a relatively young age. The early steps of infection are suspected to take place in the mucosal membranes within the digestive tract, where APCs constitute potential target cells.
Few and conflicting data on DC infection by HTLV-1 have been reported. It was concluded in a previous study (48) that DCs are not susceptible to virus infection, as no evidence of virus uptake was observed after coculture with HTLV-1-releasing cell lines. In contrast, other studies showed that both monocytes/macrophages and DCs are susceptible to HTLV-1 infection, in addition to CD4+ T cells (20, 24, 39).
The present study was undertaken to explore the events in HTLV-1 infection of primary monocyte-derived human DCs and to follow the infection transfer from infected T cells to uninfected DCs, as is expected to take place during natural infections. Our data indicate that DC-SIGN does not interact with the HTLV-1 envelope glycoprotein but rather with ICAM-2 and ICAM-3 molecules expressed on infected T cells. These interactions facilitate the contact between effector and target cells and increase the efficiency of syncytium formation by fusion due to the HTLV-1 envelope protein.
MATERIALS AND METHODS
Cells, reagents, and antibodies.
Dendritic cells were prepared using a VacCell processor as previously described (43). Briefly, peripheral blood mononuclear cells from leukapheresis were grown for 7 days in serum-free VacCell medium (Invitrogen, Frederick, MD) supplemented with 500 U/ml granulocyte-macrophage colony-stimulating factor (Novartis, Huninge, France) and 50 ng/ml interleukin-13 (Sanofi-Synthelabo, Paris, France). DCs were isolated by elutriation. This procedure yielded CD1a+, MHC-I+, MHC-II+, CD64−, CD83−, CD80-low, and CD86-low cells. DC purity was >95%.
Different cell lines expressing DC-SIGN after transduction by a lentiviral vector (32) were used: HEK(293T) epithelial cells containing an integrated HTLV-1 long terminal repeat (LTR) coupled to a green fluorescent protein (GFP) reporter gene (40) and HeLa (43), Raji (32), and C1R-HLA-A*0201+ (C1R-A2) (32) cells. The cells were grown in Dulbecco's modified Eagle's medium (Gibco BRL, Gaithersburg, MD) supplemented with glutamine (1 mM), 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum (Gibco BRL).
Lymphoid cells.
MT2, C91-PL, and C81-66 infected cell lines were used as sources of HTLV-1. CEM, an HTLV-1 negative T-cell line derived from an acute lymphoblastic leukemia, was used as a negative control. The cells were maintained in suspension in RPMI 1640 cell growth medium (Gibco BRL) supplemented with glutamine (1 mM), 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum (Gibco BRL). The cells were adjusted to 5 × 105 cells/ml 18 h before the start of each experiment.
Flow cytometric, confocal analysis, and blocking experiments were performed with monoclonal antibodies (MAbs) to human DC-SIGN (CD209/DC-SIGN1; R&D Systems, Minneapolis, MN), ICAM-2 (CD102; Diaclone, Besançon, France), ICAM-3 (CD50; Immunotech, Marseille, France); or neurofilament M145K (Chemicon International, Temecula, CA) as an irrelevant control. An anti-Gag (p24) MAb (Cambridge Biotech, Worcester, MA) or a human HTLV-1-positive serum with a high immunofluorescence assay titer (1/10,000), as well as an HTLV-1-negative human serum, was used in some experiments. Fluorescein isothiocyanate- or Texas Red-conjugated secondary antibodies were obtained from Vector Laboratories. For immunofluorescence and flow cytometry experiments, antibodies were used according to the manufacturers' instructions. For blocking experiments, the different antibodies were incubated at 15 mg/ml for 30 min at room temperature.
Fusion assay.
T-lymphocyte fusion with DCs or adherent HEK or HeLa cells expressing DC-SIGN (5 × 104 cells/well grown in LabTek; Nunc-Nalge, Hereford, United Kingdom) was assessed by coculture at a 1:1 ratio. The cells were incubated for 7 h at 37°C, fixed for 20 min at room temperature in 4% paraformaldehyde, stained with Giemsa (5% in phosphate-buffered saline [PBS] for 5 min) and hematoxylin (5 min), and rinsed in tap water. Syncytium formation after fusion of infected T lymphocytes with uninfected DCs was observed 7, 24, and 48 h after contact.
Interaction of HTLV-1-Env glycoprotein with DC-SIGN.
The envelope expression vectors (kindly provided by J. L. Battini, Montpellier, France) used in this study were HPRR (HTLV-1 proline-rich region) and H2PRR (HTLV-2 proline-rich region) plasmids encoding the 215 and 211 N-terminal amino acids of the surface regions (including the receptor-binding domain [RBD]and the proline-rich region) of HTLV-1 and HTLV-2 Env proteins, respectively, fused to a C-terminal rabbit immunoglobulin Fc (rFc) tag (28). The ARBD (amphotropic murine leukemia virus [MuLV] RBD) plasmid encodes the 397 N-terminal amino acids of the amphotropic MuLV Env surface region fused to the same rFc tag (28). The H2 plasmid encodes the full-length HTLV-2 Env (28).
Envelope binding assays were carried out as described previously (28); HEK cells were transfected with the HPRR, H2PRR, and ARBD envelope expression vectors using the calcium phosphate method. After overnight incubation, fresh medium was added. Media were harvested the next day, clarified by centrifugation (5 min) at 3,000 rpm at 4°C, and filtered through a 0.45-μm-pore-size filter. The indicated target cells (5 × 105 cells/point) were washed with PBS containing 1% bovine serum albumin and 0.1% sodium azide; incubated with 300 μl of control, HPRR, H2PRR, or ARBD supernatant for 30 min at 37°C; washed; labeled for 30 min on ice with a phycoerythryn-conjugated mouse anti-rFc antibody (1:200 dilution; Southern Biotechnology Associates, Birmingham, AL), and analyzed by flow cytometry on a FACSCalibur (Becton Dickinson).
Binding of HIV pseudotypes: HIV virions pseudotyped with HIV, vesicular stomatitis virus glycoprotein (VSV-G), or HTLV-2 Env were prepared as described previously (29). Viral pseudotypes were infectious in single-cycle infectivity assays, confirming that they correctly incorporated the Env glycoproteins (not shown). For the binding assay, CR1A2-DC-SIGN-positive (and control) target cells (5 × 105 cells/point) were exposed for 2 h at 37°C to the indicated HIV pseudotypes (50 ng p24/106 cells) (35). The cells were then washed in cold phosphate-buffered saline, pelleted, and lysed in 0.5% Triton. The Gag p24 concentrations in cell lysates were analyzed by enzyme-linked immunosorbent assay (NEN, Perkin-Elmer Life Sciences, Boston, MA).
Immunofluorescence microscopy.
DCs were grown on glass coverslips and exposed to infected or uninfected lymphoid cell lines for different times at 37°C. The cells were then fixed with paraformaldehyde for 20 min. After immunofluorescence staining, observation was performed on a Leica DMRB fluorescence microscope.
Transmission electron microscopy and immunogold labeling.
DCs were incubated with infected (C91-PL or MT2) or uninfected (CEM) lymphoid cells for 3 h at 37°C (2 × 106 cells of each cell type) in round-bottom tubes (Becton Dickinson). The cells were then fixed in 2.5% glutaraldehyde and 1% paraformaldehyde in 0.15 M cacodylate buffer complemented with MgCl2, CaCl2, and sucrose at 0.1 M. After 2 days at 4°C, the cells were washed for 2 h in cacodylate buffer and postfixed for 1 h at room temperature in 1% osmium tetroxide-1% potassium ferrocyanide solution. The cells were dehydrated in ethanol and embedded in epoxy resin at 60°C for 48 h. Ultrathin sections were cut on a microtome (Leica Ultracut UCT). The sections were then examined in a JEOL 1200 EX electron microscope.
For immunolabeling, cocultures were fixed for 1 h at 4°C in a 0.1 M Sörensen buffer (pH 7.2) containing 4% paraformaldehyde and 0.1% glutaraldehyde. The filters were washed in 0.1 M Sörensen buffer and then briefly washed three times in distilled water. The samples were stained for 1 h with 0.5% uranyl acetate at 4°C, dehydrated, and embedded in Lowicryl K4M resin. After polymerization under UV light, thin sections were cut, collected on Formvar carbon-coated nickel grids, and immunolabeled. The grids were floated for 30 min in protein block solution (Aurion, Utrecht, The Netherlands) and then incubated in Tris buffer containing 0.5% bovine serum albumin and normal goat serum for 1 h at room temperature with anti-p24 antibody (1/5 dilution). The grids were washed and incubated with goat anti-mouse immunoglobulin G (IgG) plus IgM antibodies diluted at 1/25 and coupled to 10-nm colloidal-gold particles (BioCell, Cardiff, United Kingdom). After 1 h of incubation, the grids were washed, postfixed in 1% glutaraldehyde diluted in Tris-buffered saline, washed, stained with 4% uranyl acetate with lead citrate, and observed under a JEOL 1200 EX electron microscope operating at 80 kV.
Scanning electron microscopy.
DCs were incubated on coverslips overnight (3 × 105 DCs/coverslip), and then 3 × 105 infected or uninfected lymphocytes were added to the DCs and incubated for 3 h. The cells were washed in PBS and fixed in 2.5% (vol/vol) glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) overnight at 4°C. The cells were then washed three times in 0.2 M cacodylate buffer (pH 7.2), postfixed for 1 h in 1% (wt/vol) osmium tetroxide in 0.2 M cacodylate buffer (pH 7.2), and then rinsed with distilled water and dehydrated through a graded series of 25, 50, 75, 95, and 100% ethanol, followed by critical-point drying with CO2. The dried specimens were sputter coated twice with carbon with a Baltec Med evaporator and examined with a JEOL JSM 6700F field emission scanning electron microscope operating at 5 kV.
RESULTS
HTLV-1 transfer in DCs after contact with HTLV-1-infected T cells.
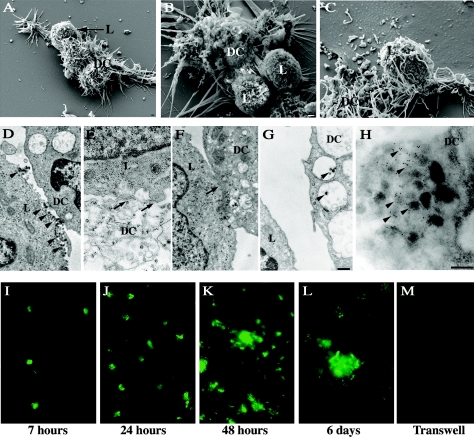
Monocyte-derived DCs were incubated with HTLV-1-infected lymphoid cells (MT2 or C91-PL) to observe cell interactions. Scanning electron microscopy analysis 3 h after contact showed recruitment and clustering of infected T cells in close contact with DCs (Fig. 1A, B, and C). In some images, DC processes were observed around HTLV-1-infected cells (Fig. 1C). In contrast, under similar experimental conditions, we did not observe clustering of uninfected CEM cells around DCs, although rare uninfected CEM cells could be seen in contact with DCs (data not shown). Under our culture conditions, clustering was specific to the HTLV-1-infected lymphocytes, with close intercellular contact with target DCs, as shown by transmission electron microscopy. The contact zone was characterized either by an apposition zone, forming a discontinuous interface (Fig. 1E), or by a tight contact (Fig. 1F). Viral particles were observed between infected T cells and uninfected DCs (Fig. 1D), with some particles internalized into DC vacuolar structures, in the lumen of the vesicle, or in contact with vesicular membranes (Fig. 1G). Immunogold detection of HTLV-1 p24 Gag protein showed positive signals in these vesicles (Fig. 1H), indicating that the virus might enter DCs by endocytosis.
FIG. 1.
Interaction of DCs with HTLV-1-infected T cells. (A to H) Analysis by electron microscopy at 3 h after contact between DCs and HTLV-1-infected cells (MT2). (A, B, and C) By scanning electron microscopy, MT2 cells could be detected in close contact with DCs (bar = 1 μm). (D and G) By transmission electron microscopy, virions were observed (arrowheads) located either between cells (D) or in the lumens of the DC vacuoles (G). (H) This observation was confirmed at a higher magnification with immunogold labeling for the viral p24 protein (arrowheads) (bar = 200 nm). (E and F) A close-contact zone between MT2 cells and DCs could be observed (arrows). (I to M) Assessment of DC infection by immunofluorescence directed to p24 at 7 h (I), 24 h (J), 48 h (K), and 6 days (L) after contact. No signal was detected when infected cells were placed in the upper chamber and DCs in the lower chamber of Transwell-Clear inserts until 6 days postcontact (M). Magnification, ×400.
In order to clarify whether DCs are permissive for HTLV-1 infection, the cytosolic HTLV-1 p24 antigen was identified by immunofluorescence soon after contact (7 h) and 1, 2, and 6 days after coculture. An increase in the fluorescence signal corresponding to the p24 capsid protein was detected over time after contact (Fig. 1I, J, K, and L). At day 6, large syncytia immunoreactive for p24 were observed (Fig. 1L). In contrast, when infected cells were placed in the upper chamber of Transwell-Clear inserts and DCs were grown in the lower chamber, neither infection of DCs nor syncytium formation was found, even at 6 days after contact (Fig. 1M). These results indicate that DCs become infected after contact with HTLV-1-infected T cells.
Fusion between HTLV-1-infected cells and DC target cells: role of DC-SIGN.
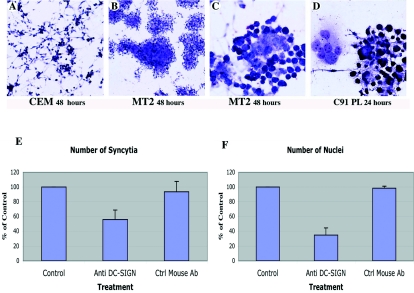
Syncytium formation was evaluated 7, 24, and 48 h after contact between DCs and HTLV-1-infected C91-PL or MT2 cells and in control (CEM) cells. Little clustering and no fusion were observed in mixed cultures of DCs and control CEM cells (Fig. 2A). In contrast, MT2 cells readily clustered with DCs, and syncytium formation was observed 48 h after contact (Fig. 2B and C). Similar results were obtained with C91-PL at an earlier time (24 h after contact) (Fig. 2D).
FIG. 2.
Fusion between HTLV-1-infected cells (MT2 and C91-PL) and uninfected DCs: role of DC-SIGN in syncytium formation. Fusion and syncytium formation were assessed by Giemsa staining. At 48 h postcontact, no clustering was observed in mixed cultures of DCs and uninfected CEM cell controls (A). Representative fields of clustering and syncytia formed between HTLV-1-infected lymphocytes and DCs: syncytia formed with MT2 cells at 48 h (B and C) and C91-PL cells at 24 h (D) postcontact. Magnification, ×100 (A and B) and ×200 (C and D). (E and F) The role of DC-SIGN in syncytium formation was assessed by determining the number of syncytia and the number of nuclei involved in these structures in DCs pretreated with an anti-DC-SIGN MAb (15 μg/ml, 30 min at room temperature) before coculture with HTLV-1-infected cells (C91-PL). At 24 h postcontact, the cells were fixed and processed for Giemsa staining. The number of syncytia (E) and the number of nuclei involved in syncytia (F) were determined in three different experiments on 15 to 30 microscopic fields (i.e., around 5,000 cells). The results represent the percentages of untreated controls (no antibody). An irrelevant MAb (anti-neurofilament) was used as a second control (Ctrl mouse Ab). The error bars indicate standard deviations.
We examined the role of DC-SIGN in HTLV-1-induced syncytium formation by cocultivating DCs in the presence or absence of anti-DC-SIGN MAb and counting the numbers of syncytia and of nuclei per syncytium. Blind analysis of randomly selected microscopic fields was performed, and the number of syncytia and the number of nuclei involved in syncytia were determined in three different experiments on 15 to 30 microscopic fields (i.e., around 5,000 cells). Syncytium formation decreased after preincubation of DCs with anti-DC-SIGN MAb. As shown in Fig. 2E, the number of syncytia was almost half that in control cultures in the absence of MAb or after incubation with an irrelevant MAb. The total number of nuclei involved in syncytia was also dramatically reduced (by about 70%) in anti-DC-SIGN MAb-treated cells compared with controls (Fig. 2F).
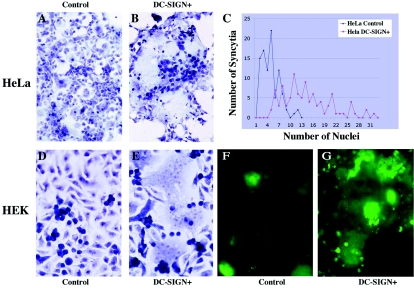
To further document the role of DC-SIGN in HTLV-1-induced fusion, we used two adherent cell lines, HeLa and HEK(293T) cells, each expressing or not expressing the lectin; HEK cells contain an integrated HTLV-1 LTR coupled with a GFP reporter gene. Cell fusion was evaluated by syncytium formation between adherent cells and infected lymphocytes (C91-PL and MT2). C91-PL cells fused strongly with HeLa cells that expressed DC-SIGN. Very large syncytia that involved most of the cell monolayer were observed as early as 7 h after contact (Fig. 3B), whereas much smaller syncytia were observed when C91-PL cells were cultivated with control HeLa cells (Fig. 3A). Expression of DC-SIGN increased both the number of syncytia and their relative size (the number of nuclei per syncytium) (Fig. 3C). The activity of DC-SIGN in HTLV-1-induced syncytium formation was associated with increased virus capture by the target cells: 24 h after contact, 28% of DC-SIGN-positive (1,731 cells counted), but only 10.5% of DC-SIGN-negative (1,773 cells counted), cells were immunoreactive for p24.
FIG. 3.
Fusion between HTLV-1-infected cells (C91-PL) and DC-SIGN-expressing cells (HeLa and HEK): role of DC-SIGN in syncytium formation. (A to C) HeLa cells, transduced (B) or not (A) by DC-SIGN expression vector, were incubated with HTLV-1-infected lymphocytes (C91-PL) for 7 h (ratio, 1:1) and processed for Giemsa staining. In DC-SIGN-positive HeLa cells, the number and the size of syncytia were greater (B) than in negative controls (HeLa cells that did not express DC-SIGN) (A). (Magnification, ×200.) (C) Quantification of the numbers of syncytia and the numbers of nuclei in DC-SIGN-positive cells and controls (DC-SIGN negative). (D to G) Similar results were obtained with HEK cells bearing an HTLV-1 LTR-GFP and transduced (E and G) or not (D and F) with DC-SIGN expression vector. In DC-SIGN-positive HEK cells, the number and the size of syncytia were greater (E and G) than in negative controls (HeLa cells that did not express DC-SIGN) (D and F), as shown by Giemsa staining (D and E) or by GFP fluorescence signal at 7 h after contact (F and G) (magnification, ×400).
Fusion between DC-SIGN-positive HeLa cells and MT2 cells was less efficient and slower than with C91-PL cells. Nevertheless, on day 6 after contact, expression of DC-SIGN increased HTLV-1-mediated fusion: the percentages of syncytia per number of cells counted in 15 randomly selected microscope fields were 23.8% (60 syncytia/252 cells) in DC-SIGN-positive cells but only 3.1% (9 syncytia/290 cells) in control HeLa cells.
Similar results were obtained with HEK(293T) cells containing an integrated HTLV-1 LTR coupled with a GFP reporter gene (40). Fusion with infected C91-PL cells was estimated either by Giemsa staining or by detection of a GFP fluorescent signal 7 h after contact. After fusion, the viral Tax-1 protein expressed in infected cells efficiently transactivated the LTR-driven reporter gene. The degree of syncytium formation, determined by phase-contrast microscopy and Giemsa staining, was greater in DC-SIGN-positive cells (Fig. 3E) than in control cells (Fig. 3D). The number of GFP-positive cells or syncytia counted in 30 randomly selected microscopic fields from two different cultures was fivefold greater in cultures in which DC-SIGN was expressed (112 in DC-SIGN-positive and 23 in DC-SIGN-negative cells). More GFP expression was observed 7 h after contact in HEK cells that expressed DC-SIGN (Fig. 3G) than in control cells (Fig. 3F). Syncytium formation increased with time: in many instances, a cytopathic effect linked to fusion led to detachment of the syncytia in DC-SIGN-positive cells 24 h after contact (data not shown).
C81-66 cells, which carry a defective HTLV-1 genome and express only the viral Tax protein, did not fuse with HeLa cells, regardless of whether they expressed DC-SIGN (data not shown). Similarly, no fusion was observed in control experiments in which HeLa and HEK cells were incubated with an uninfected lymphoid CEM cell line (data not shown).
Interaction of HTLV-1 Env glycoprotein with DC-SIGN.
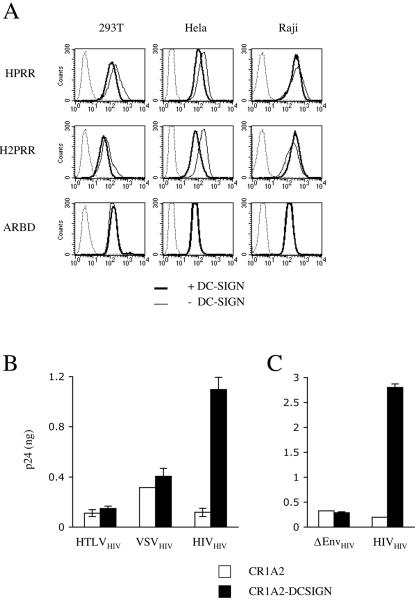
We examined whether the enhancement of HTLV fusion observed in DC-SIGN-expressing cells is mediated by direct binding of viral envelope glycoproteins to lectin. Two different binding assays were used, which were initially designed to identify the cellular glucose transporter Glut-1 as a receptor for HTLV (27). We studied the binding of truncated, soluble, recombinant HTLV envelopes and of HIV particles pseudotyped with full-length HTLV Env to target cells that expressed or did not express lectin.
We first used soluble envelopes encompassing the RBD of HTLV-1 (HPRR construct), of HTLV-2 (H2PRR), and, as a control, of amphotropic MuLV (ARBD). These envelopes were fused to a carboxy-terminal rabbit immunoglobulin Fc, allowing their detection by anti-rabbit Ig antibodies. Three human cell lines (293T, HeLa, and Raji cells) that express or do not express DC-SIGN were incubated at 37°C with these chimeric envelopes, and binding was measured by flow cytometry (Fig. 4A). With the HPRR and H2PRR constructs, efficient binding was detected in the absence of DC-SIGN, which was probably mediated by the interaction of HTLV envelopes with Glut-1 (27). The signal was not increased in the presence of lectin, strongly suggesting that DC-SIGN does not significantly interact with the RBD of HTLV envelope glycoproteins. Notably, the signal varied according to the concentration of envelope constructs in the culture medium and was not affected by DC-SIGN, whatever the concentration tested (data not shown). As expected, binding of amphotropic MuLV envelope glycoproteins, which use an unrelated cellular receptor (Pit-1), was unaffected by the lectin (Fig. 4A).
FIG. 4.
Interaction of DC-SIGN with HTLV envelope glycoproteins. (A) Binding of soluble HTLV-1 (HPRR), HTLV-2 (H2PRR), and amphotropic MuLV (ARBD) envelope glycoproteins to DC-SIGN-expressing cells. 293T, HeLa, and Raji cells, expressing or not expressing DC-SIGN, were incubated for 2 h at 37°C with the indicated envelope proteins fused to a carboxy-terminal rabbit immunoglobulin Fc tag. Binding was then measured by flow cytometry, using an anti-rabbit IgG. Soluble envelopes were omitted to determine background staining (dotted lines). The mock reaction on 293, HeLa, and Raji cells lacking DC-SIGN (mean fluorescence intensity, 35, 40, and 34, respectively) was superposable on the “+DC-SIGN” graph (MFI, 39, 42, and 35, respectively). (B) Binding of HIV pseudotypes carrying HTLV (HTLVHIV), VSV-G (VSVHIV), or HIV-1 (HIVHIV) Env glycoproteins to DC-SIGN-expressing cells. C1RA2 and C1RA2-DC-SIGN+ cells were incubated for 2 h at 37°C with the indicated pseudotypes. Cell-associated HIV p24 contents (ng of p24/0.5 × 106 cells) were then measured by enzyme-linked immunosorbent assay. The data are means (± standard deviations) of duplicates. Viral pseudotypes were infectious in single-cycle infectivity assays, confirming that they correctly incorporated the Env glycoproteins (not shown). (C) Binding of virions expressing or not expressing HIV Env glycoproteins (HIVHIV and ΔEnvHIV, respectively) to CR1A2 or CR1A2-DC-SIGN+ cells. HIV Env particles (HIVHIV) bound strongly to C1RA2-DC-SIGN+ cells, whereas HIV devoid of Env (ΔEnvHIV) bound equally to C1RA2 and C1RA2-DC-SIGN cell lines.
As the binding of truncated, soluble glycoproteins might differ from that of membrane-associated envelope, we examined whether HIV virions harboring HTLV envelope bind more efficiently to DC-SIGN-expressing cells. Binding of virions pseudotyped with HTLV-2 and of control HIV particles pseudotyped with VSV-G (which does not bind DC-SIGN) (35) or with HIV Env (which does bind DC-SIGN) was analyzed. We used a human B-cell line (C1RA2), expressing the lectin or not, as a target (32). C1RA2 and C1RA2-DC-SIGN cells were incubated at 37°C with VSVHIV, HTLVHIV, or HIVHIV virions, and viral binding was assessed after 2 h by measuring cell-associated HIV Gag p24 (Fig. 4B). We observed strong binding of virions expressing HIV Env glycoproteins to DC-SIGN-expressing cells (a ninefold increase with C1RA2-DC-SIGN+ cells in comparison with C1RA2 cells). This binding was mediated by HIV Env, since HIV particles devoid of viral envelope bound equally to C1RA2 or C1RA2-DC-SIGN+ cells (Fig. 4C) (36). Of particular interest, VSVHIV and HTLVHIV pseudotypes bound to C1RA2 cells to the same extent regardless of the presence of DC-SIGN (Fig. 4B). Similar results were obtained at 4°C (data not shown). Therefore, we did not observe direct binding of HTLV Env glycoproteins to DC-SIGN, using two different assays and four target cell lines expressing or not expressing the lectin.
Effects of anti-ICAM antibodies on syncytium formation.
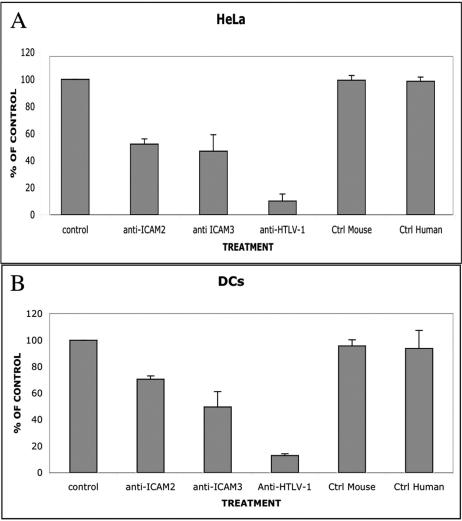
We tested whether the role of DC-SIGN in syncytium formation is due to binding to its known receptors, ICAM-2 and ICAM-3. When C91-PL cells were preincubated with MAbs specific for ICAM-2 and ICAM-3 (for 30 min at room temperature), around 50% inhibition of syncytium formation was observed in HeLa DC-SIGN-positive and infected T cells compared with controls (Fig. 5A and Table 1). In contrast, preincubation of lymphoid cells with serum from an HTLV-1-infected patient (with a high anti-Env response, as assessed by Western blotting) (data not shown) for 30 min led to a greater than 80% inhibition of syncytium formation in HeLa DC-SIGN-positive cells. Syncytium formation was not blocked by HTLV-1-negative control human serum (Fig. 5A).
FIG. 5.
Effects of anti-ICAM-2 and -ICAM-3 antibodies on HTLV-1-induced syncytium formation. (A) The roles of ICAM-2 and ICAM-3 in syncytium formation were assessed by counting the syncytia in HeLa DC-SIGN-positive cells at 7 h after contact with HTLV-1-infected lymphocytes; preincubation of lymphocytes for 30 min (at room temperature) with 15 μg/ml MAbs directed against ICAM-2 or ICAM-3 was performed. Cultures were stained with Giemsa, and the numbers of syncytia were evaluated in 12 different microscopic fields from two or three different cultures. The results are expressed as percentages of the control (without MAb); a second control was performed by using an irrelevant MAb (anti-neurofilament) (Ctrl Mouse). Strong inhibition was observed when an anti-HTLV-1 patient serum was used, whereas serum from an HTLV-1-negative patient (Ctrl Human) was inefficient. The error bars represent standard errors of the mean values. Inhibition of syncytium formation by anti-ICAM-2 or -ICAM-3 MAb was also observed in experiments performed with DC cultures (compiling data from two different donors) under the same conditions described above (B).
TABLE 1.
Effects of anti-ICAM-2 and ICAM-3 antibodies on HTLV-1-induced syncytium formation
| Cell type | Syncytium formationa
|
|||||
|---|---|---|---|---|---|---|
| Control | Anti- ICAM-2 | Anti- ICAM-3 | Anti- HTLV-1 | Control mouse | Control human | |
| HeLa | 100 | 52.28 | 46.93 | 10.18 | 99.4 | 98.70 |
| DC | 100 | 70.67 | 49.61 | 12.81 | 95.80 | 93.85 |
The roles of ICAM- and ICAM-3 in syncytium formation were assessed by counting the syncytia in HeLa DC-SIGN-positive cells or DCs at 7 h after contact with HTLV-1-infected lymphocytes; preincubation of lymphocytes for 30 min (at room temperature) with 15 μg/ml MAbs directed against ICAM-2 or ICAM-3 was performed. Cultures were stained with Giemsa, and the numbers of syncytia were evaluated in 12 different microscopic fields from two different cultures (or from two donors in the case of DCs). The results are expressed as percentages of the control (without MAb); a second control was performed by using an irrelevant MAb (anti-neurofilament [Control mouse]). Strong inhibition was observed when an anti-HTLV-1 patient serum was used, whereas serum from an HTLV-1-negative patient (Control human) was inefficient.
The inhibitory effect of anti-ICAM-2 and -3 on syncytium formation was also observed after incubation of infected lymphocytes before coculture with DCs from two donors. As shown in Fig. 5B, a 30% decrease in syncytium formation was observed with anti-ICAM-2 and a 50% decrease with anti-ICAM-3 compared to controls 24 h after contact. At later stages, a severe cytopathic effect, accompanied by syncytium detachment, was observed. As with DC-SIGN-positive HeLa cells, a polyclonal antiserum from an HTLV-1-infected patient induced a decrease of about 75% in DC syncytium formation (Fig. 5B).
DISCUSSION
In this study, we investigated the interactions between DCs and HTLV-1-infected T-cell lines. Entry of HTLV-1 during blood transfusion probably leads to the infection of T cells (CD4+ and, to a lesser extent, CD8+ T cells) according to the relative proportions of T cells and APCs in the peripheral blood and at mucosal surfaces. Alternatively, HTLV-1 entry at mucosal surfaces, during breastfeeding or by sexual transmission, might lead to infection of target APCs, such as DCs (4), as DCs are directly infected by viruses of various families, including Adenoviridae, Herpesviridae (e.g., herpes simplex virus, varicella-zoster virus, Epstein-Barr virus, and cytomegalovirus), Poxviridae, Orthoviridae, Paramyxoviridae, Filoviridae, and Retroviridae (HIV) (21). They might, therefore, be a key target for further spread of HTLV-1 within the host.
In the few papers devoted to the HTLV-1 infection of DCs, contradictory results have been reported. It was shown previously that DCs are susceptible to HTLV-1 infection in vitro (1, 25, 26), but another report indicated that DCs are not susceptible to HTLV-1 infection (48), as no evidence of virus uptake was observed after coculture with HTLV-1-infected cell lines. In the present study, we show that, after coculture of HTLV-1-infected cell lines with human DCs, viral particles can be detected within DC vacuoles as early as 3 h after contact and infection of DCs can be observed by immunofluorescence. DC infection is compatible with data obtained in vivo, which demonstrate infection of DCs in HTLV-1-infected asymptomatic carriers (12) and in TSP/HAM patients (20, 24, 25). HTLV-1 infection does not generally occur through cell-free virions, but rather by direct cell-cell transfer between lymphocytes (13), with involvement of cytoskeletal polarization (5). HTLV-1 disseminates from infected to uninfected cells by the formation of an intimate contact zone, termed the “virological synapse” (13), also described for HIV (17, 18), a structure in which exchanges of various factors take place, like those between lymphocytes and APCs (45). Accordingly, in our experiments, virus transfer was observed only upon cell-cell contact, whereas coculture with HTLV-1-infected cell lines seeded in the upper compartment of Transwell devices was inefficient in transmitting infection. Such cell-cell dissemination in vivo could explain the high proviral load found in infected patients despite good immune responses, as the virus might evade antibody-mediated host defenses.
In this study, virions were detected by electron microscopy within DC vacuoles. This pathway could provide an entry for exogenous presentation of HTLV-1 antigens in DCs. This process has been hypothesized to explain the activation of cytotoxic T lymphocytes in the absence of HIV replication (30). Moreover, a fraction of HTLV-1 particles might escape degradation and reach the cytosol, leading to productive infection. DCs might provide a reservoir of Tax-producing APCs, resulting in stimulation of a large number of CD8+ T cells, as observed during TSP/HAM (15, 16, 19). Infected progenitor cells in the bone marrow of TSP/HAM patients could provide a constant pool of infected DCs (14, 22). Moreover, Tax-induced maturation of DCs has been demonstrated during infection, implicating Tax-specific CTL in the genesis of TSP/HAM (33).
In the present study, we demonstrated the involvement of DC-SIGN in efficient syncytium formation. Our results indicate that fusion of infected cells with target cells increases when DC-SIGN is expressed in the target cells. This process can be inhibited by preincubation with anti-DC-SIGN MAbs. The numbers and sizes of syncytia were clearly higher in target cells that expressed this lectin. This was demonstrated by using different cell types expressing DC-SIGN (HeLa and HEK cell lines and human monocyte-derived DCs). As previously shown by some of us, DC-SIGN expression enhances HIV binding and transfer to HeLa CD4+ cells (35). The cell-type dependence of DC-SIGN enhancement of HIV transfer was shown to be correlated with the ability of HIV to replicate at a low level in some cells and not in others (36).
The data reported in our study are not due to binding of the HTLV-1 envelope glycoprotein to DC-SIGN, in contrast to several viruses, such as Marburg virus (31), Ebola virus (2), dengue virus (34), HIV (8), cytomegalovirus (11), and hepatitis C virus (23). Using target cell lines that either express or do not express the lectin in two different approaches, we did not observe direct binding of HTLV-1 Env glycoproteins to DC-SIGN. Thus, HTLV-1 has particular features that differentiate it from viruses known to bind to DC-SIGN.
DC-SIGN might act as a cofactor, helping to maintain a stable interaction between infected T cells and target cells. HTLV-1 Env gp46 glycoprotein has been reported to interact directly with its target cell receptor, Glut-1 (27), and with heparan sulfate proteoglycans (38). This interaction might explain the efficient binding of chimeric envelopes on the different cell lines and primary DCs used in our study. Under our experimental conditions, we cannot exclude the possibility that such interactions could disguise Env binding to DC-SIGN. However, in the case of HIV Env, binding to DC-SIGN can be detected on target cells in the absence, as well as in the presence, of CD4 and chemokine receptors (35). Furthermore, our data, obtained with primary monocyte-derived DCs, reflect the natural cell environment.
As DC-SIGN is known to interact with ICAM-2 and ICAM-3 adhesion molecules (7, 9), it might contribute to bringing together DCs and infected cells, increasing the adhesion between them and facilitating their fusion. Nevertheless, syncytium formation takes place only in coculture with infected cells, demonstrating the absolute requirement for HTLV-1 Env glycoprotein for fusion. We observed a large decrease in the number of fusion events when cells were preincubated with anti-ICAM antibodies. As shown by Daenke and colleagues (6), the fact that syncytium formation in mixed cultures could not be inhibited completely by blocking antibodies (directed to ICAM-1, ICAM-3, and VCAM-1) suggests that other molecules are involved in interactions between DCs and HTLV-1-infected cells.
Our study indicates that HTLV-1-infected cells can use surface adhesion molecules to regulate fusion with target cells, with the involvement of DC-SIGN and its ICAM ligands. This mechanism could have consequences for the regulation of both infection of DCs and dissemination of HTLV-1, but also for immune regulation. Further studies in vivo might allow us to confirm the role of such interactions, especially of the transmigration of DCs across endothelia, which express ICAMs (44, 46), and might have further implications in inflammatory disorders.
Acknowledgments
This work was supported by Association Française contre les Myopathies (AFM) and Institut Pasteur. F.D. is financially supported by a fellowship from Sidaction.
We thank N. Sol-Foulon and I. Romero for helpful discussions, F. Porrot for help in some experiments, and C. Schmitt and S. Guadagnini (Plate-Forme Microscopie Electronique, Institute Pasteur, Paris, France) for technical help in electron microscopy studies. J. L. Battini (CNRS, Montpellier, France) is acknowledged for the gift of plasmids used in the envelope binding assays.
REFERENCES
- 1.Ali, A., S. Patterson, K. Cruickshank, P. Rudge, A. G. Dalgleish, and S. C. Knight. 1993. Dendritic cells infected in vitro with human T cell leukaemia/lymphoma virus type-1 (HTLV-1); enhanced lymphocytic proliferation and tropical spastic paraparesis. Clin. Exp. Immunol. 94:32-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez, C. P., F. Lasala, J. Carrillo, O. Muniz, A. L. Corbi, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangham, C. R. 2003. The immune control and cell-to-cell spread of human T-lymphotropic virus type 1. J. Gen. Virol. 84:3177-3189. [DOI] [PubMed] [Google Scholar]
- 4.Barmak, K., E. Harhaj, C. Grant, T. Alefantis, and B. Wigdahl. 2003. Human T cell leukemia virus type I-induced disease: pathways to cancer and neurodegeneration. Virology 308:1-12. [DOI] [PubMed] [Google Scholar]
- 5.Barnard, A. L., T. Igakura, Y. Tanaka, G. P. Taylor, and C. R. Bangham. 2005. Engagement of specific T cell surface molecules regulates cytoskeletal polarization in HTLV-1-infected lymphocytes. Blood 106:988-995. [DOI] [PubMed] [Google Scholar]
- 6.Daenke, S., S. A. McCracken, and S. Booth. 1999. Human T-cell leukaemia/lymphoma virus type 1 syncytium formation is regulated in a cell-specific manner by ICAM-1, ICAM-3 and VCAM-1 and can be inhibited by antibodies to integrin β2 or β7. J. Gen. Virol. 80:1429-1436. [DOI] [PubMed] [Google Scholar]
- 7.Geijtenbeek, T. B., D. J. Krooshoop, D. A. Bleijs, S. J. van Vliet, G. C. van Duijnhoven, V. Grabovsky, R. Alon, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 1:353-357. [DOI] [PubMed] [Google Scholar]
- 8.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 10.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 11.Halary, F., A. Amara, H. Lortat-Jacob, M. Messerle, T. Delaunay, C. Houles, F. Fieschi, F. Arenzana-Seisdedos, J. F. Moreau, and J. Dechanet-Merville. 2002. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 17:653-664. [DOI] [PubMed] [Google Scholar]
- 12.Hishizawa, M., K. Imada, T. Kitawaki, M. Ueda, N. Kadowaki, and T. Uchiyama. 2004. Depletion and impaired interferon-alpha-producing capacity of blood plasmacytoid dendritic cells in human T-cell leukaemia virus type I-infected individuals. Br. J. Haematol. 125:568-575. [DOI] [PubMed] [Google Scholar]
- 13.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson, S., M. Krichavsky, N. Flerlage, and M. Levin. 1997. Immunopathogenesis of HTLV-I associated neurologic disease: massive latent HTLV-I infection in bone marrow of HAM/TSP patients. Leukemia 11(Suppl. 3):73-75. [PubMed] [Google Scholar]
- 15.Jacobson, S., D. E. McFarlin, S. Robinson, R. Voskuhl, R. Martin, A. Brewah, A. J. Newell, and S. Koenig. 1992. HTLV-I-specific cytotoxic T lymphocytes in the cerebrospinal fluid of patients with HTLV-I-associated neurological disease. Ann. Neurol. 32:651-657. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson, S., H. Shida, D. E. McFarlin, A. S. Fauci, and S. Koenig. 1990. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature 348:245-248. [DOI] [PubMed] [Google Scholar]
- 17.Jolly, C., K. Kashefi, M. Hollinshead, and Q. J. Sattentau. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 199:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jolly, C., and Q. J. Sattentau. 2004. Retroviral spread by induction of virological synapses. Traffic 5:643-650. [DOI] [PubMed] [Google Scholar]
- 19.Kannagi, M., S. Harada, I. Maruyama, H. Inoko, H. Igarashi, G. Kuwashima, S. Sato, M. Morita, M. Kidokoro, M. Sugimoto, et al. 1991. Predominant recognition of human T cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int. Immunol. 3:761-767. [DOI] [PubMed] [Google Scholar]
- 20.Knight, S. C., S. E. Macatonia, K. Cruickshank, P. Rudge, and S. Patterson. 1993. Dendritic cells in HIV-1 and HTLV-1 infection. Adv. Exp. Med. Biol. 329:545-549. [DOI] [PubMed] [Google Scholar]
- 21.Larsson, M., A. S. Beignon, and N. Bhardwaj. 2004. DC-virus interplay: a double edged sword. Semin. Immunol. 16:147-161. [DOI] [PubMed] [Google Scholar]
- 22.Levin, M. C., M. Krichavsky, R. J. Fox, T. Lehky, S. Jacobson, C. Fox, F. Kleghorn, J. White, N. Young, R. J. Edwards, N. E. Jack, and C. Bartholomew. 1997. Extensive latent retroviral infection in bone marrow of patients with HTLV-I-associated neurologic disease. Blood 89:346-348. [PubMed] [Google Scholar]
- 23.Lozach, P. Y., H. Lortat-Jacob, A. de Lacroix de Lavalette, I. Staropoli, S. Foung, A. Amara, C. Houles, F. Fieschi, O. Schwartz, J. L. Virelizier, F. Arenzana-Seisdedos, and R. Altmeyer. 2003. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J. Biol. Chem. 278:20358-20366. [DOI] [PubMed] [Google Scholar]
- 24.Macatonia, S. E., J. K. Cruickshank, P. Rudge, and S. C. Knight. 1992. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res. Hum. Retrovir. 8:1699-1706. [DOI] [PubMed] [Google Scholar]
- 25.Makino, M., S. Shimokubo, S. I. Wakamatsu, S. Izumo, and M. Baba. 1999. The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis. J. Virol. 73:4575-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makino, M., S. Wakamatsu, S. Shimokubo, N. Arima, and M. Baba. 2000. Production of functionally deficient dendritic cells from HTLV-I-infected monocytes: implications for the dendritic cell defect in adult T cell leukemia. Virology 274:140-148. [DOI] [PubMed] [Google Scholar]
- 27.Manel, N., F. J. Kim, S. Kinet, N. Taylor, M. Sitbon, and J. L. Battini. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115:449-459. [DOI] [PubMed] [Google Scholar]
- 28.Manel, N., S. Kinet, J. L. Battini, F. J. Kim, N. Taylor, and M. Sitbon. 2003. The HTLV receptor is an early T-cell activation marker whose expression requires de novo protein synthesis. Blood 101:1913-1918. [DOI] [PubMed] [Google Scholar]
- 29.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marechal, V., M. C. Prevost, C. Petit, E. Perret, J. M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marzi, A., T. Gramberg, G. Simmons, P. Moller, A. J. Rennekamp, M. Krumbiegel, M. Geier, J. Eisemann, N. Turza, B. Saunier, A. Steinkasserer, S. Becker, P. Bates, H. Hofmann, and S. Pohlmann. 2004. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 78:12090-12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moris, A., C. Nobile, F. Buseyne, F. Porrot, J. P. Abastado, and O. Schwartz. 2004. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood 103:2648-2654. [DOI] [PubMed] [Google Scholar]
- 33.Mostoller, K., C. Norbury, P. Jain, and B. Wigdahl. 2004. Human T-cell leukemia virus type I Tax induces the expression of dendritic cell markers associated with maturation and activation. J. Neurovirol. 10:358-371. [DOI] [PubMed] [Google Scholar]
- 34.Navarro-Sanchez, E., R. Altmeyer, A. Amara, O. Schwartz, F. Fieschi, J. L. Virelizier, F. Arenzana-Seisdedos, and P. Despres. 2003. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 4:723-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nobile, C., A. Moris, F. Porrot, N. Sol-Foulon, and O. Schwartz. 2003. Inhibition of human immunodeficiency virus type 1 Env-mediated fusion by DC-SIGN. J. Virol. 77:5313-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 79:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 38.Pinon, J. D., P. J. Klasse, S. R. Jassal, S. Welson, J. Weber, D. W. Brighty, and Q. J. Sattentau. 2003. Human T-cell leukemia virus type 1 envelope glycoprotein gp46 interacts with cell surface heparan sulfate proteoglycans. J. Virol. 77:9922-9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G. Dalgleish. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Royer-Leveau, C., E. Mordelet, F. Delebecque, A. Gessain, P. Charneau, and S. Ozden. 2002. Efficient transfer of HTLV-1 tax gene in various primary and immortalized cells using a flap lentiviral vector. J. Virol. Methods 105:133-140. [DOI] [PubMed] [Google Scholar]
- 41.Satomi, M., M. Shimizu, E. Shinya, E. Watari, A. Owaki, C. Hidaka, M. Ichikawa, T. Takeshita, and H. Takahashi. 2005. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. J. Infect. Dis. 191:174-181. [DOI] [PubMed] [Google Scholar]
- 42.Simmons, G., J. D. Reeves, C. C. Grogan, L. H. Vandenberghe, F. Baribaud, J. C. Whitbeck, E. Burke, M. J. Buchmeier, E. J. Soilleux, J. L. Riley, R. W. Doms, P. Bates, and S. Pohlmann. 2003. DC-SIGN and DC-SIGNR bind Ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 305:115-123. [DOI] [PubMed] [Google Scholar]
- 43.Sol-Foulon, N., A. Moris, C. Nobile, C. Boccaccio, A. Engering, J. P. Abastado,J. M. Heard, Y. van Kooyk, and O. Schwartz. 2002. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16:145-155. [DOI] [PubMed] [Google Scholar]
- 44.Szekanecz, Z., G. K. Haines, T. R. Lin, L. A. Harlow, S. Goerdt, G. Rayan, and A. E. Koch. 1994. Differential distribution of intercellular adhesion molecules (ICAM-1, ICAM-2, and ICAM-3) and the MS-1 antigen in normal and diseased human synovia. Their possible pathogenetic and clinical significance in rheumatoid arthritis. Arthritis Rheum. 37:221-231. [DOI] [PubMed] [Google Scholar]
- 45.Trautmann, A., and S. Valitutti. 2003. The diversity of immunological synapses. Curr. Opin. Immunol. 15:249-254. [DOI] [PubMed] [Google Scholar]
- 46.van Kooyk, Y., and T. B. Geijtenbeek. 2002. A novel adhesion pathway that regulates dendritic cell trafficking and T cell interactions. Immunol. Rev. 186:47-56. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zucker-Franklin, D., M. Fraig, and G. Grusky. 1995. Interaction of human immunodeficiency virus type 1, human T-cell leukemia/lymphoma virus type I (HTLV-I), and HTLV-II with in vitro-generated dendritic cells. Clin. Diagn. Lab. Immunol. 2:343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]