Abstract
The ORF57 gene of Kaposi's sarcoma-associated herpesvirus (KSHV) encodes a nuclear protein expressed during the lytic phase of KSHV replication. An ORF57 homolog is present in all known human herpesviruses and many animal herpesviruses. Many of these proteins have been demonstrated to have essential transcriptional and posttranscriptional regulatory functions. ORF57 enhances expression of reporter genes posttranscriptionally in vitro and may synergize with transcription factors to enhance gene transcription. However, the biologic role of ORF57 in KSHV replication has not been established. In this study, we demonstrate that ORF57 is essential for productive KSHV lytic replication by constructing a recombinant KSHV in which ORF57 expression has been specifically inactivated. The ORF57-null KSHV recombinant was unable to produce virion progeny or fully express several other lytic KSHV genes except when ORF57 was provided in trans. The Epstein-Barr virus (EBV) homolog of ORF57, SM, was unable to rescue lytic KSHV virion production, although EBV SM does enhance KSHV lytic gene expression from the ORF57-null mutant. Conversely, ORF57 did not rescue an SM-null recombinant EBV, indicating the existence of virus-specific functions for the ORF57 family of genes.
The Kaposi's sarcoma-associated herpesvirus (KSHV, also called human herpesvirus 8) ORF57 gene product is a member of a highly conserved family of proteins present in most mammalian herpesviruses (1, 2, 6, 10, 16, 24). It is not known whether ORF57 is essential for lytic KSHV replication. In three human herpesviruses where the question has been directly addressed by recombinant molecular genetics, the ORF57 homologs Epstein-Barr virus (EBV) SM, varicella-zoster virus (VZV) ORF4, and herpes simplex virus (HSV) ICP27 have been shown to be essential for lytic virus replication (11, 15, 33). Although each human herpesvirus expresses a homologous protein that is important in the life cycle of the virus, the structure and function of these proteins vary significantly, and each protein has unique properties that are specific to the biology of the virus. For example, the EBV SM protein induces specific cell genes that facilitate EBV replication, and it is incapable of fully substituting for the HSV ICP27 homolog (3, 27). In addition, there is a significant amount of functional and sequence divergence among the different homologs encoded by the human herpesviruses, including differences in mechanism of action and varying effects on splicing and transcription (28, 37, 43, 44). Thus, it is likely that ORF57 has unique functions related to KSHV replication and pathogenesis.
The ORF57 gene, which is expressed early during lytic KSHV replication, has a regulatory function. ORF57 posttranscriptionally enhances expression of KSHV intronless genes and represses several intron-containing genes in reporter assays (2, 16, 20). The ORF57 protein appears to have synergistic effects with the KSHV immediate-early transcriptional activator ORF50 in reporter assays, enhancing expression of ORF50-dependent genes, although the exact mechanism of this effect remains to be established (20, 22). ORF57 may also possess gene specificity in its enhancing function, differentially increasing expression of selected cellular and viral target genes (16, 20). Furthermore, it has been reported that ORF57 interacts with the cellular export factor Ref/Aly and may thereby facilitate export of intronless mRNAs (23). Based on these properties, it is likely that ORF57 has specific effects on gene expression, preferentially stimulating intronless lytic KSHV genes and inhibiting or activating particular host cell genes. Such a process is hypothesized to be essential for the orderly progression of the lytic cycle from latency to early and late lytic replication. However, the role of ORF57 in lytic replication in the context of actual KSHV infection has not been studied.
In order to study the role of the ORF57 protein in KSHV replication and in regulating gene expression, we generated a recombinant KSHV in which the ORF57 gene was specifically interrupted. The production of this recombinant KSHV mutant allowed us to determine whether the ORF57 protein was required for KSHV lytic gene expression and infectious virion production. The KSHV ORF57-null mutant recombinant virus should be useful in further analysis of the role of ORF57 in KSHV replication and pathogenesis.
MATERIALS AND METHODS
Cells and plasmids.
293 cells are human embryonic kidney cells transformed with adenovirus type 5 DNA (14). The Vero cell line was originally initiated from the kidney of a normal adult African green monkey (39).
The ORF57 coding region was cloned in the cytomegalovirus (CMV) promoter-driven expression vector pCDNA3 (Invitrogen) as previously described (16). The KSHV ORF59 open reading frame (ORF) was amplified from BCBL1 cells (29), sequenced, and cloned in pCDNA3. The ORF50 expression plasmid DD267, in which ORF50 is cloned in pCDNA3, has been previously described (19).
ORF57-null KSHV construction. (i) Construction of the ORF57-targeting cassette.
A DNA fragment comprising the Tn5 prokaryotic promoter and the kanamycin phospho-transferase gene flanked by 60 bp of ORF57 coding sequence was amplified from the vector PCR 2.1-TOPO (Invitrogen) using primers with the sequence 5′-ATGATAATTGACGGTGAGAGCCCCCGCTTCGACGACTCGATCATCCCCCGGGGCGCAAGGGCTGCTAAAGG-3′ and 5′-TGACCTCGCCAAGAAGGTTACATGCCTCTACTAAGCGGTTTCCCATCGCTTCAGAAGAACTCGTCAAGAAG-3′. The 5′ portions of these primers are derived from nucleotides 82717 to 82766 and 83229 to 83278 of the KSHV genome. The remaining 20 bp of each primer consists of the beginning and end of the Kanr cassette derived from bp 989 to 1009 and 2093 to 2113 of the PCR 2.1 sequence. Insertion of this cassette into the KSHV genome by homologous recombination is predicted to result in truncation of ORF57 and fusion of the first 197 amino acids of ORF57 to 23 adventitious amino acids from the sequence upstream of the Kanr coding region. Amplified DNA was gel purified prior to electroporation into bacteria containing KSHV BACmid.
(ii) Generation of ORF57-null recombinant BACmid in Escherichia coli.
A BACmid containing the wild-type (wt) KSHV genome and hygromycin and chloramphenicol resistance genes (BAC36), which was previously constructed and characterized, was kindly provided by S. J. Gao (45). The BAC36 BACmid also expresses green fluorescent protein (GFP), allowing direct visualization of BACmid-infected cells by microscopy. Rec(-) bacteria carrying BAC36 were transformed with the plasmid pGETREC, which encodes the bacteriophage λ recE recombinase and ampicillin resistance (26). The λ recombinase in pGETREC is under the control of an arabinose-inducible promoter. Bacteria carrying pGETREC therefore become recombination proficient in the presence of arabinose. Bacteria containing BAC36 and pGETREC were grown to the exponential growth phase with the addition of 0.2% (wt/vol) l-arabinose during the final 40 min of growth. Cells were then washed and made electrocompetent.
Electrocompetent bacteria, which remain recombination proficient for a period of several hours after withdrawal of l-arabinose, were electroporated with 0.2 μg of the ORF57-targeting cassette DNA fragment and briefly grown in liquid culture in the absence of ampicillin to promote loss of the pGETREC plasmid and expression of kanamycin resistance. The cultures were then plated on plates with chloramphenicol and kanamycin to select for clones carrying BACmids which had undergone recombination.
Kanamycin- and chloramphenicol-resistant colonies were dispersed in double-distilled H2O and analyzed by PCR with one primer from the ORF57 ORF upstream of the targeted region and one primer from the Kanr ORF designed to yield a diagnostic 700-bp fragment only if homologous recombination between the targeting cassette and the wt BACmid had occurred. Positive colonies were restreaked on kanamycin-chloramphenicol plates, and BACmid DNA was prepared by a modification of the alkaline lysis method in which cleared lysates were digested sequentially with RNase A and protease K followed by phenol extraction and column purification.
Transfection and adenovirus infection.
Transfections were performed with Lipofectamine Plus (Invitrogen) as per the manufacturer's protocol. All transfections were performed with equal amounts of DNA normalized with empty vector DNA. Adenovirus expressing ORF50 (Ad50; kind gift of Don Ganem) was grown in 293 cells and purified from concentrated supernatant over CsCl gradients, and titers were determined. Infections were performed at a multiplicity of infection of 1,000 per cell by incubation for 3 h in medium with 2% fetal calf serum. Cells were then washed twice and reincubated in medium with 10% fetal calf serum.
For generation of stably BACmid-infected cell lines, Vero cells were transfected with ORF57-null BACmid using Lipofectamine Plus. Two days after transfection, cells were examined with a fluorescence microscope and the number of GFP-expressing cells was determined. Cells were trypsinized, and approximately 100 green cells were plated per 100-mm tissue culture dish in growth medium with 100 to 200 μg/ml hygromycin B. Medium was changed every 3 days until discrete colonies of hygromycin-resistant cells were identified. Single GFP-expressing colonies were transferred using glass cloning cylinders and expanded under hygromycin selection.
Induction of lytic gene expression and virus replication.
To induce lytic replication of KSHV lytic gene expression or virus production in Vero and 293 cells, ORF57-null KSHV-infected cells were either transfected with ORF50 expression vector or infected with Ad50 as described above. As appropriate, ORF57 plasmid or empty vector was included in the transfection. Sodium butyrate was added to the growth medium at a concentration of 2 mM the day after transfection to enhance lytic replication. Cells were harvested for either immunofluorescence or RNA isolation 48 h after transfection. For virus passage, supernatant was harvested from the cultures 5 days after transfection, cleared by centrifugation twice, and filtered through a 0.45-μm cellulose acetate filter. Uninfected Vero cells were infected by incubation in virus-containing supernatant for a minimum of 4 hours. Cells were examined daily and photographed under fluorescence microscopy to detect GFP expression in newly infected cells.
Immunofluorescence microscopy, immunoblotting, and RNA analysis.
Cells for immunofluorescence microscopy were grown on glass coverslips. Where indicated, cells were transfected 48 h prior to fixation. Cells were washed and fixed in 100% methanol at −20°C for 10 minutes, air dried, and stored at −20°C. Fixed cells were blocked with 20% goat serum and stained with monoclonal antibody to ORF59 protein and AlexaFluor 594-conjugated anti-mouse immunoglobulin G antibodies to visualize ORF59 protein or polyclonal rabbit anti-ORF57 antibodies and Texas Red-conjugated goat anti-rabbit immunoglobulin G. Nuclei were counterstained, and slides were mounted for microscopy with ProLong Gold antifade reagent (Molecular Probes).
RNA was isolated from washed cell pellets using QIAGEN RNeasy columns as per the manufacturer's protocols. Five micrograms of RNA was electrophoresed in denaturing formaldehyde-agarose gels and transferred to charged nylon membranes prior to hybridization with 32P-labeled probes. Gene-specific probes were generated by gel purification of fragments excised from gene expression plasmids described above or by specific amplification of ORFs from BCBL1 DNA. Probes were 32P labeled using random primers and Klenow DNA polymerase and column purified. Northern blotting was performed as previously described (32).
Southern blotting was performed exactly according to published protocols (34). Briefly, restriction digests of BACmid DNA were electrophoresed, depurinated, denatured, neutralized, transferred to membrane, cross-linked, and hybridized with a probe generated from the ORF57 ORF.
Immunoblotting was performed with polyclonal anti-ORF57 antibodies and horseradish peroxidase-conjugated secondary antibody followed by chemiluminescence detection (Amersham).
RESULTS
Construction of an ORF57-null KSHV.
In order to investigate the role of ORF57 in KSHV replication, we constructed recombinant KSHV with ORF57 functionally deleted. The ORF57 gene was interrupted by the insertion of a kanamycin phosphotransferase gene into the KSHV genome. The recombination strategy employed a previously described KSHV BACmid (45). Briefly, a targeting cassette was generated by PCR amplification of the kanamycin resistance gene with a prokaryotic promoter from a commercially available cloning vector. The primers were constructed to be complementary to the Kanr gene at their 3′ ends and to ORF57 sequence at their 5′ ends. These primers generated a 1,227-bp fragment containing the Kanr cassette flanked by 60 bases of the ORF57 gene on either side (Fig. 1). The flanking sequences in the targeting construct were located 561 bp apart, so that homologous recombination between the targeting cassette and the KSHV genome would result in deletion of approximately 560 bp of the ORF57 gene and replacement with the Kanr gene.
FIG. 1.
Strategy for generation of ORF57-null mutant KSHV BACmid. A DNA fragment capable of homologous recombination with the endogenous ORF57 gene was generated by PCR amplification of a kanamycin resistance cassette using primers incorporating ORF57 sequence at the 5′ termini (dark gray). The fragments were electroporated into E. coli carrying a KSHV BACmid (BAC36) to allow homologous recombination mediated by transiently expressed λ bacteriophage recombinases. Recombinants that had incorporated the targeting cassette were selected by plating on chloramphenicol-kanamycin plates. BAC36 also encodes GFP, hygromycin resistance, and chloramphenicol resistance, as shown.
In order to regulate the extent of recombination in bacteria, an inducible recombinase system was employed (18, 26). The targeting cassette was purified to avoid contamination with the kanamycin-resistant parent plasmid and then electroporated into Rec(-) bacteria carrying an arabinose-inducible recombinase. These bacteria were rendered temporarily recombination proficient by arabinose induction prior to being made competent for electroporation. Bacteria containing potential recombinant KSHV were selected by plating electroporated bacteria on chloramphenicol (to select for the KSHV BACmid) and kanamycin (to select for integration of the ORF57-targeting cassette). DNA was isolated from viable colonies and screened by PCR using one primer from the ORF57 gene and another within the Kanr gene. Clones that were verified by PCR were further analyzed by restriction enzyme digestion and Southern blotting. In the wild-type BACmid, the probe hybridizes to two HindIII fragments of approximately 2 and 3 kb in which ORF57 is encoded. Due to the insertion of the Kanr cassette in the mutant ORF57-null BACmid, the 2-kb fragment becomes approximately 1 kb larger (Fig. 2).
FIG. 2.
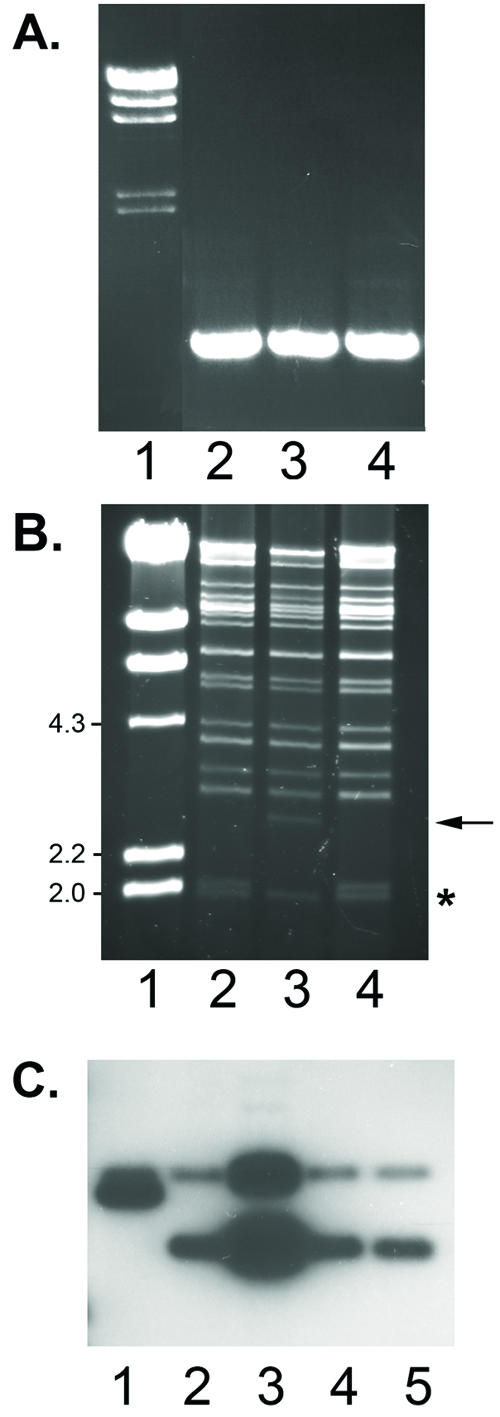
Screening and verification of recombinant ORF57-null virus. A. DNA from bacterial colonies isolated by chloramphenicol and kanamycin selection was screened for the presence of sequences diagnostic of an insertion of the Kanr cassette into ORF57 by PCR amplification, with one primer in ORF57 and one primer in Kanr. Amplification of a 700-bp fragment is shown in DNA from three independent isolates (lanes 2 to 4), and molecular weight markers are shown in lane 1. B. Restriction analysis of recombinant BACmids was performed with HindIII digestion and electrophoresis. In addition to the presence of all expected fragments, recombinant BACmids contain a 3-kb HindIII fragment, as seen in lane 3 (arrow), instead of a 2-kb fragment seen in wild-type BACmid digests (*) (lanes 2 and 4), consistent with insertion of the Kanr cassette in ORF57. C. Southern analysis of DNA from recombinant BACmid clones was used to verify correct insertion of Kanr into ORF57. There is a HindIII site in the ORF57 gene which results in two fragments that hybridize to the ORF57 probe in wt KSHV (lanes 2 to 5). Homologous recombination and insertion of Kanr into the smaller fragment result in two fragments approximately 3 kb in size (lane 1). Molecular weight standards from a HindIII digest of bacteriophage lambda DNA are shown in lane 1.
ORF57 is required for KSHV replication.
In order to ask whether ORF57 is required for KSHV replication, we attempted to passage infectious KSHV from cells transfected with ORF57-null KSHV. A variety of cell types were transfected with the ORF57-null BACmid and an ORF50 expression plasmid. Parallel transfections were performed with the addition of an ORF57 expression plasmid to rescue the ORF57-null BACmid. Supernatants were harvested at various times from 48 to 120 h after transfection and used in an attempt to infect monolayers of target cells. Despite testing a variety of manipulations and conditions, only occasional passage of virus was detected by microscopic examination of the target cells for GFP expression from the recombinant KSHV (data not shown). Notably, such occasional positive cells were only detected in supernatant from ORF57 transfected cells.
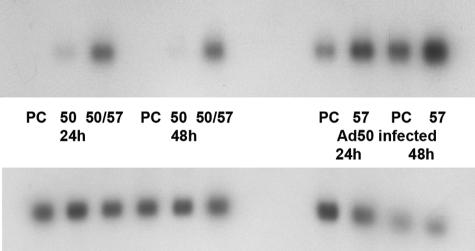
Because rescue of infectious virus production by transient transfection of BACmid into 293 cells was relatively inefficient, we wished to determine whether significant expression of KSHV lytic replicative genes was achieved under these conditions. We therefore transfected 293 cells with ORF57-null BACmid and ORF50 plasmid, with or without ORF57 rescue. RNA was harvested from the cells 48 h after transfection and analyzed by Northern blotting to measure ORF59 expression. ORF59 was chosen as a representative early lytic gene, since it is expressed within 6 hours after initiation of the lytic cycle and encodes the DNA polymerase processivity factor, a protein critical for DNA replication (46). As shown in Fig. 3, ORF59 mRNA is detectable in BACmid-transfected cells, but only when ORF57 is expressed. A similar result was obtained with Vero cells (Fig. 3B). The length of time required to obtain a similar degree of intensity on the autoradiograms was significantly shorter with mRNA from Vero cells than with 293 cells, although transfection was performed under identical conditions. These findings suggested that ORF57 was likely to be required for lytic KSHV replication and that rescue of infectious KSHV from ORF57-null BACmid-infected cells should be possible, especially in Vero cells.
FIG. 3.
Expression of an early lytic gene (ORF59) by ORF57-null KSHV. A. 293 cells were transfected with an ORF57-null KSHV BACmid in combination with either empty vector (C), an ORF50 expression vector (50), or both ORF50 and ORF57 expression vectors (50/57). RNA was harvested and analyzed by Northern blotting with an ORF59 probe. RNA from transfections with two independent BACmid isolates is shown. Blots were probed with glyceraldehyde-3-phosphate dehydrogenase probes as a loading control (bottom panels). B. Vero cells were transfected with ORF57-null BACmid and either empty vector (C), ORF50 (50), or ORF50 and ORF57 plasmids (50/57) and analyzed as for panel A.
With the aim of increasing the efficiency of virus production, we generated Vero cell lines stably infected by recombinant KSHV with ORF57 deleted. Vero cells were transfected with recombinant ORF57-null KSHV BACmid and selected with hygromycin. Individual clones were expanded and verified to be 100% GFP positive. To confirm that these ORF57-null mutants did not express any detectable ORF57, we examined the cells by immunofluorescence microscopy, Northern blotting, and immunoblotting. In parallel, we examined Vero cells infected with the parental wt ORF57 KSHV. Cells were first induced into lytic replication by transfection of ORF50 or empty vector as a control. As shown in Fig. 4A and B, wt ORF57-infected cells express ORF57 protein and mRNA when transfected with ORF50, whereas ORF57 mutant infected cells do not. After transfection with ORF50, approximately 5% of wt ORF57-infected cells were ORF57 positive by immunofluorescence, but no ORF57 expression was detectable in any of four independently derived ORF57 mutant-infected cell lines. Representative micrographs are shown in Fig. 4C.
FIG. 4.
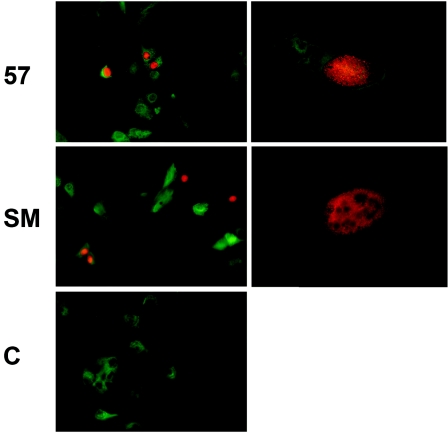
ORF57 is not expressed in cells infected with ORF57-null KSHV. A. Vero cells stably infected with either wt KSHV derived from the parent BACmid (wt) or infected with ORF57-null KSHV (57KO) were transfected with either empty vector (PC) or ORF50 expression vector (50). Protein lysates were analyzed by immunoblotting with anti-ORF57 serum to detect ORF57 protein expression. Lysate from HeLa cells transfected with ORF57 was used as a positive control. Cells are shown above the panel, and the transfected plasmids are shown below it. B. RNA was prepared from Vero cells stably infected with either wt ORF57 KSHV or ORF57-null KSHV and analyzed for expression of ORF57 by Northern blotting. Lanes are labeled as for panel A. C. Cells from transfections in panel B were also analyzed by immunofluorescence microscopy for ORF57 nuclear expression. ORF57 expression was only detected in wt ORF57 KSHV-infected cells (top right) and not in ORF57-null KSHV-infected cells (bottom right). DAPI-stained cells are shown on the left.
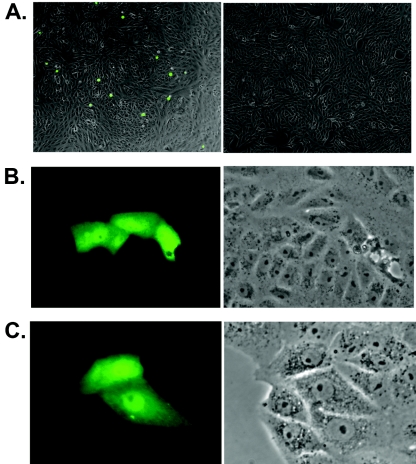
To maximize induction of lytic replication, these stably infected cells were infected with Ad50 and transfected with either empty vector plasmid or ORF57 plasmid. Supernatants were harvested from cells 5 days after induction of lytic replication and used to infect Vero cell monolayers. Infected monolayers were examined daily for GFP expression, indicating successful passage of ORF57-null KSHV. Infected cells were easily detected 48 h after infection by supernatants from wt-infected cells transfected with empty vector. In contrast, no infected cells could be derived from supernatants of any ORF57-null-infected cells transfected with empty vector. However, transfection of ORF57-null-infected cells with ORF57 yielded supernatant that gave rise to GFP-positive cells after incubation with uninfected Vero monolayers (Fig. 5). Overall, when measured by passage to uninfected Vero cells, supernatant from wt ORF57-infected cells yielded approximately 15% GFP-positive cells. Supernatant from ORF57-null-infected clones yielded 3 to 5% GFP-positive cells. Thus, the level of reconstitution by ORF57 transfection was 20 to 30% of wt ORF57 virus but undetectable in the absence of ORF57, indicating that ORF57 was required for production of infectious KSHV. In no case was virus passage observed without Ad50 infection (data not shown).
FIG. 5.
Rescue and passage of ORF57-null KSHV to uninfected cells. Vero cells stably infected with ORF57-null KSHV or wt ORF57 KSHV were induced to permit lytic replication by infection with Ad50 and treatment with sodium butyrate. Cells were also transfected with either empty vector or ORF57 expression plasmid. Five days after induction, cell supernatant was harvested and incubated with Vero cell monolayers. Infected cells were observed and photographed 48 hours after infection. A. GFP expression from ORF57-null KSHV was only observed when cells were infected with supernatant from ORF57-transfected cells (left panel), not from cells transfected with empty vector (right panel). B. Infected cells from passage of ORF57-null KSHV are shown at higher magnification (left panel) and a corresponding phase-contrast view (right panel). C. GFP-expressing virus detected in supernatants of wt ORF57 KSHV cells infected with Ad50 and transfected with empty vector (left panel), with the corresponding phase-contrast view at right.
KSHV lytic gene expression is ORF57 dependent.
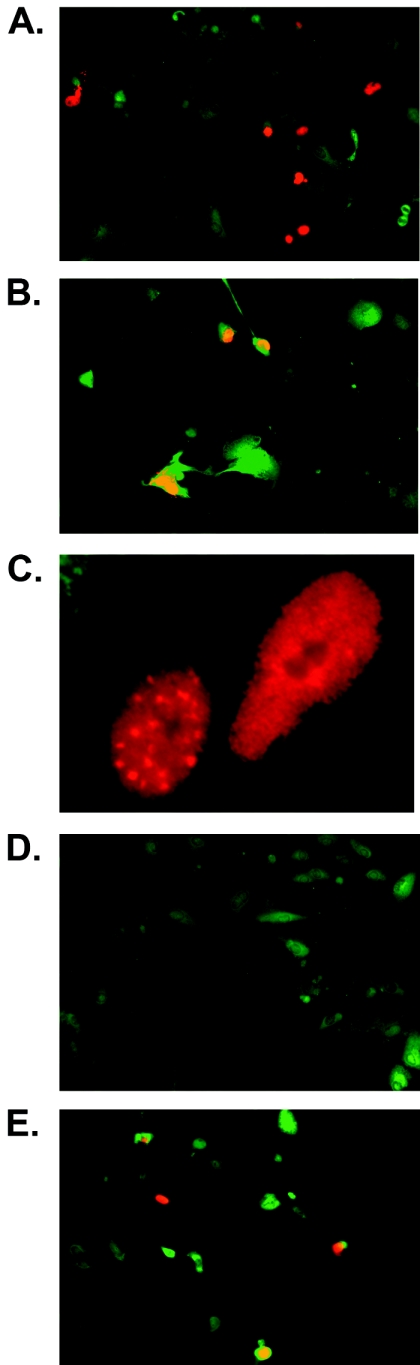
The dependence of infectious KSHV virion production on ORF57 expression suggested that one or more stages of lytic KSHV replication were ORF57 dependent. We therefore examined the expression of early and late lytic genes in the presence and absence of ORF57. Vero/ORF57-null KSHV cells were induced by transfection with ORF50 and transfected with either empty vector or ORF57 plasmid. We then assessed ORF59 protein expression from ORF57-null virus in the presence or absence of ORF57 by immunofluorescence microscopy after fixation and staining with monoclonal antibodies specific for ORF59 protein (kind gift of B. Chandran) (5). Only Vero/ORF57-null cells transfected with ORF57 expressed characteristic ORF59 nuclear staining. These results were repeated with four independent ORF57-null mutant-infected cell lines, and results from two representative experiments are shown in Fig. 6. By comparison, wt ORF57-infected cells expressed ORF59 upon transfection with ORF50 alone (Fig. 6E).
FIG. 6.
Expression of ORF59 protein by ORF57-null KSHV. Vero cells stably infected with ORF57-null KSHV were induced to permit lytic replication by transfection with ORF50. Cells were also transfected with either empty vector or ORF57 plasmid. Cells were fixed and stained with anti-ORF59 monoclonal antibody and visualized by immunofluorescence microscopy. In ORF57-null cells, ORF59 expression was only detected when ORF57 was provided by transfection (A and B). Typical punctate nuclear expression of ORF59 is seen at higher power (C). In contrast, ORF57-null cells transfected with ORF50 and empty vector did not express ORF59 (D). wt ORF57 cells transfected with ORF50 and empty vector, however, did express ORF59 (E).
Another lytic KSHV gene product whose expression is potentially regulated by ORF57 is the small polyadenylated nuclear RNA PAN (nut-1; T1.1). In cotransfection assays, PAN expression is highly activated by ORF50 at the transcriptional level, and the PAN promoter has been shown to be ORF50 dependent (42). It has also been reported that ORF57 acts synergistically with ORF50 to increase PAN expression (20). It has been suggested that the synergy is based on a physical interaction between ORF57 and ORF50 proteins, leading to increased PAN transcription. However, it is not known whether the levels of PAN RNA are actually affected by ORF57 in KSHV-infected cells. The ORF57 mutant we constructed allowed us to directly examine the dependence of PAN expression on ORF57. Lytic expression was induced in Vero/ORF57-null cells by either ORF50 transfection or Ad50 infection, and PAN expression was compared with or without ORF57 rescue. As shown in Fig. 7, although PAN RNA is transcribed when ORF50 alone is expressed, PAN accumulation is significantly increased by ORF57 expression.
FIG. 7.
Expression of lytic PAN RNAs by ORF57-null KSHV. Vero cells stably infected with ORF57-null KSHV were induced to permit lytic replication by either transfection with ORF50 (left) or infection with Ad50 (right). Cells were also transfected with either empty vector (PC) as a control or ORF57 (57) to provide ORF57 in trans. RNA was harvested from transfected cells 24 and 48 h after transfection and analyzed by Northern blotting with a probe specific for PAN transcripts. Northern blotting with a glyceraldehyde-3-phosphate dehydrogenase probe is shown in the bottom panel as a loading control.
ORF57 and its EBV homolog SM are functionally distinct.
The ability to rescue productive virus replication allows one to determine whether various herpesvirus homologs are able to complement each other or are functionally distinct. Attempts to rescue mutant virus replication in other herpesviruses in which the cognate ORF57 homologs are deleted have been generally unsuccessful (3, 11, 15, 25). Since EBV is the human herpesvirus most closely related to KSHV, we asked whether SM, the EBV homolog of ORF57, could substitute for the essential functions of ORF57. We attempted to rescue productive replication of ORF57-null KSHV by transfection of SM. Vero cells carrying ORF57-null KSHV were transfected with either empty vector, ORF57, or SM and induced into lytic replication with Ad50. Supernatants were harvested and used to infect uninfected target cells exactly as described above. SM transfection did not yield infectious progeny detectable by GFP expression in target cells, whereas ORF57 transfection resulted in easily detectable infection, as shown above in Fig. 5.
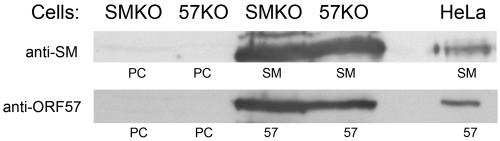
The converse experiment was performed by transfection of 293 cells carrying SM-null EBV (SM-KO EBV) (15) to determine whether ORF57 could functionally replace SM in rescuing EBV replication. SM-null EBV-infected cells were transfected with either SM, empty vector, or ORF57, and the EBV immediate-early transactivator Z (Zta; BZLF1) was included in all transfections to induce lytic EBV replication. The supernatant was used to infect EBV-negative Raji cells, and passage was monitored by examination of the target cells for GFP expression by microscopy. Although SM transfection resulted in the release of numerous infectious EBV progeny, no GFP expression was detected in cells incubated with supernatant from ORF57-transfected cells (data not shown). These experiments indicate that although similar in transactivation function in reporter assays, EBV SM and KSHV ORF57 are unable to substitute for each other in rescuing virus production. One possible reason for the failure of ORF57 and SM to cross-complement each other could have been inadequate expression of one of these proteins on transfection of the heterologous mutant-infected cells. We therefore compared SM expression in ORF57-null KSHV-infected Vero cells and vice versa, ORF57 expression in SM-KO-infected 293 cells. As shown in Fig. 8, both proteins were expressed in similar amounts in both types of cells.
FIG. 8.
Expression levels of transfected SM and ORF57 proteins in mutant virus-infected cells. 293 cells carrying SM-KO EBV and Vero cells infected with ORF57-null KSHV were transfected with either empty vector (PC), SM, or ORF57 expression vectors, and lysates were immunoblotted with anti-SM antibodies (top panel) or anti-ORF57 antibodies (bottom panel). HeLa cells transfected with either SM or ORF57 were included as a control.
EBV SM is capable of enhancing accumulation of essential KSHV lytic transcripts.
The mechanism of gene activation by ORF57 has been reported to be primarily posttranscriptional but gene specific (16, 20). The basis by which ORF57 discriminates among genes to enhance the accumulation of some genes but not others remains under investigation. Since the inability of SM to replace ORF57 in rescuing replication might have been due to an inability of SM to act on KSHV gene targets, it was of interest to determine the effect of SM on accumulation of lytic KSHV mRNAs. We therefore measured the effects of SM on the expression of early and late lytic KSHV genes, which are likely to be required for the production of infectious virions, and compared them to the effects of ORF57. Lytic virus replication was induced in Vero cells carrying ORF57-null KSHV by transfection with ORF50. Cells were also transfected with either empty vector, SM, or ORF57, exactly as was done in the virus passage experiments. First, ORF59 expression was examined by immunofluorescence of ORF57-null KSHV-infected cells. Cells were transfected with empty vector, ORF50, ORF 50 plus ORF57, or ORF50 plus SM. As expected, no ORF59 was detected with vector or ORF50 alone, consistent with previous experiments. However, when either ORF57 or SM was transfected in addition to ORF50, ORF59 expression was detectable in approximately 1 to 3% of cells (Fig. 9).
FIG. 9.
Effect of EBV SM on expression of KSHV ORF59 protein in ORF57-null virus-infected cells. Vero cells stably infected with ORF57-null KSHV were induced to permit lytic replication by transfection with ORF50. Cells were also transfected with either empty vector (C), ORF57 (57), or EBV SM plasmid (SM). Cells were fixed and stained with anti-ORF59 monoclonal antibodies and visualized by immunofluorescence microscopy. ORF59 expression was only detected when either ORF57 or SM was provided by transfection. Typical punctate nuclear expression of ORF59 is easily seen at higher power and was similar with ORF57 or SM transfection.
We also measured expression of minor capsid protein (mCP, a late gene) and ORF9, the KSHV DNA polymerase (another early gene) by Northern blotting. In order to maximize lytic gene expression, cells were induced by infection with Ad50 and transfected with either empty vector, ORF57, or SM. These experiments revealed that while mCP was detectable with ORF50 alone, ORF57 significantly enhanced mCP expression (Fig. 10). Somewhat surprisingly, EBV SM was similar to ORF57 in its ability to enhance mCP expression under these same conditions. Furthermore, SM appeared to be superior to ORF57 in enhancing expression of ORF9, the KSHV DNA polymerase. Finally, since ORF57 has been suggested to enhance PAN transcription by a specific interaction with ORF50, it was of interest to ask whether SM could also replace ORF57 in enhancing PAN expression. We therefore examined the effect of SM on PAN levels in ORF57-null KSHV-infected cells. Northern analysis of RNAs as performed in the previous experiments again demonstrated that SM rescues PAN accumulation as efficiently as ORF57 itself. These data suggest that ORF57 acts on both early and late KSHV lytic genes and that the inability of SM to rescue ORF57-null KSHV virus replication is not due to a general inability to act on KSHV target mRNAs. Finally, although these cells were infected with Ad50 as a source of ORF50 for lytic induction, to rule out the possibility that the observed effects of ORF57 and SM might have been mediated indirectly by enhancing ORF50 RNA accumulation, we directly measured the levels of ORF50 transcripts in cells which had been transfected with either empty vector, ORF57, or SM. As shown in Fig. 10, SM and ORF57 did not significantly affect ORF50 mRNA levels in these cells.
FIG. 10.

Effects of ORF57 and EBV SM on early and late lytic KSHV transcripts. Vero cells stably infected with ORF57-null KSHV were induced to permit lytic replication by infection with Ad50. Cells were also transfected with either empty vector (PC) as a control, ORF57 (57), or SM expression vector. RNA was harvested from transfected cells 48 h after transfection and analyzed by Northern blotting with a probe specific for PAN, ORF9, ORF50, or mCP transcripts. Blots were also probed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control.
DISCUSSION
In this study, we have shown that ORF57 is essential for KSHV virion production and lytic gene expression after induction of the lytic cycle by constructing a KSHV recombinant in which the ORF57 gene was insertionally inactivated. ORF57 is the KSHV gene belonging to the herpesvirus gene family of multifunctional gene regulatory proteins that also includes HSV ICP27, EBV SM (Mta, EB2, and BMLF1), VZV ORF4, CMV UL69, and herpesvirus saimiri ORF57 (for review, see reference 37). The general importance of these genes in lytic herpesvirus replication is suggested by the presence of an ORF57 homolog in a large number of mammalian herpesviruses whose genomes have been sequenced, including ovine, equine, bovine, alcelaphine, murid, and various primate species. In addition, the requirement of these genes for virus replication has been directly tested in VZV, HSV, and EBV (11, 15, 33). In these three viruses and in KSHV, as we have shown here, ORF57 or its homolog is essential. The ability of ORF57 transfection to reconstitute virus production to only 20 to 30% of wt levels is likely due at least in part to the fact that, whereas all wt KSHV-infected cells were transduced by ORF50, only a fraction of cells was transfected by ORF57.
As we have shown here, early and late lytic KSHV gene expression is highly dependent, at the mRNA level, on KSHV ORF57. The essential character of the ORF57 family of genes is likely to derive from their role in facilitating accumulation of lytic mRNA transcripts. ICP27 and EBV SM have been shown to interact with various components of the cellular export machinery, facilitating export of unspliced mRNAs (4, 7-9, 13, 17, 21, 30, 35, 36, 38, 41). The majority of early and late lytic herpesvirus genes are intronless and may therefore lack the ability to independently recruit components of the cellular exon junction complex, a multiprotein complex deposited near the exon junction of processed mRNAs (for review, see reference 12). Components of the exon junction complex, particularly REF/Aly, interact with TAP, the primary mediator of nuclear mRNA export, which interacts with the nuclear pore complex and facilitates cytoplasmic mRNA transfer. Current models for the mechanism of action of ORF57 include an interaction of ORF57 with viral mRNA and recruitment of REF/Aly by direct protein-protein interaction, and published evidence indicates that one region of ORF57 directly binds to REF/Aly (23).
We have also demonstrated that ORF57 and EBV SM are each unable to substitute for the other in rescuing productive replication of SM-null and ORF57-null recombinant viruses, respectively. Although the ORF57 family proteins are homologous, there are significant differences in sequence and function, particularly among the alpha-, beta-, and gammaherpesviruses. In no case has one member of this family been able to substitute for another in rescuing virus replication. EBV SM has been inserted into an HSV genome with ICP deleted, and the recombinant, although it does replicate, is almost as defective in replication as the parent Δ27 mutant (3). Similarly, CMV UL69 and ICP27 were not capable of trans-complementing a recombinant EBV with SM deleted to efficiently rescue virion production (15). This exclusive requirement for the specific regulatory gene of each virus also applies within herpesvirus families, since VZV ORF4 mutants cannot be complemented by HSV ICP27 (11, 25). Although these viral proteins behave similarly in reporter assays, transactivating reporter genes, they are clearly not equivalent in biologic function. Somewhat surprisingly, EBV SM was as efficient as ORF57 in enhancing expression of ORF57 and mCP genes from ORF57-null virus and even more active than ORF57 on the ORF9 gene. The inability of SM to rescue infectious KSHV production despite apparent activity on KSHV transcripts has also been observed in other systems, where ICP27 cannot rescue EBV SM or VZV ORF4 mutants despite activity on heterologous viral mRNAs.
These functional differences are likely to be due to one or more aspects of the mechanism of action of ORF57 homologs. Most importantly, there is some degree of specificity in terms of the responsiveness of various target genes to the ORF57 family of proteins. Such differences have been observed both in reporter assays and with viral genes (30). For example, some EBV lytic genes are more highly SM dependent than others (15, 38). It is likely that these differences in target gene responsiveness are at least partly due to the regulatory proteins having different affinities for different mRNA species, as suggested by yeast three-hybrid experiments with ICP27, which demonstrated that ICP27 has a preferential affinity for a subset of HSV transcripts (40). Thus, although SM does act on several essential KSHV lytic genes, it may be that its mRNA specificity is distinctive enough that it does not permit appropriate accumulation of all heterologous KSHV transcripts. This hypothesis can now be directly tested by using the ORF57-null and EBV SM-null recombinants in combination with viral gene arrays to examine the differences in viral transcript accumulation when trans-complemented with the heterologous regulatory gene. It should be noted that the dependence of late lytic genes on ORF57 and its homologs is complicated by the likely involvement of these proteins in regulation of genes involved in viral DNA replication. Thus, ORF57 and its homologs are likely to affect late gene expression via both direct effects (on mRNA levels) and indirect effects (on DNA template numbers).
A second important difference between the various herpesvirus homologs that does not allow them to be functionally interchangeable may lie in their effects on host cell gene expression. For example, EBV SM has significant growth-inhibitory effects, whereas we have not observed such an effect with ORF57 (unpublished observations). In addition, SM induces a specific subset of host cell genes, and some of these induced gene products may be important in lytic EBV gene expression (27, 31). Thus, the functional specificity of the various ORF57 homologous proteins may also derive from unique effects on host cell gene expression that are required for efficient replication.
Finally, the utility of producing this specific ORF57-null mutant is demonstrated by the ability to directly determine whether ORF57 is involved in regulation of a specific pathway or expression of a particular gene. It has been hypothesized that ORF57 may be involved in regulation of PAN expression (20, 22), and using the ORF57-null mutant, we have demonstrated that maximal PAN expression during lytic replication does in fact require ORF57. While this finding is compatible with the interpretation that ORF57 synergizes with ORF50 to stimulate PAN transcription, it is also possible that ORF57 enhances nuclear stability of PAN posttranscriptionally. The actual role of a physical interaction between ORF57 and ORF50 proteins in PAN gene transcription in vivo is also called into question by the finding that EBV SM is as effective in enhancing PAN accumulation from the ORF57-null KSHV as ORF57 itself. The ORF57-null recombinant will allow us to address these and other mechanistic questions regarding the regulation of gene expression during lytic KSHV replication in the context of the entire viral genome.
Acknowledgments
This work was supported by Public Health Service grants CA-81133 and CA-119905 from the National Cancer Institute.
REFERENCES
- 1.Albrecht, J. C., J. Nicholas, D. Billler, K. R. Cameron, B. C. N. Beisinger, S. Wittman, M. A. Craxton, and H. Coleman. 1992. Primary structure of the herpesvirus saimiri genome. J. Virol. 66:5047-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bello, L. J., A. J. Davison, M. A. Glenn, A. Whitehouse, N. Rethmeier, T. F. Schulz, and J. Barklie Clements. 1999. The human herpesvirus-8 ORF 57 gene and its properties. J. Gen. Virol. 80:3207-3215. [DOI] [PubMed] [Google Scholar]
- 3.Boyer, J. L., S. Swaminathan, and S. J. Silverstein. 2002. The Epstein-Barr virus SM protein is functionally similar to ICP27 from herpes simplex virus in viral infections. J. Virol. 76:9420-9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle, S. M., V. Ruvolo, A. K. Gupta, and S. Swaminathan. 1999. Association with the cellular export receptor CRM 1 mediates function and intracellular localization of Epstein-Barr virus SM protein, a regulator of gene expression. J. Virol. 73:6872-6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, S. R., C. Bloomer, and B. Chandran. 1998. Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology 240:118-126. [DOI] [PubMed] [Google Scholar]
- 6.Chee, M., and B. Barrell. 1990. Herpesviruses: a study of parts. Trends Genet. 6:86-91. [DOI] [PubMed] [Google Scholar]
- 7.Chen, I. H., L. Li, L. Silva, and R. M. Sandri-Goldin. 2005. ICP27 recruits Aly/REF but not TAP/NXF1 to herpes simplex virus type 1 transcription sites, although TAP/NXF1 is required for ICP27 export. J. Virol. 79:3949-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, I. H., K. S. Sciabica, and R. M. Sandri-Goldin. 2002. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J. Virol. 76:12877-12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung, P., K. S. Ellison, R. Verity, and J. R. Smiley. 2000. Herpes simplex virus ICP27 induces cytoplasmic accumulation of unspliced polyadenylated alpha-globin pre-mRNA in infected HeLa cells. J. Virol. 74:2913-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho, M. S., K. T. Jeang, and S. D. Hayward. 1985. Localization of the coding region for an Epstein-Barr virus early antigen and inducible expression of this 60-kilodalton nuclear protein in transfected fibroblast cell lines. J. Virol. 56:852-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen, J. I., T. Krogmann, J. P. Ross, L. Pesnicak, and E. A. Prikhod'ko. 2005. Varicella-zoster virus ORF4 latency-associated protein is important for establishment of latency. J. Virol. 79:6969-6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreyfuss, G., V. N. Kim, and N. Kataoka. 2002. Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell Biol. 3:195-205. [DOI] [PubMed] [Google Scholar]
- 13.Farjot, G., M. Buisson, M. Duc Dodon, L. Gazzolo, A. Sergeant, and I. Mikaelian. 2000. Epstein-Barr virus EB2 protein exports unspliced RNA via a Crm-1-independent pathway. J. Virol. 74:6068-6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 15.Gruffat, H., J. Batisse, D. Pich, B. Neuhierl, E. Manet, W. Hammerschmidt, and A. Sergeant. 2002. Epstein-Barr virus mRNA export factor EB2 is essential for production of infectious virus. J. Virol. 76:9635-9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta, A. K., V. Ruvolo, C. Patterson, and S. Swaminathan. 2000. The human herpesvirus 8 homolog of Epstein-Barr virus SM protein (KS-SM) is a posttranscriptional activator of gene expression. J. Virol. 74:1038-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiriart, E., G. Farjot, H. Gruffat, M. V. Nguyen, A. Sergeant, and E. Manet. 2003. A novel nuclear export signal and a REF interaction domain both promote mRNA export by the Epstein-Barr virus EB2 protein. J. Biol. Chem. 278:335-342. [DOI] [PubMed] [Google Scholar]
- 18.Jamsai, D., M. Nefedov, K. Narayanan, M. Orford, S. Fucharoen, R. Williamson, and P. A. Ioannou. 2003. Insertion of common mutations into the human beta-globin locus using GET recombination and an EcoRI endonuclease counterselection cassette. J. Biotechnol. 101:1-9. [DOI] [PubMed] [Google Scholar]
- 19.Jeong, J. H., R. Hines-Boykin, J. D. Ash, and D. P. Dittmer. 2002. Tissue specificity of the Kaposi's sarcoma-associated herpesvirus latent nuclear antigen (LANA/orf73) promoter in transgenic mice. J. Virol. 76:11024-11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirshner, J. R., D. M. Lukac, J. Chang, and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 57 encodes a posttranscriptional regulator with multiple distinct activities. J. Virol. 74:3586-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koffa, M. D., J. B. Clements, E. Izaurralde, S. Wadd, S. A. Wilson, I. W. Mattaj, and S. Kuersten. 2001. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 20:5769-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik, P., D. J. Blackbourn, M. F. Cheng, G. S. Hayward, and J. B. Clements. 2004. Functional co-operation between the Kaposi's sarcoma-associated herpesvirus ORF57 and ORF50 regulatory proteins. J. Gen. Virol. 85:2155-2166. [DOI] [PubMed] [Google Scholar]
- 23.Malik, P., D. J. Blackbourn, and J. B. Clements. 2004. The evolutionarily conserved Kaposi's sarcoma-associated herpesvirus ORF57 protein interacts with REF protein and acts as an RNA export factor. J. Biol. Chem. 279:33001-33011. [DOI] [PubMed] [Google Scholar]
- 24.McGeoch, D., M. A. Dalrymple, A. J. Davidson, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 25.Moriuchi, H., M. Moriuchi, H. A. Smith, and J. I. Cohen. 1994. Varicella-zoster virus open reading frame 4 protein is functionally distinct from and does not complement its herpes simplex virus type 1 homolog, ICP27. J. Virol. 68:1987-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayanan, K., R. Williamson, Y. Zhang, A. F. Stewart, and P. A. Ioannou. 1999. Efficient and precise engineering of a 200 kb beta-globin human/bacterial artificial chromosome in E. coli DH10B using an inducible homologous recombination system. Gene. Ther. 6:442-447. [DOI] [PubMed] [Google Scholar]
- 27.Nicewonger, J., G. Suck, D. Bloch, and S. Swaminathan. 2004. Epstein-Barr virus (EBV) SM protein induces and recruits cellular Sp110b to stabilize mRNAs and enhance EBV lytic gene expression. J. Virol. 78:9412-9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perera, L. P., S. Kaushal, P. R. Kinchington, J. D. Mosca, G. S. Hayward, and S. E. Straus. 1994. Varicella-zoster virus open reading frame 4 encodes a transcriptional activator that is functionally distinct from that of herpes simplex virus homolog ICP27. J. Virol. 68:2468-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 30.Ruvolo, V., A. K. Gupta, and S. Swaminathan. 2001. Epstein-Barr virus SM protein interacts with mRNA in vivo and mediates a gene-specific increase in cytoplasmic mRNA. J. Virol. 75:6033-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruvolo, V., L. Navarro, C. E. Sample, M. David, S. Sung, and S. Swaminathan. 2003. The Epstein-Barr virus SM protein induces STAT1 and interferon-stimulated gene expression. J. Virol. 77:3690-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruvolo, V., E. Wang, S. Boyle, and S. Swaminathan. 1998. The Epstein-Barr virus nuclear protein SM is both a post-transcriptional inhibitor and activator of gene expression. Proc. Natl. Acad. Sci. USA 95:8852-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacks, W. R., C. C. Greene, D. P. Aschman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55:796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 35.Sandri-Goldin, R. M. 1998. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12:868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandri-Goldin, R. M. 1998. Interactions between a herpes simplex virus regulatory protein and cellular mRNA processing pathways. Methods 16:95-104. [DOI] [PubMed] [Google Scholar]
- 37.Sandri-Goldin, R. M. 2004. Viral regulation of mRNA export. J. Virol. 78:4389-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semmes, O. J., L. Chen, R. T. Sarisky, Z. Gao, L. Zhong, and S. D. Hayward. 1998. Mta has properties of an RNA export protein and increases cytoplasmic accumulation of Epstein-Barr virus replication gene mRNA. J. Virol. 72:9526-9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simizu, B., J. S. Rhim, and N. H. Wiebenga. 1967. Characterization of the Tacaribe group of arboviruses. I. Propagation and plaque assay of Tacaribe virus in a line of African green monkey kidney cells (Vero). Proc. Soc. Exp. Biol. Med. 125:119-123. [DOI] [PubMed] [Google Scholar]
- 40.Sokolowski, M., J. E. Scott, R. P. Heaney, A. H. Patel, and J. B. Clements. 2003. Identification of herpes simplex virus RNAs that interact specifically with regulatory protein ICP27 in vivo. J. Biol. Chem. 278:33540-33549. [DOI] [PubMed] [Google Scholar]
- 41.Soliman, T. M., and S. J. Silverstein. 2000. Identification of an export control sequence and a requirement for the KH domains in ICP27 from herpes simplex virus type 1. J. Virol. 74:7600-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song, M. J., H. J. Brown, T.-T. Wu, and R. Sun. 2001. Transcription activation of polyadenylated nuclear RNA by Rta in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:3129-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkler, M., T. aus Dem Siepen, and T. Stamminger. 2000. Functional interaction between pleiotropic transactivator pUL69 of human cytomegalovirus and the human homolog of yeast chromatin regulatory protein SPT6. J. Virol. 74:8053-8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winkler, M., and T. Stamminger. 1996. A specific subform of the human cytomegalovirus transactivator protein pUL69 is contained within the tegument of virus particles. J. Virol. 70:8984-8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu, F. X., T. Cusano, and Y. Yuan. 1999. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:5556-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]