Abstract
MicroRNAs (miRNAs) are a class of ∼22-nucleotide noncoding RNAs that inhibit the expression of specific target genes at the posttranscriptional level. Recently, 11 miRNAs encoded by the pathogenic human herpesvirus Kaposi's sarcoma-associated herpesvirus (KSHV) were cloned from latently infected cells. While the expression of these miRNAs has been confirmed by Northern analysis, their ability to inhibit target gene expression has not been demonstrated. We have devised a novel assay for miRNA function that uses lentiviral indicator vectors carrying two perfectly complementary target sites for each given miRNA in the 3′ untranslated region of the Renilla luciferase gene. This assay allowed us to demonstrate the activity of each viral miRNA upon cotransduction of cells with the Renilla luciferase indicator vector together with a firefly luciferase control vector. In KSHV-infected BC-1 and BCBL-1 cells, but not uninfected control cells, Renilla luciferase expression was selectively reduced up to 10-fold. Interestingly, one of the viral miRNAs (miR-K5) exhibited much higher activity in BC-1 cells than in BCBL-1 cells. Sequence analysis of both viral genomes revealed a single nucleotide polymorphism in the miR-K5 precursor stem-loop, which inhibits the expression of mature miR-K5 in BCBL-1 cells. We show that the primary miR-K5 sequence present in BCBL-1 results in diminished processing by Drosha both in vivo and in vitro. This is the first report of a naturally occurring sequence polymorphism in an miRNA precursor that results in reduced processing and therefore lower levels of mature miRNA expression and function.
Animals, plants, and some viruses encode microRNAs (miRNAs), a class of small (∼22-nucleotide [nt]) regulatory RNAs (1). As part of RNA-induced silencing complexes (RISCs), miRNAs guide the sequence-specific posttranscriptional repression of target mRNAs containing sequences with various degrees of complementarity to the miRNA (1, 11). The RISC was initially discovered as the mediator of RNA interference (15), and it is now clear that, at least in vertebrates, miRNAs and small interfering RNAs utilize overlapping cellular pathways (10, 19, 37). miRNAs are initially transcribed as part of long primary miRNA (pri-miRNA) precursors in which they form part of one arm of an RNA stem-loop (4, 8, 23). In animals, the processing of the pri-miRNA is initiated by the RNase III enzyme Drosha, which acts in concert with its partner, DGCR8, to release a ∼65-nt pre-miRNA hairpin characterized by a 2-nt 3′ overhang and an imperfect ∼20-bp stem (9, 12, 16, 20, 22, 34). The 3′ overhang and RNA stem allow the recognition and nuclear export of the pre-miRNA by exportin 5 (25, 32, 35). Once in the cytoplasm, cleavage of the pre-miRNA by another RNase enzyme, Dicer, yields an imperfectly base-paired RNA duplex (2, 14, 18). Generally, one strand of this duplex is then selectively incorporated into the RISC to form the mature miRNA, while the other strand, referred to as the passenger strand, is typically degraded (17). Target recognition by the RISC results in target degradation if the miRNA is perfectly complementary to the target (15). While this is mainly the case in the related RNA interference pathway, this seems to be unusual for animal miRNAs (1). However, it has been shown that miRNAs can direct the cleavage of mRNAs containing perfectly matched target sequences (19, 37). More commonly, the miRNA and its cellular target sequences are imperfectly matched, and the binding of the RISC to such targets leads mainly to a block of mRNA translation (1). It has been suggested that ∼30% of mammalian genes are subject to regulation by miRNAs (24).
Given the emerging importance of miRNA-mediated gene regulation, it is perhaps not surprising that several viruses also encode miRNAs. The gammaherpesvirus Epstein-Barr virus was the first virus shown to express miRNAs, and subsequently, the gammaherpesviruses Kaposi's sarcoma-associated herpesvirus (KSHV) and murine herpesvirus 68 as well as the betaherpesvirus human cytomegalovirus (CMV) were also shown to express miRNAs (5, 13, 26, 27, 30). Moreover, the polyomavirus simian virus 40 encodes a miRNA that targets and downregulates the expression of simian virus 40 T antigens, the coding region of which overlaps the pri-miRNA gene in the reverse orientation (31). While several groups have previously identified 11 KSHV miRNAs that are expressed in latently KSHV-infected BC-1 and BCBL-1 cells, the biological activity of these KSHV miRNAs, or indeed any herpesviral miRNA, has not been demonstrated (5, 26, 30). Here, we utilize a novel indicator system for miRNA function to show that the majority of the KSHV miRNAs expressed in BC-1 and BCBL-1 cells can induce robust downregulation of an mRNA bearing artificial target sequences. We also provide evidence for differential expression of one KSHV miRNA, miR-K5, in the latently infected human cell lines BC-1 and BCBL-1. We demonstrate that this difference arises from a sequence polymorphism in KSHV that results in the inefficient processing of the pri-miR-K5 precursor in BCBL-1 cells.
MATERIALS AND METHODS
Molecular clones.
Full-length firefly luciferase (FLuc) and Renilla luciferase (RLuc) cDNAs were amplified by PCR and used to replace the SnaB1-XhoI fragment of the previously described human immunodeficiency virus type 1-based vector pNL-SIN-CMV-BLR (21). Oligonucleotides bearing two target sequences were annealed and inserted between the ClaI and XbaI sites of pNL-SIN-CMV-RL. The N-terminal ClaI site was destroyed by ligation of the insert, and a second ClaI site 4 nt upstream of the XbaI site was used to insert two more target sites in the case of the vectors carrying four target sequences. KSHV miRNA expression constructs for miR-K1 (bp 121762 to 122003), miR-K3 (bp 121455 to 121703), miR-K8 (bp 119855 to 120100), and miR-K9 (bp 119212 to 119447) were cloned by PCR amplification of the genomic KSHV DNA present in BC-1 cells (29) and inserted into the BglII and HindIII sites of a modified pSUPER construct (3). Termination was achieved by a run of five T's inserted 5′ to the HindIII site. pTre-miR-K5(BC-1) and pTre-miR-K5(BCBL-1) were cloned by the insertion of PCR-generated DNA fragments corresponding to bp 121418 to 120947 of the KSHV genome into the BamHI and SalI sites of pTre2hyg (BD Clontech). All PCR-amplified fragments were verified by sequencing.
Virus production and transduction and target site assays.
293T, BJAB, BC-1, and BCBL-1 cells were maintained as previously described (5). Transfections were carried out using the calcium phosphate method. Virus was produced from 293T cells by cotransfection of the reporter vectors with pcRev, pcTat, and pHIT/G, as previously described (21). Virus was harvested 2 days after transfection, and control virus and indicator virus were mixed at a 2:1 ratio and used to transduce BJAB, BC-1, and BCBL-1 cells in the presence of polybrene. Twenty-four hours after transduction, the cells were recovered by centrifugation, washed with phosphate-buffered saline, and subjected to a dual luciferase assay (Promega). For the transient cotransfection of indicator constructs with KSHV expression constructs, cells were transfected in 24-well plates with 2.5 ng control vector, 2.5 ng indicator vector, and 0.4 μg of the KSHV miRNA expression construct. Dual luciferase assays were carried out 3 days after transfection.
Northern blotting and in vitro Drosha cleavage.
Mature miRNAs were detected by Northern blotting as previously described (5). The primary transcript from the pTre-miR-K5 constructs was detected using a ∼250-bp PCR product derived from the KSHV genome present in BC-1 cells, which was labeled using a random-primed DNA labeling kit (Roche). For in vitro Drosha processing, 293T cells were cotransfected using Drosha-Flag (22) and DGCR8-Flag (12) expression plasmids at a 9:1 ratio. After 48 h, Flag-tagged Drosha and DGCR8 proteins were copurified as described previously (33). Drosha processing experiments were performed as described previously except that drained beads were used directly in the reaction mixture (33). The substrate was prepared by in vitro transcription from T7-added, ∼270-bp PCR products, encompassing the entire pre-miRNA stem-loop as well as flanking regions on both sites.
RESULTS AND DISCUSSION
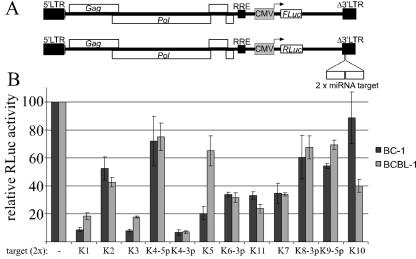
Previously established assays for miRNA activity rely on the transfection of indicator constructs bearing multiple copies of complementary target sites in the 3′ untranslated region (3′UTR) of a reporter gene (10, 36, 37). This approach is difficult to apply to hard-to-transfect cell lines or primary cells. While KSHV-infected B cells can be transfected by electroporation, this method is not suitable for large sample numbers and/or small sample volumes. We therefore established a new indicator assay for miRNA activity that relies on the delivery of indicator constructs by transduction rather than by transfection. This assay is based on the previously described self-inactivating human immunodeficiency virus type 1-based lentiviral vector pNL-SIN-CMV (21), which was reengineered to express either FLuc or RLuc under the control of the CMV immediate-early promoter. The resulting vectors were designated pNL-SIN-CMV-FL and pNL-SIN-CMV-RL. Unique ClaI and XbaI sites within pNL-SIN-CMV-RL allow the insertion of target site sequences into the 3′UTR of the Renilla luciferase gene (Fig. 1A). In the presence of the miRNA in question, the ratio of RLuc activity to FLuc activity is expected to be reduced compared to an RLuc indicator vector containing no target site or an unrelated target site. Similarly, this same ratio should also be reduced compared to the value obtained from control cell lines that do not express the relevant miRNA.
FIG. 1.
(A) Schematic of the control vector pNL-SIN-CMV-FL, which encodes FLuc, and the indicator vector pNL-SIN-CMV-RL, which encodes RLuc. In each RLuc-based indicator construct, two perfectly complementary target sequences for one KSHV miRNA were inserted into the 3′UTR of the Renilla luciferase gene. (B) BJAB, BC-1, and BCBL-1 cells were transduced with a mixture of the control and indicator viruses, and dual luciferase assays were performed 24 h later. RLuc activities were normalized to the observed FLuc activity. Normalized RLuc activities in KSHV-positive BC-1 and BCBL-1 cells are shown relative to those obtained in KSHV-negative BJAB cells. Values from cells transduced with the parental RLuc-expressing vector were set at 100%. LTR, long terminal repeat.
To assess the biological activity of the various KSHV miRNAs, we constructed an indicator vector for each KSHV miRNA by the insertion of two perfectly complementary target sites. The parental RLuc vector, lacking any inserted target sites, served as a negative control (Fig. 1A). Production of vesicular stomatitis virus G pseudotyped lentiviral vector particles was carried out as described previously (21), and virus preparations from each of the 13 RLuc expression vectors were mixed with an aliquot of the FLuc control virus. These mixed virus stocks were then used to infect the KSHV-negative B-cell line BJAB as well as the latently KSHV-infected B-cell lines BC-1 and BCBL-1. At 24 h after transduction, dual luciferase assays were performed. Based on control transductions with a green fluorescent protein (GFP)-expressing vector, we estimate transduction efficiencies to be >90% in the case of BJAB and ∼10% in the case of BC-1 and BCBL-1 cells (data not shown). The ratios of RLuc activities to FLuc activities obtained for BC-1 and BCLB-1 cells were normalized to the ratio obtained using the control RLuc vector that did not contain any target sequences and to the ratios obtained in BJAB cells (Fig. 1B). Phenotypically, the KSHV miRNAs fell into three groups. miR-K1, miR-K3, and miR-K4-3p reduced the expression of RLuc from the corresponding target site vector by up to 10-fold. While less active, miR-K6-3p, miR-K5 in BC-1, miR-K11, and miR-K7 nevertheless induced a readily detectable inhibition (three- to fivefold) of RLuc expression, while miR-K2 induced only about a twofold inhibition. The remaining miRNAs (miR-K4-5p, miR-K5 in BCBL-1, miR-K8, miR-K9-5p, and miR-K10) induced only a modest reduction (≤40%) of RLuc activity.
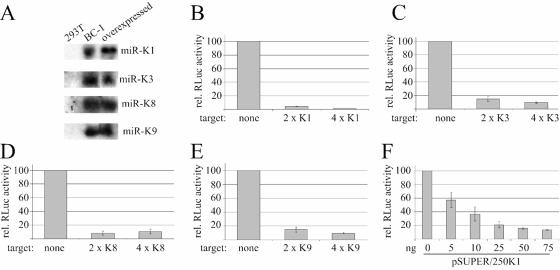
The differences between the activities measured for the various KSHV miRNAs could reflect either differences in their expression levels or inaccessibility of the target sites in a subset of the lentiviral indicator constructs. It seems unlikely, however, that some target site sequences would be readily accessible while others at the same location are not. If the difference in activity indeed reflects differences in viral miRNA expression levels, it should be possible to achieve robust reporter gene inhibition upon overexpression of the relevant miRNA in all cases. To test this, we generated expression constructs for miR-K1 and miR-K3, two miRNAs that had high levels of activity in BC-1 and BCBL-1 cells, as well as for miR-K8 and miR-K9, which were only poorly active in these cell types. In each case, we placed a ∼250-bp genomic fragment, encompassing the miRNA stem-loop at a central position, under the control of the H1 polymerase III promoter in a modified pSUPER construct (3). The miRNA signals detected by Northern blotting for 293T cells transfected with these constructs were comparable to those of BC-1 cells (Fig. 2A). However, since less than 60% of the 293T cells were transfected (estimated using GFP control transfection), the expression levels of the miRNAs in 293T cells are presumably higher, on a per-cell basis, than those in BC-1 cells.
FIG. 2.
Overexpression of selected KSHV miRNAs results in enhanced reporter gene repression. (A) Northern analysis of miR-K1, miR-K3, miR-K8, and miR-K9 expression in total RNA from untransfected 293T cells (left lane), BC-1 cells, and 293T cells transfected with the relevant miRNA expression construct (right lane). (B to E) Reporter assay using 293T cells cotransfected with the indicated RLuc target site vector, the control FLuc vector, and expression constructs for miRK-1 (B), miR-K3 (C), miR-K8 (D), or miR-K9 (E). (F) Effect of different amounts of a transfected miR-K1 expression construct (pSUPER/250K1) on indicator activity. In this case, six-well plates were transfected with 12.5 ng of each luciferase vector and the indicated amounts of pSUPER/250K1. The total amount of transfected plasmid was adjusted by adding pSUPER to 2 μg total DNA.
To test miRNA activity, we cotransfected the miRNA expression constructs with the FLuc control vector as well as RLuc vectors containing zero, two, or four perfectly complementary target sites for each miRNA in the 3′UTR of the RLuc gene. Dual luciferase assays were carried out 3 days after transfection. As predicted, we saw robust target downregulation in all cases. While miR-K1 was still the most active miRNA (Fig. 2B), we now achieved levels of target gene repression for miR-K8 and miR-K9 that were comparable to that of miR-K3 (Fig. 2C and D), which was much more active in BC-1 and BCBL-1 cells (Fig. 1B). This finding strongly suggests that miR-K8 and miR-K9 are not expressed at sufficiently high levels in BC-1 and BCBL-1 cells to induce efficient inhibition of reporter gene expression. It is also evident from this experiment that four perfectly matched target sites in the 3′UTR of the RLuc gene do not result in significantly greater inhibition of target gene expression than do two target sites. This is consistent with RLuc downregulation by mRNA cleavage, which is believed to require only one perfectly matched target site, while downregulation by translational repression may require several copies of imperfectly matched target sequences (10). To confirm that the observed RLuc activity indeed correlates with the level of miRNA expression, we performed a dose-response experiment by transfecting different amounts of an miR-K1 expression plasmid, pSUPER/250 K1 (Fig. 2F). Within a concentration range of 5 to 50 ng pSUPER/250K1 per six-well dish, the RLuc activity was found to be approximately linearly related to the plasmid input.
miR-K5 is differentially expressed in BC-1 and BCBL-1 cells.
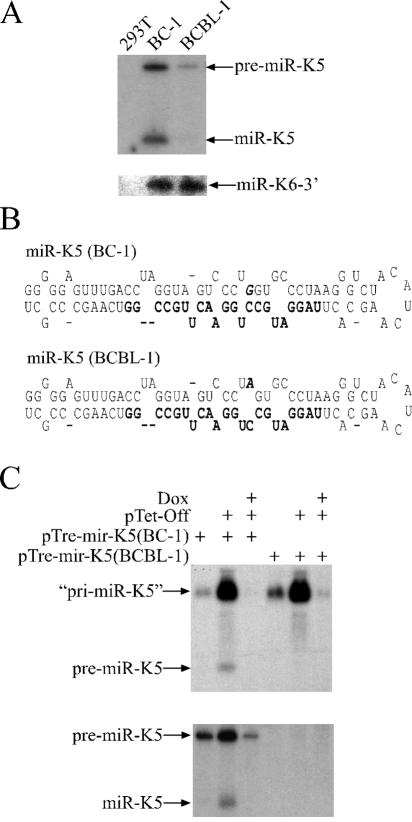
miR-K5 reduced reporter gene expression by ∼80% in BC-1 cells, while repression was much more moderate (∼35%) in BCBL-1 cells (Fig. 1B). If our target site assay accurately reflects the activity and/or expression level of viral miRNAs, one would predict that miR-K5 is less abundant in BCBL-1 cells than in BC-1 cells or that its activity is selectively inhibited by some other mechanism. We performed Northern analysis to compare miR-K5 expression levels in BC-1 and BCBL-1 cells and also probed miR-K6-3p expression as a control (Fig. 3A). Clearly, miR-K5 expression was much lower in BCBL-1 cells than in BC-1 cells, while levels of miR-K6-3p were virtually identical. The relative abundance of pre-miR-K5, which was also detected, mirrored that of the mature miR-K5. Therefore, the results obtained in the target site assay indeed accurately reflect the expression level of miR-K5 in both cell types.
FIG. 3.
Differential expression of miR-K5 in BC-1 and BCBL-1 cells. (A) Northern analysis of miR-K5 (upper panel) and miR-K6-3p expression in total RNA preparations from 293T cells, BC-1 cells, and BCBL-1 cells. (B) Predicted (Mfold) pre-miR-K5 stem-loop structures encoded by BC-1-derived (upper panel) or BCBL-1-derived (lower panel) KSHV. The mature miR-K5 sequence is shown in boldface type, while the single nucleotide difference is shown in boldface type and italics. (C) Northern analysis of total RNA preparations from 293T cells transfected with the indicated plasmids and cultured in the presence or absence of doxycycline (Dox). The miR-K5 pri-miRNA and pre-miRNA precursors as well as mature miR-K5 are indicated. The quotation marks flanking “pri-miR-K5” signify that this RNA represents a truncated form of the authentic viral pri-miR-K5 transcript.
Sequence analysis of the genomic context of the miR-K5 precursor indicated a single nucleotide polymorphism in the stem-loop structure of the miR-K5 precursor in BCBL-1 cells. This change is located in the predicted stem sequence of the pre-miR-K5 precursor, opposite the mature miR-K5, and is predicted to introduce an A:C mismatch in place of a G:C base pair. This is expected to result in an enlarged RNA bulge compared to the homologous pri-miR-K5 stem-loop structure found in the KSHV isolate present in BC-1 cells (Fig. 3B). Conceivably, the presence of this larger bulge might inhibit the processing of the miR-K5 pri-miRNA precursor by Drosha or the efficiency of nuclear export of the pre-miRNA or might alter pre-miRNA stability.
To test whether Drosha cleavage is indeed reduced, we cloned a ∼470-bp fragment of the KSHV genome encompassing the miR-K5 precursor from both BC-1 cells and BCBL-1 cells and expressed this fragment under the control of a tetracycline-responsive promoter element. These constructs were transfected into 293T cells and examined by Northern analysis for expression of the primary transcript, pre-miR-K5, and mature miR-K5. As expected, we detected high levels of pri-miRNA only when the pTet-Off transactivator was coexpressed and in the absence of doxycycline, thus providing a control for specificity (Fig. 3C). Importantly, the BC-1- or BCBL-1-derived expression constructs yielded comparable levels of the predicted primary miR-K5 transcript (Fig. 3C, upper panel). In contrast, the pre-miR-K5 precursor and mature miR-K5 were readily detected in cells transfected with the BC-1-derived KSHV sequence but not in cells transfected with the BCBL-1-derived construct (Fig. 3C, lower panel).
The lack of pre-miR-K5 expression in BCBL-1 cells could represent either a block in pri-miRNA processing by Drosha or reduced pre-miRNA stability (e.g., due to a defect in recognition by its nuclear export receptor, exportin 5). To address whether Drosha processing directly contributed to the observed phenotype, we subjected in vitro-transcribed pri-miR-K5 substrates, obtained from either the BC-1- or BCBL-1-derived KSHV genome, to processing using recombinant Drosha/DGCR8. This experiment (Fig. 4) essentially recapitulated the difference seen in RNA preparations from BC-1 and BCBL-1 cells or upon overexpression of the pTre-miR-K5 expression constructs. While the BC-1-derived substrate yielded readily detectable levels of pre-miR-K5, this was clearly not the case for the analogous BCBL-1-derived substrate. Therefore, subtle differences in the RNA structure adopted by the miR-K5 RNA stem-loop can modulate Drosha processing efficiency and thereby affect the level of miRNA expression. This is the first report of a naturally occurring polymorphism in an miRNA precursor stem-loop that modulates the level of expression of the mature miRNA.
FIG. 4.
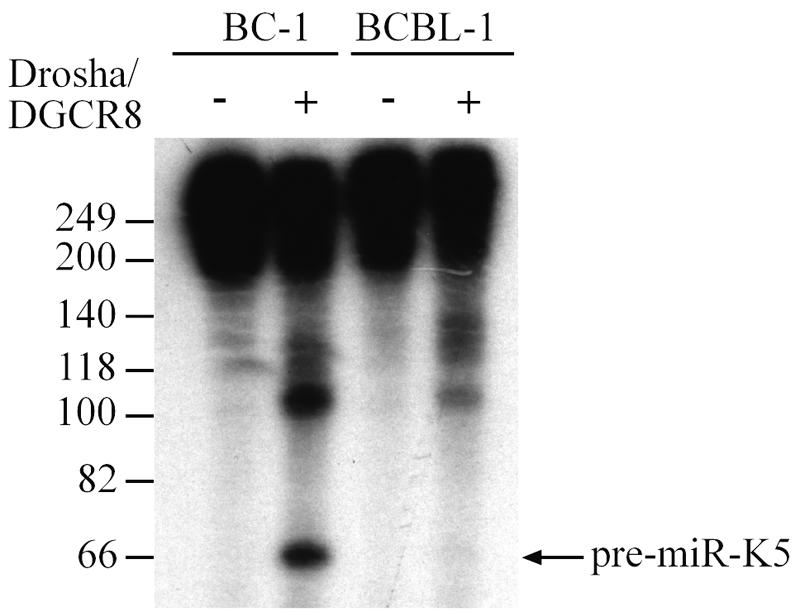
The BCBL-1-derived miR-K5 precursor is deficient in Drosha processing. BC-1- or BCBL-1-derived pri-miR-K5 transcripts were incubated in vitro with bead-bound Flag-Drosha/Flag-DGCR-8 (+) or beads incubated with extracts prepared from GFP-transfected cells (−). The processed pre-miRNA is marked by an arrow.
We conducted a BLAST search using the pre-miR-K5 sequence from the KSHV genome contained in BCBL-1 as input. Interestingly, the same sequence found in BCBL-1 was also present in the only two other previously published KSHV sequences encompassing this region, both derived from Kaposi's sarcoma biopsies (GenBank accession numbers AF148805 and U93872). We also extended our analysis to another latently Epstein-Barr/KSHV-coinfected cell line, JSC-1 (6). The biological activity of miR-K5 in JSC-1 cells was comparable to that in BCBL-1 cells (data not shown), and, consistent with this finding, sequencing of pre-miR-K5 from JSC-1 cells yielded a sequence identical to that found in BCBL-1 cells. Therefore, BC-1 cells contain the only miR-K5 sequence presently known to be fully active.
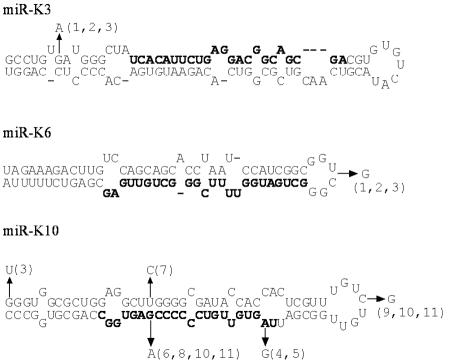
We also performed BLAST searches on the pre-miRNA sequences of all other KSHV miRNAs. For most miRNAs, only four sequences have been published. These include the BC-1 sequence (GenBank accession numbers NC_003409 and U75698), the miRNA cluster from BCBL-1 (accession number AY973824), and the two sequences from Kaposi's sarcoma biopsies mentioned above. Our analysis identified further polymorphisms in miR-K3, miR-K6, and miR-K10 (Fig. 5). In the case of miR-K3, only KSHV from BC-1 cells has a G near the base of the stem, while the remaining three sequences have an A at this position. In this case, however, the disruption of base pairing does not result in a pronounced effect on miR-K3 activity in BCBL-1 (Fig. 1B). In the case of miR-K6-3p, these same three sequences showed a 1-nt insertion in the terminal loop of the pre-miRNA. Based on previously published data, this change is not expected to affect miRNA production (34), and indeed, the levels of miR-K6-3p activity in BC-1 and BCBL-1 cells are indistinguishable (Fig. 1). For miR-K10, 15 other KSHV sequences are available in addition to the sequences mentioned above. The position of reported sequence polymorphisms is shown in Fig. 5. While a U at the base of the pre-miRNA stem may disrupt base pairing, this bulge might be too far away from the Drosha cleavage site to affect processing. The other polymorphisms in the stem actually all stabilize the stem and are therefore not predicted to negatively affect Drosha processing. As discussed above for miR-K6-3p, the sequence in the terminal loop is largely irrelevant to miRNA biogenesis. Interestingly, however, in several cases, the sequence of the mature miRNA is altered. One of these variable residues actually lies in the miRNA seed region that is expected to be most relevant for target recognition (1). While A and G might both base pair with U, it is currently unclear whether a wobble G:U base pair can function in a seed region. Notably, the previously reported sequences could also encode defective miRNAs and/or have been selected for other reasons (e.g., changes in the overlapping K12 reading frame) (5, 24).
FIG. 5.
Predicted pre-miRNA structures of BC-1-derived miR-K3 (top), miR-K6-3p (middle), and miR-K10 (bottom). Polymorphisms are indicated by arrows, and numbers refer to the accession numbers of the sequences carrying the identified nucleotides. In the case of miR-K10, a total of 18 pre-miR-K10 sequences were available. Of these sequences, nine differed from the sequence in BC-1 by at least 1 nt. GenBank accession numbers are as follows: 1, AY973824 (BCBL-1); 2, AF148805 (GK18); 3, U93872; 4, KSU66522; 5, AY157025; 6, AY042963; 7, AY042957; 8, AY042958; 9, AY042963; 10, AY042959; 11, AY042960.
In this paper, we describe a novel indicator assay in which lentivirus-based indicator vectors are delivered by transduction rather than transfection. We used this assay to show that the majority of the KSHV miRNAs are biologically active in latently KSHV-infected BC-1 and BCBL-1 cells. We predict that this assay will also be useful to evaluate the biological activity of artificially introduced miRNAs (e.g., in miRNA-expressing cell lines) or the differential expression of miRNAs in different types of primary cells, including nondividing cells such as neurons. Guided by the observed differences in the activity of miR-K5 in the indicator system, we identified a naturally occurring sequence polymorphism in the passenger strand of the miR-K5 pre-miRNA precursor that leads to reduced miR-K5 expression in BCBL-1 cells due to diminished Drosha cleavage. While our group previously showed that the artificial enlargement of an internal bulge in a pre-miRNA stem-loop can abolish miRNA expression (34), the example of KSHV miR-K5 shows that subtle changes within a pre-miRNA precursor can greatly affect the level of mature miRNA expression. Moreover, these polymorphisms can clearly arise naturally. Since the mRNA targets and biological functions of miR-K5 remain unknown, the consequences of reduced miR-K5 expression for KSHV replication and pathogenesis are unclear. While it is possible that miR-K5 function is dispensable, it is also possible that the loss of miR-K5 expression might account for differences in the properties of BCBL-1 cells compared to those of BC-1, such as the higher rate of spontaneous KSHV lytic replication in BCBL-1 cells (5, 7, 28).
As in the case of the viral miRNA miR-K5, it is easy to imagine that subtle sequence changes in cellular miRNA precursors, produced either by mutation or by RNA editing, might also either inhibit or enhance mature miRNA expression and hence affect the progression of diseases such as cancer.
Acknowledgments
This research was supported by National Institutes of Health grant GM071408 awarded to B.R.C. and by a Feodor Lynen research fellowship from the Alexander von Humboldt Foundation to E.G.
We thank V. Narry Kim and R. Shiekhattar for plasmids used in the research.
REFERENCES
- 1.Bartel, D. P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281-297. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein, E., A. A. Caudy, S. M. Hammond, and G. J. Hannon. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363-366. [DOI] [PubMed] [Google Scholar]
- 3.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 4.Cai, X., C. H. Hagedorn, and B. R. Cullen. 2004. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 10:1957-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, X., S. Lu, Z. Zhang, C. M. Gonzalez, B. Damania, and B. R. Cullen. 2005. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc. Natl. Acad. Sci. USA 102:5570-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon, J. S., D. Ciufo, A. L. Hawkins, C. A. Griffin, M. J. Borowitz, G. S. Hayward, and R. F. Ambinder. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J. Virol. 74:10187-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, S. R., C. Bloomer, and B. Chandran. 1998. Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology 240:118-126. [DOI] [PubMed] [Google Scholar]
- 8.Cullen, B. R. 2004. Transcription and processing of human microRNA precursors. Mol. Cell 16:861-865. [DOI] [PubMed] [Google Scholar]
- 9.Denli, A. M., B. B. Tops, R. H. Plasterk, R. F. Ketting, and G. J. Hannon. 2004. Processing of primary microRNAs by the microprocessor complex. Nature 432:231-235. [DOI] [PubMed] [Google Scholar]
- 10.Doench, J. G., C. P. Petersen, and P. A. Sharp. 2003. siRNAs can function as miRNAs. Genes Dev. 17:438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filipowicz, W. 2005. RNAi: the nuts and bolts of the RISC machine. Cell 122:17-20. [DOI] [PubMed] [Google Scholar]
- 12.Gregory, R. I., K. P. Yan, G. Amuthan, T. Chendrimada, B. Doratotaj, N. Cooch, and R. Shiekhattar. 2004. The microprocessor complex mediates the genesis of microRNAs. Nature 432:235-240. [DOI] [PubMed] [Google Scholar]
- 13.Grey, F., A. Antoniewicz, E. Allen, J. Saugstad, A. McShea, J. C. Carrington, and J. Nelson. 2005. Identification and characterization of human cytomegalovirus-encoded microRNAs. J. Virol. 79:12095-12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grishok, A., A. E. Pasquinelli, D. Conte, N. Li, S. Parrish, I. Ha, D. L. Baillie, A. Fire, G. Ruvkun, and C. C. Mello. 2001. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106:23-34. [DOI] [PubMed] [Google Scholar]
- 15.Hammond, S. M., E. Bernstein, D. Beach, and G. J. Hannon. 2000. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293-296. [DOI] [PubMed] [Google Scholar]
- 16.Han, J., Y. Lee, K. H. Yeom, Y. K. Kim, H. Jin, and V. N. Kim. 2004. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 18:3016-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutvagner, G. 2005. Small RNA asymmetry in RNAi: function in RISC assembly and gene regulation. FEBS Lett. 579:5850-5857. [DOI] [PubMed] [Google Scholar]
- 18.Hutvagner, G., J. McLachlan, A. E. Pasquinelli, E. Balint, T. Tuschl, and P. D. Zamore. 2001. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293:834-838. [DOI] [PubMed] [Google Scholar]
- 19.Hutvagner, G., and P. D. Zamore. 2002. A microRNA in a multiple-turnover RNAi enzyme complex. Science 297:2056-2060. [DOI] [PubMed] [Google Scholar]
- 20.Landthaler, M., A. Yalcin, and T. Tuschl. 2004. The human DiGeorge syndrome critical region gene 8 and its D. melanogaster homolog are required for miRNA biogenesis. Curr. Biol. 14:2162-2167. [DOI] [PubMed] [Google Scholar]
- 21.Lee, M. T., G. A. Coburn, M. O. McClure, and B. R. Cullen. 2003. Inhibition of human immunodeficiency virus type 1 replication in primary macrophages by using Tat- or CCR5-specific small interfering RNAs expressed from a lentivirus vector. J. Virol. 77:11964-11972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, Y., C. Ahn, J. Han, H. Choi, J. Kim, J. Yim, J. Lee, P. Provost, O. Radmark, S. Kim, and V. N. Kim. 2003. The nuclear RNase III Drosha initiates microRNA processing. Nature 425:415-419. [DOI] [PubMed] [Google Scholar]
- 23.Lee, Y., M. Kim, J. Han, K. H. Yeom, S. Lee, S. H. Baek, and V. N. Kim. 2004. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 23:4051-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis, B. P., C. B. Burge, and D. P. Bartel. 2005. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15-20. [DOI] [PubMed] [Google Scholar]
- 25.Lund, E., S. Guttinger, A. Calado, J. E. Dahlberg, and U. Kutay. 2004. Nuclear export of microRNA precursors. Science 303:95-98. [DOI] [PubMed] [Google Scholar]
- 26.Pfeffer, S., A. Sewer, M. Lagos-Quintana, R. Sheridan, C. Sander, F. A. Grasser, L. F. van Dyk, C. K. Ho, S. Shuman, M. Chien, J. J. Russo, J. Ju, G. Randall, B. D. Lindenbach, C. M. Rice, V. Simon, D. D. Ho, M. Zavolan, and T. Tuschl. 2005. Identification of microRNAs of the herpesvirus family. Nat. Methods 2:269-276. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer, S., M. Zavolan, F. A. Grasser, M. Chien, J. J. Russo, J. Ju, B. John, A. J. Enright, D. Marks, C. Sander, and T. Tuschl. 2004. Identification of virus-encoded microRNAs. Science 304:734-736. [DOI] [PubMed] [Google Scholar]
- 28.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 29.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samols, M. A., J. Hu, R. L. Skalsky, and R. Renne. 2005. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:9301-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan, C. S., A. T. Grundhoff, S. Tevethia, J. M. Pipas, and D. Ganem. 2005. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature 435:682-686. [DOI] [PubMed] [Google Scholar]
- 32.Yi, R., Y. Qin, I. G. Macara, and B. R. Cullen. 2003. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 17:3011-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng, Y., and B. R. Cullen. 2005. Efficient processing of primary microRNA hairpins by Drosha requires flanking nonstructured RNA sequences. J. Biol. Chem. 280:27595-27603. [DOI] [PubMed] [Google Scholar]
- 34.Zeng, Y., and B. R. Cullen. 2003. Sequence requirements for micro RNA processing and function in human cells. RNA 9:112-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng, Y., and B. R. Cullen. 2004. Structural requirements for pre-microRNA binding and nuclear export by exportin 5. Nucleic Acids Res. 32:4776-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng, Y., E. J. Wagner, and B. R. Cullen. 2002. Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol. Cell 9:1327-1333. [DOI] [PubMed] [Google Scholar]
- 37.Zeng, Y., R. Yi, and B. R. Cullen. 2003. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl. Acad. Sci. USA 100:9779-9784. [DOI] [PMC free article] [PubMed] [Google Scholar]