Abstract
The human epidermal growth factor receptor 2/neuregulin (HER2/neu) receptor is overexpressed in highly malignant mammary and ovarian tumors and correlates with a poor prognosis. It is a target for therapy; humanized monoclonal antibodies to HER2 have led to increased survival of patients with HER2/neu-positive breast cancer. As a first step in the design of an oncolytic herpes simplex virus able to selectively infect HER2/neu-positive cells, we constructed two recombinants, R-LM11 and R-LM11L, that carry a single-chain antibody (scFv) against HER2 inserted at residue 24 of gD. The inserts were 247 or 256 amino acids long, and the size of the gD ectodomain was almost doubled by the insertion. We report the following. R-LM11 and R-LM11L infected derivatives of receptor-negative J or CHO cells that expressed HER2/neu as the sole receptor. Entry was dependent on HER2/neu, since it was inhibited in a dose-dependent manner by monoclonal antibodies to HER2/neu and by a soluble form of the receptor. The scFv insertion in gD disrupted the ability of the virus to enter cells through HVEM but maintained the ability to enter through nectin1. This report provides proof of principle that gD can tolerate fusion to a heterologous protein almost as large as the gD ectodomain itself without loss of profusion activity. Because the number of scFv's to a variety of receptors is continually increasing, this report makes possible the specific targeting of herpes simplex virus to a large collection of cell surface molecules for both oncolytic activity and visualization of tumor cells.
Viruses are being explored as oncolytic agents and vectors. Ideally, oncolytic viruses and viral vectors should be capable of infecting and/or multiplying solely in the cells that need to be targeted. To achieve this goal, the two strategies that are being explored include the transcriptional control of an essential viral gene, e.g., by a tumor-specific promoter, or the retargeting of the virus tropism to specific receptors present exclusively or predominantly on the surfaces of the targeted cells.
A notable example of oncolytic viruses is a group of genetically engineered herpes simplex viruses (HSVs) designed to treat malignant gliomas, human tumors for which no therapy is available at present (1, 35, 47, 48). The recombinants that successfully underwent phase 1 clinical studies had been extensively debilitated by the simultaneous deletion of the γ134.5 gene, encoding the infected cell protein γ1 no. 34.5 (ICP γ134.5), and the UL39 gene, encoding the large subunit of ribonucleotide reductase (2, 24, 38). The viral γ134.5 protein is designed to preclude the total synthesis shutoff mediated by the activated protein kinase R; in its absence, the virus is blocked by the interferon pathway (25). The additional UL39 gene deletion ensures that second-site mutations to both genes are not likely to arise (35, 38). The strength of these deletion mutant viruses is that they have a significantly reduced capacity to replicate in normal, nondividing cells in vivo, and therefore they exhibit a high safety profile; the two drawbacks are that they replicate poorly even at the site of inoculation and maintain the tropism of the wild-type (wt) virus. Clearly, the availability of viruses with selective tropism to the tumor cells would allow the use of less attenuated viruses with higher oncolytic activity.
Entry of HSV into cells is a multistep process, controlled by at least four essential viral glycoproteins and cellular receptors. Following virus attachment to heparan sulfate proteoglycans, mediated by the viral glycoproteins gC and gB, gD interacts with one of two alternative protein receptors. One, designated HVEM (herpesvirus entry mediator), is a member of the family of receptors for tumor necrosis factors (40). The second group includes nectin1 and nectin2, intercellular adhesion molecules belonging to the immunoglobulin (Ig) superfamily (11, 12, 19, 32, 53). The gD ectodomain is organized into two functionally and topologically distinct domains: the N-terminal domain, carrying the receptor-binding sites and exhibiting an Ig-folded structure with extensions, and the C-terminal domain, carrying the pro-fusion domain that folds back toward the N terminus (7, 10, 18, 31). When gD interacts with one of its receptors, the pro-fusion domain is displaced from its binding site on the gD N terminus, and gD switches from a closed to an open conformation and triggers fusion (18, 31), possibly by recruiting or activating the three downstream glycoproteins, gB, gH, gL, or a subset of them. Of these, gH is the leading candidate to execute fusion of the virion envelope with cell membranes (17, 21-23, 52). Depending on the cell, fusion takes place at plasma membranes or in endocytic vesicles (20, 42).
A remarkable achievement in the design of oncolytic HSV has been the genetic engineering of gD such that it can mediate entry through a novel receptor. The first receptor selected was the interleukin 13 (IL-13) receptor α2 (IL-13Rα2), a protein specifically exhibited on the surfaces of malignant gliomas (58, 59). Retargeting of the HSV tropism was achieved by insertion of IL-13 (134 amino acids [aa]) at aa 24 of gD. The resultant IL-13-gD chimeric virus was capable of infecting cells that express IL-13Rα2 as the sole receptor. A second recombinant, engineered according to the same strategy, carried part of the urokinase plasminogen activator (uPA) inserted into gD and exhibited a tropism to the uPA receptor (30). In a totally different strategy, HSV tropism was redirected to the epidermal growth factor receptor (EGFR) by means of a soluble adapter protein comprising the N-terminal domain of nectin1 fused to a single-chain antibody (scFv) to EGFR (41).
The objective of this work was to genetically engineer an HSV capable of infecting cells through the human epidermal growth factor receptor 2/neuregulin (HER2/neu) receptor, a member of the tyrosine kinase EGFR family, also named EGFR2 (54). Overexpressed or mutated HER2/neu forms homodimers that interact with no known natural ligand, or heterodimers with other EGFRs that bind members of the EGF family. The dimers transduce positive growth signals and are thus involved in the initiation and progression of neoplastic transformation (28, 54). The clinical relevance of HER2/neu stems from the fact that it is overexpressed in 25 to 30% of human breast cancers, which account for one out of three cancer diagnoses in women, as well as in some ovarian cancers. In mammary tumors, HER-2/neu expression correlates with particular invasiveness, metastatic ability, overall aggressiveness of the tumor, and a poor prognosis (26). Because of these properties, HER2/neu is a target for therapy. The humanized anti-HER2/neu antibody called Herceptin, or Trastuzumab, applied in conjunction with standard chemotherapy, has led to an increase in the response rate, time to disease progression, and overall survival for patients with HER2/neu-positive metastatic breast cancer (16, 45, 50, 51). Despite these proven benefits, however, treatment with Herceptin fails to eradicate the tumor or its metastases, and a more effective treatment is needed (28).
Inasmuch as HER2/neu has no specific natural ligand, in order to generate an HSV recombinant specifically redirected to HER2/neu, we engineered into gD a single-chain antibody derived from monoclonal antibody (MAb) 4D5 against HER2/neu (27, 49). It is noteworthy that the gD ectodomain is composed of 310 aa residues and the scFv to HER2 (scHER2) has 250 aa, and therefore the insertion almost doubled the Mr of gD. We report that the HSV recombinants carrying chimeric gD-scHER2 infect cells that exhibit HER2/neu as the sole receptor.
MATERIALS AND METHODS
Cells and viruses.
Cells were grown in Dulbecco's modified Eagle medium supplemented with 5% fetal calf serum. The J cell line, a derivative of BHK-tk− cells that lacks gD receptors, has been described previously (12). The receptor-negative Chinese hamster ovary (CHO) cells were cultured in F-12 (Ham) nutrient mixture medium (Gibco) supplemented with 5% fetal calf serum. Viruses were grown in RS (rabbit skin) or baby hamster kidney (BHK) cells and routinely titrated by plaque assay in Vero cells. Wild-type HSV-1(F) and HSV-1(KOS)tk12, which expresses β-galactosidase from an insert in the viral thymidine kinase gene, have been described previously (15, 53). The gD− virus HSV-1(KOS)tk12/FRT-GFP (55), carrying green fluorescent protein (GFP) cDNA in place of the gD gene, was grown in R6 cells, a complementing rabbit skin cell line expressing inducible gD (57).
Construction of the J-HER2 and CHO-HER2 cell lines.
J or CHO cells were transfected with the pcDNA-HER2 plasmid (8) selected with neomycin G418 at a concentration of 400 to 800 μg/ml for 5 days. Individual clones were obtained by limiting dilution and were checked for HER2/neu expression by indirect immunofluorescence (IFA) with MAb 9G6 (Santa Cruz), diluted 1:50 in 20% newborn calf serum in phosphate-buffered saline, followed by fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (Jackson Immunoresearch).
Engineering of the scHER2 in gD.
For all gD constructs, the starting plasmid was pEA99 (36), which contains the wt gD coding sequence in pcDNA3.1(−) (Invitrogen). To allow cloning of the scHER2 sequence, two unique EcoRI and BamHI restriction sites were inserted at nucleotides 137 to 142 and nucleotides 162 to 167 of the gD coding sequence by site-directed mutagenesis with primer gD_21EcoRI_30BamHI (GCT TTC GCG GCA AAG GAA TTC CGG TCC TGG ACC AGC TGA CGG ATC CTC CGG GGG TCC). The EcoRI site insertion introduces the two D21G and L22I substitutions in mature gD. The BamHI site insertion is silent. pS2019a served as a template to PCR amplify the sequence for scHER2 (previously derived from MAb 4D5) (49). Briefly, scHER2 was amplified with primers scFv_EcoRI_f (GCA AAG GAA TTC CGG TCT CCG ATA TCC AGA TGA CCC AGT CCC CG) and scFv_BamHI_r (CGG AGG ATC CGT CAG CTG GTC CAG GGA GAC GGT GAC TAG TGT TCC TTG ACC); similarly, scHER2L (where scHER2 is followed by a 9-residue serine-glycine linker) was amplified with primers scFv_EcoRI_f (GCA AAG GAA TTC CGG TCT CCG ATA TCC AGA TGA CCC AGT CCC CG) and scFv_SGlink_BamHI_r (CGG AGG ATC CGT CAG CTG GTC CAG ACC GGA ACC AGA GCC ACC GCC ACT CGA GG). For the construction of both gD-scHER2 and gD-scHER2L, the primer sequences included the sequences encoding aa 21 to 24 and aa 25 to 30 of mature gD, such that in the final constructs gD lacked no amino acid residue and only contained the insert at aa residue 24. The gD-scHER2 and gD-scHER2L chimeras were subcloned into vectors for constitutive expression under the control of the immediate-early cytomegalovirus (CMV) promoter, generating plasmids pLM10 and pLM10L, respectively. For the generation of recombinant viruses by homologous recombination, the gD-scHER2 and gD-scHER2L chimeras were subcloned into recombination plasmids named pLM11 and pLM11L, which contained about 500 bp of the natural upstream and downstream gD-flanking sequences. All the constructs were sequenced for accuracy and checked for expression of chimeric gD by indirect immunofluorescence, as follows. BHK or RS cells transfected with pLM10 or pLM10L (carrying gD under the control of the immediate-early CMV promoter) were fixed with −20°C cold methanol for 10 min at 30 h after transfection, and reacted with monoclonal antibodies to gD: MAbs H170 and HD1 (Goodwin Institute, Plantation, FL) or MAb30 (5). Cells transfected with pLM11 or pLM11L (carrying gD under the control of the natural promoter) were superinfected with HSV-2(G) (3 PFU/cell) 6 h after transfection in order to induce gD gene expression, fixed with −20°C cold methanol for 10 min at 16 h after infection, and reacted with the type 1-specific MAb30 (5) and H1380.1 (Goodwin Institute, Plantation, FL). In all cases, primary antibodies to gD were followed by FITC-conjugated anti-mouse IgG (Jackson Immunoresearch).
Generation of recombinant viruses by homologous recombination.
BHK cells were transfected by means of Fugene 6 (Roche, Milan, Italy) with pLM11 or pLM11L and were superinfected 6 h later with the gD− virus HSV-1(KOS)tk12/FRT GFP (55) at 3 PFU/cell. At 24 h after infection, the cell lysate was plated in RS cells. Plaques were harvested and screened by PCR with primers gD_96_f (GCG GCA AAT ATG CCT TGG CGG ATG CC) and gD_200_r (GGG GCT GGA ACG GGT CCG GTA GGC CCG), flanking the site of scHER2 insertion in gD, or with primers scFv_EcoRI_f and scFv_BamHI_r or scFv_SGlink_BamHI_r, described above, which are specific for the scHER2 insert. The recombinant viruses R-LM11 and R-LM11L are derived from pLM11 and pLM11L, respectively. The relative electrophoretic mobilities of chimeric gD and wt gD were determined by Western blotting with MAb H170 against gD (Goodwin Institute, Plantation, FL), followed by peroxidase-conjugated anti mouse IgG and enhanced chemiluminescence (ECL; Amersham).
Cell-cell fusion assay.
The cell-cell fusion assays have been described previously (37, 46) and were adapted as follows. Briefly, effector COS cells were seeded in 24-well dishes (5 · 105 cells/well) and transfected with plasmid pCAGT7pol (43), plasmids encoding gB, gH, and gL (3), and either wt gD (pEA99) (36) or one of the gD-scHER2 chimeras (pLM10 and pLM10L). Target cells, namely, J-HVEM, J-Nectin1, or J-HER2 cells, seeded in T25 flasks, were transfected with the pEMCVLuc reporter construct (43). At 24 h after transfection, the target cells were seeded in a 1:1 ratio with COS effector cells, and cells were lysed after 24 h of cocultivation. The extent of fusion was measured by means of the luciferase assay system by Promega (Florence, Italy) in a TD20/20 luminometer (Turner Designs). All samples were run in triplicate.
Plating efficiency.
J, J-nectin1, J-HVEM, J-HER2, CHO, CHO-nectin1, CHO-HER2, and Vero cells were infected with serial dilutions of R-LM11, R-LM11L, or HSV-1(KOS)tk12. At 24 or 48 h after infection, plaques were visualized by in situ X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining as previously described (12, 40). The samples were run in triplicate.
Virus replication assay.
J, J-nectin1, J-HVEM, and J-HER2 cells grown in 12-well plates were infected with R-LM11, R-LM11L, or HSV-1(KOS)tk12 at 10 PFU/cell for 90 min at 37°C. Extracellular virus was inactivated by means of an acid wash (40 mM citric acid, 10 mM KCl, 135 mM NaCl [pH 3]) (6). Replicate cultures were frozen at 3, 24, or 48 h after infection, and the viral progeny (intracellular plus extracellular) was titrated on Vero cells.
Inhibition of virus infection.
To measure the effects of anti-HER2 antibodies on infection with the recombinant viruses, J-HER2 and CHO-HER2 cells grown in 96-well plates were preincubated with increasing concentrations of purified IgGs of MAb 4D5 or 9G6 (Santa Cruz Biotechnology), directed to HER-2 conformational epitopes, or of irrelevant mouse IgGs in 30 μl for 1 h at 37°C. The recombinant R-LM11 or R-LM11L was added in 3 μl (100 PFU/cell as measured in Vero cells) and allowed to absorb to cells for 90 min at 37°C.
To measure the effect of a recombinant soluble form of HER2, aliquots of R-LM11 or R-LM11L were mixed with increasing concentrations of a HER2-Fc chimera (ErbB2/Fc; R&D Systems) and CTLA4-Fc as a negative control for 1 h at 37°C and were allowed to absorb to J-HER2 and CHO-HER2 cells for 90 min at 37°C. In both types of experiments, the viral inoculum was removed at the end of the absorption interval, and the cells were rinsed twice, overlaid with medium containing the same concentration of IgGs or proteins as was present during virus absorption, and incubated for 16 h at 37°C. Expression of β-galactosidase was a direct measure of the extent of virus infection (12, 40). The optical density was read in a Bio-Rad enzyme-linked immunosorbent assay (ELISA) reader. For each antibody or protein concentration, triplicate samples were run. A value of 100% represents data obtained with infected cells not exposed to antibodies or to a recombinant receptor.
RESULTS
Genetic engineering of HSV gD carrying an scFv to HER2/Neu.
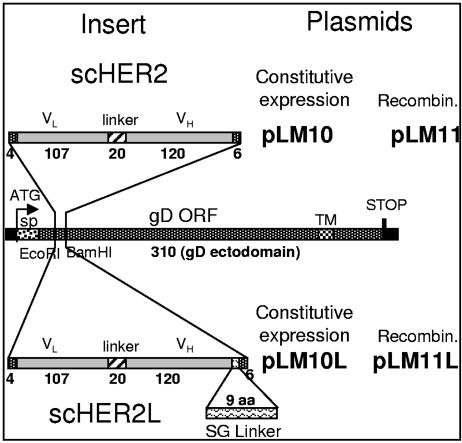
The overall objective was to insert a ligand to HER2/neu into gD. Because HER2 has no known natural ligand, the selected ligand was an scFv derived from MAb 4D5 (49), here designated scHER2. scHER2 was inserted at aa 24, a site previously reported to tolerate the IL-13 and uPA insertions. The site of insertion is very close to the binding site for HVEM receptor, which was mapped to a continuous region that includes residues 27 to 29 (7, 14). The experimental design consisted first in the insertion of two restriction sites, EcoRI and BamHI, at aa residues 21 and 30 of mature gD, respectively, and subsequently in the insertion of a fragment encoding scHER2. Downstream of it, one construct contained a 9-aa serine-glycine flexible linker and another construct contained no linker (Fig. 1). In the final constructs, named gD-scHER2 and gD-scHER2L, scHER2 was inserted between gD aa residues 24 and 25, and gD lacked no amino acid residue but carried two substitutions, D21G and L22I, as a consequence of the EcoRI restriction site insertion. gD-scHER2 and gD-scHER2L were cloned into pcDNA3.1 under the control of the CMV promoter for constitutive expression. The expression and proper folding of the chimeric forms of gD in transfected BHK or RS cells were essentially similar to those of wt gD, as detected by IFA (data not shown). Of note, the two substitutions introduced with the EcoRI restriction site did not alter the expression of gD or its ability to bind receptors and mediate cell-cell fusion (data not shown).
FIG. 1.
Schematic representation of the gD gene carrying the sequence encoding scHER2. The cDNA encoding scHER2 was inserted into the gD gene, previously modified to carry EcoRI and BamHI restriction sites. The insert was flanked by sequences encoding 4 and 6 aa residues at the 5′ and 3′ ends, respectively, to restore the complete gD sequence. pLM10L and pLM11L carried a 9-aa Ser-Gly linker (L) downstream of the insert. In pLM10 and pLM10L, the chimeric gD gene was cloned into pcDNA3.1 for constitutive expression. pLM11 and pLM11L carry the chimeric gD gene bracketed by upstream and downstream gD sequences and were employed to generate recombinant (Recombin.) viruses. Numbers indicate the length in amino acid residues of each fragment. The insertion of the EcoRI site in gD caused the D21G and L22I substitutions in mature gD. VH and VL, heavy- and light-chain variable domains of the anti-HER2/neu antibody 4D5. sp, signal peptide. Bars are drawn to scale.
Construction of cell lines stably expressing HER2/neu.
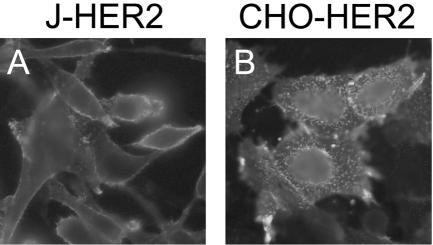
In order to construct cell lines expressing HER2/neu in the absence of any other HSV-1 entry receptors, an expression plasmid encoding HER2/neu (8) was transfected into HSV-resistant J or CHO cells (12, 40). Both cell lines lack the receptors necessary for entry of HSV. The transfected cells were cloned by limiting dilution, and individual clones were scored for HER2/neu surface expression by IFA on nonpermeabilized cells. Figure 2 shows the cell surface localization of the receptor in overexpressing stable clonal cell lines J-HER2 (Fig. 2A) or CHO-HER2 (Fig. 2B).
FIG. 2.
Expression of HER2/neu in the stable clonal cell lines J-HER2 (A) and CHO-HER2 (B). Cells were fixed with paraformaldehyde and reacted with MAb 9G6 to the HER2 ectodomain, followed by an FITC-conjugated secondary antibody. Fluorescence localizes at the plasma membrane.
Chimeric gD-scHER2 mediates cell-cell fusion through the HER2/neu receptor.
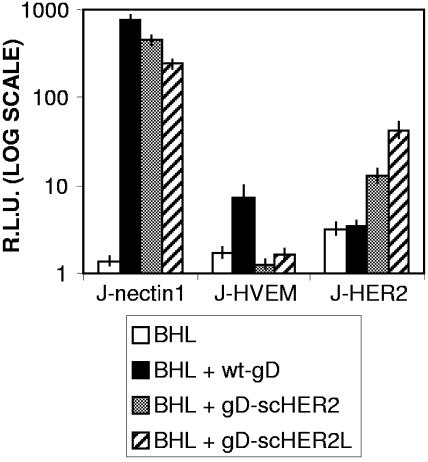
As a first assay to check whether the chimeric forms of gD-scHER2 were able to interact with the HER2/neu receptor and thus trigger fusion, we performed a cell-cell fusion assay. In this assay, the effector COS cells, cotransfected with plasmids encoding wt or chimeric gD under the control of the CMV promoter, plus gB, gH, gL, and a T7 polymerase (pCAGT7pol), fuse with receptor-positive target cells transfected with a T7 promoter-driven luciferase reporter gene. The luciferase activity is a direct measure of the fusion capacity of the transfected cells. J-HER2 cells were used as target cells, and J-nectin1 and J-HVEM cells were used as positive controls. The results in Fig. 3 show that gD-scHER2 and gD-scHER2L mediated fusion with J-HER2 cells to an extent comparable to, or slightly higher than, that exhibited by wt gD with J-HVEM cells, providing a first line of evidence that the insertion of scHER2 resulted in a functional gD exhibiting redirected tropism. The chimeric forms of gD mediated fusion with J-nectin1 cells to an extent indistinguishable from that of wt gD, providing evidence that the scHER2 insertion did not affect the binding to nectin1 or the pro-fusion activity of gD. By contrast, fusion with J-HVEM cells was almost abolished. Of note, the fusion activity of wt gD-expressing cells with J-HVEM cells was much lower than that with J-nectin1 cells, in agreement with previously published observations (46).
FIG. 3.
Cell-cell fusion mediated by chimeric scHER2-gD. The effector COS cells, cotransfected with plasmids encoding gB, gH, gL (BHL), and pCAGT7pol plus chimeric or wt gD, were cocultivated with J-nectin1, J-HVEM, or J-HER2 target cells transfected with pEMCVLuc. Luciferase activity was expressed as relative light units (RLU) on a log scale. Each experiment was performed at least three times, and samples were run in triplicate; mean values are shown. Vertical bars, standard deviations.
Construction of HSV recombinants carrying gD-scHER2.
We next generated recombinant viruses carrying gD-scHER2 or gD-scHER2L by homologous recombination between the gD deletion virus HSV-1(KOS)tk12/FRT-GFP (55) and the recombination plasmids containing gD-scHER2 and gD-scHER2L, designated pLM11 and pLM11L. To aid recombination, the plasmids carried the chimeric gD genes bracketed by the upstream and downstream sequences that flank the gD coding sequence. The recipient virus encodes the lacZ gene under the control of the α4 promoter in place of the thymidine kinase gene, and therefore, the recombinants can be traced and quantified as β-galactosidase activity.
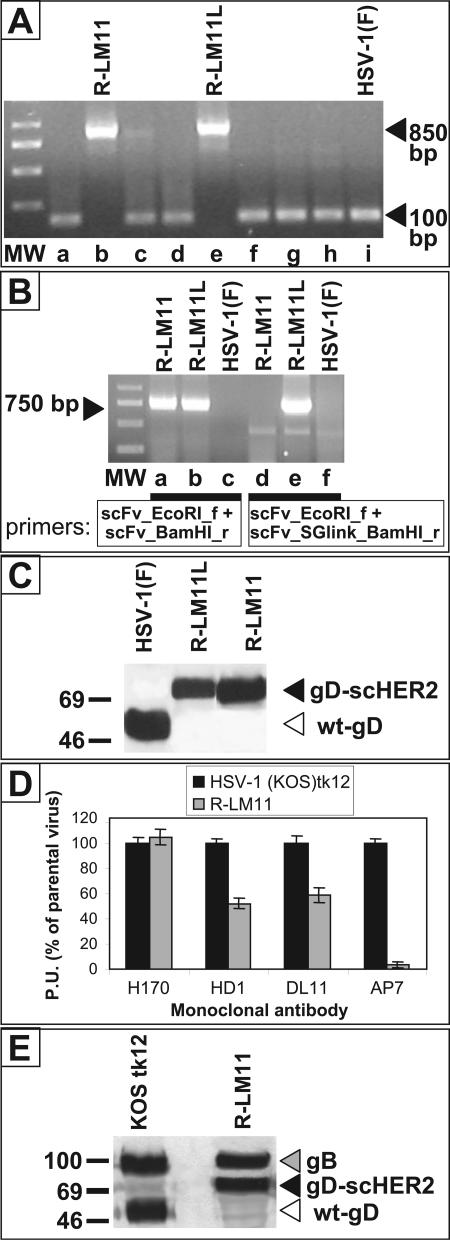
The recombinants, named R-LM11 and R-LM11L, were verified genotypically and for production and properties of the chimeric gD. First, the scHER2 sequences were amplified with primers annealing to the gD sequences flanking the insertion (Fig. 4A) or with primers specific for the scHER2 insert (Fig. 4B). In either case, the amplimers exhibited the expected size. Second, lysates of cells infected with the recombinants were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. As shown in Fig. 4C, gD from R-LM11 and R-LM11L exhibited a decrease in electrophoretic mobility and an apparent Mr consistent with the insertion. Both wt gD and the slower-migrating bands from the recombinants reacted in Western blots with MAb H170 against gD.
FIG. 4.
Recombinant viruses harbor the scFv to HER2 in gD and express a chimeric gD. (A and B) Amplification of the sequences encoding scHER2 from lysates of R-LM11- and R-LM11L-infected cells. (A) PCR was performed with primers annealing to the gD sequences that flank the site for scHER2 insertion. The presence of the insert causes an increase in the size of the amplification product from 100 bp (nonrecombinant plaques [lanes a, c, d, f, g, and h]) to 850 bp (lanes b and e). Lane i, HSV-1(F), used as a control. MW, 1-kb DNA ladder. (B) PCR was performed with primers annealing to the scHER2 insert. Lanes a to c, PCR with primers used for cloning the scHER2 insert. Lanes d to f, PCR with the same forward primer as in lanes a to c and a reverse primer annealing to the serine-glycine linker, thus amplifying R-LM11L rather than R-LM11. The amplification products from the recombinants exhibit the expected length (∼750 bp). HSV-1(F) was used as a negative control and did not give rise to any amplification product (lanes c and f). (C) Electrophoretic mobility of chimeric scHER2-gD. Lysates of cells infected with R-LM11, R-LM11L, or HSV-1(F) were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and visualized by Western blotting with MAb H170 against gD, followed by peroxidase-conjugated anti-mouse IgG and ECL. In the recombinants, the presence of scHER2 results in a slower-migrating band (black arrowhead) than that with wt gD (white arrowhead). Numbers to the left represent migration positions of molecular mass markers (in kilodaltons). (D) Reactivities of wt gD and the gD-HER2 chimera to a panel of monoclonal antibodies, measured by cell ELISA (21). Vero cells were infected with the indicated viruses. At 16 h after infection, they were reacted with the indicated antibodies, followed by a peroxidase-conjugated anti-mouse antibody and o-phenylenediamine. Binding of antibodies was quantified as peroxidase units (P.U.), and expressed as a percentage relative to the cells infected with the parental HSV-1(KOS)tk12 virus. Each assay was performed in quadruplicate. Bars represent means. Error bars, standard deviations. (E) Quantification of gD and gB present in virions. Virions were pelleted from the extracellular medium of infected Vero cells after growth for 24 h. Equal amounts of virions, measured as PFU, were loaded for SDS-PAGE separation. Amounts of gB and gD were detected by Western blotting with MAbs H1817 and H170, respectively. Arrowheads indicate migration positions.
Next, we analyzed the reactivity of the chimeric gD-scHER2 to antibodies directed to conformation-dependent epitopes by cell ELISA. Cells infected with R-LM11, or with HSV-1(KOS)tk12 as a control, were reacted with MAbs HD1 and DL11, two potent neutralizing antibodies that react with a region involved in virus entry, and with MAb AP7, which reacts to a discontinuous epitope localized in part at the N terminus and in part at the C terminus (residues 290 to 300) (9, 39). The results in Fig. 4D show that reactivity to MAb H170, which recognizes aa 1 to 23, was not modified in chimeric gD relative to wt gD, as expected. Reactivity to the neutralizing MAbs was somewhat decreased but still present, in accordance with the ability of the virus to carry out infection. The reactivity of the chimeric gD-scHER2 to MAb AP7 was lost. The results indicate that (i) critical epitopes for infection are maintained and (ii) in the receptor-unbound gD-scHER2, the ectodomain N and C termini do not interact with each other anymore. Of note, the insertion did not confer instability on the viruses; the recombinants were passaged for several months, and their properties were stable. Given that the Mr of gD was almost doubled by the insertion, we also asked whether recombinant virions were able to incorporate gD in amounts similar to those present in wt virions. Extracellular virions were pelleted and analyzed for the content of gD and for that of gB as a reference. The results in Fig. 4E show that amounts of gD were very similar in RLM11 and wt virions.
R-LM11 and R-LM11L infect cells that express HER2/neu as the sole receptor.
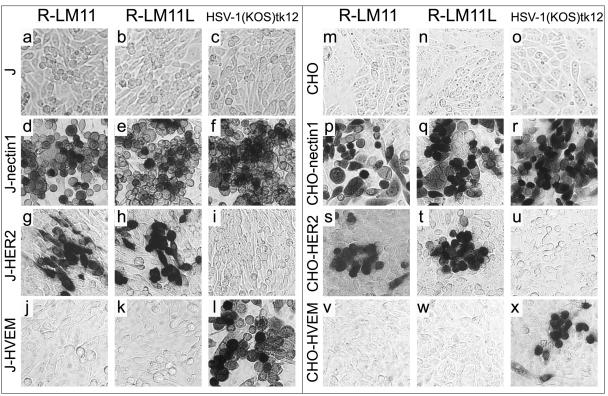
Replicate cultures of J, J-nectin1, J-HER2, and J-HVEM cells or CHO, CHO-nectin1, CHO-HER2, and CHO-HVEM cells were exposed to R-LM11 and R-LM11L viruses, or to the parental virus HSV1(KOS)tk12 as a control. After 24 h, infection was detected as β-galactosidase activity. Figure 5 shows that R-LM11 and R-LM11L, but not the parental HSV-1(KOS)tk12, were able to infect J-HER2 and CHO-HER2 cells (Fig. 5g, h, i, s, t, and u). The recombinants were still able to infect J-nectin1 and CHO-nectin1 cells (Fig. 5d, e, p, and q) but lost the ability to infect J-HVEM and CHO-HVEM cells (Fig. 5j, k, v, and w).
FIG. 5.
The recombinant viruses R-LM11 and R-LM11L infect cells via the HER2/neu receptor. Micrographs show J (a to c), J-nectin1 (d to f), J-HER2 (g to i), J-HVEM (j to l), CHO (m to o), CHO-nectin1 (p to r), CHO-HER2 (s to u), and CHO-HVEM (v to x) cells exposed to the recombinant virus R-LM11 (a, d, g, j, m, p, s, and v) or R-LM11L (b, e, h, k, n, q, t, and w) or to the parental virus HSV-1(KOS)tk12 (c, f, i, l, o, r, u, and x) at 10 PFU/cell. Infection was monitored as β-galactosidase activity by X-Gal staining 16 h following infection.
R-LM11 and R-LM11L grow and spread in cells expressing HER2/neu as the sole receptor.
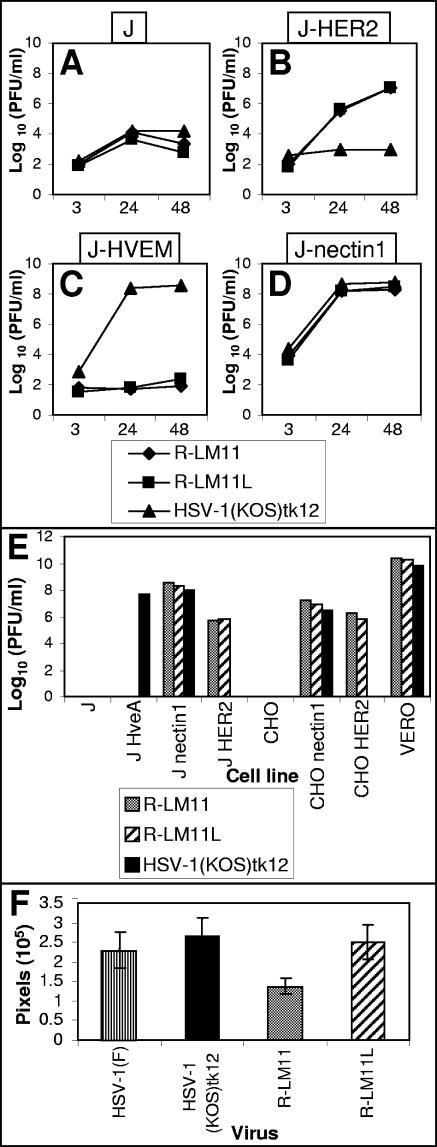
Replicate cultures of J-HER2, J-nectin1, J-HVEM, and J cells were exposed to R-LM11 and R-LM11L (10 PFU/cell). After 24 and 48 h, the cells were harvested and progeny viruses titrated on Vero cells. The results in Fig. 6A to D show that R-LM11 and R-LM11L grew in J-HER2 cells. The titer was about 20-fold lower than that in J-nectin1 cells. Both recombinants were unable to grow in J-HVEM cells, suggesting that that the HVEM binding site on gD was altered by the insertion of scHER2. As expected, the parental HSV-1(KOS)tk12 did not replicate in J-HER2 cells.
FIG. 6.
Growth and plaque formation of R-LM11 and R-LM11L recombinants. (A to D) Growth curves of R-LM11 and R-LM11L. Replicate cultures of J (A), J-HER2 (B), J-HVEM (C), or J-nectin1 (D) cells were infected with R-LM11 (♦), R-LM11L (▪), or HSV-1(KOS)tk12 (▴) at 10 PFU/cell. Progeny virus was harvested at 3, 24, or 48 h after infection and titrated on Vero cells. (E) Plaque formation of R-LM11 and R-LM11L. R-LM11 (gray bars), R-LM11L (hatched bars), and HSV-1(KOS)tk12 (black bars) were plated in the indicated cell lines. Monolayers were fixed at 24 or 48 h after infection, and plaques were visualized by X-Gal or Giemsa staining. (F) Plaques formed as shown in panel E were photographed, and the plaque areas were measured by means of the Histogram program and expressed as pixels. For each virus, the areas of at least 20 plaques were measured. Histograms represent averages; error bars, standard deviations.
In the next series of experiments, R-LM11 and R-LM11L were assayed for the ability to spread from cell to cell in J-HER2 and CHO-HER2 cells, and in J-nectin1 and CHO-nectin1 cells as controls (Fig. 6E). The plaques formed by the recombinants in J-HER2 cells were fewer in number (700-fold and 250-fold reductions) than those in J-nectin1 cells. The reduction observed in plaque numbers in CHO-HER2 versus CHO-nectin1 cells was only 10-fold. HSV-1(KOS)tk12 did not form plaques in J-HER2 and CHO-HER2 cells. The ratio between the number of plaques in Vero cells and that in J-nectin1 cells, or between that in Vero cells and that in CHO-nectin1 cells, was practically the same for the two recombinants and the wt virus. The recombinants did not form plaques in J-HVEM cells, in agreement with the lack of growth observed in Fig. 6C. As a measure of the abilities of R-LM11 and R-LM11L recombinants to spread from cell to cell, we also determined their plaque sizes. As shown in Fig. 6F, the plaque sizes of wt viruses and R-LM11L did not differ significantly from each other, whereas the plaque size of R-LM11 was 50% reduced. Cumulatively, the results of Fig. 5 and 6 provide evidence for the following conclusions. (i) R-LM11 and R-LM11L have acquired the ability to grow and spread in J-HER2 and CHO-HER2 cells, although at reduced efficiency relative to that in J-nectin1 and CHO-nectin1 cells. The reduction is more evident for the R-LM11 virus, which lacks a linker between scHER2 and gD, at least as far as cell-to-cell spread is concerned. This indicates that the recombinants exhibited a modified tropism and were effectively redirected to the HER2/neu receptor. (ii) The recombinants maintained the ability to infect through nectin1 but not through HVEM.
Entry of R-LM11 and R-LM11L into J-HER2 or CHO-HER2 cells is dependent on HER2/neu.
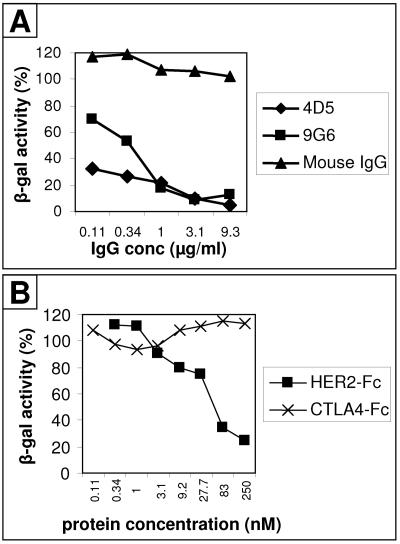
To provide evidence that entry of R-LM11 and R-LM11L recombinants into J-HER2 or CHO-HER2 cells was dependent on the HER2 receptor, we measured whether infection was inhibited by MAbs to HER2/neu or by a soluble form of HER2. In the first series of experiments, we used two MAbs, addressed to different epitopes of HER2. MAb 4D5 is addressed to domain IV and is the MAb from which the scFv was derived. MAb 9G6 is addressed to an unmapped conformational epitope. Figure 7A shows that both MAbs inhibited R-LM11L infection of J-HER2 cells in a dose-dependent manner. Mouse IgG had no effect. In the second series of experiments, we tested the effect of a recombinant form of HER2/neu in which the ectodomain is fused to the Fc portion of IgG (HER2-Fc). As shown in Fig. 7B, HER2-Fc inhibited entry of R-LM11L into CHO-HER2 cells in a dose-dependent manner, whereas an unrelated receptor, CTLA4-Fc, did not.
FIG. 7.
Infection of R-LM11 and R-LM11L recombinants in HER2/neu-expressing cells is blocked by antibodies to HER2/neu and by a soluble form of HER2, HER2-Fc. (A) J-HER2 cells were preincubated with the indicated concentrations of purified IgG of monoclonal antibody 4D5 (♦) or 9G6 (▪) against HER2/neu or of irrelevant mouse IgGs (▴) for 1 h at 37°C. Virus was added to the antibody-containing medium and allowed to infect the cells for 90 min at 37°C. Infection was monitored 16 h later as β-galactosidase activity. (B) Replicate aliquots of R-LM11L were preincubated with the indicated concentrations of purified soluble recombinant HER2-Fc (▪) or CTLA4-Fc (×) for 1 h at 37°C and allowed to absorb to CHO-HER2 cells for 90 min at 37°C. Infection was quantified 16 h later as β-galactosidase activity. Each point represents the average of triplicate assays. The standard error ranged from 0.6 to 1.9% of mean values. One hundred percent indicates the optical density measured in untreated, virus-infected cultures.
DISCUSSION
We report on the construction and properties of two HSV recombinants, R-LM11 and R-LM11L, carrying the insertion of an scFv to HER2/neu at residue 24 of gD. The key finding is that R-LM11 and R-LM11L recombinants were able to infect and replicate in cells that express HER2 as the sole receptor, called J-HER2 and CHO-HER2 cells, but not in the parental receptor-negative J and CHO cells and therefore were redirected to HER2/neu receptor. The significance of the results stems from the following considerations.
Entry of R-LM11 and R-LM11L recombinants into J-HER2 cells was dependent on HER2, as demonstrated by the observations that infection was inhibited in a dose-dependent manner by two monoclonal antibodies that recognize two different epitopes on HER2/neu and by a soluble recombinant form of the receptor. Replication of the recombinants in J-HER2 cells was about 20-fold lower than that in J-nectin1 cells. Similarly, the recombinants were able to form plaques in J-HER2 or CHO-HER2 cells, although at an efficiency several hundredfold lower than that in J-nectin1 or CHO-nectin1 cells.
The scHER2 insertion in gD abolished the ability of HSV to enter cells via the HVEM receptor. This was a beneficial effect, given that our ultimate goal is the construction of an oncolytic virus capable of infecting solely HER-2-positive cells and not the cells normally targeted by HSV. The result was deliberately searched for but somehow unexpected, given that the IL-13 insertion in gD failed to disrupt the HVEM binding site (58). In either case, the site of insertion is adjacent to the binding site for HVEM receptor, which was mapped to a continuous region that includes residues 27 to 29 (7, 14). A major difference between the current and previous results was the size of the insert, which was 247 or 256 aa in our constructs and 134 aa in the IL-13-gD chimera. We speculate that the larger size of the scHER2 insert may hinder the flexibility of the gD N terminus such that it prevents the formation of the N-terminal hairpin and therefore the HVEM binding site. By contrast, the scHER2 insertion did not modify the capacity of the recombinants to enter cells via the nectin1 receptor. The nectin1 binding site on gD has yet to be fully characterized. A number of substitutions or insertions, at residues 34, 38, 126, 151, 187, 215, 222, 223, 243, 246, etc. (13, 33, 56), were reported to affect entry through nectin1, as measured by an infectivity complementation assay. However, recombinants specifically defective in entry through nectin1 and still capable of pro-fusion activity remain to be constructed. Given that R-LM11 and R-LM11L have lost the ability to enter cells through HVEM, knocking down the ability to infect through nectin1 is our next goal in order to obtain a fully retargeted oncolytic virus with a high safety profile.
This is the third demonstration that gD can be modified to effectively use a cellular protein as a receptor and that entry of HSV can be totally dependent on receptors selected for the purpose of targeting the virus to specific cells (30, 58, 59). In the first two instances, the inserts were 134 and 136 aa long, respectively. The remarkable aspect of our results is that gD can tolerate an insert almost as big as gD itself and that its Mr can be doubled without loss of key functions. In particular, in view of the current model of gD action (10, 18, 31), results presented here and elsewhere imply that the ability of gD to modify its conformation and trigger fusion takes place independently of whether gD binds to its natural receptors or to foreign receptors for which it carries a ligand.
In the past few years, several efforts to construct viruses retargeted to a number of different cellular receptors have been described. To our knowledge, only two reports illustrated the generation of viruses retargeted to the HER2/neu receptor. In one instance, retroviral vector particles derived from spleen necrosis virus were pseudotyped with the antigen binding site of an antibody to HER2/neu (29). Compared to the pseudotyped retrovirus, R-LM11 and R-LM11L are genetically engineered to express the chimeric gD, and therefore any progeny virus at any replication cycle will carry the retargeted envelope. This ensures that the redirected tropism and, consequently, the possible oncolytic activity are maintained for as many viral replication cycles as needed. The second instance concerns a vesicular stomatitis virus recombinant exhibiting a Sindbis virus glycoprotein modified with the scFv to HER2/neu (4). Compared to small RNA viruses, such as vesicular stomatitis viruses, oncolytic viruses based on HSV have the advantage of a large genome capacity and genetic stability and therefore provide the possibility to deliver to the tumor cells additional heterologous therapeutic or immunomodulatory gene products (e.g., IL-12 or granulocyte-macrophage colony-stimulating factor) (34, 44).
A remarkable aspect of this work is that it provides proof of principle that retargeting HSV tropism by fusion of an scFv to gD is feasible. In recent years, a high number of single-chain antibodies have been generated, some of which have entered clinical practice. Our finding and the availability of a large number of scFv's greatly increases the collection of potential receptors to which HSV can be redirected.
It has been proposed that HSV recombinants that target specific surface markers can be used to visualize the distribution of tumor cells and their metastases in tissues by at least two methods (59). The first involves the viral thymidine kinase-dependent incorporation of a radioactive precursor. A more attractive approach is to fuse a noncritical tegument protein present in high numbers per virion to GFP or similar molecules capable of being visualized in vivo. The studies presented in this report make the specific targeting of HSV for both oncolytic activity and visualization of tumor cells possible for a large variety of cell surface molecules to which antibodies and scFv's are available.
ADDENDUM IN PROOF
After this paper was accepted, G. Zhou and B. Roizman (Proc. Natl. Acad. Sci. USA, 103:5508-5513, 2006) reported the first HSV-1 recombinant debilitated in nectin1 binding, generated through the V34S substitution.
Acknowledgments
We thank Genentech (San Francisco, Calif.) and S. Pupa (Istituto Nazionale Tumori, Milan, Italy) for the gift of cDNAs encoding the scFv to HER2/neu and for pcDNA3/HER2, and P.G. Spear (Northwestern University), Pier-Luigi Lollini, and Carla De Giovanni (University of Bologna) for gifts of viruses and antibodies. We are indebted to Elisabetta Romagnoli for invaluable assistance.
This work was supported by FIRB autonomous and coordinated project PRIN-MIUR (grant 40%), by the University of Bologna (grant 60%), and by Fondo Pallotti.
REFERENCES
- 1.Advani, S. J., R. R. Weichselbaum, R. J. Whitley, and B. Roizman. 2002. Friendly fire: redirecting herpes simplex virus-1 for therapeutic applications. Clin. Microbiol. Infect. 8:551-563. [DOI] [PubMed] [Google Scholar]
- 2.Andreansky, S., L. Soroceanu, E. R. Flotte, J. Chou, J. M. Markert, G. Y. Gillespie, B. Roizman, and R. J. Whitley. 1997. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 57:1502-1509. [PubMed] [Google Scholar]
- 3.Avitabile, E., G. Lombardi, and G. Campadelli-Fiume. 2003. Herpes simplex virus glycoprotein K, but not its syncytial allele, inhibits cell-cell fusion mediated by the four fusogenic glycoproteins, gD, gB, gH, and gL. J. Virol. 77:6836-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman, I., P. Whitaker-Dowling, Y. Gao, and J. A. Griffin. 2004. Preferential targeting of vesicular stomatitis virus to breast cancer cells. Virology 330:24-33. [DOI] [PubMed] [Google Scholar]
- 5.Brandimarti, R., T. Huang, B. Roizman, and G. Campadelli Fiume. 1994. Mapping of herpes simplex virus 1 genes with mutations which overcome host restrictions to infection. Proc. Natl. Acad. Sci. USA 91:5406-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunetti, C. R., R. L. Burke, B. Hoflack, T. Ludwig, K. S. Dingwell, and D. C. Johnson. 1995. Role of mannose-6-phosphate receptors in herpes simplex virus entry into cells and cell-to-cell transmission. J. Virol. 69:3517-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carfi, A., S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8:169-179. [DOI] [PubMed] [Google Scholar]
- 8.Casalini, P., L. Botta, and S. Menard. 2001. Role of p53 in HER2-induced proliferation or apoptosis. J. Biol. Chem. 276:12449-12453. [DOI] [PubMed] [Google Scholar]
- 9.Chiang, H. Y., G. H. Cohen, and R. J. Eisenberg. 1994. Identification of functional regions of herpes simplex virus glycoprotein gD by using linker-insertion mutagenesis. J. Virol. 68:2529-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocchi, F., D. Fusco, L. Menotti, T. Gianni, R. J. Eisenberg, G. H. Cohen, and G. Campadelli-Fiume. 2004. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. USA 101:7445-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocchi, F., M. Lopez, L. Menotti, M. Aoubala, P. Dubreuil, and G. Campadelli-Fiume. 1998. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc. Natl. Acad. Sci. USA 95:15700-15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin superfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connolly, S. A., D. J. Landsburg, A. Carfi, C. J. Whitbeck, Y. Zuo, D. C. Wiley, G. H. Cohen, and R. J. Eisenberg. 2005. Potential nectin-1 binding site on herpes simplex virus glycoprotein D. J. Virol. 79:1282-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly, S. A., D. J. Landsburg, A. Carfi, D. C. Wiley, G. H. Cohen, and R. J. Eisenberg. 2003. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface. J. Virol. 77:8127-8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 16.Finn, R. S., and D. J. Slamon. 2003. Monoclonal antibody therapy for breast cancer: herceptin. Cancer Chemother. Biol. Response Modif. 21:223-233. [DOI] [PubMed] [Google Scholar]
- 17.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusco, D., C. Forghieri, and G. Campadelli-Fiume. 2005. The pro-fusion domain of herpes simplex virus glycoprotein D (gD) interacts with the gD N terminus and is displaced by soluble forms of viral receptors. Proc. Natl. Acad. Sci. USA 102:9323-9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 20.Gianni, T., G. Campadelli-Fiume, and L. Menotti. 2004. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J. Virol. 78:12268-12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simpex virus glycoprotein H contains a membrane α-helix with attributes of an internal fusion peptide, positionally conserved in the Herpesviridae family. J. Virol. 79:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianni, T., L. Menotti, and G. Campadelli-Fiume. 2005. A heptad repeat in herpes simplex virus gH, located downstream of the α-helix with attributes of a fusion peptide, is critical for virus entry and fusion. J. Virol. 79:7042-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianni, T., A. Piccoli, C. Bertucci, and G. Campadelli-Fiume. 2006. Heptad repeat 2 in herpes simplex virus 1 gH interacts with heptad repeat 1 and is critical for virus entry and fusion. J. Virol. 80:2216-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrow, S., V. Papanastassiou, J. Harland, R. Mabbs, R. Petty, M. Fraser, D. Hadley, J. Patterson, S. M. Brown, and R. Rampling. 2004. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 11:1648-1658. [DOI] [PubMed] [Google Scholar]
- 25.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holbro, T., and N. E. Hynes. 2004. ErbB receptors: directing key signaling networks throughout life. Annu. Rev. Pharmacol. Toxicol. 44:195-217. [DOI] [PubMed] [Google Scholar]
- 27.Hudziak, R. M., G. D. Lewis, M. Winget, B. M. Fendly, H. M. Shepard, and A. Ullrich. 1989. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell. Biol. 9:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hynes, N. E., and H. A. Lane. 2005. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5:341-354. [DOI] [PubMed] [Google Scholar]
- 29.Jiang, A., T. H. Chu, F. Nocken, K. Cichutek, and R. Dornburg. 1998. Cell-type-specific gene transfer into human cells with retroviral vectors that display single-chain antibodies. J. Virol. 72:10148-10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamiyama, H., G. Zhou, and B. Roizman. 2006. Herpes simplex virus 1 recombinant virions exhibiting the amino terminal fragment of urokinase-type plasminogen activator can enter cells via the cognate receptor. Gene Ther. 13:621-629. [DOI] [PubMed] [Google Scholar]
- 31.Krummenacher, C., V. M. Supekar, J. C. Whitbeck, E. Lazear, S. A. Connolly, R. J. Eisenberg, G. H. Cohen, D. C. Wiley, and A. Carfi. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez, M., F. Cocchi, L. Menotti, E. Avitabile, P. Dubreuil, and G. Campadelli-Fiume. 2000. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J. Virol. 74:1267-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manoj, S., C. R. Jogger, D. Myscofski, M. Yoon, and P. G. Spear. 2004. Mutations in herpes simplex virus glycoprotein D that prevent cell entry via nectins and alter cell tropism. Proc. Natl. Acad. Sci. USA 101:12414-12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markert, J. M., G. Y. Gillespie, R. R. Weichselbaum, B. Roizman, and R. J. Whitley. 2000. Genetically engineered HSV in the treatment of glioma: a review. Rev. Med. Virol. 10:17-30. [DOI] [PubMed] [Google Scholar]
- 35.Markert, J. M., M. D. Medlock, S. D. Rabkin, G. Y. Gillespie, T. Todo, W. D. Hunter, C. A. Palmer, F. Feigenbaum, C. Tornatore, F. Tufaro, and R. L. Martuza. 2000. Conditionally replicating herpes simplex virus mutant, G207, for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 7:867-874. [DOI] [PubMed] [Google Scholar]
- 36.Menotti, L., M. Lopez, E. Avitabile, A. Stefan, F. Cocchi, J. Adelaide, E. Lecocq, P. Dubreuil, and G. Campadelli-Fiume. 2000. The murine homolog of human Nectin1δ serves as a species nonspecific mediator for entry of human and animal alphaherpesviruses in a pathway independent of a detectable binding to gD. Proc. Natl. Acad. Sci. USA 97:4867-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milne, R. S., S. L. Hanna, A. H. Rux, S. H. Willis, G. H. Cohen, and R. J. Eisenberg. 2003. Function of herpes simplex virus type 1 gD mutants with different receptor-binding affinities in virus entry and fusion. J. Virol. 77:8962-8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mineta, T., S. D. Rabkin, T. Yazaki, W. D. Hunter, and R. L. Martuza. 1995. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1:938-943. [DOI] [PubMed] [Google Scholar]
- 39.Minson, A. C., T. C. Hodgman, P. Digard, D. C. Hancock, S. E. Bell, and E. A. Buckmaster. 1986. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J. Gen. Virol. 67:1001-1013. [DOI] [PubMed] [Google Scholar]
- 40.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 41.Nakano, K., R. Asano, K. Tsumoto, H. Kwon, W. F. Goins, I. Kumagai, J. B. Cohen, and J. C. Glorioso. 2005. Herpes simplex virus targeting to the EGF receptor by a gD-specific soluble bridging molecule. Mol. Ther. 11:617-626. [DOI] [PubMed] [Google Scholar]
- 42.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okuma, K., M. Nakamura, S. Nakano, Y. Niho, and Y. Matsuura. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235-244. [DOI] [PubMed] [Google Scholar]
- 44.Parker, J. N., G. Y. Gillespie, C. E. Love, S. Randall, R. J. Whitley, and J. M. Markert. 2000. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc. Natl. Acad. Sci. USA 97:2208-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pegram, M. D., T. Pienkowski, D. W. Northfelt, W. Eiermann, R. Patel, P. Fumoleau, E. Quan, J. Crown, D. Toppmeyer, M. Smylie, A. Riva, S. Blitz, M. F. Press, D. Reese, M. A. Lindsay, and D. J. Slamon. 2004. Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J. Natl. Cancer Inst. 96:759-769. [DOI] [PubMed] [Google Scholar]
- 46.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 47.Rampling, R., G. Cruickshank, V. Papanastassiou, J. Nicoll, D. Hadley, D. Brennan, R. Petty, A. MacLean, J. Harland, E. McKie, R. Mabbs, and M. Brown. 2000. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 7:859-866. [DOI] [PubMed] [Google Scholar]
- 48.Shah, A. C., D. Benos, G. Y. Gillespie, and J. M. Markert. 2003. Oncolytic viruses: clinical applications as vectors for the treatment of malignant gliomas. J. Neurooncol. 65:203-226. [DOI] [PubMed] [Google Scholar]
- 49.Sidhu, S. S., B. Li, Y. Chen, F. A. Fellouse, C. Eigenbrot, and G. Fuh. 2004. Phage-displayed antibody libraries of synthetic heavy chain complementarity determining regions. J. Mol. Biol. 338:299-310. [DOI] [PubMed] [Google Scholar]
- 50.Slamon, D. J., B. Leyland-Jones, S. Shak, H. Fuchs, V. Paton, A. Bajamonde, T. Fleming, W. Eiermann, J. Wolter, M. Pegram, J. Baselga, and L. Norton. 2001. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344:783-792. [DOI] [PubMed] [Google Scholar]
- 51.Tripathy, D., D. J. Slamon, M. Cobleigh, A. Arnold, M. Saleh, J. E. Mortimer, M. Murphy, and S. J. Stewart. 2004. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J. Clin. Oncol. 22:1063-1070. [DOI] [PubMed] [Google Scholar]
- 52.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]
- 54.Yarden, Y., and M. X. Sliwkowski. 2001. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2:127-137. [DOI] [PubMed] [Google Scholar]
- 55.Yoon, M., and P. G. Spear. 2004. Random mutagenesis of the gene encoding a viral ligand for multiple cell entry receptors to obtain viral mutants altered for receptor usage. Proc. Natl. Acad. Sci. USA 101:17252-17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou, G., E. Avitabile, G. Campadelli-Fiume, and B. Roizman. 2003. The domains of glycoprotein D required to block apoptosis induced by herpes simplex virus 1 are largely distinct from those involved in cell-cell fusion and binding to nectin1. J. Virol. 77:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou, G., and B. Roizman. 2005. Characterization of a recombinant herpes simplex virus 1 designed to enter cells via the IL13Rα2 receptor of malignant glioma cells. J. Virol. 79:5272-5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou, G., G. J. Ye, W. Debinski, and B. Roizman. 2002. Engineered herpes simplex virus 1 is dependent on IL13Rα2 receptor for cell entry and independent of glycoprotein D receptor interaction. Proc. Natl. Acad. Sci. USA 99:15124-15129. [DOI] [PMC free article] [PubMed] [Google Scholar]