Abstract
The koala retrovirus (KoRV) is a gammaretrovirus closely related to the gibbon ape leukemia virus and induces leukemias and immune deficiencies associated with opportunistic infections, such as chlamydiosis. Here we characterize a KoRV newly isolated from an animal in a German zoo and show infection of human and rat cell lines in vitro and of rats in vivo, using immunological and PCR methods for virus detection. The KoRV transmembrane envelope protein (p15E) was cloned and expressed, and p15E-specific neutralizing antibodies able to prevent virus infection in vitro were developed. Finally, evidence for immunosuppressive properties of the KoRV was obtained.
Retroviruses have long been known to be capable of infecting new host species by transspecies transmission; human immunodeficiency viruses (HIV) types 1 and 2 are the products of such a transspecies transmission (12, 13). The koala retrovirus (KoRV) is an example of a recent transspecies transmission and endogenization, whereas the closely related gibbon ape leukemia virus (GaLV) remains exogenous in gibbons. Although both viruses are related to endogenous retroviruses of South East Asian mice (18), the transmission routes are still unknown. Koalas in Australia as well as in many zoos suffer from leukemia (1, 14) and from infections such as chlamydiosis (2). Since Chlamydia infections are characteristically opportunistic infections commonly associated with retroviral infections, such as HIV and feline immunodeficiency virus (4, 5, 20), it is likely that KoRV, as with many other retroviruses, is able to induce immunosuppression.
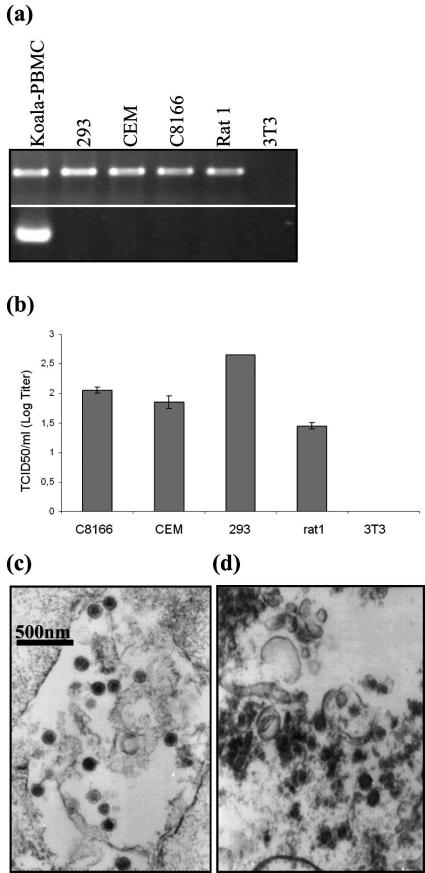
A new KoRV isolate was obtained from mitogen-stimulated peripheral blood mononuclear cells (PBMCs) of a healthy male animal from the Duisburg Zoo, Duisburg, Germany (designated KoRV Duisburg-Berlin [KoRVD-B]). This strategy has been used previously to isolate porcine endogenous retroviruses (PERVs) from pig PBMCs (24). To study the host range of KoRV, human 293 kidney cells and the human T lymphocyte lines C8166 and CEM, as well as rat and mouse fibroblasts (rat1 and NIH 3T3, respectively), were used. Provirus integration was shown by PCR in all cell lines except NIH 3T3 (Fig. 1a), and the release of infectious virus was shown by the titration of cell-free supernatant on uninfected 293 (Fig. 1b) or C8166 cells. In addition, virus production was demonstrated by electron microscopy (Fig. 1c and d). Whereas virus particles produced after three passages on human 293 cells were characterized by a uniform morphology, particles from a lymphoma from an animal at the Antwerp Zoo (Fig. 1d) showed pleomorphic particles as described previously for the cells of a leukemic koala (26).
FIG. 1.
Infection studies with KoRV. (a) Detection of provirus in different cell lines before and after infection with KoRV using PCR. PBMCs from a healthy koala were taken as the positive control. (b) Fifty percent tissue culture infective dose (TCID50) values (means and standard deviations) of KoRV produced from different cell lines and titrated on human 293 cells. (c) Transmission electron microscopy of KoRV produced by human 293 kidney cells and (d) KoRV particles in koala lymphoma cells.
The entire env gene (p15E and gp70) was amplified from the DNA of the animal from which KoRVD-B was isolated and sequenced. The sequence revealed an almost perfect match (substitutions at positions 408 [Arg to His], 459 [Ser to Pro], and 647 [Arg to Lys]) to the sequence of an Australian animal described previously (14). When the sequences of the ectodomains of p15E amplified from the DNA of one other healthy animal and one diseased animal were compared, no differences were found.
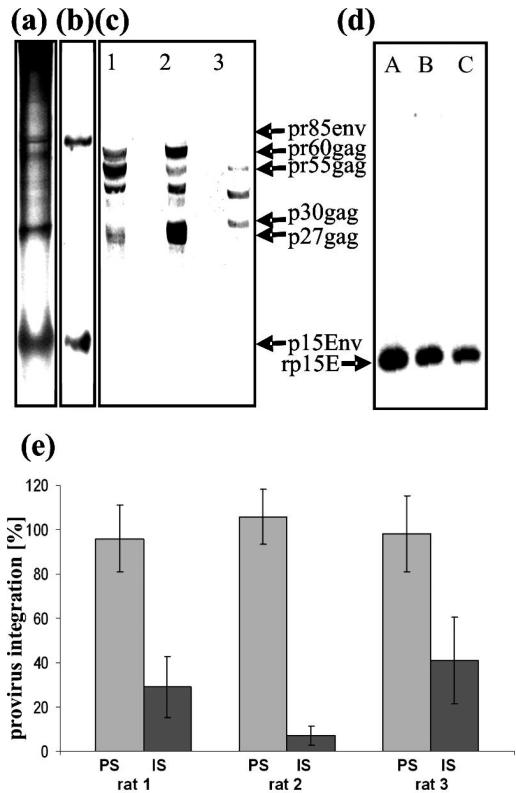
Biochemical and immunological characterizations of KoRVD-B were performed using virus produced by 293 cells, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (for the major viral proteins, see Fig. 2a), and Western blot analysis with two antisera. One antiserum was generated by immunizing with the recombinant ectodomain of p15E of KoRV (Fig. 2b), and the other was specific for p27Gag of PERV (15) and cross-reacted with p27Gag of KoRV (Fig. 2c). In contrast to murine leukemia viruses (MuLV), which contain p30Gag, PERV (25) and KoRV (Fig. 2c) contain a p27Gag.
FIG. 2.
Biochemical and immunological characterization of KoRV. (a) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of sucrose gradient-purified KoRV produced by human 293 cells with Coomassie blue staining. (b) Western blot analysis of the same material using a rat antiserum specific for KoRV p15E that recognized p15E and the precursor gp85. (c) Western blot analysis of cell lysates from human 293 cells producing KoRV from (lane 1) human 293 cells producing PERV and (lane 2) mouse NIH 3T3 cells producing MuLV and (lane 3) using a goat antiserum specific for PERV p27Gag. (d) Western blot analyses using recombinant p15E of KoRV as antigens and sera from three different rats (A, B, C) immunized with recombinant p15E (rp15E) of KoRV. (e) Neutralizing activity of rat sera (immune sera [IS]) in comparison to that of preimmune sera (PS) at a dilution of 1:8. Infection was measured as the percentage of provirus integration using real-time PCR. Results are expressed as means and standard deviations.
The p15E-specific antiserum was shown to neutralize KoRV in a neutralization assay based on the use of 293 cells and measurement of infection as provirus integration with real-time PCR. The inhibition of provirus integration by between 60 and 95% was detected at a serum dilution of 1:8 (Fig. 2e). This strategy may provide the basis for a vaccine preventing KoRV replication, as described previously for PERV (11) and feline leukemia virus (FeLV) (16, 17).
As it was possible to productively infect rat cells with KoRV in vitro (Fig. 1a and b), Wistar rats were inoculated with cell-free KoRV (grown either on rat1 cells or 293 cells) or with KoRV-producing rat1 cells. Eleven of 12 animals were positive for p15E-specific antibodies, and four animals showed high levels of provirus integration in PBMCs at day 21 (>2.5 × 105 copies/ml whole blood) (Table 1), indicating a productive infection in all 11 animals. The cell-associated virus load decreased, however, and 63 days postinoculation, no provirus was detected in the PBMCs of all inoculated rats. Despite this, coincubation of cell-free plasma and mitogen-stimulated PBMCs from these animals with 293 cells yielded infectious virus (except with rat 14). When organs (spleen, ovary, lymph node, lung, liver, and kidney) or PBMCs from two rats, 6 and 8, were analyzed for provirus integration on day 70, no KoRV sequences were detected. Whereas in 11 animals the virus load decreased after an initial peak, the situation was different for rat 14, in which KoRV-specific antibodies and provirus in PBMCs were not detected at days 21 and 63 and no infectious virus was released from mitogen-triggered PBMCs. However, 200 days after inoculation, rat 14 developed a fibrosarcoma on its back, and high levels of proviral DNA were detected in the tumor as well as all organs investigated (spleen, lymph node, lung, liver, kidney whole blood, and spinal cord), indicating that, finally, all 12 animals were infected. Further investigations are needed to clarify whether the KoRV caused this tumor.
TABLE 1.
In vivo infection of rats with three different sources of KoRV
| Animal | Virus source | p15E-specific Ab detectiona | Provirus detection onb:
|
Virus isolation on day 42/63c | |
|---|---|---|---|---|---|
| Day 21 | Day 63 | ||||
| 4 | Cell-free supernatant | + | − | − | + |
| 5 | from KoRV- | + | − | − | + |
| 6 | infected 293 cells | + | + | − | + |
| 7 | + | − | − | + | |
| 8 | Cell-free supernatant | + | + | − | + |
| 9 | from KoRV- | + | − | − | + |
| 10 | infected rat1 cells | + | − | − | + |
| 11 | + | − | − | + | |
| 12 | KoRV-infected rat1 | + | − | − | + |
| 13 | cells | + | + | − | + |
| 14 | − | − | − | − | |
| 15 | + | + | − | + | |
Antibodies (Ab) specific for p15E were detected by Western blot assay.
Provirus was measured in PBMC by PCR.
Virus was isolated by incubating plasma and PBMCs from infected rats with human 293 cells and measuring subsequent provirus integration.
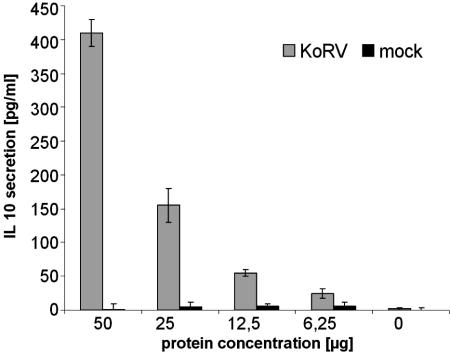
Many retroviruses, including HIV, MuLV, and FeLV, induce immunosuppression in the infected host (7), leading to opportunistic infections. Although the mechanism of the immunopathogenesis of these retroviruses is still unclear, there are indications that the highly conserved so-called immunosuppressive domains of their transmembrane envelope proteins are involved (8). The sequences of the immunosuppressive domains are identical for KoRV, MuLV, and FeLV (3, 7). Purified viruses, specifically their transmembrane envelope proteins, as well as synthetic peptides corresponding to immunosuppressive domains of HIV and of FeLV (and therefore automatically also of KoRV), inhibited lymphocyte proliferation and modulated cytokine production, e.g., by an increase in interleukin-10 (IL-10) production (3, 7, 9). When sucrose gradient-purified KoRV produced from 293 cells was incubated with PBMCs from healthy human blood donors, an increased production of IL-10 was observed (Fig. 3). Using a cytokine array, elevated levels of IL-10, growth-related oncogene GRO, IL-6, and MCP-1 expression were found, while the expression of 18 other cytokines remained unchanged. Similar changes have been observed with HIV and FeLV, both of which induce immunodeficiencies in vivo (7).
FIG. 3.
Influence of purified KoRV and mock preparations on IL-10 production. Supernatants from 293 cells producing KoRV and from uninfected 293 cells (mock) were ultracentrifuged, and pellets were incubated overnight at different concentrations with human PBMCs from healthy donors. IL-10 was measured in the supernatant using an enzyme-linked immunosorbent assay, and results are expressed as means (and standard deviations) after subtracting the concentration of IL-10 released by untreated cells.
This report shows that KoRV, like its nearest relative GaLV, as well as PERV (23), is able to infect a variety of human cells. These data confirm recently published findings showing infection of human, rat, hamster, and bovine cells by KoRV (21). In contrast to PERV, which does not infect rat cells (22), KoRV productively infected rat cells in vitro (Fig. 1a and b). However, both PERV (15) and KoRV (Fig. 1a and b) did not infect mouse cells, and in the case of PERV, the absence of a specific receptor was demonstrated (10). Whereas, for lentiviruses, experimental transspecies transmissions have been reported repeatedly (7), this is the first report showing an experimental transspecies transmission of a gammaretrovirus in vivo. KoRV infection of rats represents an animal model that allows study of the efficacies of vaccines, based on neutralizing antibodies induced by immunization with p15E and gp70 of KoRV, as well as of antiviral drugs under in vivo conditions.
GaLV, as the nearest relative of KoRV, is an example of a natural transspecies transmission (18, 19). The fact that gibbons and koalas live on different continents suggests that the zoonosis may have involved an intermediate vector. The transfer probably occurred recently, because the level of sequence divergency between GaLV and KoRV is similar to that between two different strains of GaLV, SEATO and SF (6). Although it has been suggested that GaLV originated from South East Asian mice, such as Mus caroli (18), it is unlikely that the KoRV came from the same source in recent times. The evidence presented here that rats can be infected in vivo with KoRV may suggest a possible mode of transmission.
Protein structure accession number.
The nucleotide sequence of the entire env gene (p15E and gp70) from the animal from which KoRVD-B was isolated has been submitted to GenBank under accession no. DQ174772.
Acknowledgments
We thank Francis Vercammen, Royal Zoological Society of Antwerp, for material from diseased koalas and S. Klein, M. Lau, M. Pack, and F. Kaulbars for technical assistance.
REFERENCES
- 1.Booth, R. J., and W. H. Blanshard. 1999. Diseases of koalas, p. 321-333. In M. E. Fowler and R. E. Miller (ed.), Zoo and wild animal medicine: current therapy. W. B. Saunders, Philadelphia, Pa.
- 2.Brown, A. S., A. A. Girjes, M. F. Lavin, P. Timms, and J. B. Woolcock. 1987. Chlamydial disease in koalas. Aust. Vet. J. 64:346-350. [DOI] [PubMed] [Google Scholar]
- 3.Cianciolo, G. J., T. D. Copeland, S. Oroszlan, and R. Snyderman. 1985. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science 230:453-455. [DOI] [PubMed] [Google Scholar]
- 4.Comandini, U. V., P. Maggi, P. Santopadre, R. Monno, G. Angarano, and V. Vullo. 1997. Chlamydia pneumoniae respiratory infections among patients infected with the human immunodeficiency virus. Eur. J. Clin. Microbiol. Infect. Dis. 16:720-726. [DOI] [PubMed] [Google Scholar]
- 5.Contini, C. 2003. Molecular identification and antibody testing of Chlamydophila pneumoniae in a subgroup of patients with HIV-associated dementia complex. Preliminary results. J. Neuroimmunol. 136:172-177. [DOI] [PubMed] [Google Scholar]
- 6.Delassus, S., P. Sonigo, and S. Wain-Hobson. 1989. Genetic organization of gibbon ape leukemia virus. Virology 173:205-213. [DOI] [PubMed] [Google Scholar]
- 7.Denner, J. 1998. Immunosuppression by retroviruses: implications for xenotransplantation. Ann. N. Y. Acad. Sci. 862:75-86. [DOI] [PubMed] [Google Scholar]
- 8.Denner, J. 2000. How does HIV induce AIDS? The virus protein hypothesis. J. Hum. Virol. 3:81-82. [PubMed] [Google Scholar]
- 9.Denner, J., S. Norley, and R. Kurth. 1994. The immunosuppressive peptide of HIV-1: functional domains and immune response in AIDS patients. AIDS 8:1063-1072. [PubMed] [Google Scholar]
- 10.Ericsson, T. A. 2003. Identification of receptors for pig endogenous retrovirus. Proc. Natl. Acad. Sci. USA 27:6759-6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiebig, U., O. Stephan, R. Kurth, and J. Denner. 2003. Neutralizing antibodies against conserved domains of p15E of porcine endogenous retroviruses (PERVs): basis for a vaccine for xenotransplantation? Virology 307:406-413. [DOI] [PubMed] [Google Scholar]
- 12.Gao, F., L. Yue, D. C. Robertson, S. C. Hill, H. Hui, R. J. Biggar, A. E. Neequaye, T. M. Whelan, D. D. Ho, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J. Virol. 68:7433-7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, F. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397:436-441. [DOI] [PubMed] [Google Scholar]
- 14.Hanger, J. J., L. D. Bromham, J. J. McKee, T. M. O'Brien, and W. F. Robinson. 2000. The nucleotide sequence of koala (Phascolarctos cinereus) retrovirus: a novel type C endogenous virus related to gibbon ape leukemia virus. J. Virol. 74:4264-4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irgang, M., A. Karlas, C. Laue, V. Specke, S. J. Tacke, R. Kurth, J. Schrezenmeir, and J. Denner. 2005. Porcine endogenous retroviruses PERV-A and PERV-B do not infect mouse cells in vitro and SCID mice in vivo. Intervirology 48:167-173. [DOI] [PubMed] [Google Scholar]
- 16.Langhammer, S., J. Hübner, R. Kurth, and J. Denner. 2005. Antibodies neutralizing feline leukemia virus (FeLV) in cats immunized with the transmembrane envelope protein p15E. Immunology 117:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langhammer, S., U. Fiebig, R. Kurth, and J. Denner. 2005. Neutralising antibodies against the transmembrane protein of feline leukaemia virus (FeLV). Vaccine 23:3341-3348. [DOI] [PubMed] [Google Scholar]
- 18.Lieber, M. M. 1975. Isolation from the Asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc. Natl. Acad. Sci. USA 72:2315-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin, J., E. Herniou, J. Cook, R. W. O'Neill, and M. Tristem. 1999. Interclass transmission and phyletic host tracking in murine leukemia virus-related retroviruses. J. Virol. 73:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Dair, H. A., C. D. Hopper, T. J. Gruffydd-Jones, D. A. Harbour, and L. Waters. 1994. Clinical aspects of Chlamydia psittaci infection in cats infected with feline immunodeficiency virus. Vet. Rec. 134:365-368. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira, N. M., K. B. Farrell, and M. V. Eiden. 2006. In vitro characterization of a koala retrovirus. J. Virol. 80:3104-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Specke, V., S. Tacke, K. Boller, J. Schwendemann, and J. Denner. 2001. Porcine endogenous retroviruses (PERVs): in vitro host range and attempts to establish small animal models. J. Gen. Virol. 82:837-844. [DOI] [PubMed] [Google Scholar]
- 23.Specke, V., S. Rubant, and J. Denner. 2001. Productive infection of human primary cells and cell lines with porcine endogenous retroviruses (PERVs). Virology 285:177-180. [DOI] [PubMed] [Google Scholar]
- 24.Tacke, S. J., R. Kurth, and J. Denner. 2000. Porcine endogenous retroviruses inhibit human immune cell function: risk for xenotransplantation? Virology 268:87-93. [DOI] [PubMed] [Google Scholar]
- 25.Tacke, S. J., K. Bodusch, A. Berg, and J. Denner. 2001. Sensitive and specific detection methods for porcine endogenous retroviruses applicable to experimental and clinical xenotransplantation. Xenotransplantation 8:125-135. [PubMed] [Google Scholar]
- 26.Tarlinton, R., J. Meers, J. Hanger, and P. Young. 2005. Real-time reverse transcriptase PCR for the endogenous koala retrovirus reveals an association between plasma viral load and neoplastic disease in koalas. J. Gen. Virol. 86:783-787. [DOI] [PubMed] [Google Scholar]