Abstract
Varicella-zoster virus (VZV) is an alphaherpesvirus that causes varicella and herpes zoster. Using human cellular DNA microarrays, we found that many nuclear factor kappa B (NF-κB)-responsive genes were down-regulated in VZV-infected fibroblasts, suggesting that VZV infection inhibited the NF-κB pathway. The activation of this pathway causes a cellular antiviral response, including the production of alpha/beta interferon, cytokines, and other proteins that restrict viral infection. In these experiments, we demonstrated that VZV interferes with NF-κB activation in cultured fibroblasts and in differentiated epidermal cells in skin xenografts of SCIDhu mice infected in vivo. VZV infection of fibroblasts caused a transient nuclear translocation of p50 and p65, the canonical NF-κB family members. In a process that was dependent upon the presence of infectious VZV, these proteins rapidly became sequestered in the cytoplasm of VZV-infected cells. Exclusion of NF-κB proteins from nuclei was associated with the continued presence of IκBα, which binds p50 and p65 and prevents their nuclear accumulation. IκBα levels did not diminish even though the protein became phosphorylated and ubiquitinated, as determined based on detection of the characteristic high-molecular-weight form of the protein, and the 26S proteasome remained functional in VZV-infected cells. VZV infection also inhibited the characteristic degradation of IκBα that is induced by exposure of fibroblasts to tumor necrosis factor alpha. As expected, herpes simplex virus 1 caused the persistent nuclear translocation of NF-κB proteins, which has been shown to facilitate its replication, whereas VZV infection progressed without persistent NF-κB nuclear localization. We suggest that VZV has evolved a mechanism to limit host cell antiviral defenses by sequestering NF-κB proteins in the cytoplasm, a strategy that appears to be unique among the herpesviruses.
Primary infection with varicella-zoster virus (VZV) causes varicella (4, 15). VZV pathogenesis requires replication in skin, which appears to be initiated by transfer of infectious virus in T cells from respiratory epithelial sites of inoculation (30) and results in typical varicella lesions after a prolonged incubation period of 10 to 21 days. The cell-cell spread of the virus in skin results in cutaneous vesicles that contain high concentrations of infectious virus (24, 38). VZV infection of epidermal cells disrupts the interferon (IFN) pathway, as shown by the inhibition of the phosphorylation and nuclear translocation of STAT1 and by down-regulation of IFN-α production (30). At the same time, alpha IFN (IFN-α) expression is induced in skin cells that surround foci of VZV-infected cells. These observations suggest a model of VZV pathogenesis in skin in which lesions appear at the skin surface through a carefully modulated process during which VZV inhibits innate antiviral responses in each new skin cell that it infects while adjacent cells are signaled to up-regulate these defenses.
Members of the nuclear factor kappa B (NF-κB) family of proteins, which includes NF-κB1 (p50), NF-κB2 (p52), Rel A (p65), Rel B, and c-rel, are key cellular transcription factors that function as heterodimers or homodimers to trigger the expression of many genes involved in innate immunity and inflammation, including the type I IFNs (6). Our previous microarray studies demonstrated that many NF-κB-responsive genes were down-regulated in VZV-infected cells and that the expression of MCP-1, a representative NF-κB-induced protein, was decreased (25, 26). These observations led to this analysis of the effects of VZV on the expression and intracellular fates of proteins in the NF-κB pathway, which was of particular interest because other herpesviruses, including the related alphaherpesviruses herpes simplex virus type 1 (HSV-1) and HSV-2, elicit sustained NF-κB activation.
The most abundant form of NF-κB is a heterodimer composed of p50 and p65/RelA. In resting cells, NF-κB proteins are sequestered in the cytoplasm by the binding of members of the inhibitor of NF-κB (IκB) family to the nuclear localization signals of p50 and p65 (52). The NF-κB pathway is activated by cytokines, growth factors, and many microbial pathogens. These stimuli typically activate the IκB kinase complex (IKK), which causes the phosphorylation of IκB family members at conserved serine residues (17, 52). Phosphorylated IκB proteins are ubiquitinated and targeted for degradation by the 26S proteasome (6). Degradation of IκB releases the NF-κB dimers, which translocate to the nucleus, where p50 and p65 bind to promoters containing NF-κB response elements consisting of a loose consensus sequence of GGGRNNYYCC and initiate gene expression (20). Maintaining the transcription of target genes in the pathway requires the continued trafficking of NF-κB proteins between the cytoplasm and nucleus and cycles of p65 phosphorylation and dephosphorylation (44). Since the IκBα gene is induced by NF-κB, IκBα can be regenerated in stimulated cells (23). IκBα shuttling between the cytoplasm and the nucleus mediates the export of NF-κB proteins from the nucleus and causes IκBα to act as the major feedback inhibitor of the NF-κB pathway (5), while other IκB family members augment NF-κB signaling (23).
HSV-1 induces a persistent nuclear translocation of NF-κB proteins, associated with the breakdown of IκBα and -β (48). HSV-1 also activates IKKα and IKKβ upstream of IκBα, which is necessary for NF-κB activation (3), as well as double-stranded RNA protein kinase (PKR) (56). The degradation of IκBα in HSV-1-infected cells begins within 6 h and depends upon viral replication; binding of NF-κB to DNA is reduced in cells infected with viruses that lack ICP4 or ICP27 (51, 54). However, soluble HSV glycoprotein D and UV-inactivated HSV also signal NF-κB activation (36). These results suggest that exposure to HSV-1 proteins or particles activates NF-κB initially but that continued activation requires viral replication. Contact with cytomegalovirus (CMV) particles also induces nuclear localization of NF-κB proteins (60). Immune response genes that are induced in HSV-infected cells include IFN-γ, nitric oxide synthase (NOS), interleukin 12, tumor necrosis factor alpha (TNF-α), and RANTES/CCL5 (34, 37, 46, 47). NF-κB activation appears to be necessary for fully efficient HSV replication; inhibition results in lower viral yields, coincident with decreased transcription of viral genes that have NF-κB response elements in their promoters (3, 21, 48, 57).
While these and other reports indicate that herpesviruses activate NF-κB, some viruses inhibit the pathway, thereby directly reducing the inflammatory response to infection. These include poxviruses, which interfere with the upstream activators of NF-κB signaling, PKR, MyD88, and TNF-α (10, 13, 45), influenza A virus, which encodes the NS1 protein that prevents virus and/or double-stranded RNA-mediated activation of the NF-κB pathway and IFN-β synthesis (58), African swine fever virus, which encodes an IκBα homolog that inhibits the NF-κB pathway by acting as a dominant-negative IκBα protein (49, 50), and measles virus, which can inhibit the phosphorylation of IκBα specifically in cells of neuronal lineage (9, 16).
In this study, we found that VZV, in contrast to HSV-1(F) and HSV-1(KOS), inhibited the NF-κB pathway by sequestering p50 and p65 in the cytoplasm of infected cells after a transient nuclear translocation. IκBα levels remained constant in infected cells, even though the protein was phosphorylated and ubiquitinated, as determined on the basis of protein size. VZV also inhibited the degradation of IκBα after exogenous stimulation of the NF-κB pathway by TNF-α signaling. Infectious VZV elicited these effects, whereas inactivated VZV induced persistent nuclear localization of p50 and p65. Importantly, NF-κB was retained in the cytoplasm of VZV-infected epidermal cells in human skin xenografts of SCIDhu mice, suggesting that NF-κB inhibition facilitates VZV pathogenesis in vivo.
MATERIALS AND METHODS
Viruses and cells.
Recombinant VZV vaccine Oka (rOka) was grown in human foreskin fibroblasts (HFF), and titers of the virus were determined on melanoma (MeWo) cells by infectious focus assay (39). Fibroblasts or melanoma cell monolayers were inoculated with infected cell suspensions. HSV-1(KOS) and HSV-1(F) strains were maintained in MeWo cells, and cell-free virus was harvested by sonication for 1 min followed by centrifugation at 500 × g for 5 min at 4°C; titers of the virus were determined on Vero cells, and cell-free virus was used to infect fibroblasts or melanoma cells. Cells were grown in modified Eagle's medium (Cellgro) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 U penicillin/ml, and 50 μg streptomycin/ml at 37°C. For some experiments, rOka-infected fibroblasts were sonicated for 1 min to disrupt any intact cells, or infectious rOka was inactivated by exposure of infected cells in 6-well plates in 1 ml medium to UV light in a tissue culture hood for 25 min; elimination of infectious virus was confirmed by infectious focus assay. Growth curves were determined by infecting human foreskin fibroblast or MeWo cells with VZV or Vero cells with HSV-1(F) strain in the presence of 0, 5, or 10 μM pyrrolidinedithiocarbamate ammonium salt (PDTC) (Sigma-Aldrich). Higher concentrations of PDTC were toxic to the cells.
NF-κB GFP reporter assay.
Fibroblast monolayers in six-well plates were transfected with the pNF-κB-hrGFP plasmid (Stratagene) (1 μg), which contains a humanized green fluorescent protein (GFP) expressed from a promoter containing only a TATA box and five sequential κB binding elements. The GFP construct was used to facilitate flow cytometry sorting of virus-infected, plasmid-transfected cells. NF-κB reporter plasmid or MEKK-expressing positive-control plasmid (supplied by Stratagene). DNA was diluted in serum-free media (250 ul) and combined with Lipofectamine 2000 transfection reagent (5 ul) (Invitrogen) in serum-free medium (250 ul). This mixture was incubated at room temperature for 30 min, added to cells for 5 h, and then replaced with fresh medium. After 24 h, cells were inoculated with rOka-infected cell inoculum or cell-free HSV-1 (2.0 × 105 PFU) or with UV-inactivated rOka or were mock infected. Cells were harvested at 5, 24, and 48 h postinfection by trypsinization and centrifugation, resuspended in 1× phosphate-buffered saline (PBS) with 2% paraformaldehyde, and stained for analysis by flow cytometry. VZV infection was detected with high-titer human anti-VZV sera (39) and an anti-human phycoerythrin-conjugated secondary antibody; HSV infection was detected with mouse anti-HSV antibodies (DAKO) and an anti-mouse phycoerythrin-conjugated secondary antibody. The criteria for judging the activation of the NF-κB pathway as assayed by fluorescence of the GFP reporter were as follows: upon transfection of fibroblasts with the reporter plasmid, the cells could be segregated into two distinct populations based on their FL-1 (GFP) channel fluorescent intensity, with the high-signal-intensity population having more than a 1-log difference from the low-signal-intensity population. Standard gates were set and kept constant for all experiments, and the number of cells within each population was counted. The number of GFP-positive cells in each sample was normalized to the number of GFP-positive cells in mock-infected control samples in each of three experiments in order to determine the percentage of cells with GFP-reporter expression relative to the mock-infected control. The shift in GFP intensity was also measured in each experiment to identify any decrease in reporter expression when cells were inoculated with infectious VZV.
Western blot analysis.
Cell lysates were prepared from VZV-infected or mock infected fibroblasts by use of PhosphoSafe lysis buffer (Novagen); proteins were separated on 4 to 15% gradient polyacrylamide gels (Bio-Rad) and transferred to nitrocellulose membranes, which were blocked with 5% milk or bovine serum albumin in 1× PBS or 1× Tris-buffered saline (TBS). Blots were probed with primary antibodies against the p50 NF-κB subunit (NeoMarkers), p65 NF-κB subunit (NeoMarkers or Santa Cruz Biotechnologies), IκBα (Cell Signaling Technology), phospho-IκBα (Cell Signaling Technology), ubiquitin (Cell Signaling Technology), IKK-β (NeoMarkers), PKR (Cell Signaling Technology), VZV gE (monoclonal 3B3 kindly provided by Charles Grose), or α-tubulin (Sigma). Blots were washed, probed with secondary antibodies conjugated to horseradish peroxidase, and exposed with an ECL Plus Western blotting detection system (Amersham Biosciences). Blots were stripped between primary antibody exposures by treatment with 0.2 M NaOH for 15 min. In some experiments, VZV-infected and uninfected fibroblasts were treated with the protease inhibitor, MG132 (Biomol), at 100 nM, a concentration at which the cell death rate was less than 10% after a 24-h exposure. In other experiments, cells were treated with the NF-κB inhibitor PDTC (Sigma-Aldrich) (10 μM) or treated with recombinant TNF-α (Genzyme) (20 nM), a potent agonist of the NF-κB pathway. A GS710 densitometer and Quantity One software (Bio-Rad) were used to quantify protein amounts on Western blots.
Immunofluorescence confocal microscopy.
Fibroblasts were grown on coverslips and inoculated with VZV-infected cells, cell-free (sonicated) VZV, UV-inactivated VZV, or HSV-1. The cells were fixed with 2% paraformaldehyde in PBS, permeabilized with 0.2% Triton X-100 in PBS, and stained with primary antibodies against VZV IE62, VZV gE, HSV-1 (DAKO), p65, p50, and phospho-IκBα. Fluorescein isothiocyanate-conjugated secondary antibodies were used to detect the NF-κB proteins, while Texas Red-conjugated secondary antibodies were used to detect VZV and HSV proteins. The coverslips were washed, mounted on slides by use of VECTASHIELD with DAPI (4′,6′diamidino-2-phenylindole) (Vector Laboratories), and examined with a Zeiss LSM 510 microscope. The frequencies of infected and uninfected cells that showed NF-κB nuclear localization were determined by counting the number of cells expressing viral proteins, with and without nuclear NF-κB p65, in 10 separate fields per slide.
Immunohistochemistry.
Human skin xenografts were infected with rOka and harvested 21 days after infection; specimens were mounted in paraffin and sectioned. Protocols for animal studies were approved by the Stanford University Administrative Panel on Laboratory Animal Care; human fetal tissues were obtained with informed consent according to federal and state regulations. For staining, the tissue sections were deparaffinized, the endogenous peroxides were quenched with 3% hydrogen peroxide, and sections were treated for antigen retrieval by boiling in a pressure cooker in antigen unmasking solution (Vector Laboratories). Sections were blocked with 10% normal goat serum in TBS-0.1% Tween-20 and probed with primary antibodies against VZV IE62, VZV gE, p50, and p65. Biotinylated goat secondary antibodies and streptavidin-peroxidase label (LabVision) were added, and DAB or VIP substrate kits were used for peroxidase detection (Vector Laboratories).
Proteasome assay.
Fibroblasts in T25 flasks were infected with rOka or HSV-1 and harvested after 5, 24, or 48 h in 500 μl radioimmunoprecipitation buffer without protease inhibitors. Proteasome activity was determined using a peptide that releases a fluorogenic 7-amino-4-methylcoumarin (AMC) molecule when cleaved by the proteasome (Chemicon). Briefly, 50 μl of sample with or without 50 nM MG132 was added in duplicate to the reaction mixture provided by the manufacturer in a 96-well plate and allowed to react at 37°C for 2 h. Dilutions of AMC and 20S enzyme controls were added in duplicate along with the test samples to determine the linear range of sensitivity. Fluorescence was read using 380 nm excitation and 460 nm emission filters in a fluorometer. The background was subtracted, and standard curves were established. Fluorescence curves of samples were averaged and compared to the standard curves to ensure that the values were within the linear range of detection, and standard errors were calculated. Values for proteasome activity were based on the averages of three independent experiments.
RESULTS
VZV infection reduces GFP expression from an NF-κB-responsive promoter.
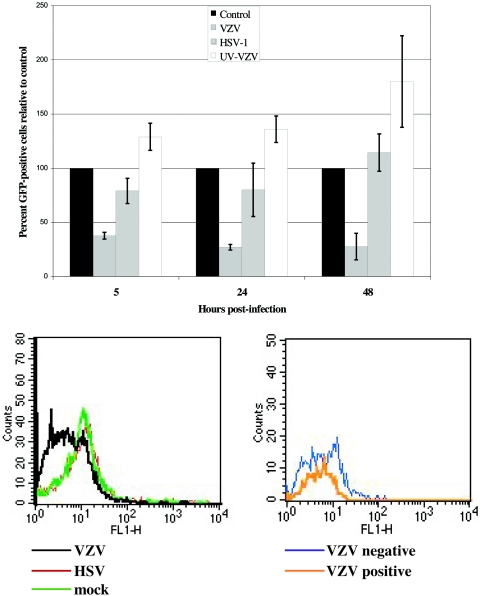
VZV infection of fibroblasts caused the down-regulation of NF-κB-responsive genes when evaluated using 42,000-spot human cellular gene microarrays (25, 26). To demonstrate that VZV infection specifically inhibited transcriptional activation by NF-κB, an NF-κB GFP reporter plasmid was transfected into fibroblasts, which were then inoculated with VZV-infected cells, UV-inactivated VZV-infected cells, HSV-1-infected cells, or mock-infected cells. This GFP reporter construct was used to facilitate fluorescence-activated cell sorter separation of VZV-infected and uninfected cells from the same monolayer harvested at each time point, which is important because of the difficulty of achieving a uniform initial inoculation of cells with VZV. As further validation, we used parallel experiments with two strains of HSV-1, HSV(F) and HSV(KOS), to assure that the anticipated effects of HSV on NF-κB-regulated gene transcription were observed under the same experimental conditions. The GFP reporter assays were conducted as three independent experiments, with the number of GFP-positive cells normalized to the number of GFP-positive cells in mock-infected controls for each experiment. By 48 h after inoculation, >90% of cells expressed VZV or HSV proteins (data not shown). VZV infection caused a decrease in the number of GFP-positive cells compared to expression in mock-infected cells, but inoculation with UV-inactivated VZV-infected cells or with HSV-1(F) did not (Fig. 1). The number of GFP-positive cells was reduced significantly in VZV-infected cells compared to the mock-infected control results when measured at 5 h (P < 0.001), 24 h (P < 0.01), and 48 h (P < 0.001) after inoculation. Inoculation of transfected cells with UV-inactivated VZV or HSV-1(F) was not associated with any significant differences between the experimental and control means for numbers of GFP-positive cells. As in cells infected with HSV-1(F), expression of the reporter was not inhibited in cells infected with HSV-1(KOS) (data not shown). Representative histogram plots of the flow cytometric analysis are shown from one experiment at 24 h after infection. Though relatively few cells were GFP positive, infection with VZV caused a decrease in the intensity of the GFP reporter (FL1-H) compared to HSV- or mock-infected cell results (Fig. 1, lower left panel). Furthermore, in a VZV-infected monolayer at 24 h, the VZV-positive-staining cells had a lower GFP intensity than the VZV-negative cells from the same monolayer (Fig. 1, lower right), indicating that VZV infection of fibroblasts inhibited the NF-κB signaling pathway and, consequently, NF-κB-responsive gene expression.
FIG. 1.
NF-κB reporter gene expression in VZV-infected fibroblasts. Fibroblasts were transfected with a NF-κB GFP reporter plasmid, pNF-κB-hrGFP (1 μg). After 24 h, the cells were infected with VZV or HSV-1, exposed to UV-inactivated VZV, or mock infected. Cells were harvested at 5, 24, and 48 h after infection and examined for expression of GFP and VZV or HSV proteins by flow cytometry. The number of GFP-positive cells in each sample was normalized to mock-infected cells in each of three experiments to assess the percent of GFP reporter expression relative to the control, with the control equal to 100% (top panel). Representative flow cytometry histogram plots for these data are also shown. At 24 h, infection with VZV causes decreased intensity of the GFP reporter (FL1-H) compared to HSV or mock-infected cell results (bottom left panel). Furthermore, in a VZV-infected monolayer at 24 h, the VZV-positive-staining cells had a lower GFP intensity than the VZV-negative cells in that population (bottom right panel).
VZV infection induces expression of NF-κB proteins with a predominantly cytoplasmic localization.
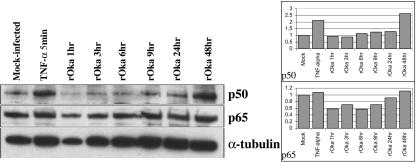
To determine whether the inhibition of NF-κB signaling suggested by the cellular DNA microarrays and the NF-κB reporter experiments was due to decreased levels of NF-κB proteins, the expression of p50 and p65 in VZV-infected fibroblasts was assessed by Western blotting and quantified by densitometry over a 48-h period (Fig. 2). The p50 and p65 proteins were detected at levels at least equivalent to those in mock-infected fibroblasts and appeared to increase over the course of infection to the levels that were observed in TNF-α-treated, uninfected fibroblasts. These results suggested that inhibition of the NF-κB pathway in VZV-infected fibroblasts was not due to lower concentrations of p50 or p65, since these proteins remained abundant at 48 h when >90% of cells were infected.
FIG. 2.
NF-κB protein expression in VZV-infected fibroblasts. Cell lysates were prepared from fibroblast monolayers at 1, 3, 6, 9, 24, and 48 h after infection and from TNF-α-treated or mock-infected cells, and proteins were separated by polyacrylamide gel electrophoresis (left). Proteins were transferred to nitrocellulose membranes and probed with antibodies against p50 and p65; α-tubulin was used as a loading control. The differences in the amount of p50 or p65 present in each sample were quantified using a densitometer, where the area of each p50 or p65 band was normalized to the area of the corresponding tubulin band. These ratios were then set relative to the mock-infected control, so that mock-infected results = 100%.
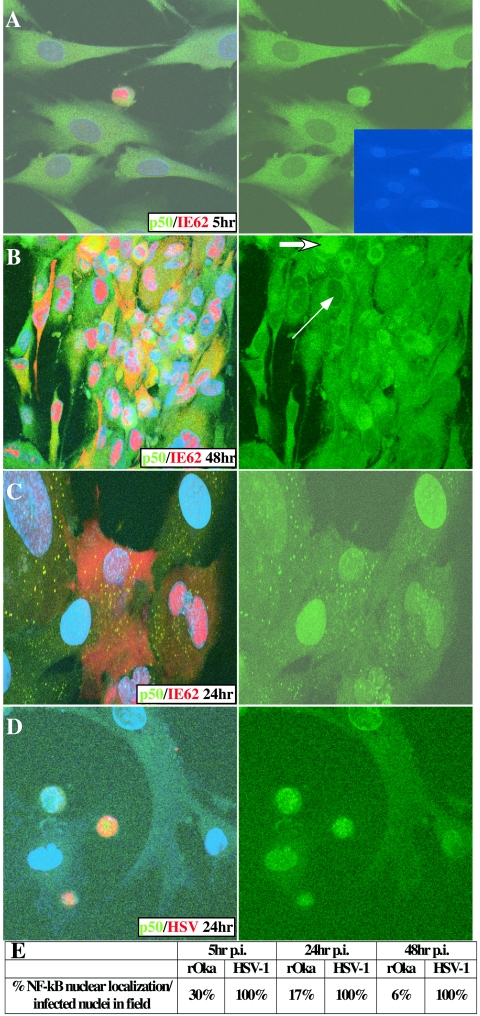
When expression and localization of p50 and p65 were examined by confocal microscopy, p50 and p65 levels appeared to increase, and both proteins were cytoplasmic in most VZV-infected cells at 5, 24, and 48 h, as shown in these representative images of p50 localization (Fig. 3). The pattern of intracellular distribution of p65 was identical to that of p50 (data not shown). The distribution of p65 was examined visually in single, VZV-positive cells at 5, 24, and 48 h after infection. Nuclear localization of p65 was detected in 30% of VZV-infected cells at the early time point (108 total nuclei counted) but decreased to 17% by 24 h (198 nuclei counted) and 6% at 48 h (204 nuclei counted) (Fig. 3E). In the few cells in which p50 and p65 were located in the nucleus, VZV IE62 was observed only in the nucleus (Fig. 3A). Nuclear IE62 is a marker of the initial phase of VZV infection; as infection progresses, IE62 becomes predominantly cytoplasmic. The localization of p50 or p65 was restricted to the cytoplasm in VZV-infected cells within the large, multinucleated syncytia that had formed by 48 h; some cells at the margins of these syncytia showed nuclear p50 or p65, consistent with an initial transient nuclear translocation of the proteins (Fig. 3B). (This image is shown at a slightly lower magnification in order to display the complete multi-nucleated cell.) These images were resolved in greater detail by z-stack analysis to confirm NF-κB localization (data not shown). Mock-infected cells exhibited a low frequency of NF-κB nuclear localization, ranging from 1 to 3%, which was observed predominantly in dividing cells (data not shown). The induction and transient nuclear localization of p50 and p65 were also observed in fibroblasts 5 h after inoculation with sonicated VZV-infected cells, indicating that NF-κB protein expression was being observed in newly infected cells and not in intact cells remaining from the infected cell inoculum (data not shown). These experiments indicated that VZV infection caused a transient nuclear localization of p50 and p65, followed by rapid cytoplasmic sequestration.
FIG. 3.
Intracellular localization of NF-κB proteins in VZV-infected fibroblasts. VZV-infected fibroblasts at 5 h (A) and 48 h (B) and UV-inactivated VZV-inoculated fibroblasts at 24 h (C) were stained with antibodies against NF-κB p50 (green), VZV IE62 (red), and DAPI nuclei (blue); HSV-1-infected fibroblasts at 24 h (D) were stained with antibodies against NF-κB p50 (green), HSV (red), and DAPI nuclei (blue). Three-color images are shown on the left, and NF-κB staining alone is shown on the right. In panel A, DAPI nuclear staining is displayed in the inset, indicating that only the single infected cell contained nuclear p50 at 5 h. In panel B, the thin arrow indicates an infected cell nucleus without p50, while the thick arrow indicates an infected cell nucleus containing p50. In this multinucleated cell, nearly all nuclei were without p50, while NF-κB proteins were nuclear in all HSV-infected cells and most cells inoculated with UV-inactivated VZV. (E) The percentages of VZV- and HSV-infected cells that had nuclear localization of the NF-κB proteins were determined as the averages of 10 high-power fields. p.i., postinfection.
In contrast to these effects of infectious VZV, exposure to UV-inactivated VZV-infected cells induced nuclear localization of the NF-κB proteins in fibroblasts that persisted throughout the 48-h period (Fig. 3C). No cytoplasmic sequestration of NF-κB proteins was observed, indicating that the shift from nuclear to cytoplasmic localization of p50 and p65 in VZV-infected fibroblasts depended upon the presence of infectious VZV. The anti-p50 antibody used in these experiments may also detect the p50 precursor protein, p105, which is thought to be expressed solely in the cytoplasm (8). However, detection of p105 along with p50 in the cytoplasm would not influence the interpretation of these results, since p50 only functions to transduce NF-κB signaling when it is in the nucleus. In addition, p65 is not processed from such a precursor, and p65 and p50 localization were examined in parallel in all experiments.
Because the prominent cytoplasmic sequestration of p50 and p65 in VZV-infected cells differed from reports about NF-κB proteins in HSV-infected cells, we examined fibroblasts infected with HSV-1(F) and HSV-1(KOS) by confocal microscopy. The localization of p50 and p65 was uniformly nuclear at 5, 24, and 48 h, as illustrated for p50 in HSV-1(F)-infected cells at 24 h (Fig. 3D). Counting HSV-infected cells showed 100% nuclear localization of NF-κB proteins from the earliest measurement (Fig. 3E).
VZV infection of skin cells in vivo induces cytoplasmic localization of NF-κB proteins.
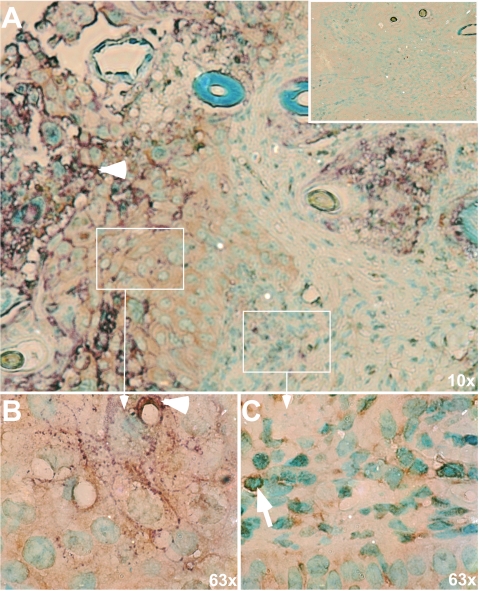
In order to determine whether the effects of VZV on NF-κB proteins observed in vitro were relevant to VZV pathogenesis, p50 and p65 expression was examined in VZV-infected human skin xenografts of SCIDhu mice. VZV gE was used to identify infected cells within VZV lesions. As illustrated with p65, the NF-κB proteins were induced in VZV-infected epidermal and dermal cells and showed a predominantly cytoplasmic distribution (Fig. 4A and B); p50 expression was also cytoplasmic in gE-positive skin cells (data not shown). Some epidermal cells adjacent to the foci of infected cells forming the VZV skin lesion exhibited nuclear localization of p65. This pattern was similar to the detection of nuclear p50 and p65 in fibroblasts at the margins of syncytia at early times after infection (Fig. 3C). Controls in which sections were stained with normal rabbit or mouse immunoglobulin G had no background reactivity, as shown for p65 (Fig. 4A, inset).
FIG. 4.
Intracellular localization of NF-κB in VZV-infected skin cells in vivo. A representative section of a VZV-infected skin xenograft is shown in which late VZV protein expression was detected with monoclonal anti-gE (purple) and NF-κB protein expression was detected with rabbit anti-p65 (brown); methyl green was used to stain cell nuclei. The inset in panel A shows a serial section of skin stained with anti-rabbit serum and anti-mouse immunoglobulin G as a control. Panels B and C are magnifications of the approximate regions indicated in panel A. Panel B represents an area well within the infected lesion, and the purple gE staining is indicated by the white arrowhead. The p65 antibody (brown) has a diffuse, cytoplasmic staining in this region, without any nuclear localization. In comparison, in panel C, which represents an area at the edge of the lesion, p65 is contained within many, but not all, nuclei. One such p65-containing nucleus is indicated by the white arrow.
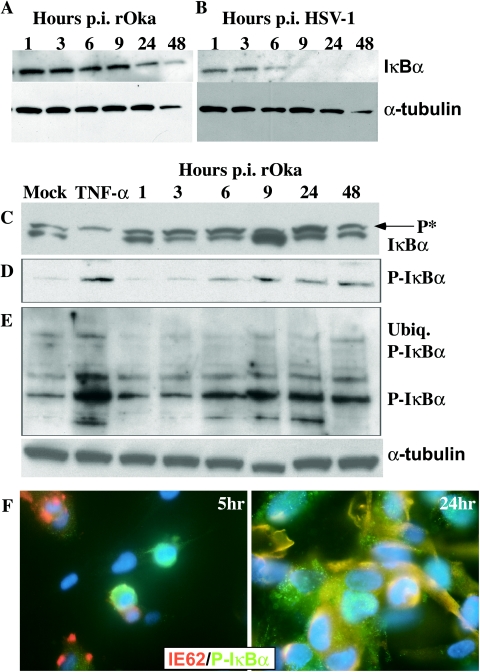
IκBα levels are stabilized in VZV-infected cells.
The observation that p50 and p65 were sequestered in the cytoplasm in VZV-infected fibroblasts and in skin cells in vivo was investigated further in VZV-infected fibroblasts. Phosphorylation of IκBα by the IKK complex targets this inhibitory protein for proteasome-mediated degradation, which allows translocation of the NF-κB p50/p65 heterodimer to the nucleus and transactivation of NF-κB-responsive genes, including IκBα. The persistence of IκBα would be expected to lead to the cytoplasmic retention of NF-κB proteins, as was demonstrated by confocal microscopy (Fig. 3A and B). As shown in Fig. 5A, I κBα levels in infected cell lysates were comparable at an early time after VZV inoculation, when relatively few fibroblasts were infected, and at 48 h, when >90% of cells expressed VZV proteins. In contrast, and in accordance with published observations, IκBα was rapidly degraded in HSV-1-infected cells (Fig. 5B). Immunoprecipitation of VZV-infected cell lysates with IκBα showed that some p65 was associated with IκBα (data not shown).
FIG. 5.
Analysis of IκBα levels and the state of phosphorylation and ubiquitination in VZV-infected fibroblasts. Cell lysates were prepared from VZV (A, C, D, and F)- or HSV-1 (B)-infected fibroblasts at 1, 3, 6, 9, 24, and 48 h, from mock-infected fibroblasts, or from fibroblasts treated with 20 nM TNF-α for 5 min. Proteins were separated by polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and probed with antibodies against IκBα (A, B, and C), phospho-IκBα (P-IκBα) (D and E), or α-tubulin as a loading control. “P*” in panel C indicates the band characteristic of the phosphorylated form of IκBα, and “ubiq. P-IκBα” indicates the high-molecular-weight form characteristic of ubiquitinated IκBα. p.i., postinfection. Panel F shows confocal immunofluorescence images of VZV-infected fibroblasts at 5 h (left) and 24 h (right), probed with antibodies against phospho-IκBα (green), VZV IE62 (red), and DAPI nuclei (blue), which indicated the presence of phospho-IκBα in most infected cells.
Blocking IκBα phosphorylation could explain its persistence in VZV-infected fibroblasts. However, phosphorylation of IκBα was not inhibited when VZV-infected cell lysates were prepared using TBS buffer to detect both the phosphorylated and unphosphorylated forms of IκBα (Fig. 5C). Both the higher- and lower-molecular-weight forms of IκBα were observed in VZV-infected cells throughout the 48-h time course. Using TNF-α exposure for 5 min as a control for these experiments showed degradation of most IκBα, with only some phosphorylated protein remaining detectable. Analysis with a phosphospecific antibody to IκBα confirmed that phosphorylated IκBα persisted in VZV-infected cells (Fig. 5D). Confocal microscopy using this phosphospecific antibody showed that phosphorylated IκBα was within VZV-infected cells (Fig. 5F). IκBα also appeared to be ubiquitinated in VZV-infected fibroblasts, as determined on the basis of detection of the characteristic very-high-molecular-weight forms by Western blotting using the phosphospecific anti-IκBα antibody, and this form persisted over the course of VZV infection (2, 28, 43) (Fig. 5E). This high-molecular-weight, ubiquitinated IκBα was induced in TNF-α-treated fibroblasts, which were used as a positive control in these experiments.
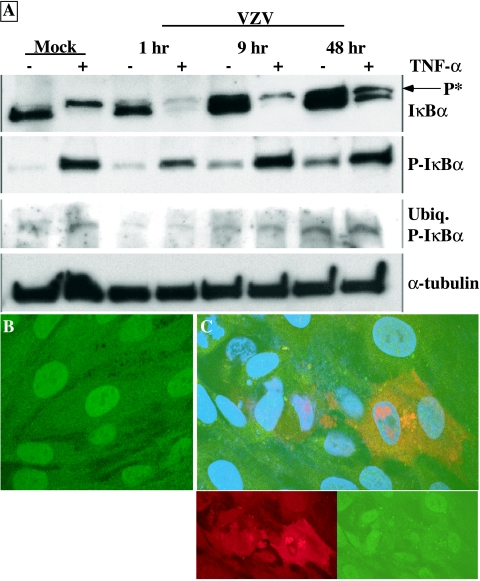
Interference with the NF-κB pathway in VZV-infected cells could represent a passive process in which the initial stimulus from viral replication disappears and the cell reverts to its resting state with respect to IκBα levels and p50 and p65 cytoplasmic localization. Alternatively, the process may be actively mediated by the virus to ensure the inhibition of NF-κB signaling throughout the replication cycle. To test these possibilities VZV-infected cells were challenged with TNF-α at 1, 9, and 48 h after infection, and phosphorylation and degradation was assessed for IκBα (Fig. 6A). TNF-α treatment early after infection caused the degradation of most IκBα in VZV-infected cells. As infection progressed, TNF-α treatment was associated with much less IκBα degradation, even though IκBα was phosphorylated and ubiquitinated. Nuclear localization of p50 was evident in uninfected fibroblasts after TNF-α treatment (Fig. 6B). In contrast, when fibroblast monolayers were infected with VZV before TNF-α exposure, p50 was cytoplasmic in cells that expressed VZV IE62 protein but it was nuclear in adjacent uninfected cells (Fig. 6C). These experiments indicated that VZV infection inhibited the degradation of IκBα that was triggered by TNF-α signaling.
FIG. 6.
Analysis of IκBα levels in VZV-infected cells challenged with TNF-α. (A) Total lysate was harvested from mock-infected or VZV-infected fibroblasts either mock treated or treated with TNF-α for 5 min after the indicated times postinfection, and lysates from preparations with equal cell numbers were loaded on polyacrylamide gels. Proteins were transferred to membranes and probed with antibodies against IκBα, phospho-IκBα (P-IκBα) (which also detected the high-molecular-weight ubiquitinated form of IκBα), or α-tubulin. “P*” indicates the band characteristic of the phosphorylated form of IκBα. (B and C) Mock-infected fibroblasts (B) or fibroblasts infected with VZV for 24 h (C) were treated with TNF-α (20 nM) for 15 min. After fixation and permeabilization, the cells were stained with antibodies against p50 (green) and VZV IE62 (red), while the nuclei were stained with DAPI (blue). IE62 (lower left) and p50 (lower right) staining of VZV-infected cells alone is also shown. All cells except those infected with VZV had nuclear localized p50.
VZV infection does not inhibit proteasome function.
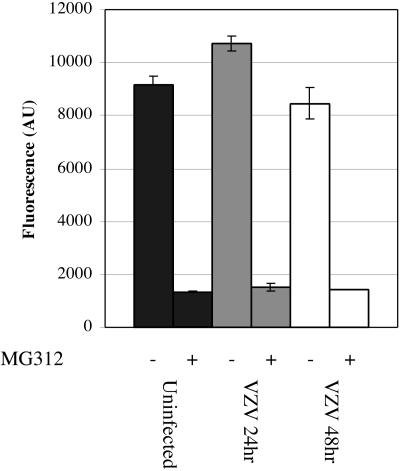
Since phosphorylated IκBα typically becomes ubiquitinated, which targets proteins for degradation by the 26S proteasome, persistence of phosphorylated IκBα in VZV-infected cells could be due to altered proteasome activity. When a peptide substrate that releases a fluorogenic AMC molecule when cleaved by the proteasome was used to evaluate proteosome function, no loss of function was detected in VZV-infected cells compared to mock-infected cell results (Fig. 7). The small differences between the fluorescence signal from VZV-infected cells at 24 and 48 h and that of uninfected cells were not statistically significant. Adding the proteasome inhibitor, MG132, during the assay inhibited AMC release equally in VZV-infected and uninfected cell lysates (Fig. 7). Proteasome function was also inhibited by MG132 in cell lysates from fibroblasts that were incubated with MG132 for 24 h before VZV infection (data not shown). Furthermore, Western blot analysis using an antiubiquitin antibody revealed an accumulation of total ubiquitinated proteins in uninfected fibroblasts that were treated with MG132 but not in untreated VZV-infected fibroblasts (data not shown). These experiments suggested that VZV infection did not inhibit proteasome function.
FIG. 7.
Analysis of proteasome function in VZV-infected cell lysates. Cell lysates were prepared from mock-infected or VZV-infected fibroblasts at 24 or 48 h after infection by use of 500 μl lysis buffer without protease inhibitors. Cell lysates (50 μl) ± MG132 (50 nM) were added to the fluorogenic peptide substrate reaction mixture (Chemicon) in 96-well plates and allowed to react for 2 h at 37°C. Duplicate sets of standards and samples were assayed for fluorescence at 460 nm. Data are presented as the background-subtracted fluorescence level (in arbitrary units [AU]) ± standard error.
Inhibition of the NF-κB pathway does not affect VZV growth.
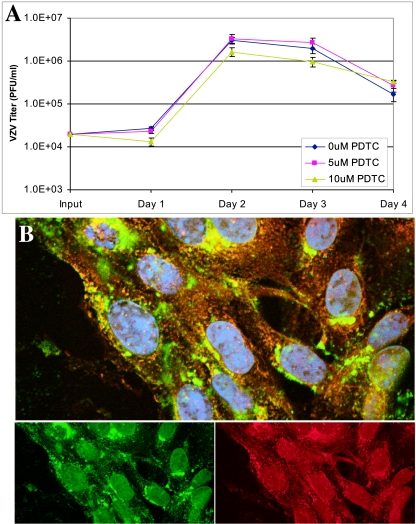
To determine whether activation of the NF-κB pathway was necessary for VZV replication, the virus was grown in the presence of PDTC, a potent chemical inhibitor of the NF-κB pathway that blocks IκBα phosphorylation (7). PDTC had no significant effect on yields of infectious virus in fibroblasts over a 4-day period (Fig. 8A) or in MeWo cells (data not shown). PDTC reduced the growth of HSV-1(F) strain in Vero cells (data not shown) and prevented the induction and nuclear translocation of p50 in HSV-infected fibroblasts (Fig. 8B). While a small fraction of p50 translocated to the nucleus, p50 was retained at the perinuclear region in a pattern that was not observed in untreated HSV-1-infected fibroblasts (Fig. 3D). These experiments indicated that persistent activation of the NF-κB pathway is not required for VZV replication but is necessary for normal HSV-1 growth, as has been reported previously (21, 48, 57).
FIG. 8.
Growth of VZV and HSV in presence of PDTC. (A) Fibroblasts were pretreated with 0, 5, or 10 μM PDTC for 1 h before VZV infection. Cells were harvested and titers of the virus were determined over 4 days, with no significant differences among the treatments. (B) Fibroblasts were pretreated with 10 μM PDTC for 1 h before infection with HSV-1 (multiplicity of infection, ∼0.1). At 24 h after infection, cells were fixed and permeabilized and stained with antibodies against NF-κB p50 (green) or HSV-1 (red).
DISCUSSION
These observations indicate that VZV inhibits the NF-κB pathway directly through the stabilization of IκBα levels and rapid cytoplasmic sequestration of p50 and p65. Retention of NF-κB proteins in the cytoplasm appears to be significant for VZV pathogenesis, since it occurred in infected epidermal cells in human skin xenografts in vivo. The cytoplasmic localization of p50 and p65 associated with VZV infection contrasted with the well-documented early and sustained nuclear translocation of NF-κB proteins in HSV-infected cells, which we confirmed in parallel analyses of HSV-1(KOS) and HSV-1(F). Since human CMV, Epstein-Barr virus, and Kaposi's sarcoma-associated herpesvirus also persistently activate the NF-κB pathway (5, 11, 14, 19, 29, 32, 33, 35, 59), these experiments suggest that VZV has evolved a mechanism to counteract cellular antiviral defenses that is unique among the herpesviruses. This hypothesis was supported by the observation that VZV inhibited transcription from an NF-κB reporter construct while HSV-1(KOS) and HSV-1(F) did not. VZV replication was required to achieve cytoplasmic sequestration of p50 and p65, as UV-irradiated VZV induced nuclear translocation of NF-κB proteins only, as is observed when cells are exposed to noninfectious HSV or CMV (21, 29, 48). Thus, transient NF-κB activation appears to be a conserved cellular response to herpesvirus particles, but VZV differs from other herpesviruses in that viral replication leads to inhibition, not persistent activation, of the NF-κB pathway. Importantly, although p50 and p65 were sequestered in the cytoplasm in epidermal cells that expressed VZV proteins in vivo, NF-κB proteins were observed in the nuclei of neighboring cells at the margins of lesions, which can be attributed to exposure to VZV proteins synthesized by adjacent VZV-infected cells. This mechanism could allow VZV to spread from cell to cell in a controlled manner without overwhelming the host.
Because IκBα binds to nuclear localization signal motifs in p50 and p65 and inhibits their nuclear translocation, the stabilization of IκBα in VZV-infected cells accounts for the cytoplasmic sequestration of p50 and p65 (52). The stabilization of IκBα levels in VZV-infected cells may be caused by inhibiting degradation or by increased synthesis to replenish degraded IκBα. However, since IκBα transcription is activated primarily by NF-κB, the persistence of IκBα, even at late times after VZV infection, seems more likely to represent a block of IκBα degradation. Protection from degradation was also suggested by IκBα persistence in VZV-infected cells despite TNF-α signaling, which is known to cause the degradation of IκBα.
IκBα is typically phosphorylated in response to stimuli that activate the NF-κB pathway (52). Phosphorylation allows the protein to be ubiquitinated, which usually targets proteins for 26S proteasome-mediated degradation. Interestingly, our experiments indicated that IκBα levels were maintained despite IκBα phosphorylation and the apparent ubiquitination of the protein in VZV-infected cells. IκBα phosphorylation appeared to be enhanced in TNF-α-treated cells compared to VZV-infected cell results, but this difference may result from more uniform signaling by TNF-α. However, the possibility that IκBα was not fully phosphorylated in VZV-infected cells and was therefore degraded less efficiently cannot be excluded. The ubiquitination of proteins does not always lead to their degradation, as the addition of ubiquitin can regulate other protein functions (1). Phosphorylated IκBα could also persist if it is not completely polyubiquitinated or undergoes specific deubiquitination in VZV-infected cells. IκBα has been shown to be phosphorylated without being degraded in cells infected with cowpox and certain vaccinia virus strains, although the ubiquitination state of IκBα was not determined (45). Thus, VZV and some poxviruses may encode proteins that share a common mechanism in which the NF-κB pathway is inhibited by blocking IκBα degradation.
Dysfunction of the 26S proteosome could also explain IκBα stabilization despite ubiquitination. However, this possibility was not supported by experiments showing that proteasomes from VZV-infected cell lysates were capable of degrading a known peptide target, and treatment with MG312, a proteasome inhibitor, severely inhibited its degradation in VZV-infected cells. Although the peptide reporter may not accurately represent the capacity of the proteasome to degrade full-length proteins, other observations also indicated that proteasome function was intact. Total ubiquitinated protein levels were not increased in VZV-infected cells, while MG132 treatment of these cells caused a marked increase in total ubiquitinated protein levels. Furthermore, STAT-1α, which is degraded by the ubiquitin-proteasome pathway (55), was reduced in VZV-infected cells in culture and in VZV-infected epidermal cells in vivo (30). Alternatively, degradation of IκBα could be inhibited by the addition of an ubiquitin-like moiety, such as small ubiquitin-like modifier, or by the binding of a VZV protein to IκBα, which may block IκBα entry into the proteasome.
NF-κB activation appears to be necessary for optimal replication of HSV-1 and human CMV, since blocking the NF-κB pathway decreased viral yields and inhibited the activation of viral genes with NF-κB response elements in their promoters (3, 21, 48, 57). In contrast, persistent activation of the NF-κB pathway was not required for the progression of VZV infection in fibroblasts in vitro or in human skin cells in vivo. We speculate that this difference could be related to the relative lack of NF-κB response elements in the promoters of VZV IE genes, as suggested from bioinformatics predictions. By this analysis, HSV has multiple κB sites in the promoters of the genes encoding VP16, ICP0, ICP4, and ICP22, while VZV has only one such NF-κB site, located in the noncoding region between ORF62, which encodes the major IE protein, and ORF63, the ICP22 homolog. Mutagenesis of this NF-κB binding site in a dual promoter reporter cassette did not reduce transcription of either ORF62 or ORF63 reporter genes during VZV infection in primary fibroblasts or skin xenografts (27). Inhibiting the NF-κB pathway might not affect VZV gene transcription as much as it alters HSV gene transcription and replication, with the caveat that some VZV may have functional NF-κB sites not predicted from the consensus sequence. Furthermore, HSV-1 and other herpesviruses have evolved strategies to avoid the downstream effects of NF-κB-responsive genes, which may be less effective or absent in VZV. For example, the HSV virion host shutoff protein acts as a nuclease to specifically degrade mRNAs, including those encoding NF-κB-responsive genes (18) whereas mRNA cleavage by the VZV homolog, ORF17 protein, appears to be significantly less efficient (53). HSV-1 also has many mechanisms to avoid the effects of the IFN response, which can be activated by the NF-κB pathway, such as enhancement of proteasome-dependent degradation of IFN-stimulated gene products by ICP0 (31, 40-42). While HSV-1 ICP34.5 can bypass the phosphorylation of eIF2-α by activated PKR, which would otherwise inhibit cellular protein synthesis (22), VZV does not encode an ICP34.5 homolog. Additionally, the RNase L pathway is inhibited in HSV-1-infected cells (12). Therefore, VZV may need to interfere with NF-κB signaling directly in order to avoid downstream effects of NF-κB activation that are circumvented through secondary mechanisms by other herpesviruses. The observation that IκBα degradation was diminished when VZV-infected fibroblasts were treated with TNF-α suggests that the strategy by which VZV inhibits the NF-κB pathway may also protect VZV-infected cells from antiviral responses induced by exogenous inflammatory cytokines, many of which act through NF-κB signaling pathways.
The finding that NF-κB proteins were excluded from the nuclei of VZV-infected epidermal cells but that they could be translocated to the nuclei of neighboring uninfected cells in vivo is consistent with our observations concerning the type I IFN response in VZV-infected skin xenografts (30). In epidermal cells that expressed VZV proteins, IFN-α expression was inhibited and STAT1 was localized to the cytoplasm. Since NF-κB is a major inducer of IFN-α transcription, viral interference with NF-κB signaling should limit IFN production within VZV-infected cells. In the surrounding uninfected cells, IFN-α expression was increased and STAT-1 was phosphorylated and translocated into the nuclei (30). Since NF-κB proteins are stimulated and translocate to the nucleus in cells immediately adjacent to the VZV lesion, the activation of the NF-κB pathway in these cells could lead to the increased expression of IFN-α and, in turn, the paracrine effects of IFN-α could activate STAT signaling and amplify the IFN response in the surrounding uninfected cells.
In summary, we have shown that VZV infection restricts NF-κB signaling in infected cells in culture and in vivo by sequestering NF-κB proteins in the cytoplasm, likely by the stabilization of IκBα. In contrast to HSV, VZV replication does not depend on maintaining NF-κB functions in the nuclei of infected cells. VZV appears to have evolved a unique, direct mechanism to inhibit the antiviral effects of NF-κB induction.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (AI20459 and AI053846) (A.M.A.) and from the National Cancer Institute (CA49605) (A.M.A.) and by a graduate fellowship from the National Science Foundation (J.O.J.).
We thank Anne Schaap and Reija Matheson for assistance with experiments. We also thank Chia-Chi Ku, Leigh Zerboni, and Marvin Sommer for advice and technical assistance.
We have no conflicting financial interests.
REFERENCES
- 1.Aguilar, R. C., and B. Wendland. 2003. Ubiquitin: not just for proteasomes anymore. Curr. Opin. Cell Biol. 15:184-190. [DOI] [PubMed] [Google Scholar]
- 2.Alkalay, I., A. Yaron, A. Hatzubai, A. Orian, A. Ciechanover, and Y. Ben-Neriah. 1995. Stimulation-dependent I kappa B alpha phosphorylation marks the NF-kappa B inhibitor for degradation via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 92:10599-10603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of I kappa b kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759-28766. [DOI] [PubMed] [Google Scholar]
- 4.Arvin, A. (ed.). 2001. Varicella-zoster virus, 4th ed., vol. 2. Lippincott-Raven, Philadelphia, Pa.
- 5.Atkinson, P. G., H. J. Coope, M. Rowe, and S. C. Ley. 2003. Latent membrane protein 1 of Epstein-Barr virus stimulates processing of NF-kappa B2 p100 to p52. J. Biol. Chem. 278:51134-51142. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin, A. S. 2001. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J. Clin. Investig. 107:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beauparlant, P., and J. Hiscott. 1996. Biological and biochemical inhibitors of the NF-kappa B/Rel proteins and cytokine synthesis. Cytokine Growth Factor Rev. 7:175-190. [DOI] [PubMed] [Google Scholar]
- 8.Beinke, S., and S. C. Ley. 2004. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem. J. 382:393-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bour, S., C. Perrin, H. Akari, and K. Strebel. 2001. The human immunodeficiency virus type 1 Vpu protein inhibits NF-kappa B activation by interfering with beta TrCP-mediated degradation of Ikappa B. J. Biol. Chem. 276:15920-15928. [DOI] [PubMed] [Google Scholar]
- 10.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caposio, P., M. Dreano, G. Garotta, G. Gribaudo, and S. Landolfo. 2004. Human cytomegalovirus stimulates cellular IKK2 activity and requires the enzyme for productive replication. J. Virol. 78:3190-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cayley, P. J., J. A. Davies, K. G. McCullagh, and I. M. Kerr. 1984. Activation of the ppp(A2′p)nA system in interferon-treated, herpes simplex virus-infected cells and evidence for novel inhibitors of the ppp(A2′p)nA-dependent RNase. Eur. J. Biochem. 143:165-174. [DOI] [PubMed] [Google Scholar]
- 13.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhary, P. M., A. Jasmin, M. T. Eby, and L. Hood. 1999. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene 18:5738-5746. [DOI] [PubMed] [Google Scholar]
- 15.Cohen, J. I., and S. E. Strauss. 2001. Varicella-zoster virus and its replication, p. 2707-2730. In D. M. Knipe, P. M. Howley, D. E. Griffith, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 2. Lipincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 16.Dhib-Jalbut, S., J. Xia, H. Rangaviggula, Y. Y. Fang, and T. Lee. 1999. Failure of measles virus to activate nuclear factor-kappa B in neuronal cells: implications on the immune response to viral infections in the central nervous system. J. Immunol. 162:4024-4029. [PubMed] [Google Scholar]
- 17.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 18.Everly, D. N., Jr., P. Feng, I. S. Mian, and G. S. Read. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76:8560-8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field, N., W. Low, M. Daniels, S. Howell, L. Daviet, C. Boshoff, and M. Collins. 2003. KSHV vFLIP binds to IKK-gamma to activate IKK. J. Cell Sci. 116:3721-3728. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 21.Gregory, D., D. Hargett, D. Holmes, E. Money, and S. L. Bachenheimer. 2004. Efficient replication by herpes simplex virus type 1 involves activation of the IκB kinase-IκB-p65 pathway. J. Virol. 78:13582-13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He, B., M. Gross, and B. Roizman. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann, A., A. Levchenko, M. L. Scott, and D. Baltimore. 2002. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 298:1241-1245. [DOI] [PubMed] [Google Scholar]
- 24.Ito, H., M. H. Sommer, L. Zerboni, H. He, D. Boucaud, J. Hay, W. Ruyechan, and A. M. Arvin. 2003. Promoter sequences of varicella-zoster virus glycoprotein I targeted by cellular transactivating factors Sp1 and USF determine virulence in skin and T cells in SCIDhu mice in vivo. J. Virol. 77:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, J. O., and A. M. Arvin. 2003. Microarray analysis of host cell gene transcription in response to varicella-zoster virus infection of human T cells and fibroblasts in vitro and SCIDhu skin xenografts in vivo. J. Virol. 77:1268-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, J. O., and A. M. Arvin. 2005. Viral and cellular gene transcription in fibroblasts infected with small plaque mutants of varicella-zoster virus. Antiviral Res. 68:56-65. [DOI] [PubMed] [Google Scholar]
- 27.Jones, J. O., M. Sommer, S. Stamatis, and A. M. Arvin. 2006. Mutational analysis of the varicella-zoster virus ORF62/63 intergenic region. J. Virol. 80:3116-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovalenko, A., C. Chable-Bessia, G. Cantarella, A. Israel, D. Wallach, and G. Courtois. 2003. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 424:801-805. [DOI] [PubMed] [Google Scholar]
- 29.Kowalik, T. F., B. Wing, J. S. Haskill, J. C. Azizkhan, A. S. Baldwin, Jr., and E. S. Huang. 1993. Multiple mechanisms are implicated in the regulation of NF-kappa B activity during human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 90:1107-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ku, C. C., L. Zerboni, H. Ito, B. S. Graham, M. Wallace, and A. M. Arvin. 2004. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-alpha. J. Exp. Med. 200:917-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 78:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, L., M. T. Eby, N. Rathore, S. K. Sinha, A. Kumar, and P. M. Chaudhary. 2002. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the Ikappa B kinase complex. J. Biol. Chem. 277:13745-13751. [DOI] [PubMed] [Google Scholar]
- 33.Luftig, M., E. Prinarakis, T. Yasui, T. Tsichritzis, E. Cahir-McFarland, J. Inoue, H. Nakano, T. W. Mak, W. C. Yeh, X. Li, S. Akira, N. Suzuki, S. Suzuki, G. Mosialos, and E. Kieff. 2003. Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6. Proc. Natl. Acad. Sci. USA 100:15595-15600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malmgaard, L., S. R. Paludan, S. C. Mogensen, and S. Ellermann-Eriksen. 2000. Herpes simplex virus type 2 induces secretion of IL-12 by macrophages through a mechanism involving NF-kappaB. J. Gen. Virol. 81:3011-3020. [DOI] [PubMed] [Google Scholar]
- 35.Matta, H., and P. M. Chaudhary. 2004. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP). Proc. Natl. Acad. Sci. USA 101:9399-9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medici, M. A., M. T. Sciortino, D. Perri, C. Amici, E. Avitabile, M. Ciotti, E. Balestrieri, E. De Smaele, G. Franzoso, and A. Mastino. 2003. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor kappaB. J. Biol. Chem. 278:36059-36067. [DOI] [PubMed] [Google Scholar]
- 37.Melchjorsen, J., and S. R. Paludan. 2003. Induction of RANTES/CCL5 by herpes simplex virus is regulated by nuclear factor kappa B and interferon regulatory factor 3. J. Gen. Virol. 84:2491-2495. [DOI] [PubMed] [Google Scholar]
- 38.Moffat, J., H. Ito, M. Sommer, S. Taylor, and A. M. Arvin. 2002. Glycoprotein I of varicella-zoster virus is required for viral replication in skin and T cells. J. Virol. 76:8468-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moffat, J. F., M. D. Stein, H. Kaneshima, and A. M. Arvin. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mossman, K. L. 2002. Activation and inhibition of virus and interferon: the herpesvirus story. Viral Immunol. 15:3-15. [DOI] [PubMed] [Google Scholar]
- 41.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 76:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neish, A. S., A. T. Gewirtz, H. Zeng, A. N. Young, M. E. Hobert, V. Karmali, A. S. Rao, and J. L. Madara. 2000. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science 289:1560-1563. [DOI] [PubMed] [Google Scholar]
- 44.Nelson, D. E., A. E. Ihekwaba, M. Elliott, J. R. Johnson, C. A. Gibney, B. E. Foreman, G. Nelson, V. See, C. A. Horton, D. G. Spiller, S. W. Edwards, H. P. McDowell, J. F. Unitt, E. Sullivan, R. Grimley, N. Benson, D. Broomhead, D. B. Kell, and M. R. White. 2004. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science 306:704-708. [DOI] [PubMed] [Google Scholar]
- 45.Oie, K. L., and D. J. Pickup. 2001. Cowpox virus and other members of the orthopoxvirus genus interfere with the regulation of NF-kappaB activation. Virology 288:175-187. [DOI] [PubMed] [Google Scholar]
- 46.Paludan, S. R., S. Ellermann-Eriksen, V. Kruys, and S. C. Mogensen. 2001. Expression of TNF-alpha by herpes simplex virus-infected macrophages is regulated by a dual mechanism: transcriptional regulation by NF-kappa B and activating transcription factor 2/Jun and translational regulation through the AU-rich region of the 3′ untranslated region. J. Immunol. 167:2202-2208. [DOI] [PubMed] [Google Scholar]
- 47.Paludan, S. R., S. Ellermann-Eriksen, and S. C. Mogensen. 1998. NF-kappaB activation is responsible for the synergistic effect of herpes simplex virus type 2 infection on interferon-gamma-induced nitric oxide production in macrophages. J. Gen. Virol. 79(Pt. 11):2785-2793. [DOI] [PubMed] [Google Scholar]
- 48.Patel, A., J. Hanson, T. I. McLean, J. Olgiate, M. Hilton, W. E. Miller, and S. L. Bachenheimer. 1998. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology 247:212-222. [DOI] [PubMed] [Google Scholar]
- 49.Powell, P. P., L. K. Dixon, and R. M. Parkhouse. 1996. An IκB homolog encoded by African swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J. Virol. 70:8527-8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Revilla, Y., M. Callejo, J. M. Rodriguez, E. Culebras, M. L. Nogal, M. L. Salas, E. Vinuela, and M. Fresno. 1998. Inhibition of nuclear factor kappaB activation by a virus-encoded IkappaB-like protein. J. Biol. Chem. 273:5405-5411. [DOI] [PubMed] [Google Scholar]
- 51.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 64:1704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richmond, A. 2002. Nf-kappa B, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato, H., L. D. Callanan, L. Pesnicak, T. Krogmann, and J. I. Cohen. 2002. Varicella-zoster virus (VZV) ORF17 protein induces RNA cleavage and is critical for replication of VZV at 37°C but not 33°C. J. Virol. 76:11012-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shepard, A. A., and N. A. DeLuca. 1991. Activities of heterodimers composed of DNA-binding- and transactivation-deficient subunits of the herpes simplex virus regulatory protein ICP4. J. Virol. 65:299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shuai, K., and B. Liu. 2003. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3:900-911. [DOI] [PubMed] [Google Scholar]
- 56.Taddeo, B., T. R. Luo, W. Zhang, and B. Roizman. 2003. Activation of NF-kappaB in cells productively infected with HSV-1 depends on activated protein kinase R and plays no apparent role in blocking apoptosis. Proc. Natl. Acad. Sci. USA 100:12408-12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taddeo, B., W. Zhang, F. Lakeman, and B. Roizman. 2004. Cells lacking NF-κB or in which NF-κB is not activated vary with respect to ability to sustain herpes simplex virus 1 replication and are not susceptible to apoptosis induced by a replication-incompetent mutant virus. J. Virol. 78:11615-11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. García-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiong, A., R. H. Clarke-Katzenberg, G. Valenzuela, K. M. Izumi, and M. T. Millan. 2004. Epstein-Barr virus latent membrane protein 1 activates nuclear factor-kappa B in human endothelial cells and inhibits apoptosis. Transplantation 78:41-49. [DOI] [PubMed] [Google Scholar]
- 60.Yurochko, A. D., T. F. Kowalik, S. M. Huong, and E. S. Huang. 1995. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J. Virol. 69:5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]