Abstract
The importance of the GABAergic system in spinal nociceptive processing has long been appreciated but we have only recently begun to understand how this system is modulated by the regulation of anion gradients. In neuronal tissues, cation-chloride cotransporters regulate Cl- homeostasis and the activity and/or expression of these transporters has important implications for the direction and magnitude of anion flow through GABA-A channels. Here we review recent evidence that two cation-chloride cotransporters, NKCC1 and KCC2 are involved in pain and enhanced nociception. On the one hand, NKCC1 activity is upregulated in primary afferents following an inflammatory insult and this produces excessive GABAergic depolarization in primary afferents leading to cross excitation between low and high threshold afferents. On the other hand, KCC2 expression is reduced in dorsal horn neurons following peripheral nerve injury resulting in a loss of GABA-/glycinergic inhibitory tone and, in some cases, inverting its action into net excitation. Pharmacological targeting of these cation chloride cotransporters to restore normal GABA-/glycinergic transmission in the spinal cord represents an entirely novel approach to the development of analgesics.
Keywords: Pain, hyperalgesia, chloride cotransporters, GABA, glycine
INTRODUCTION
Traditional pharmacological approaches to the treatment of pain and to the development of new analgesics have focused on the manipulation of receptor-transmitter targets through direct action at membrane receptors (such as opioids) or on the antagonism of enzymes responsible for transmitter production (such as NSAIDs). This approach has been successful in many areas but there are still numerous pain conditions resistant to current pharmacological treatments. In this paper we review a novel approach to the development of analgesic procedures based on the manipulation of anion gradients at the interface of the peripheral and central nervous systems and in spinal cord neurons. Intracellular Cl- anion gradients in neurons are largely controlled by cation-chloride cotransporters and these cotranporters are responsible for setting the reversal potential for GABA-A and glycine receptor channels as well as other Cl- channels [1]. The relevance of GABA-/glycinergic mechanisms in the control of afferent messages in the dorsal horn of the spinal cord, where the first synaptic contacts in the pain pathway take place, make cation-chloride cotransporters a novel target for the manipulation of neural transmission in the pain pathway. This article will review the current evidence that cation-chloride cotransporters play a role in animal models of pain and will present a rationale for how these molecules could be modified to achieve pain control in humans.
CATION-CHLORIDE COTRANSPORTERS AND NEURONAL CHLORIDE BALANCE
Cation-chloride cotransporters play a number of physiological roles ranging from ion secretion and fluid homeostasis between luminal cavities to volume regulation in response to osmotic alterations [2-4]. In nervous tissue, cation-chloride transporters play a key role in modulating GABA-A and glycine-mediated currents because they are a dominant mechanism for regulating intracellular Cl- concentrations [1]. Potassium chloride cotransporters (KCCs) and sodium potassium chloride cotransporters (NKCCs) comprise the two classes of cation-chloride transporters and are integrally involved in Cl- transport in neurons. KCCs normally reduce intracellular K+ and Cl- ions while NKCCs increase intracellular Na+, K+ and Cl- ions. Hence, KCCs and NKCCs have characteristically opposing effects on the Cl- reversal potential for the flow of anions through GABA-A and other anion channels. Because these transporters are electroneutral they do not require energy themselves and are driven by Na+-K+ ATPase pump gradients. The known KCC and NKCC genes are summarized in (Table 1). In nervous tissue KCC2 and NKCC1 are thought to be the major players in maintaining Cl- balance [1], hence, this review will focus on these two cotranporters.
Table 1.
| Protein Name | Accession # | Tissue Distribution | KO phenotype | Rank Order Potency of Antagonists |
|---|---|---|---|---|
| NKCC1 | NM_001046 | kidney, epithelium, CNS, sensory neurons (DRG, TG, olfactory) | Male infertility, deafness, shaker, hypoalgesia | bumetanide >> piretanide > furosemide |
| NKCC2 | NM_000338 | Kidney specific | Renal insufficiency, Barrter syndrome | |
| KCC1 | NM_005072 | Ubiquitous | No KO mice | |
| KCC2 | AF208159 | CNS, not DRG, TG | CNS hyperexcitability, epileptic activity | piretanide > furosemide ≈ bumetanide |
| KCC3 | AF314956 | Ubiquitous | Sensorymotor neuropathy | |
| KCC4 | NM_006598 | Ubiquitous, little in CNS | Deafness |
NKCC1 and KCC2 are regulated by a number of kinases which modulate their activity. However, their predominant influence on neuronal intracellular Cl- levels appears to be regulated mainly by gene expression (e.g. whether or not the gene is expressed by a given neuron and to what extent). It is generally accepted that in early developmental stages GABA acts primarily as an excitatory neurotransmitter in the brain [5-7]. This is due, to a large extent, to the developmental regulation of cation-chloride transporters [8-11]. At early developmental stages, NKCC1 expression levels are high and KCC2 levels are low, making NKCC1 the dominant cation-chloride cotransporter in the brain. Therefore, intracellular Cl- is maintained at a high level and this causes a membrane depolarization when GABA-A receptors are activated due to a net outward flow of anions. Eventually NKCC1 expression diminishes, and KCC2 expression increases, causing a shift in Cl- ion gradients leading to a reduction in the concentration of intracellular Cl-. This results in a reversal of GABA-A receptor mediated currents which now produce a net inflow of anions and an inward hyperpolarizing potential [12]. In certain experimental paradigms, such as peripheral nerve injury (as reviewed below [13]) or experimentally induced seizure [14], KCC2 expression is decreased leading to a decrease in the hyperpolarizing GABA-A mediated currents and, in some cases, its conversion to depolarization leading to net excitation (Fig. 1). This mechanism, which appears to be regulated by BDNF in the adult CNS [14-16], has been proposed as the molecular mechanism of the pathology associated with the loss of GABAergic inhibition. Interestingly BDNF appears to act in opposite directions in the adult vs the immature CNS [14, 16] and this may be due to a differential coupling of trkB receptors to intracellular pathways [17]. On the other hand, NKCC1 expression in the peripheral nervous system (PNS) is maintained throughout development and into adulthood leading to depolarizing GABA-A responses in primary sensory neurons throughout the life of the animal.
Fig. (1).
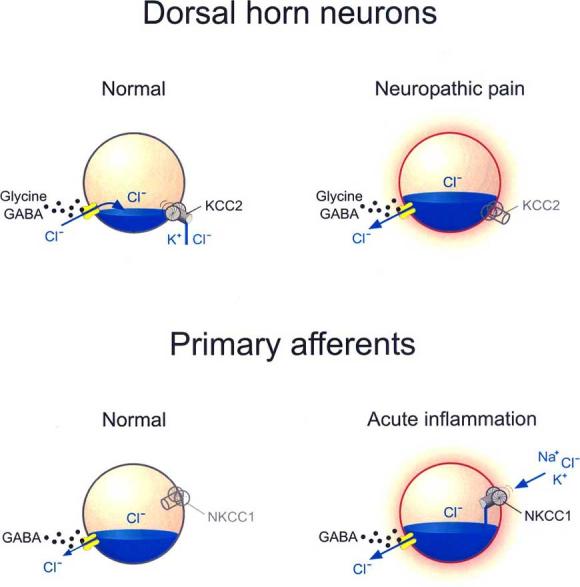
Role of Cation Chloride Cotransporters in regulation of Cl− gradients and flux. Cation-chloride cotransporters are responsible for setting the reversal potential for GABA-A or glycine receptor channels. In adult dorsal horn neurons, the anion reversal potential is maintained hyperpolarizing due to Cl− extrusion via KCC2. Trans-synaptic loss of KCC2 expression following peripheral nerve injury causes an intracellular Cl− accumulation which inverts the anion flux upon GABA-A or glycine receptor activation, and thus reverses their action. In primary afferents, GABA-A receptor activation is already depolarizing due to a slightly depolarizing Cl− reversal potential, maintained by the lack of KCC2 expression and by a weak NKCC1 expression. This depolarization remains inhibitory by causing Na+ channel inactivation and decreasing Ca++ influx upon action potential invasion in the terminal. Following acute inflammation, upregulation or enhanced activity of NKCC1 causes further accumulation of intracellular Cl− which produces an enhanced GABA-A receptor-mediated depolarization that can trigger action potentials effectively converting presynaptic inhibition into cross excitation between afferents.
PRIMARY AFFERENT DEPOLARIZATION (PAD), DORSAL ROOT REFLEXES (DRRs) AND GABA
The “Gate Control Theory [18]” of pain mechanisms proposed that large, myelinated Aβ-fibers could antagonize nociceptive primary afferent inputs to the dorsal horn through inhibitory mechanisms mediated by interneurons. The physiological substrate for this mechanism was proposed to be primary afferent depolarization (PAD). PAD refers to the commonly observed phenomena that a barrage of impulses in primary afferents evokes depolarization in other primary afferent terminals in the spinal cord. Because PAD shunts the magnitude of incoming action potentials and decreases excitatory amino acid release, this mechanism has been proposed to explain the presynaptic inhibition that occurs in the dorsal spinal cord (for review see [19, 20]). Importantly, the pharmacological mechanism underlying PAD appears to be GABA as GABA-A antagonists reverse PAD [21-25].
It is known that under normal conditions, Aβ-fibers conduct action potentials to the spinal dorsal horn where glutamate mediates excitation of GABAergic interneurons [24, 26]. These interneurons, in turn, release GABA which activates GABA-A receptors on primary afferents nerve terminals in juxtaposition to these GABAergic interneurons [27] (Fig. 2). Importantly, both myelinated and unmyelinated primary afferents make synaptic contacts with spinal GABAergic interneurons and these interneurons, in turn, make axo-axonic and dendro-axonic contacts with primary afferent central terminals [28, 29]. Furthermore, GABAergic interneurons appear to be presynaptic to unmyelinated axons in the dorsal horn suggesting that GABA is involved in presynaptic inhibition of these fibers as well [30]. Together, these findings indicate that GABAergic interneurons might constitute the morphological substrate for PAD.
Fig. (2).

Three mechanisms of altered Cl− homeostasis that enhance nociception by facilitating low threshold input to nociceptive neurons. The general diagram on the left illustrates how gating of the nociceptive input at spinal level can affect pain perception at the cortical level. The enlarged diagram on the right serves to illustrate distinct mechanisms by which low threshold (innocuous) input from large caliber afferents may be conveyed to nociceptive relay neurons, offering a substrate for touch evoked allodynia. For the sake of simplicity, the green GABA- and or glycinergic interneuron is represented as a single cell, but different interneuronal pathways may be involved. The numbers next to the interneuron represent one of the three possible mechanisms: 1) Exaggerated Primary Afferent Depolarization (PAD): Activity in low threshold non-nociceptive afferents activates GABAergic interneurons (shown in green) that release GABA onto the nociceptive afferent terminals. The activation of GABA-A receptors opens Cl− channels and Cl− ions flow out, partially depolarizing the membrane of the primary afferents (PAD). Enhanced activity of NKCC1 in the nociceptive afferent terminal would lead to an increased intracellular Cl− concentration, and therefore to a larger GABA-induced depolarization. This enhanced depolarization can reach firing threshold, producing action potentials in the nociceptive afferent terminal evoked by impulses in the low threshold afferent. 2) Disinhibition of polysynaptic pathways to nociceptive neurons: Low threshold afferents can excite feed-forward excitatory interneurons contacting nociceptive projection neurons. Excitability of these neurons can be maintained low by local GABA/glycinergic interneurons. Decreased KCC2 expression/activity in both the feed-forward and/or the nociceptive projecting neuron can unmask normally subliminal input to the latter from low threshold afferents. 3) Direct relay of low threshold input to nociceptive neurons via GABA/glycinergic interneuron: Following decreased KCC2 expression/activity in nociceptive projecting neurons input from low threshold afferents that would normally cause inhibition of the relay cell can now cause a net excitation of this neuron.
An important development in theories concerning the functional significance of PAD was the observation that primary afferents have the unusual property of maintaining a high intracellular Cl- concentration [31, 32]. Due to this, activation of GABA-A receptors produces an outward anion flux, leading to depolarization [31, 33]. This outward anion current is absent in NKCC1 knockout mice, indicating that NKCC1 expression is responsible for maintaining GABA-A reversal potentials (Fig. 1). Depolarizing GABA-A currents reduce the magnitude of incoming action potentials, leading to a reduction of neurotransmitter release in the dorsal horn [20].
As explained above, under normal conditions, an incoming afferent barrage from Aβ-fibers leads to inhibition of nociceptive afferents through PAD. However, in inflamed conditions PAD might be enhanced such that it now leads to excessive depolarization of A∂- and C-fibers above their thresholds for action potential generation (Fig. 1). This activation, termed dorsal root reflexes (DRRs), could produce a novel Aβ input to the nociceptive channel which it has been hypothesized to be a potential mechanism for touch-evoked pain in inflammatory conditions (Fig. 2) [34, 35]. Multiple lines of evidence suggest that the generation of DRRs is enhanced in inflammatory conditions which provoke touch-evoked allodynia [24, 36-38]. This process confers the ability to Aβ-fibers of stimulating A∂- and C-fiber terminals thus evoking anti and orthodromic action potential generation in small afferent fibers. Importantly, Lin et al. [38] have demonstrated that A∂- and C-fiber DRRs are evoked by mechanical stimulation of the hindpaw following intraplantar injection of capsaicin. In experiments designed to demonstrate that Aβ-fibers can evoke DRRs in C- and A∂-fibers, the sural nerve was stimulated at Aβ-fiber strength and blood flow was measured in the immediately surrounding area before and after mustard oil- or capsaicin-induced inflammation [36]. Aβ-fiber stimulation led to increases in blood flow, following capsaicin or mustard oil application, that were attenuated by dorsal root transection, sciatic nerve cut and peripheral calcitonin gene-related peptide (CGRP) receptor antagonism. This finding is consistent with the hypothesis that Aβ-fiber stimulation can lead to centrally-mediated antidromic action potential generation in A∂- and C-fibers. Additionally, DRRs modulate the propagation of signaling of A∂- and C-fibers onto ascending neurons of the outer spinal lamina. This effect was demonstrated by recordings from neurons in lamina I of the dorsal horn of anesthetized rats [39]. Prior to capsaicin or mustard oil application, Aβ-fiber stimulation was incapable of inducing action potential generation in lamina I neurons. However, following capsaicin or mustard oil application Aβ-fiber stimulation produced lamina I neuron excitation [39]. This excitation of lamina I neurons was dose-dependently reversed by topical spinal application of the GABA-A antagonist bicuculline, indicating that the alteration in lamina I neuron activity was dependent on GABAergic mechanisms. Together, these lines of evidence support the hypothesis that conditions leading to touch-evoked allodynia involve a GABA-A receptor-dependent unmasking of low threshold excitatory input to lamina I neurons and ascending activation of nociceptive pathways.
INVOLVEMENT OF NKCC1 IN SENSORY PROCESSING AND NOCICEPTION
NKCC1 is expressed by both DRG and trigeminal (TG) neurons as demonstrated by in situ hybridization and immunohistochemistry [32, 40-42]. These results indicate that many sensory neurons express NKCC1 but the precise population of NKCC1-expressing neurons have yet to be defined. While depolarizing responses to GABA are generally observed in all DRG or TG neurons this could be due to alterations in expression due to culturing techniques or a lack of KCC2 expression in sensory neurons [40, 41]. In that regard, GABA-A agonists applied directly to the dorsal root suppress the compound action potential mostly in slowly conducting fibers suggesting a possible heterogeneity in NKCC1 expression within the ganglion [42]. NKCC2, on the other hand, is not expressed by sensory neurons [1], and has not been detected in neuronal tissue to date. NKCC1 is also expressed in the spinal cord of adult animals; however, its expression appears to be mostly restricted to the inner lamina of the dorsal horn and neurons of the ventral grey areas [11, 43].
The first indication that NKCC1 might be involved in the pain pathway originated from the demonstration that NKCC1 knockout mice have deficits in thermal nociceptive thresholds [31]. Subsequently it was shown that NKCC1-KO mice display a decrease in Aβ-fiber-mediated touch-evoked allodynia following capsaicin injection into the hindpaw [44]. These results are consistent with the hypothesis that NKCC1 is involved in alterations in spinal processing leading to Aβ-fiber-mediated touch-evoked allodynia. In human skin, bumetanide and furosamide inhibit itch and flare responses to histamine [45]. Moreover, in the formalin model of tissue injury induced pain, peripheral bumetanide attenuated phase I and II behavioral responses and intrathecal delivery attenuated phase II responses [46]. These spinal effects were mimicked by piretanide and furosemide (inhibitors of both NKCCs and KCCs) and the effects of bumetanide were not blocked by naloxone. A number of findings have also been reported in abstract form from the Willis group indicate that bumetanide inhibits neurogenic inflammation [47] and DRRs [48] evoked by mechanical and electrical stimulation following capsaicin injection into the hind paw. Together, these results indicate that NKCC1 is involved in altered spinal processing in pain states.
REGULATION OF NKCC1 IN PAIN STATES
Neuronal Cl- balance is not only maintained by differential expression of cation-chloride cotransporters but is altered by the state-dependent transport kinetics of cation-chloride cotransporters. Phosphorylation has been pinpointed as an important regulator of NKCC1-mediated Cl- transport. NKCC1 is phosphorylated on the intracellular N-terminus of the protein [49] and phosphorylation leads to an increase in transport of ions through the cotransporter [50, 51]. Furthermore, NKCC1-dependent Cl- accumulation has been shown to be upregulated by a number of kinases including JNK [51], PKCδ [52, 53], CamKIIα [54] and ERK [52]. NKCC1 activity can be modified by mGluR and AMPA agonists and the mGluR-mediated upregulation of NKCC1 activity is attenuated by CamKIIα inhibition [54]. The afferent barrage associated with central sensitization increases the activity of multiple kinases in the spinal dorsal horn [55-58] suggesting that an increase in NKCC1 activity may accompany this enhanced afferent discharge and facilitate the transition of PAD into DRRs in primary afferent terminals under conditions that generate touch-evoked allodynia (Figs. 1 and 2). In support of this hypothesis, intra-colonic capsaicin injection in mice induced a significant increase in dorsal, spinal phosphorylated NKCC1 that was rapid (occurred within 10 min of injection) and transient [59]. Long lasting changes where also observed in membrane delivery of NKCC1 in the dorsal spinal cord lasting for at least 180 min after the stimulus and were likely not dependent on transcriptional alterations [59]. These results suggest that phosphorylation of NKCC1 might play a role in the initiation of hyperalgesia whereas trafficking is involved in maintenance of the hyperalgesic state. Additionally, they provide a novel therapeutic potential in that proteins involved in targeting NKCC1 to the membrane might be advantageously antagonized in a manner analogous to the targeting of scaffolding proteins for the NMDA-SRC complex [60, 61].
There are also indications that NKCC1 expression is altered in models of persistent inflammation. In a rodent model of arthritis NKCC1 mRNA expression was unchanged in ipsilateral DRG while increases were observed in the spinal dorsal horn [43]. Although the neurons showing an increase in NKCC1 expression were not identified it is tempting to speculate that these increases might accompany KCC2 downregulation in the same neurons, which will produce a direct GABAergic excitation in projection neurons (see below). This finding also raises the intriguing possibility that regulation of NKCC1 activity in primary sensory neurons might be mediated by kinases or alterations in membrane trafficking (as discussed above) whilst increases in spinal NKCC1 expression could lead to dual hyperexcitability of the spinal GABAergic system through alterations in anion gradients in the neurons of the superficial layers of the dorsal horn.
NKCC1 AND NON-GABAergic CL- CHANNELS
In olfactory neurons, GPCRs that respond to chemical stimulation stimulate cAMP accumulation which leads to the activation of cAMP gated Ca++ channels. This Ca++ influx then leads to the gating of a Ca++-activated Cl- channel. Because olfactory neurons express NKCC1, activation of Ca++-gated Cl- channels transduces an outward Cl- flux leading to depolarization of the neuron and propagation of the signal to the brain [62, 63]. The finding that NKCC1 antagonism in the periphery attenuates formalin induced nociceptive responses [46] and histamine-induced itch and flare [45] raises the possibility that NKCC1 might be involved in more than just GABAergic responses in sensory neurons. Although exogenous GABA leads to depolarization of DRG fibers in the periphery [64], the source of endogenous GABA in the periphery is unclear. On the other hand, inflammatory mediators couple to second messenger pathways that lead to both Ca++ entry and release of Ca++ from intracellular stores. Since sensory neurons also express Ca++-activated Cl- channels [65-67] and NKCC1 is found in the peripheral axons of sensory neurons [42, 68] this might provide a mechanism for NKCC1-mediated hyperexcitability in the periphery (as proposed by [46]).
GABA-A/GLYCINE-MEDIATED INHIBITION OF DORSAL HORN NEURONS
While inhibition of central endings of sensory nerves in the spinal dorsal horn (via PAD) constitutes a first level of control of input and appears to be largely mediated by GABA acting on GABA-A receptors, as mentioned above, a large proportion GABA-A and glycine receptor mediated inhibition is via a postsynaptic action on dorsal horn neurons [69-74]. Thus, another important site where modulating the Cl- reversal potential can have a dramatic impact is on the postsynaptic action of GABA and glycine on dorsal horn neurons. Indeed, disinhibition of local dorsal horn circuits can unmask polysynaptic inputs (normally repressed by local inhibitory neurons) from low threshold afferents to nociceptive pathways [75, 76] (Fig. 2). Furthermore, the conversion of GABA/glycine mediated transmission into net excitation may, itself, provide a direct excitatory link from low threshold input to nociceptive neurons (Fig. 2).
A ROLE FOR KCC2 IN NOCICEPTION AND DEVELOPMENT
As mentioned above, KCC2 expression increases throughout development leading to conversion of GABA-A / glycine mediated mechanisms from being net excitatory to inhibitory [77, 78]. In correlation with this developmental change, the threshold for nociceptive withdrawal reflexes is low in neonates and increases with age [79-82]. Because cutaneous primary afferent properties at birth are similar to those in adults, it has been suggested that central rather than peripheral mechanisms are responsible for the post-natal changes in cutaneous sensory reflexes [83]. A likely mechanism is that of disinhibition [84]. It appears however that the shift in reversal occurs at a different time scale (∼postnatal day 7) than that of the change in nociceptive threshold (>postnatal day 14) [85]. Yet, it is interesting to note in this context that quantitative measurements of the Cl- extrusion of the neurons reveal that full extrusion capacity is only achieved beyond postnatal day 14 in spinal lamina I [86]. This is functionally relevant because this deficiency in Cl- extrusion capacity is associated with a greater susceptibility of the cells to a collapse of the Cl- gradient in response to Cl- influx through GABA-A or glycine receptor themselves when activated. This leads to an inversion of the anion currents during the response to GABA or glycine from inhibitory to excitatory. This type of mechanism has been extensively described in other systems and suggested to underlie conditions of hyperexcitability such as epilepsy [87]. Thus, beyond changes in Cl- reversal potential affecting the extent and polarity of GABA-A / glycine receptor-mediated responses, incomplete extrusion capacity can affect the excitability of the tissue via a facilitated dynamic switch in Cl- flux during GABA / glycine responses.
A ROLE FOR KCC2 IN NEUROPATHIC PAIN
A depolarizing shift in Cl- reversal potential has also recently been shown to occur in the adult spinal dorsal horn neurons following peripheral nerve injury [13]. This shift occurred in lamina I neurons, one of the main spinal nociceptive output pathways, and it was due to a reduction in KCC2 expression in these cells (Fig. 1). The net effect of a depolarizing shift in Cl- reversal was disinhibition, and, in a subset of cells, the resulting shift could cause GABAergic mechanisms to switch to excitatory. Indeed, responses to applied GABA as well as GABA-A mediated synaptic potentials could trigger Ca++ influx as well as actions potentials in lamina I neurons. Local blockade or knockdown of the spinal KCC2 exporter in intact rats markedly reduced nociceptive threshold, confirming that the reported disruption of anion homeostasis in lamina I neurons was sufficient to cause neuropathic pain. As mentioned above, two mechanisms can be considered to contribute to this: 1) disinhibition of polysynaptic A-fiber input to lamina I neurons as well as 2) conversion of an inhibitory pathway activated by low-threshold inputs into an excitatory one (Fig. 2).
Particularly relevant to therapeutics is the question of whether endogenous factors are involved in the regulation of anion reversal potentials in dorsal horn neurons. Recent results are promising in this respect. Indeed, consistent with findings in the hippocampus, acute BDNF action also caused a depolarizing shift in anion reversal potential in spinal lamina I neurons and, inversely, blocking endogenous BDNF action with a function blocking antibody [88] reversed the depolarizing shift in anion reversal in animals with peripheral nerve injury [16]. The latter result showed not only that it is possible to modulate anion reversal potential in spinal lamina I neurons, it also shows that the depolarizing shift in anion reversal is due to an on-going modulatory mechanism. More recently, it has also been shown that ATP-stimulated microglia, which have been suggested to underlie nerve injury-induced allodynia [89] produce a depolarizing shift in anion reversal potential in adult neurons [90], suggesting that the latter may be the biophysical mechanism by which microglia alter neuronal excitability in these conditions.
Beyond disinhibition and or net excitation, GABA/glycine-mediated depolarization may also serve as a gating mechanism to enable or facilitate other excitatory mechanisms such as those mediated by voltage sensitive Ca++ channels (VSCCs) and NMDA receptor/channels [87]. Given that Ca++ influx via these channels is thought to be critical for the sensitization of spinal neurons [91, 92], KCC2, and possibly NKCC1, in spinal dorsal horn neurons may be critical targets to manipulate these forms of plasticity. Indeed, use of non-competitive NMDA antagonists [93] have been associated with undesirable side effects. Because GABA/glycine-mediated depolarization may be upstream of these mechanisms, this may provide a more adequate means to control activation of Ca++ influx.
PHARMACOLOGICAL CONSIDERATIONS
The only known specific inhibitor of NKCCs, bumetanide, is both an NKCC1 and NKCC2 antagonist. This loop diuretic would undoubtedly cause unwanted side effects in patients without renal difficulties and this may outweigh its potential analgesic properties in a clinical setting. Bumetanide has equal potency at both NKCC1 and NKCC2 (IC50 ∼ 100-300nM); however, these measurements are derived from studies of hypertonically activated (presumably phosphorylated [51]) NKCC1. NKCC1, at basal activity levels, has a reduced bumetanide sensitivity (IC50 ∼ 3μM [94]). This finding potentially supports an interesting route of pharmacological treatment in that specific antagonists for NKCC1 with enhanced activity at phosphorylated NKCC1 might be feasible. On the other hand, furosemide (NKCC1 IC50 ∼ 10μM) and piretanide (NKCC1 IC50 ∼1 μM [94]) are only slightly more potent antagonists of KCCs than NKCCs. Bumetanide and furosemide have similar potencies at KCCs [1]. Considering that blocking spinal KCC activity may raise excitability, blockers acting on both classes of transporters are unlikely to be useful therapeutics for pain. From another perspective, the pharmacology of many experiments is potentially muddled by the possibility that bumetanide also alters GABA-A channel function through a direct antagonist action [31]. Clearly, the development of improved NKCC1 antagonists are needed not only for experimental use but for potential therapeutics as a role for NKCC1 is emerging not only in the pain pathway but in neuroprotection following ischemic injury [95, 96].
In the case of KCC2, the fact that it is a loss of expression that is associated with the neuropathic pain condition poses a greater challenge for therapeutics. Potential avenues include positive modulators of the efficacy of the transporter and/or it expression. The results obtained with BDNF suggest that targeting intracellular second messenger systems may be viable avenues for therapeutic intervention. From a therapeutic stand point, it is also worthwhile stressing that targeting KCC2 or NKCC1 activity does not affect neuronal excitability directly, but, in turn, will modulate the efficacy of endogenous inhibition, similar to allosteric modulators such as benzodiazepines. Restoring endogenous inhibition rather than imposing inhibition may yield strategies that are more specific to the neuronal nuclei affected as well as providing safer therapeutic windows (e.g. comparison between benzodiazepines and barbiturates).
GENERAL CONCLUSIONS AND FUTURE DIRECTIONS
In this review we presented three general mechanism by which Cation Chloride Cotransporters may affect neuronal excitability in the spinal dorsal horn and cause hyperalgesia and allodynia: 1) cross talk between low and high threshold afferents via exaggerated PAD due to enhanced NKCC1 expression / activity, 2) disinhibition of low threshold input via polysynaptic pathways to nociceptive neurons via decreased KCC2 expression/activity and possibly enhanced NKCC1 expression/activity and 3) direct relay of low threshold input to nociceptive neurons via inverted inhibitory interneuron pathways following decreased KCC2 expression/activity and possibly enhanced NKCC1 expression/activity. These three mechanisms are summarized in (Fig. 1). Manipulation of anion gradients at these levels represent entirely novel therapeutic avenues for the treatment and prevention of chronic pain.
Several issues remain unsolved which will be important for the design of novel therapeutics targeting cation-chloride cotransporters. These include: do changes in KCC2 and NKCC1 expression parallel all phases of the chronic pain conditions in which they are involved or do they play transient roles in the etiology of the syndromes? Which kinases modulate the activity of KCC2 and NKCC1 and are these kinases under the control of endogenous spinal and peripheral neuromodulators? Moreover, what directs the trafficking of KCC2 and NKCC1 to the membrane and might scaffolding proteins represent unique targets such as has been suggested for the NMDA-SRC kinase complex? Finally, what is the role of NKCC1 in the periphery and might peripheral GABA play a role in inflammatory or nerve injury induced pain or might other Cl- channels, such as Ca++ activiated Cl- channels also be involved.
ACKNOWLEDGEMENTS
Work from the authors' laboratories was supported by grants from the Canadian Foundation for Innovation (CFI), the Canadian Institutes of Health Research (CIHR) and the Fonds de la recherche en santé du Québec (FRSQ). TJP is a NIH NRSA Fellow (NS049772), FC is the holder of a CIHR Research Chair and YDK is a senior Scholar of the FRSQ. The authors are grateful to Lisa Krawec for technical assistance and to Sylvain Côté for expert artwork in producing the illustrations.
REFERENCES
- 1.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26(4):199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 2.Mount DB, Gamba G. Renal potassium-chloride cotransporters. Curr. Opin. Nephrol. Hypertens. 2001;10(5):685–691. doi: 10.1097/00041552-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Hebert SC, Mount DB, Gamba G. Molecular physiology of cation-coupled Cl- cotransport, the SLC12 family. Pflugers Arch. 2004;447(5):580–593. doi: 10.1007/s00424-003-1066-3. [DOI] [PubMed] [Google Scholar]
- 4.Delpire E, Mount DB. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu. Rev. Physiol. 2002;64:803–843. doi: 10.1146/annurev.physiol.64.081501.155847. [DOI] [PubMed] [Google Scholar]
- 5.Barker JL, Behar T, Li YX, Liu QY, Ma W, Maric D, Maric I, Schaffner AE, Serafini R, Smith SV, Somogyi R, Vautrin JY, Wen XL, Xian H. GABAergic cells and signals in CNS development. Perspect Dev. Neurobiol. 1998;5(23):305–322. [PubMed] [Google Scholar]
- 6.Maric D, Liu QY, Maric I, Chaudry S, Chang YH, Smith SV, Sieghart W, Fritschy JM, Barker JL. GABA expression dominates neuronal lineage progression in the embryonic rat neocortex and facilitates neurite outgrowth via GABA(A) autoreceptor/Cl- channels. J. Neurosci. 2001;21(7):2343–2360. doi: 10.1523/JNEUROSCI.21-07-02343.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6(3):333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- 8.Hubner CA, Lorke DE, Hermans-Borgmeyer I. Expression of the Na-K-2Cl-cotransporter NKCC1 during mouse development. Mech. Dev. 2001;102(12):267–269. doi: 10.1016/s0925-4773(01)00309-4. [DOI] [PubMed] [Google Scholar]
- 9.Mikawa S, Wang C, Shu F, Wang T, Fukuda A, Sato K. Developmental changes in KCC1.; KCC2 and NKCC1 mRNAs in the rat cerebellum. Brain Res. Dev. Brain Res. 2002;136(2):93–100. doi: 10.1016/s0165-3806(02)00345-0. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1.; KCC2.; and NKCC1 mRNA expressions in the rat brain. Brain Res. Dev. Brain Res. 2002;139(1):59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Tornberg J, Kaila K, Airaksinen MS, Rivera C. Patterns of cation-chloride cotransporter expression during embryonic rodent CNS development. Eur J Neurosci. 2002;16(12):2358–2370. doi: 10.1046/j.1460-9568.2002.02419.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl- uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J. Physiol. 2004;557(Pt 3):829–841. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424(6951):938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 14.Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl- extrusion. J. Cell Biol. 2002;159(5):747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling, the K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 2005;562(Pt 1):27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coull JA, Boudreau D, Bachand K, De Koninck Y. Anion reversal potential in rat spinal lamina I neurons is modulated via the TrkB receptor. Soc. Neurosci. Abstr. 2003;(29) [Google Scholar]
- 17.Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, Payne JA, Minichiello L, Saarma M, Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 2004;24(19):4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melzack R, Wall PD. Pain mechanisms, a new theory. Science. 1965;150(699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 19.Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res. 1999;129(1):1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- 20.Willis WD., Jr. Dorsal root potentials and dorsal root reflexes, a double-edged sword. Exp. Brain Res. 1999;124(4):395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- 21.Eccles JC, Schmidt R, Willis WD. Pharmacological Studies on Presynaptic Inhibition. J. Physiol. 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mokha SS, McMillan JA, Iggo A. Dorsal root potentials in the cat, effects of bicuculline. Brain Res. 1983;259(2):313–318. doi: 10.1016/0006-8993(83)91265-9. [DOI] [PubMed] [Google Scholar]
- 23.Nishi S, Minota S, Karczmar AG. Primary afferent neurones, the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974;13(3):215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- 24.Rees H, Sluka KA, Westlund KN, Willis WD. The role of glutamate and GABA receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetized rat. J. Physiol. 1995;484(Pt 2):437–445. doi: 10.1113/jphysiol.1995.sp020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher JP, Higashi H, Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J. Physiol. 1978;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou X, Lin Q, Willis WD. NMDA or non-NMDA receptor antagonists attenuate increased Fos expression in spinal dorsal horn GABAergic neurons after intradermal injection of capsaicin in rats. Neuroscience. 2001;106(1):171–182. doi: 10.1016/s0306-4522(01)00175-0. [DOI] [PubMed] [Google Scholar]
- 27.Labrakakis C, Tong CK, Weissman T, Torsney C, MacDermott AB. Localization and function of ATP and GABAA receptors expressed by nociceptors and other postnatal sensory neurons in rat. J. Physiol. 2003;549(Pt 1):131–142. doi: 10.1113/jphysiol.2002.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribeiro-da-Silva A, Cuello AC. Choline acetyltransferase-immunoreactive profiles are presynaptic to primary sensory fibers in the rat superficial dorsal horn. J. Comp. Neurol. 1990;295(3):370–384. doi: 10.1002/cne.902950303. [DOI] [PubMed] [Google Scholar]
- 29.Olave MJ, Puri N, Kerr R, Maxwell DJ. Myelinated and unmyelinated primary afferent axons form contacts with cholinergic interneurons in the spinal dorsal horn. Exp. Brain Res. 2002;145(4):448–456. doi: 10.1007/s00221-002-1142-5. [DOI] [PubMed] [Google Scholar]
- 30.Todd AJ, Lochhead V. GABA-like immunoreactivity in type I glomeruli of rat substantia gelatinosa. Brain Res. 1990;514(1):171–174. doi: 10.1016/0006-8993(90)90454-j. [DOI] [PubMed] [Google Scholar]
- 31.Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J. Neurosci. 2000;20(20):7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J. Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudo Y, Abe N, Goto S, Fukuda H. The chloride-dependent depression by GABA in the frog spinal cord. Eur. J. Pharmacol. 1975;32(02):251–259. doi: 10.1016/0014-2999(75)90290-3. [DOI] [PubMed] [Google Scholar]
- 34.Cervero F, Laird JM, Garcia-Nicas E. Secondary hyperalgesia and presynaptic inhibition, an update. Eur. J. Pain. 2003;7(4):345–351. doi: 10.1016/s1090-3801(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 35.Cervero F, Laird JM. Mechanisms of touch-evoked pain (allodynia), a new model. Pain. 1996;68(1):13–23. doi: 10.1016/S0304-3959(96)03165-X. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Nicas E, Laird JM, Cervero F. Vasodilatation in hyperalgesic rat skin evoked by stimulation of afferent A beta-fibers, further evidence for a role of dorsal root reflexes in allodynia. Pain. 2001;94(3):283–291. doi: 10.1016/S0304-3959(01)00365-7. [DOI] [PubMed] [Google Scholar]
- 37.Rees H, Sluka KA, Westlund KN, Willis WD. Do dorsal root reflexes augment peripheral inflammation? Neuroreport. 1994;5(7):821–824. doi: 10.1097/00001756-199403000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Lin Q, Zou X, Willis WD. Adelta and C primary afferents convey dorsal root reflexes after intradermal injection of capsaicin in rats. J. Neurophysiol. 2000;84(5):2695–2698. doi: 10.1152/jn.2000.84.5.2695. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Nicas E, Laird JM, Cervero F. Sensitization of nociceptor-specific neurons by capsaicin or mustard oil, effect of GABAA-receptor blockade. In: Dostrovsky JO, D.B. C, Koltzenburg M, editors. Proceedings of the 10th World Congress on Pain. X. IASP Press; Seattle: 2003. pp. 327–335. [Google Scholar]
- 40.Toyoda H, Yamada J, Ueno S, Okabe A, Kato H, Sato K, Hashimoto K, Fukuda A. Differential functional expression of cation-Cl(−) cotransporter mRNAs (KCC1.; KCC2.; and NKCC1) in rat trigeminal nervous system. Brain Res. Mol. Brain Res. 2005;133(1):12–18. doi: 10.1016/j.molbrainres.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1.;2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104(4):933–946. doi: 10.1016/s0306-4522(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez-Leefmans FJ, Leon-Olea M, Mendoza-Sotelo J, Alvarez FJ, Anton B, Garduno R. Immunolocalization of the Na(+)-K(+)-2Cl(−) cotransporter in peripheral nervous tissue of vertebrates. Neuroscience. 2001;104(2):569–582. doi: 10.1016/s0306-4522(01)00091-4. [DOI] [PubMed] [Google Scholar]
- 43.Morales-Aza BM, Chillingworth NL, Payne JA, Donaldson LF. Inflammation alters cation chloride cotransporter expression in sensory neurons. Neurobiol. Dis. 2004;17(1):62–69. doi: 10.1016/j.nbd.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Laird JM, Garcia-Nicas E, Delpire EJ, Cervero F. Presynaptic inhibition and spinal pain processing in mice, a possible role of the NKCC1 cation-chloride co-transporter in hyperalgesia. Neurosci. Lett. 2004;361(13):200–203. doi: 10.1016/j.neulet.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Willis EF, Clough GF, Church MK. Investigation into the mechanisms by which nedocromil sodium.; frusemide and bumetanide inhibit the histamine-induced itch and flare response in human skin in vivo. Clin. Exp. Allergy. 2004;34(3):450–455. doi: 10.1111/j.1365-2222.2004.01898.x. [DOI] [PubMed] [Google Scholar]
- 46.Granados-Soto V, Arguelles CF, Alvarez-Leefmans FJ. Peripheral and central antinociceptive action of Na+-K+-2Cl− cotransporter blockers on formalin-induced nociception in rats. Pain. 2005;114(12):231–238. doi: 10.1016/j.pain.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Valencia De Ita S, Lawand NB, Gondensen KJ, Willis WD. The role of the Na+-K+-2Cl− cotransporter in the development of capsaicin-induced neurogenic inflammation. Soc. Neurosci. Abstr. 2003 doi: 10.1152/jn.01091.2005. [DOI] [PubMed] [Google Scholar]
- 48.Valencia De Ita S, Lawand NB, Castaneda-Hernandez G, Willis WD. Contribution of the Na+-K+-2Cl− cotransporter to the generation of dorsal root reflexes in rats. Soc. Neurosci. Abstr. 2004 [Google Scholar]
- 49.Darman RB, Forbush B. A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J. Biol. Chem. 2002;277(40):37542–37550. doi: 10.1074/jbc.M206293200. [DOI] [PubMed] [Google Scholar]
- 50.Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl otransporter NKCC1 detected with a phospho-specific antibody. J. Biol. Chem. 2002;277(40):37551–37558. doi: 10.1074/jbc.M206294200. [DOI] [PubMed] [Google Scholar]
- 51.Klein JD, Lamitina ST, O'Neill WC. JNK is a volume-sensitive kinase that phosphorylates the Na-K-2Cl cotransporter in vitro. Am. J. Physiol. 1999;277(3 Pt 1):C425–431. doi: 10.1152/ajpcell.1999.277.3.C425. [DOI] [PubMed] [Google Scholar]
- 52.Liedtke CM, Cole TS. Activation of NKCC1 by hyperosmotic stress in human tracheal epithelial cells involves PKC-delta and ERK. Biochim. Biophys. Acta. 2002;1589(1):77–88. doi: 10.1016/s0167-4889(01)00189-6. [DOI] [PubMed] [Google Scholar]
- 53.Liedtke CM, Cole TS. PKC signaling in CF/T43 cell line, regulation of NKCC1 by PKC-delta isotype. Biochim. Biophys. Acta. 2000;1495(1):24–33. doi: 10.1016/s0167-4889(99)00146-9. [DOI] [PubMed] [Google Scholar]
- 54.Schomberg SL, Su G, Haworth RA, Sun D. Stimulation of Na-K-2Cl cotransporter in neurons by activation of Non-NMDA ionotropic receptor and group-I mGluRs. J. Neurophysiol. 2001;85(6):2563–2575. doi: 10.1152/jn.2001.85.6.2563. [DOI] [PubMed] [Google Scholar]
- 55.Galan A, Lopez-Garcia JA, Cervero F, Laird JM. Activation of spinal extracellular signaling-regulated kinase-1 and -2 by intraplantar carrageenan in rodents. Neurosci. Lett. 2002;322(1):37–40. doi: 10.1016/s0304-3940(02)00078-2. [DOI] [PubMed] [Google Scholar]
- 56.Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, Tachibana T, Liu Y, Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J. Neurosci. 2002;22(17):7737–7745. doi: 10.1523/JNEUROSCI.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pezet S, Malcangio M, Lever IJ, Perkinton MS, Thompson SW, Williams RJ, McMahon SB. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol. Cell Neurosci. 2002;21(4):684–695. doi: 10.1006/mcne.2002.1205. [DOI] [PubMed] [Google Scholar]
- 58.Fang L, Wu J, Lin Q, Willis WD. Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J. Neurosci. 2002;22(10):4196–4204. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galan A, Cervero F. Painful stimuli induced in vivo phosphorylation and membrane mobilization of mouse spinal cord NKCC1 co-transporter. Neuroscience. 2005 doi: 10.1016/j.neuroscience.2005.02.025. in press. [DOI] [PubMed] [Google Scholar]
- 60.Gingrich JR, Pelkey KA, Fam SR, Huang Y, Petralia RS, Wenthold RJ, Salter MW. Unique domain anchoring of Src to synaptic NMDA receptors via the mitochondrial protein NADH dehydrogenase subunit 2. Proc. Natl. Acad. Sci. USA. 2004;101(16):6237–6242. doi: 10.1073/pnas.0401413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salter MW, Kalia LV. Src kinases, a hub for NMDA receptor regulation. Nat. Rev. Neurosci. 2004;5(4):317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 62.Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T. Chloride accumulation in mammalian olfactory sensory neurons. J. Neurosci. 2004;24(36):7931–7938. doi: 10.1523/JNEUROSCI.2115-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reisert J, Lai J, Yau KW, Bradley J. Mechanism of the excitatory cl(−) response in mouse olfactory receptor neurons. Neuron. 2005;45(4):553–561. doi: 10.1016/j.neuron.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carlton SM, Zhou S, Coggeshall RE. Peripheral GABA(A) receptors, evidence for peripheral primary afferent depolarization. Neuroscience. 1999;93(2):713–722. doi: 10.1016/s0306-4522(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 65.Mayer ML. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J. Physiol. 1985;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andre S, Boukhaddaoui H, Campo B, Al-Jumaily M, Mayeux V, Greuet D, Valmier J, Scamps F. Axotomy-induced expression of calcium-activated chloride current in subpopulations of mouse dorsal root ganglion neurons. J. Neurophysiol. 2003;90(6):3764–3773. doi: 10.1152/jn.00449.2003. [DOI] [PubMed] [Google Scholar]
- 67.Bader CR, Bertrand D, Schlichter R. Calcium-activated chloride current in cultured sensory and parasympathetic quail neurones. J. Physiol. 1987;394:125–148. doi: 10.1113/jphysiol.1987.sp016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na(+)-K(+)-2Cl- cotransporter BSC2 in the nervous system. Am. J. Physiol. 1997;272(1 Pt 1):C173–183. doi: 10.1152/ajpcell.1997.272.1.C173. [DOI] [PubMed] [Google Scholar]
- 69.Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I-III of the mammalian spinal dorsal horn. Prog. Neurobiol. 1993;41(5):609–645. doi: 10.1016/0301-0082(93)90045-t. [DOI] [PubMed] [Google Scholar]
- 70.Hongo T, Jankowska E, Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the lumbosacral cord. Exp. Brain Res. 1966;1(4):338–358. doi: 10.1007/BF00237706. [DOI] [PubMed] [Google Scholar]
- 71.Brown AG, Koerber HR, Noble R. An intracellular study of spinocervical tract cell responses to natural stimuli and single hair afferent fibres in cats. J. Physiol. 1987;382:331–354. doi: 10.1113/jphysiol.1987.sp016370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Koninck Y, Henry JL. Prolonged GABAA-mediated inhibition following single hair afferent input to single spinal dorsal horn neurones in cats. J. Physiol. 1994;476(1):89–100. [PMC free article] [PubMed] [Google Scholar]
- 73.Salter MW, De Koninck Y, Henry JL. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Prog. Neurobiol. 1993;41(2):125–156. doi: 10.1016/0301-0082(93)90006-e. [DOI] [PubMed] [Google Scholar]
- 74.Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices, pharmacological properties of synaptic currents. Neuroscience. 1993;53(2):519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]
- 75.Sherman SE, Luo L, Dostrovsky JO. Spinal strychnine alters response properties of nociceptive-specific neurons in rat medial thalamus. J. Neurophysiol. 1997;78(2):628–637. doi: 10.1152/jn.1997.78.2.628. [DOI] [PubMed] [Google Scholar]
- 76.Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol. Cell Neurosci. 2003;24(3):818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 77.Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA.; NMDA and AMPA receptors, a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20(11):523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- 78.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− cotransporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 79.Teng CJ, Abbott FV. The formalin test, a dose-response analysis at three developmental stages. Pain. 1998;76(3):337–347. doi: 10.1016/S0304-3959(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 80.Falcon M, Guendellman D, Stolberg A, Frenk H, Urca G. Development of thermal nociception in rats. Pain. 1996;67(1):203–208. doi: 10.1016/0304-3959(96)03070-9. [DOI] [PubMed] [Google Scholar]
- 81.Fitzgerald M, Gibson S. The postnatal physiological and neurochemical development of peripheral sensory C fibres. Neuroscience. 1984;13(3):933–944. doi: 10.1016/0306-4522(84)90107-6. [DOI] [PubMed] [Google Scholar]
- 82.Jiang MC, Gebhart GF. Development of mustard oil-induced hyperalgesia in rats. Pain. 1998;77(3):305–313. doi: 10.1016/S0304-3959(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 83.Fitzgerald M. Cutaneous primary afferent properties in the hind limb of the neonatal rat. J. Physiol. 1987;383:79–92. doi: 10.1113/jphysiol.1987.sp016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fitzgerald M. The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. J. Physiol. 1985;364:1–18. doi: 10.1113/jphysiol.1985.sp015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J. Neurosci. 2004;24(20):4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coredero-Erausquin M, Coull JA, Boudreau D, Rolland M, De Koninck Y. Ontogeny and mechanisms of GABA receptor-mediated rise in intracellular Ca2+ in spinal lamina I neurons of immature rats. 4th Forum of European Neuroscience. 2004;(A0106) [Google Scholar]
- 87.Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269(5226):977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- 88.Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J. Neurosci. 2000;20(19):7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 90.Beggs S, Coull JA, Biovin D, Boudreau D, Salter MW, De Koninck Y. Activated Microglia induce tactile allodynia in the rat through a disruption of anion homeostasis in dorsal horn lamina I neurons. Soc. Neurosci. Abstr. 2004;(292):13. [Google Scholar]
- 91.Woolf CJ, Salter MW. Neuronal plasticity, increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 92.Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J. Neurosci. 1992;12(9):3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parsons CG. NMDA receptors as targets for drug action in neuropathic pain. Eur. J. Pharmacol. 2001;429(13):71–78. doi: 10.1016/s0014-2999(01)01307-3. [DOI] [PubMed] [Google Scholar]
- 94.Hannaert P, Alvarez-Guerra M, Pirot D, Nazaret C, Garay RP. Rat NKCC2/NKCC1 cotransporter selectivity for loop diuretic drugs. Naunyn Schmiedebergs Arch. Pharmacol. 2002;365(3):193–199. doi: 10.1007/s00210-001-0521-y. [DOI] [PubMed] [Google Scholar]
- 95.Chen H, Luo J, Kintner DB, Shull GE, Sun D. Na(+)-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2005;25(1):54–66. doi: 10.1038/sj.jcbfm.9600006. [DOI] [PubMed] [Google Scholar]
- 96.Yan Y, Dempsey RJ, Flemmer A, Forbush B, Sun D. Inhibition of Na(+)-K(+)-Cl(−) cotransporter during focal cerebral ischemia decreases edema and neuronal damage. Brain Res. 2003;961(1):22–31. doi: 10.1016/s0006-8993(02)03832-5. [DOI] [PubMed] [Google Scholar]


