Abstract
Group B coxsackieviruses can initiate rapid onset type 1 diabetes (T1D) in old nonobese diabetic (NOD) mice. Inoculating high doses of poorly pathogenic CVB3/GA per mouse initiated rapid onset T1D. Viral protein was detectable in islets shortly after inoculation in association with beta cells as well as other primary islet cell types. The virulent strain CVB3/28 replicated to higher titers more rapidly than CVB3/GA in the pancreas and in established beta cell cultures. Exchange of 5′-nontranslated regions between the two CVB3 strains demonstrated a variable impact on replication in beta cell cultures and suppression of in vivo replication for both strains. While any CVB strain may be able to induce T1D in prediabetic NOD mice, T1D onset is linked both to the viral replication rate and infectious dose.
Insulin-dependent (type 1) diabetes mellitus (T1D) is an autoimmune, largely T-cell-mediated disease typically diagnosed before the end of the teen years (5, 6). A predisposing, multigenic component has been described but accounts for fewer than 50% of cases (7, 35, 45); environmental factors (e.g., viral infections) have therefore been proposed to explain the remaining cases of T1D (3, 6, 32, 57, 58) that cannot be ascribed solely to host-driven pathogenic autoimmunity. Human enterovirus (HEV) infections have long been suspected as environmental triggers of human T1D (12, 22, 27); infections by common HEVs, such as the group B coxsackieviruses (CVB 1 to 6) and diverse echoviruses, have been implicated as triggers of T1D onset at the time of or shortly after infection (8, 9, 13, 19, 28, 37, 39, 40, 42, 52, 60). Nonetheless, it remains unclear whether HEV initiates T1D in humans (18, 21, 24, 26); evidence supporting an etiologic connection between HEV infection and T1D onset is not as well-established as the links between, for example, specific HEV infections and poliomyelitis, aseptic meningitis, or myocarditis (43).
The nonobese diabetic (NOD) mouse is widely used as a model for the study of T1D. Previous work demonstrated that CVB inoculation protects young NOD mice significantly better from T1D as they age than no treatment (mock infected) (55). Unlike all other treatments that protect NOD mice from T1D (reviewed in references 4 and 46), the CVB are widely thought to be instigators of T1D onset with no protective effect. The extent of protection from T1D onset correlates directly with replication efficiency of the specific CVB strain that is employed: CVB strains that replicate to higher titers protect more mice than do CVB strains which replicate to lower titers. As NOD mice age, naturally occurring, pathogenic autoimmune insulitis develops rapidly (4) with widespread islet inflammation (insulitis) present by week 12 to 15 of age, when T1D also begins to occur. T1D rapidly ensues following inoculation of old (>12 weeks of age) NOD mice with virulent CVB (14), a model that recapitulates observations of sudden T1D onset in humans reported to occur during or shortly after an infection (37, 52). Initiation of rapid T1D onset in older mice also appears to correlate directly with the viral replication phenotype: CVB strains that replicate more rapidly to higher titers in NOD mouse pancreatic tissue initiate rapid T1D onset, whereas viruses which replicate to lower titers do not trigger T1D at rates different from mock-infected animals (14).
The CVB are described as diabetogenic on the basis of reports associating infections with human T1D onset and the ability of CVB to infect isolated human and murine islets in vitro (10, 47, 48, 61), although it is not known if all CVB strains can initiate T1D or whether it is a phenotype expressed only by rarely occurring strains. The latter possibility would be consistent with a paucity of known epidemic/outbreak T1D cases in the presence of common and widespread HEV (and CVB) circulation (1, 2, 11, 20). However, if all CVB were potentially capable of initiating T1D, then CVB-caused T1D would either itself be rare and/or involve other factors. The same CVB3 strains which effectively suppress T1D incidence when inoculated into healthy young NOD mice (55) can rapidly trigger T1D in older, prediabetic NOD mice (14). This observation, which obscures a simple definition of a diabetogenic CVB phenotype, is consistent with CVB diabetogenicity being modulated by the host environment. The impact of the host environment on viral replication is well-established: expression of CVB phenotypes are modulated by a variety of influences, including immune status, age, strain, and gender of the mouse host (14, 23, 25, 49, 50).
To test the hypothesis whether diabetogenicity is a common phenotype, we used a well-characterized, poorly pathogenic CVB3 strain, CVB3/GA (33, 55, 56), reasoning that rapid T1D onset should not be observed if the virus lacks a putative specific genetic determinant necessary to trigger T1D onset. The precedent for considering this hypothesis is the case for CVB3-induced myocarditis in mice: the primary structure of domain II in the 5′-nontranslated region (NTR) of the viral genome determines a myocarditic phenotype (15, 16). Furthermore, CVB3/GA does not induce T1D onset shortly after inoculation of 5 × 105 50% tissue culture infective doses (TCID50) into old NOD mice (14), sharply contrasting with another virulent strain, CVB3/28, that induces ≥70% T1D incidence at this dose within 1 to 2 weeks postinoculation (p.i.). All virus stocks used in these experiments were derived from infectious cDNA copies of the genomes (33, 55) by transfection of HeLa cells. Virus stocks were collected by ultracentrifugation through 30% (wt/vol) sucrose in 1 M NaCl, 10 mM Tris HCl pH 7.6, 0.1 mM MgCl2 into a glycerol button and resuspended in 100 mM NaCl, titers were determined on HeLa cells (TCID50/ml), and stocks were stored aliquoted at −75°C.
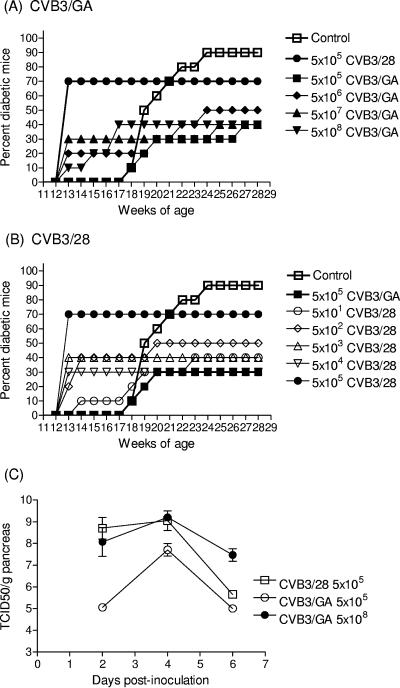
In the first experiment, groups of female NOD mice (Taconic, Germantown, NY) were inoculated intraperitoneally (i.p.) at 12 weeks of age with different doses of CVB3/GA (5 × 105 to 5 × 108 TCID50 per mouse). Other groups of mice were inoculated with decreasing doses of CVB3/28 (5 × 105 to 5 × 101 TCID50 per mouse); greater doses of CVB3/28 were not used, as preliminary experiments indicated that ≥5 × 106 TCID50 CVB3/28 per mouse resulted in high mortality (data not shown). Mice were maintained and assayed weekly for urine glucose levels to indicate T1D onset as described previously (14, 55).
Mock-infected (injected with sterile 100 mM NaCl, used as the virus diluent) mice first showed T1D onset at 18 weeks of age. However, T1D developed in each group of CVB3-inoculated mice 4 weeks earlier and within 1 to 2 weeks p.i. (Fig. 1A and B). The sole exception were mice inoculated with the lowest CVB3/GA dose (5 × 105 TCID50); like control mice, T1D onset in these mice first occurred at 18 weeks of age. In mice inoculated with higher CVB3/GA doses per mouse (5 × 106 to 5 × 108 TCID50), accelerated T1D onset was observed within 1 week p.i. (Fig. 1A; the results of mice inoculated with CVB3/28 at 5 × 105 TCID50 per mouse are included for comparison to Fig. 1B). T1D incidences in CVB3/GA-inoculated mice eventually reached 30 to 50%. T1D in CVB3/28-inoculated mice also first occurred within 1 week p.i. with the exception of mice inoculated with the lowest dose (50 TCID50), in which T1D first occurred at week 2 p.i. (Fig. 1B; results from CVB3/GA inoculated at 5 × 105 TCID50 per mouse are included for reference to Fig. 1A). After the initial burst of rapid onset T1D induced by CVB3/28, incidences remained constant for weeks, similar to results with CVB3/GA. With the exception of mice inoculated with the highest CVB3/28 dose (5 × 105 TCID50 per mouse), all CVB3/28-inoculated groups developed a 30 to 50% incidence by 18 to 20 weeks of age, the time when mock-infected mice were rapidly developing host-driven autoimmune T1D. Regardless of the CVB3 strain used and despite rapid onset of T1D observed in all but the group inoculated with 5 × 105 TCID50 CVB3/GA, T1D incidences remained lower than those of the mock-infected control group through the end of the experiment.
FIG.1.
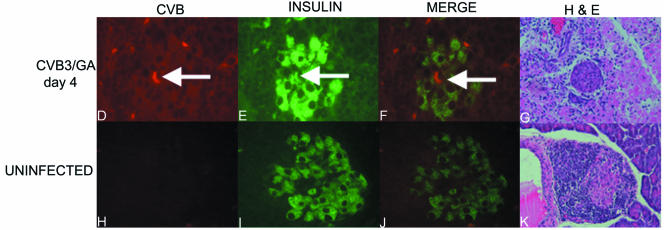
Inoculation of older NOD mice with high doses of CVB3/GA initiates T1D, increases pancreas titers, and permits detection of virus in islets prior to T1D onset. (A and B) Groups of 10 female NOD mice at 12 weeks of age were inoculated i.p. with different doses of CVB3/GA or CVB3/28. Doses (as TCID50 per mouse) are shown. T1D onset was assayed by urinalysis (55). The experiment was terminated when mice were 28 weeks old, 4 weeks after mock-infected control mice achieved 90% T1D incidence. (C) NOD mice at 4 to 5 weeks of age were inoculated i.p. with CVB3/GA or CVB3/28 at the dose shown and then killed (three to four mice per point) at various times. Pancreata were weighed and homogenized, and titers were determined on HeLa cell monolayers as described earlier (55). (D to K) Prediabetic mice at 21 weeks of age were inoculated with 1 × 108 TCID50 CVB3/GA per mouse (D to G) or saline (control mice [H to K]) and killed 4 days later. Formalin-fixed, paraffin-embedded pancreas sections (6 μm thick) were stained with antibody against viral capsid protein (D and H) or insulin (E and I). The merged image (F) shows an example (arrow) of colocalization of CVB3/GA with insulin; no virus signal was detected in control sections (H and J). Original magnification, ×480. Panels G and K show hematoxylin and eosin-stained sections. Original magnification, ×120 (G) and ×240 (K).
To test that rapid T1D induction by either of the two virus strains was related to a threshold infectious titer that may need to be achieved shortly after inoculation, 5 × 105 (CVB3/28 or CVB3/GA) or 5 × 108 TCID50 (CVB3/GA only) were inoculated per mouse, after which the infectious titer in pancreatic tissue was assayed. Following inoculation at 5 × 105 TCID50 per mouse, CVB3/28 replicated to 3 to 4 logs higher titer per g of pancreas tissue than CVB3/GA (Fig. 1C) within 2 days of inoculation and remained higher than CVB3/GA at day 4 p.i., confirming previous results (55). At this dose, CVB3/28 also initiated rapid T1D onset in 70% of mice (Fig. 1B) (14), whereas CVB3/GA caused no rapid onset. By increasing the CVB3/GA inoculum 1,000-fold to 5 × 108 TCID50 per mouse, CVB3/GA pancreas titers were equivalent to those generated by CVB3/28 when inoculated at 1,000-fold fewer infectious units within 2 days of inoculation (Fig. 1C). This correlated with observations that rapid onset T1D occurred in older mice inoculated with 5 × 106 to 5 × 108 TCID50 CVB3/GA (Fig. 1B). The results show that when sufficient virus is inoculated per mouse, raising the pancreatic virus titer above an apparent threshold, even a poorly pathogenic clinical isolate like CVB3/GA (33, 56) can initiate rapid onset T1D in older NOD mice.
We then asked if higher pancreatic CVB3/GA titers that occur as a result of inoculating higher doses per mouse and which can initiate rapid T1D onset might result in the detection of CVB3/GA in islets. Earlier work showed that CVB3/28 was detectable in islets of old mice 4 days p.i., immediately prior to T1D onset (14), supporting the hypothesis that CVB infection within remaining intact islet tissue in older, prediabetic NOD mice leads to rapid T1D onset. Four nondiabetic NOD mice (21 weeks of age) were inoculated with 1 × 108 TCID50 CVB3/GA i.p. and killed 4 days later; none of the mice were diabetic when killed. Immunohistochemical staining was performed as previously described (14) on formalin-fixed, paraffin-embedded pancreas sections (6 μm thick) using antibodies against enterovirus capsid protein (Dako, Carpinteria, CA) and insulin (Sigma, St. Louis, MO). Alexa-Flour-labeled secondary antibodies (Invitrogen, Carlsbad, CA) were used for detection. CVB3/GA capsid protein was visualized within intact regions of islets (Fig. 1D), colocalizing with insulin expression (Fig. 1E and F, merged image). These results were similar to results that had been described for virulent CVB3/28 (14). No virus protein was detected in sections from control, mock-infected mice, as expected (Fig. 1H and J, merged image). Insulin staining demarcates intact regions of inflamed islets in each section shown (Fig. 1E and I), while the extent of islet inflammation is evident in the hematoxylin and eosin-stained serial sections (Fig. 1G and K; panel G is shown at a lower magnification to better illustrate the extent of insulitis). Thus, inoculating mice with sufficient TCID50 CVB3/GA to initiate T1D in older mice can also result in the detection of virus replicating in islets prior to T1D onset. This result supports the hypothesis that any CVB strain may induce T1D under the correct conditions.
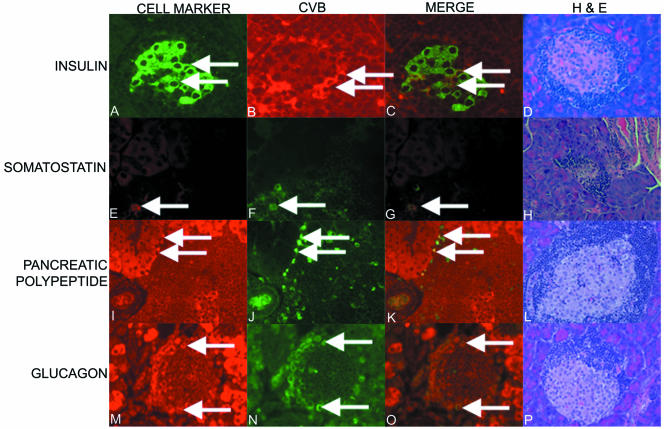
Colocalization of CVB3/GA with insulin in islets and the rapid T1D onset that can occur following inoculation of older, prediabetic NOD mice with CVB are consistent with a mechanism involving viral infection of insulin-producing beta cells in most or all islets. Islets cultured in vitro can be infected with diverse HEVs, and outcome can vary as a function of the virus strain used (48, 53, 59). In addition to insulin-producing beta cells, three primary endocrine cell types are recognized in the pancreatic islet of Langerhans: alpha (producing glucagon), delta (somatostatin), and PP (pancreatic polypeptide) (54). To determine whether CVB colocalizes only with beta cells in the NOD mouse islet, we assayed CVB-infected islets by immunohistochemistry. Staining was carried out on formalin-fixed, paraffin-embedded pancreas sections from CVB3/28-inoculated prediabetic (12-week-old) NOD mice killed 4 days p.i. Serial sections were first stained with antibodies against insulin (beta cells), somatostatin (delta cells; Chemicon, Temecula CA), glucagon (alpha cells; Chemicon), and pancreatic polypeptide (PP cells; DakoCytomation, Carpinteria, CA) to verify that each antibody detected different cells (data not shown). The antiviral antibody and the other antibodies were then used in pairs on sections from CVB3/28-inoculated NOD mouse pancreas tissue. As before, Alexa-Flour-labeled secondary antibodies were used for detection. Virus capsid protein was not detected, as expected, in the control (mock-infected mice) (data not shown) but was detected in noninflamed islet tissue of CVB-inoculated mice (Fig. 2B, F, J, and N). Separate and merged images show staining for virus protein and the specific islet cell product protein (Fig. 2A to C, E to G, I to K, and M to O). Hematoxylin and eosin-stained sections of the islets are also shown (Fig. 2D, H, L, and P; panel H is shown at a lower magnification to better illustrate the extent of insulitis in this islet). These results demonstrated that CVB infection of the islet in vivo appears to be a generalized infection, involving not only the insulin-producing beta cells but also other primary islet cell types.
FIG. 2.
CVB infections in islets of 12-week-old, prediabetic NOD mice colocalize with different islet cell types. A dose of 5 × 105 TCID50 CVB3/28 was inoculated into mice; mice were killed 4 days later, and serial pancreas sections were stained for insulin (A), somatostatin (E), pancreatic polypeptide (I), and glucagon (M) or virus capsid protein (B, F, J, and N). Merged images (C, G, K, and O) show examples of virus protein colocalized with the specific cell marker protein (arrows) at an original magnification of ×480. Hematoxylin and eosin-stained sections (D, H, L, and P) show the extent of islet inflammation at an original magnification of ×480 (D, L, and P) or ×240 (H).
The 5′-NTR RNA is a key genetic region that controls the extent of cardiac titer in mice and in cardiac cell culture as well as the expression of CVB cardiovirulence in mice (15, 16). We therefore wished to determine whether the 5′-NTR sequences of CVB3/GA and CVB3/28 similarly impacted replication in beta cell cultures and in mice. CVB3/GA- and CVB3/28-based genomes were created using standard techniques, and infectious cDNA copies of the CVB3/GA and CVB3/28 genomes (33, 55), in which the complete 5′-NTR sequences of each virus were exchanged (CVB3/28 with the 5′ NTR replaced by that from CVB3/GA [CVB3/28-5NTRGA] or CVB3/GA with the 5′ NTR replaced by that from CVB3/28 [CVB3/GA-5NTR28]). The 5′-NTR replacements were precise in both cases, leaving the start of translation beginning at nucleotide 740 intact. For each virus stock, the 5′-NTR sequences were verified by sequence analysis (data not shown). We also characterized the NOD mouse pancreatic beta cell line MIN6 (38) for permissiveness to CVB replication. MIN6 cultures were graciously provided by M. A. Permutt, Washington University School of Medicine, by permission of J.-I. Miyazaki, Kumamoto University Medical School, Kumamoto, Japan. Monolayer cultures inoculated with CVB1-6 (ATCC, Manassas, VA) at a multiplicity of infection of 20 TCID50 per cell showed significant or complete cytopathic effects (cell rounding and cell detachment) within 48 to 72 h p.i. depending upon the virus strain (data not shown).
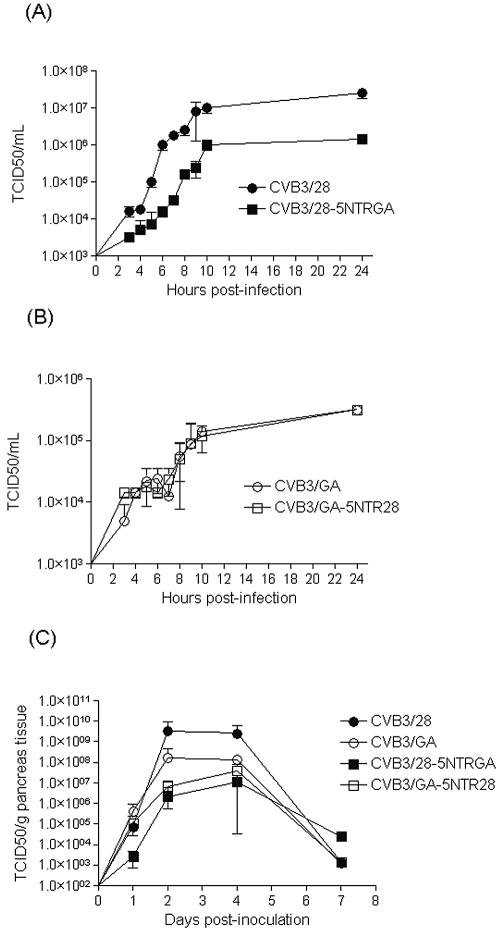
Single-step growth curves were then performed to compare viral replication rates. Duplicate cultures (1 × 105 MIN6 cells in 0.5 ml medium in 24-well plates) were inoculated with parental CVB3/28, CVB3/GA, or either of the two 5′-NTR chimeric strains at a multiplicity of infection of 20 TCID50 per cell, washed 1 hour after infection, and then provided fresh medium. Cultures were harvested by freezing, and then titers were determined on HeLa cell monolayers. Parental CVB3/28 replicated to a nearly 100-fold higher titer in MIN6 cultures than CVB3/GA at 10 h p.i. (compare Fig. 3A and B), consistent with and extending results obtained from CVB3/28 and CVB3/GA infections of murine pancreas tissue (Fig. 1C). Replication of the CVB3/28-5NTRGA chimeric strain was slowed relative to parental CVB3/28 (Fig. 3A) by 3 to 4 h; the final titer was 10-fold lower than that of the parental strain. Despite this, CVB3/28-5NTRGA replicated to a 1 log higher titer at 10 h p.i. than either parental CVB3/GA or CVB3/GA-5NTR28 (Fig. 3B). Replacement of the CVB3/GA 5′ NTR with that from CVB3/28 had no effect on the replication of CVB3/GA-5NTR28 relative to the parental CVB3/GA strain in MIN6 cultures: both strains replicated with similar rates to equivalent titers. Thus, the CVB3/GA-based strain was unaffected by exchange of the 5′-NTR sequence, while the CVB3/GA 5′ NTR suppressed replication of the chimeric strain relative to parental CVB3/28.
FIG. 3.
Replication of parental and 5′-NTR chimeric CVB3/28 and CVB3/GA strains in MIN6 cell cultures and in NOD mice. Single-step growth curves were carried out in monolayer MIN6 cultures (38) using CVB3/28 and CVB3/28-5NTRGA (in which the 5′ NTR from CVB3/GA replaced that of CVB3/28) (A) or CVB3/GA and CVB3/GA-5NTR28 (in which the 5′ NTR from CVB3/28 replaced that of CVB3/GA) (B). Cells (5 × 104 per well) were plated in 24-well clusters, inoculated the following day at a multiplicity of infection of 20 TCID50 per cell, and washed after 1 h incubation at 37°C, and then 0.5 ml fresh medium was added to each well. Plates were harvested by freezing; infectious titers were determined on HeLa cell monolayers. Results have been separated into two panels for clarity. (C) Four- to 5-week-old NOD mice were inoculated with 5 × 105 TCID50 CVB3/28, CVB3/GA, or the chimeric strains CVB3/28-5NTRGA or CVB3/GA-5NTR28. Mice were killed on days 1, 2, 4, and 7 p.i., and infectious virus titers in the pancreas were determined on HeLa cells.
We then compared replication of the four CVB3 strains in NOD mouse pancreas tissue following i.p. inoculation of female NOD mice (4 to 5 weeks old) with 5 × 105 TCID50 of virus. Infectious virus titers in the pancreas were determined on HeLa cell monolayers. CVB3/28 replicated to a higher titer than CVB3/GA by days 2 to 4 p.i. with clearance of both viruses well under way by day 7 p.i., similar to previous observations (55). Both 5′-NTR chimeric strains replicated with nearly equivalent rates to closely similar titers in the pancreas. Although chimeric viruses initially replicated more slowly than parental strains, the chimeric strains achieved titers close to that of parental CVB3/GA by day 4 p.i. In contrast to replication in MIN6 cultures, 5′ NTR exchange resulted in lowering the initial titers achieved by the chimerics relative to their parental strains. However, as before, CVB3/28-5NTRGA was more crippled relative to the parental strain than CVB3/GA-5NTR28.
We demonstrate here that even a poorly pathogenic, clinical virus isolate such as CVB3/GA can initiate rapid T1D in older, prediabetic NOD mice, providing that the dose of virus (number of infectious CVB virions inoculated per mouse) is sufficient. If inoculated at a dose which promotes rapid T1D onset, CVB3/GA could also be colocalized with insulin-producing beta cells in islets prior to T1D onset. Consistent with the necessity of inoculating higher doses of CVB3/GA than CVB3/28 per mouse to achieve similar results, CVB3/GA was shown to replicate more slowly than CVB3/28 both in a mouse beta cell culture and in total pancreas tissue. These results support the hypothesis that the CVB replication rate is the key factor in defining a diabetogenic viral phenotype in this system and suggest that any CVB strain can be diabetogenic in old, prediabetic NOD mice, providing that the mouse is inoculated with a sufficient infectious dose. Previous observations (51, 55) that sporadic cases of rapid T1D onset, well in advance of that in control groups, can occur when younger (8-week-old) NOD mice are inoculated with different CVB strains further support this hypothesis.
While both CVB3 strains induce equivalent T1D incidences in older mice, significantly different ranges of infectious virus were needed to achieve this end: inoculation of mice with lower CVB3/28 doses (5 × 101 to 5 × 104 TCID50 per mouse) resulted in T1D incidences in each group similar to those induced by higher CVB3/GA doses (5 × 106 to 5 × 108 TCID50 per mouse). Our working hypothesis to explain this observation is that an initially high pancreatic titer of CVB3/GA resulting from injecting at least 5 × 106 TCID50 per mouse can compensate for the slower CVB3/GA replication rate, leading to infection and killing of more beta cells than that which occurs following inoculation with fewer infectious virus particles per mouse. CVB3/28, on the other hand, replicates more rapidly to higher doses and so can initiate rapid T1D onset with even fewer virus particles in the infectious dose. The observation that CVB can also associate with other islet cells suggests islet infections may be generalized events, not limited solely to the beta cells. However, the present data cannot determine the efficiency with which CVB replicates in cells other than beta cells.
We theorize that the reason for equivalent T1D incidences in nearly every group results from a combination of two factors: the residual resistance of pancreatic islets to CVB infection, shown to be due to an islet-specific production of innate interferons (10, 17), and the remaining number of intact, functional beta cells. It is reasonable that only an overwhelming initial virus presence (such as inoculation with 5 × 105 TCID50 of virulent CVB3 strains [14]) may destroy enough beta cells within a short length of time to induce rapid T1D onset incidences of ≥70%. Lower doses of the specific virus strain do not achieve this extent of damage in the face of the residual islet-specific innate immune response and a rapidly activating antiviral adaptive immune response, with the result that fewer mice become diabetic. We would predict on this basis that more cases of CVB-initiated T1D would be observed following inoculation of even older mice with less intact islet tissue mass. Even with 108 TCID50 CVB3/GA inoculated per mouse, the slower replication rate of this strain compared to CVB3/28 cumulatively slows the impact of this less pathogenic strain upon islet biology, a hypothesis supported by the slower rates and lower titers achieved by CVB3/GA relative to CVB3/28 in MIN6 cells. We propose that rapidly replicating HEV strains are more likely to be pathogenic during a human infection than strains which replicate more slowly, thereby providing a practical definition of diabetogenic. These observations further suggest grounds for why cases of HEV-associated T1D onset may be rare: even when islets are significantly inflamed by one's autoimmune disease, the infecting virus must rapidly attain high titer and deplete residual beta cell activity to initiate T1D prior to the rise of the antiviral immune response. Because T1D incidences remained lower than those of mock-infected control mice through the end of the experiments, regardless of the CVB3 strain or dose employed, the protective impact upon the immune system that has been shown in young mice (55) may still function even in the prediabetic stage of life, thereby helping to suppress long-term T1D levels.
Despite the characterization of diabetogenic CVB4 strains (30, 60) and infectious CVB4 cDNA clones (29, 44), the mapping of viral genetics that determine a diabetogenic CVB phenotype has not been carried out, as has been accomplished for other CVB virulence phenotypes (15, 31, 44). This is in part due to the lack of a suitable mouse model in which irreversible, lethal T1D can be reliably induced, although such a model is now available (14). Data presented here argue that CVB serology per se appears to be of little consequence, consistent with the growing understanding that genetic recombination among HEV-B is commonplace (34, 36, 41). The established link between the primary and predicted secondary structures of domain II in the CVB3 5′ NTR and the capacity to induce myocarditis (15, 33) suggested the possibility that the 5′ NTR might also influence CVB3 replication in beta cells and pancreatic tissue. However, a clearly defined association was not observed in these experiments. The observation that either 5′ NTR suppressed replication of the respective chimeric CVB3 strain relative to the parental strain in vivo may be indicative of replication in a variety of cell types, whereas results from studies in mouse beta cell cultures may reflect different requirements of the virus during replication in beta cells. Analysis of replication of other chimeric viral strains in specific host cells and tissues will be required to fully illuminate this issue.
Acknowledgments
We thank P. Karki and B. Henley for excellent technical assistance. T. Kanno and K. Kim contributed equally to this work.
Support was in part by grants from the Juvenile Diabetes Foundation International and the American Diabetes Association (S.T.), the American Heart Association (N.M.C.), and the USPHS and the Multiple Sclerosis Society (K.M.D.). T.K. was supported in part by a grant from the National Agriculture and Bio-oriented Research Organization (Japan). K. Kim was supported in part by the Postdoctoral Fellowship Program of the Korea Science & Engineering Foundation. K. Kono was supported in part by a grant from Chugai Pharmaceuticals.
REFERENCES
- 1.Akerblom, H. K., O. Vaarala, H. Hyoty, J. Ilonen, and M. Knip. 2002. Environmental factors in the etiology of type 1 diabetes. Am. J. Med. Genet. 115:18-29. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2000. Enterovirus surveillance, United States, 1997-1999. Morb. Mortal. Wkly. Rep. 49:913-916. [PubMed] [Google Scholar]
- 3.Anonymous. 2006. Enterovirus surveillance, United States, 2002-2004. Morb. Mortal. Wkly. Rep. 55:153-156. [PubMed] [Google Scholar]
- 4.Atkinson, M. A., and E. H. Leiter. 1999. The NOD mouse model of type 1 diabetes: as good as it gets? Nat. Med. 5:601-604. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson, M. A., and N. Maclaren. 1994. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 331:1428-1436. [DOI] [PubMed] [Google Scholar]
- 6.Baekkeskov, S., and B. Hansen. 1990. Human diabetes: genetic, environmental, and autoimmune etiology. Curr. Top. Microbiol. Immunol. 164:143-168. [DOI] [PubMed] [Google Scholar]
- 7.Barnett, A. H., C. Eff, R. Leslie, and D. Pyke. 1981. Diabetes in identical twins: a study of 200 pairs. Diabetologia 20:87-93. [DOI] [PubMed] [Google Scholar]
- 8.Cabrera-Rode, E., L. Sarmiento, G. Molina, C. Perez, C. Arranz, J. A. Galvan, M. Prieto, J. Barrios, R. Palomera, M. Fonseca, P. Mas, O. Diaz-Diaz, and O. Diaz-Horta. 2005. Islet cell related antibodies and type 1 diabetes associated with echovirus 30 epidemic: a case report. J. Med. Virol. 76:373-377. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera-Rode, E., L. Sarmiento, C. Tiberti, G. Molina, J. Barrios, D. Hernandez, O. Diaz-Horta, and U. Di Mario. 2003. Type 1 diabetes islet associated antibodies in subjects infected by echovirus 16. Diabetologia 46:1348-1353. [DOI] [PubMed] [Google Scholar]
- 10.Chehadeh, W., J. Kerr-Conte, F. Pattou, G. Alm, J. Lefebvre, N. Waugh, and D. Hober. 2000. Persistent infection of human pancreatic islets by coxsackievirus B is associated with alpha interferon synthesis in beta cells. J. Virol. 74:10153-10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Control, C. F. D. 2000. Non-polio enterovirus surveillance, United States, 1993-1996. Morbid. Mortal. Wkly. Rep. 46:748-750. [Google Scholar]
- 12.Craighead, J. E. 1975. The role of viruses in the pathogenesis of pancreatic disease and diabetes mellitus. Prog. Med. Virol. 19:161-214. [PubMed] [Google Scholar]
- 13.Diaz-Horta, O., M. Bello, E. Cabrera-Rode, J. Suarez, P. Mas, I. Garcia, I. Abalos, R. Jofra, G. Molina, O. Diaz-Diaz, and U. Di Mario. 2001. Echovirus 4 and type 1 diabetes mellitus. Autoimmunity 34:275-281. [DOI] [PubMed] [Google Scholar]
- 14.Drescher, K. M., K. Kono, S. Bopegamage, S. D. Carson, and S. Tracy. 2004. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology 329:381-394. [DOI] [PubMed] [Google Scholar]
- 15.Dunn, G., S. Bradrick, N. Chapman, S. Tracy, and J. Romero. 2003. The stem loop II within the 5′ nontranslated region of clinical coxsackievirus B3 genomes determines cardiovirulence phenotype in a murine model. J. Infect. Dis. 15:1552-1561. [DOI] [PubMed] [Google Scholar]
- 16.Dunn, J. J., N. M. Chapman, S. Tracy, and J. R. Romero. 2000. Natural genetics of cardiovirulence in coxsackievirus B3 clinical isolates: localization to the 5′ nontranslated region. J. Virol. 74:4787-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flodstrom, M., A. Maday, B. Balakrishna, M. Cleary, A. Yoshimura, and N. Sarvetnick. 2002. Target cell defense prevents the development of diabetes after viral replication. Nat. Immunol. 3:373-382. [DOI] [PubMed] [Google Scholar]
- 18.Foulis, A. K., M. Farquharson, S. Cameron, M. McGill, H. Schoenke, and R. Kandolf. 1990. A search for the presence of enteroviral capsid protein VP1 in pancreases of patients with type 1 diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetologia 33:290-298. [DOI] [PubMed] [Google Scholar]
- 19.Frisk, G., and T. Tuvemo. 2004. Enterovirus infections with beta-cell tropic strains are frequent in siblings of children diagnosed with type 1 diabetes children and in association with elevated levels of GAD65 antibodies. J. Med. Virol. 73:450-459. [DOI] [PubMed] [Google Scholar]
- 20.Froeschle, J., P. Feorino, and H. M. Gelfand. 1966. A continuing surveillance of enterovirus infection in healthy children in six United States cities. II. Surveillance enterovirus isolates 1960-1963 and comparison with enterovirus isolates from cases of acute central nervous system disease. Am. J. Epidemiol. 83:455-469. [DOI] [PubMed] [Google Scholar]
- 21.Fuchtenbusch, M., A. Irnstetter, G. Jager, and A.-G. Ziegler. 2001. No evidence for an association of coxsackievirus infections during pregnancy and early childhood with development of islet autoantibodies in offspring of mothers or fathers with type 1 diabetes. J. Autoimmun. 17:333-340. [DOI] [PubMed] [Google Scholar]
- 22.Gamble, D. R. 1976. A possible virus etiology for juvenile diabetes, p. 95-105. In W. Kreutzfeld, J. Kobberling, and J. Neel (ed.), The genetics of diabetes mellitus. Springer Verlag, Berlin, Germany.
- 23.Gauntt, C. J. 1997. Roles of the humoral response in coxsackievirus B-induced disease. Curr. Top. Microbiol. Immunol. 223:259-282. [DOI] [PubMed] [Google Scholar]
- 24.Hierholzer, J. C., and W. Farris. 1974. Follow-up of children infected in a coxsackievirus B3 and B4 outbreak: no evidence of diabetes mellitus. J. Infect. Dis. 129:741-746. [DOI] [PubMed] [Google Scholar]
- 25.Huber, S. H. 1988. The role of immune mechanisms in pathogenesis, p. 103-116. In M. Bendinelli and H. Friedman (ed.), Coxsackieviruses: a general update. Plenum Press, New York, N.Y.
- 26.Huff, J. C., J. C. Hierholzer, and W. Farris. 1974. An “outbreak” of juvenile diabetes mellitus: consideration of a viral etiology. Am. J. Epidemiol. 100:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyoty, H., M. Hiltunen, and M. Lonnrot. 1998. Enterovirus infections and insulin dependent diabetes mellitus—evidence for causality. Clin. Diagn. Virol. 9:77-84. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki, T., N. Monma, R. Satodate, R. Kawana, and T. Kurata. 1985. An immunofluorescent study of generalized coxsackie virus B3 infection in a newborn infant. Acta Pathol. Jpn. 35:741-748. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins, O., J. D. Booth, P. D. Minor, and J. W. Almond. 1987. The complete nucleotide sequence of coxsackievirus B4 and its comparison to other members of the picornaviridae. J. Gen. Virol. 68:1835-1848. [DOI] [PubMed] [Google Scholar]
- 30.Kang, J., N. Chatterjee, M. Nodwell, and J. Yoon. 1994. Complete nucleotide sequence of a strain of coxsackie B4 virus of human origin that induces diabetes in mice and its comparison with nondiabetogenic coxsackie B4 JBV strain. J. Med. Virol. 44:353-361. [DOI] [PubMed] [Google Scholar]
- 31.Kanno, T., D. Mackay, G. Wilsden, and P. Kitching. 2001. Virulence of swine vesicular disease virus is determined at two amino acids in capsid protein VP1 and 2A protease. Virus Res. 80:107. [DOI] [PubMed] [Google Scholar]
- 32.Knip, M., and H. K. Akerblom. 1999. Environmental factors in the pathogenesis of type 1 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 107(Suppl. 3):S93-S100. [DOI] [PubMed] [Google Scholar]
- 33.Lee, C.-K., K. Kono, E. Haas, K.-S. Kim, K. M. Drescher, N. M. Chapman, and S. Tracy. 2005. Characterization of an infectious cDNA copy of the genome of a naturally-occurring, avirulent coxsackievirus B3 clinical isolate. J. Gen. Virol. 86:197-210. [DOI] [PubMed] [Google Scholar]
- 34.Lindberg, A., P. Andersoon, C. Savolainen, M. N. Mulders, and T. Hovi. 2003. Evolution of the genome of human enterovirus B: incongruence between phylogenies of the VP1 and 3CD regions indicates frequent recombination within the species. J. Gen. Virol. 84:1223-1235. [DOI] [PubMed] [Google Scholar]
- 35.Lo, S., R. Tun, M. Hawa, and R. Leslie. 1991. Studies of diabetic twins. Diabetes Metab. Rev. 7:223-228. [DOI] [PubMed] [Google Scholar]
- 36.Lukashev, A. N., V. A. Lashkevich, O. E. Ivanova, G. A. Koroleva, A. E. Hinkkanen, and J. Ilonen. 2005. Recombination in circulating human enterovirus B: independent evolution of structural and non-structural genome regions. J. Gen. Virol. 86:3281-3290. [DOI] [PubMed] [Google Scholar]
- 37.Maria, H., A. Elshebani, O. Anders, T. Torsten, and F. Gun. 2005. Simultaneous type 1 diabetes onset in mother and son coincident with an enteroviral infection. J. Clin. Virol. 33:158-167. [DOI] [PubMed] [Google Scholar]
- 38.Miyazaki, J., K. Araki, E. Yamato, H. Ikegami, T. Asano, Y. Shibasaki, Y. Oka, and K. Yamamura. 1990. Establishment of a pancreatic beta cell line that retains glucose inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127:126-132. [DOI] [PubMed] [Google Scholar]
- 39.Moya-Suri, V., M. Schlosser, K. Zimmermann, I. Rjasanowski, L. Gurtler, and R. Mentel. 2005. Enterovirus RNA sequences in sera of schoolchildren in the general population and their association with type 1-diabetes-associated autoantibodies. J. Med. Microbiol. 54:879-883. [DOI] [PubMed] [Google Scholar]
- 40.Nigro, G., M. E. Pacella, E. Patane, and M. Midulla. 1986. Multi-system coxsackievirus B-6 infection with findings suggestive of diabetes mellitus. Eur. J. Pediatr. 145:557-559. [DOI] [PubMed] [Google Scholar]
- 41.Oberste, M. S., S. Penaranda, and M. Pallansch. 2004. RNA recombination plays a major role in genomic change during circulation of coxsackie B viruses. J. Virol. 78:2948-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otonkoski, T., M. Roivainen, O. Vaarala, B. Dinesen, J. Leipala, L. Hix, and M. Knip. 2000. Neonatal type I diabetes associated with maternal echovirus 6 infection: a case report. Diabetologia 43:1235-1238. [DOI] [PubMed] [Google Scholar]
- 43.Pallansch, M., and R. P. Roos. 2001. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p. 723-776. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 44.Ramsingh, A., A. Hixson, B. Duceman, and J. Slack. 1990. Evidence suggesting that virulence maps to the P1 region of the coxsackievirus B4 genome. J. Virol. 64:3078-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redondo, M., L. Yu, M. Hawa, T. Mackenzie, D. Pyke, G. Eisenbarth, and R. Leslie. 2001. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia 44:354-362. [DOI] [PubMed] [Google Scholar]
- 46.Roep, B., M. A. Atkinson, and M. G. von Herrath. 2004. Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat. Rev. Immunol. 4:989-997. [DOI] [PubMed] [Google Scholar]
- 47.Roivainen, M., S. Rasilainen, P. Ylipaasto, R. Nissinen, J. Ustinov, L. Bouwens, D. Eizirik, T. Hovi, and T. Otonkoski. 2000. Mechanism of coxsackievirus-induced damage to human pancreatic beta cells. J. Clin. Endocrinol. Metab. 85:432-440. [DOI] [PubMed] [Google Scholar]
- 48.Roivainen, M., P. Ylipaasto, C. Savolainen, J. M. Galama, T. Hovi, and T. Otonkoski. 2002. Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia 45:693-702. [DOI] [PubMed] [Google Scholar]
- 49.Rose, N. R., D. A. Neumann, and A. Herskowitz. 1988. Genetics of susceptibility to viral myocarditis in mice. Pathol. Immunopathol. Res. 7:266-278. [DOI] [PubMed] [Google Scholar]
- 50.Schwimmbeck, P. L., S. A. Huber, and H. P. Schultheiss. 1997. Roles of T cells in coxsackievirus B-induced disease. Curr. Top. Microbiol. Immunol. 223:283-303. [DOI] [PubMed] [Google Scholar]
- 51.Serreze, D. V., E. W. Ottendorfer, T. M. Ellis, C. J. Gauntt, and M. A. Atkinson. 2000. Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes 49:708-711. [DOI] [PubMed] [Google Scholar]
- 52.Smith, C., G. Clements, M. Riding, P. Collins, G. Bottazo, and K. Taylor. 1998. Simultaneous onset of type 1 diabetes mellitus in identical infant twins with enterovirus infection. Diabet. Med. 15:515-517. [DOI] [PubMed] [Google Scholar]
- 53.Szopa, T. M., D. R. Gamble, and K. W. Taylor. 1986. Coxsackie B4 virus induces short-term changes in the metabolic functions of mouse pancreatic islets in vitro. Cell Biochem. Function 4:181-187. [DOI] [PubMed] [Google Scholar]
- 54.Thomson, A. B. R., and E. A. Shaffer. 1992. The first principles of gastroenterology. University of Toronto Press, Toronto, Canada.
- 55.Tracy, S., K. M. Drescher, N. M. Chapman, K.-S. Kim, S. D. Carson, S. Pirruccello, P. H. Lane, J. R. Romero, and J. S. Leser. 2002. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J. Virol. 76:12097-12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tracy, S., K. Hofling, S. Pirruccello, P. H. Lane, S. M. Reyna, and C. Gauntt. 2000. Group B coxsackievirus myocarditis and pancreatitis in mice: connection between viral virulence phenotypes. J. Med. Virol. 62:70-81. [DOI] [PubMed] [Google Scholar]
- 57.Verge, C., N. Howard, L. Irwig, J. Simpson, D. Mackerras, and M. Silink. 1994. Environmental factors in childhood IDDM: a population-based, case-control study. Diabetes Care 17:1381-1389. [DOI] [PubMed] [Google Scholar]
- 58.Wadsworth, E., J. Shield, L. Hunt, and J. Baum. 1997. A case control study of environmental factors associated with diabetes in the under 5s. Diabet. Med. 14:390-396. [DOI] [PubMed] [Google Scholar]
- 59.Ylipaasto, P., K. Klingel, A. M. Lindberg, T. Otonkoski, R. Kandolf, T. Hovi, and M. Roivainen. 2004. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47:225-239. [DOI] [PubMed] [Google Scholar]
- 60.Yoon, J., M. Austin, T. Onodera, and A. Notkins. 1979. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N. Engl. J. Med. 300:1173-1179. [DOI] [PubMed] [Google Scholar]
- 61.Yoon, J., T. Onodera, A. Jenson, and A. Notkins. 1978. Virus induced diabetes mellitus. XI. Replication of coxsackie B3 virus in human pancreatic beta cell cultures. Diabetes 27:778-781. [DOI] [PubMed] [Google Scholar]