Abstract
The Bombyx mori nucleopolyhedrovirus (BmNPV) encodes a gene homologous to the mammalian fibroblast growth factor (FGF) family. We report the cloning of B. mori and Spodoptera frugiperda orthologous genes (Bmbtl and Sfbtl, respectively) of Drosophila melanogaster breathless (btl) encoding a receptor for Branchless/FGF and show that these genes encode the receptor for a baculovirus-encoded FGF (vFGF). Sequence analysis showed that BmBtl is composed of 856 amino acid residues, which potentially encodes a 97.3-kDa polypeptide and shares structural features and sequence similarities with the FGF receptor family. Reverse transcription-PCR experiments showed that Bmbtl was abundantly expressed in the trachea and midgut in B. mori larvae, with moderate expression observed in the hemocytes and the B. mori cultured cell line BmN. We generated Sf-9 cells that stably expressed His-tagged BmBtl. Western blot analysis revealed that BmBtl was an ∼110-kDa protein. Immunoprecipitation experiments showed that BmNPV vFGF markedly phosphorylated BmBtl in Sf-9 cells. In addition, we found that BmBtl overexpression enhanced the migration activity for BmNPV vFGF. Furthermore, we generated Sf-9 cells in which Sfbtl was knocked down by transfection with double-strand RNA-expressing plasmids. In these cells, cell motility triggered by vFGF was markedly reduced. These results strongly suggest that the Btl orthologs, BmBtl and SfBtl, are the receptors for vFGF, which mediate vFGF-induced host cell chemotaxis.
There are now 22 members of the fibroblast growth factor (FGF) family, with each having a conserved 120-amino-acid residue core with 30 to 70% amino acid sequence identity (9, 19). The FGFs appear to play important roles in both developing and adult tissues. The Baculoviridae is a large family of pathogens that are infectious for arthropods, particularly insects of the Lepidoptera. Nucleopolyhedroviruses (NPVs), a genus of the Baculoviridae, have a large circular, supercoiled, and double-stranded DNA genome packaged into rod-shaped virions. Recent sequence analysis has revealed the existence of FGF gene homologs in the NPV genome (2, 8). In our previous work, we cloned and characterized an FGF gene homolog (vfgf) of Bombyx mori NPV (BmNPV) (13). Transcriptional analysis showed that vfgf is one of the baculovirus early genes, although there are no consensus sequences for the baculovirus early gene promoters. vFGF of BmNPV has a hydrophobic amino terminus (16 amino acids), which is a typical signal sequence. Western blot analysis has revealed that vFGF is efficiently secreted from BmNPV-infected BmN cells. Also, we have shown that BmNPV vFGF is a glycosylated protein.
The Autographa californica NPV (AcMNPV) also encodes vfgf (2). Recently, Detvisitsakun et al. reported the properties of AcMNPV vfgf (4). Northern blot hybridization showed that AcMNPV vfgf was transcribed as a 0.6-kb mRNA at early times but as part of a 1.4-kb bicistronic mRNA at late times. These researchers also demonstrated that vFGF had a strong affinity to heparin, a property important for FGF signaling via an FGF receptor. AcMNPV vFGF was secreted into the extracellular fluid when expressed in Sf-21 cells, suggesting that it may act as an extracellular ligand. Furthermore, these authors showed that vFGF was able to stimulate migration of Spodptera Sf-21 cells, Trichoplusia ni TN-368 cells, and T. ni hemocytes.
FGFs are known to function by binding heparin or heparan sulfate proteoglycans to form oligomers, and this complex interacts specifically with cell-surface FGF receptors (FGFRs). FGFRs are the receptor-type tyrosine kinases that are activated upon FGF binding, leading to receptor dimerization and autophosphorylation. The activated FGFR then stimulates signal transduction pathways (21). To investigate the signaling cascade triggered by vFGF, we tried to identify the vFGF receptor. Since vFGF has a high homology to Drosophila Branchless/FGF, which is a ligand for Breathless (Btl) (22), we speculated that the B. mori Btl ortholog might be a receptor for vFGF. In the present study, we isolated B. mori btl ortholog (Bmbtl) and showed that the lepidopteran ortholog of Drosophila Btl is a receptor for vFGF.
MATERIALS AND METHODS
Insects, cell lines, and viruses.
B. mori larvae (p50T strain) were reared as described previously (12). The BmN-4 (BmN) and Sf-9 cell lines were cultured at 27°C in TC-100 medium supplemented with 10% fetal bovine serum. AcMNPV was propagated in Sf-9 cells, and virus titers of AcMNPV were determined by plaque assay on Sf-9 cells.
cDNA cloning and DNA sequencing.
To determine the sequence of the full-length cDNA of Bmbtl, we screened B. mori cDNA libraries (17; T. Shimada et al., unpublished data [http://www.ab.a.u-tokyo.ac.jp/Bombyx_EST]) and found a clone (FWDP26_H17) showing homology to Drosophila Btl from a wing disk cDNA library. Sequence analysis of FWDP26_H17 identified a 2,568-bp open reading frame (ORF) that potentially encodes a protein with 856 amino acids. Using the degenerate primer sets DegFGFR-F1 and DegFGFR-R1 (Table 1) with Sf-9 cDNA as a template, a partial fragment of Sfbtl was amplified by PCR. We isolated the full-length cDNA of Sfbtl (3,147 bp) from Sf-9 cDNA by rapid amplification of the cDNA ends (RACE) as described previously (3). Primers (Sf9FGFRGSP-F1 and Sf9FGFRGSP-R1) used in 5′- and 3′-RACE experiments of Sfbtl cDNA are shown in Table 1. The nucleotide sequence was determined by using the ABI Big Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems) and an ABI Prism 3100 DNA sequencer (Applied Biosystems).
TABLE 1.
Primers used in this study
| Name | Sequence (5′ to 3′)a | Purpose |
|---|---|---|
| FWDP26-1 | GGTGTGTCTAACTGTGGCAG | RT-PCR for Bmbtl |
| FWDP26-2 | CAAGATCGCCGACTTTGGTC | RT-PCR for Bmbtl |
| DegFGFR-F1 | AARGARGAYGARGGNTGGTA | Cloning for Sfbtl |
| DegFGFR-R1 | ATCATYTTCATCATYTCCATYTC | Cloning for Sfbtl |
| Sf9FGFRGSP-F1 | GTACAAGAAGCGCCAGACCATGGAAAGCACA | 3′-RACE for Sfbtl |
| Sf9FGFRGSP-R1 | CGCATTCCGCCTTGACGACCTTTCCAAAC | 5′-RACE for Sfbtl |
| dsSfbtl1F-EcoRI | AAAGAATTCAACCTTCGAGAACGTTACTG | dsRNA for Sfbtl |
| dsSfbtl1F-EcoRV | AAAGATATCAACCTTCGAGAACGTTACTG | dsRNA for Sfbtl |
| dsSfbtl1RL-XhoI | AAACTCGAGGTACTCCGACATCATCATTG | dsRNA for Sfbtl |
| dsSfbtl1RS-XhoI | AAACTCGAGGTGACCTTCTTCGTCCAATG | dsRNA for Sfbtl |
| dsSfbtl2F-EcoRI | AAAGAATTCCTGTGGTCGCTGTTAAGATG | dsRNA for Sfbtl |
| dsSfbtl2F-EcoRV | AAAGATATCCTGTGGTCGCTGTTAAGATG | dsRNA for Sfbtl |
| dsSfbtl2RL-XhoI | AAACTCGAGCTGTAATAGTCGTTGCTGTG | dsRNA for Sfbtl |
| dsSfbtl2RS-XhoI | AAACTCGAGTACACCGTCTAGAAGCTAAG | dsRNA for Sfbtl |
| SfbtlRTF | TGAAATATACATGCTGATGC | RT-PCR for Sfbtl |
| SfbtlRTR | TACTGTTCATTGCTACATCC | RT-PCR for Sfbtl |
| SfActinF | ACGATATGGAGAAGATCTGG | RT-PCR for Sfactin |
| SfActinR | ACCGGAGTCCAGCACGATAC | RT-PCR for Sfactin |
Underlined residues indicate enzyme recognition sites.
Generation of Sf-9 cells stably expressing His-tagged BmBtl.
Bmbtl was cloned into pIZ/V5-His (Invitrogen), generating plasmid BmBtl-His-pIZ. This vector possesses the ie2 promoter of Orgyia pseudotsugata NPV for the constitutive expression of the gene of interest and the zeocin-resistant gene for selection of stable cell lines. Sf-9 cells were transfected with pIZ/V5-His or BmBtl-His-pIZ by using Cellfectin reagent (Invitrogen). Two days after transfection, zeocin (final concentration, 500 μg/ml) was added into the medium. At 2 weeks after drug selection, we verified the expression of His-tagged BmBtl by Western blot analysis with anti-His antibody (QIAGEN). We used these cells in our experiments within 1 month after their establishment, since we observed that the expression of His-tagged BmBtl gradually decreased.
Western blotting and immunoprecipitation.
Western blot and immunoprecipitation experiments were performed as described previously (23). Exogenous His-tagged BmBtl was detected by using anti-His antibody (QIAGEN). Phosphorylated BmBtl or SfBtl was detected by using an anti-phospho-tyrosine antibody (4G10 [Upstate] or pY99 [Santa Cruz]).
Reverse transcription-PCR (RT-PCR).
Total RNA was prepared by using the TRIzol reagent (Invitrogen) as described previously (14). Then, 1 μg total RNA was reverse transcribed, diluted, and used for PCR as described elsewhere (10, 26). The primers for PCR are described in Table 1.
Expression and purification of the BmNPV vFGF.
Recombinant AcMNPVs were constructed by using a Bac-to-Bac system (Invitrogen). The coding region of BmNPV vFGF with a His tag sequence at the C terminus was amplified by PCR with the genomic DNA of BmNPV T3. The PCR product was cloned into the pFastBac1 vector (Invitrogen), and a bacmid containing the BmNPV vfgf was isolated. Recombinant AcMNPVs were generated by transfection with bacmid DNAs. Monolayers of Sf-9 cells cultured in a 150-mm dish were infected with a recombinant AcMNPV expressing His-tagged BmNPV vFGF at a multiplicity of infection of 5. After 72 h, the culture medium was centrifuged at 2,000 × g for 5 min, and the supernatant was collected. Protein purification was performed as reported previously (3, 4, 13). Purification of the BmNPV vFGF was performed by using nickel chromatography. The medium was concentrated, desalted by using a PD-10 column (Amersham Biosciences), and loaded onto a HiTrap column (Amersham Biosciences). Purification with a HiTrap column was performed twice. The eluant was then incubated with heparin-Sepharose 6 Fast Flow beads (Amersham Biosciences). Elution was performed with phosphate buffer containing 1.4 M NaCl, and proteins were desalted by using a PD-10 column. The protein concentration was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by staining with Coomassie brilliant blue. We verified that the control protein purified from Sf-9 cells infected with wild-type AcMNPV (this virus has an endogenous AcMNPV-encoded vFGF, but not His-tagged BmNPV vFGF) by the method described above neither activated BmBtl nor stimulated cell migration of Sf-9 cells, indicating that the recombinant BmNPV vFGF purified by this method did not contain endogenous AcMNPV vFGF.
Migration assay.
Cell migration was measured by using a 24-well Boyden chamber (Corning), as previously described (27). Cells (104 cells) in 100 μl of serum-free TC-100 medium per well were loaded into the upper chambers, which were then inserted into the tissue culture wells containing 1, 5, and 25 ng of vFGF/ml in 600 μl of serum-free TC-100 medium per well. After incubation at 27°C for 3 h, the filters were stained with Diff-Quick (Sysmex). The number of stained cells was counted in four fields per filter in two independent experiments.
Generation of Sf-9 cells in which Sfbtl was knocked down.
Plasmids used in Sfbtl-knockdown experiments were constructed as described by Tanaka et al. (24). Fragments of Sfbtl (nucleotides [nt] 987 to 1548, dsRNA1s) and 561 bp in the opposite direction of Sfbtl (nt 1412 to 987, dsRNA1as) were amplified from Bmbtl cDNA by using the primer sets dsSfbtl1F-EcoRI and dsSfbtl1RL-XhoI (for dsRNA1s) or dsSfbtl1F-EcoRV and dsSfbtl1RS-XhoI (for dsRNA1as) (Table 1). The dsRNA1s was designed to be 116 bp longer than the dsRNA1as. The EcoRI- and XhoI-digested dsRNA1s and EcoRV- and XhoI-digested dsRNA1as were inserted in tandem into the pIZ/V5-His vector after digestion with EcoRI and EcoRV (creating plasmid dsRNA1-pIZ). In the same way, we constructed dsRNA2-pIZ by using the primer sets dsSfbtl2F-EcoRI and dsSfbtl2RL-XhoI for dsRNA2s (nt 1679 to 2117) or dsSfbtl1F-EcoRV and dsSfbtl1RS-XhoI for dsRNA2as (nt 2011 to 1679) (Table 1).
We generated Sfbtl-knocked-down Sf-9 cells by transfection with dsRNA1-pIZ or dsRNA2-pIZ. At 2 days after transfection, zeocin (final concentration, 500 μg/ml) was added into the medium. Two weeks after drug selection, we examined the expression level of SfBtl by RT-PCR analysis by using the primers SfbtlF1 and SfbtlR1 (Table 1). We used these cells in the migration assay within 1 month after establishment, since we observed that the knock-down effect of Sfbtl gradually decreased.
Nucleotide sequence accession number.
The nucleotide sequences reported in the present study have been submitted to the DDBJ/EMBL/GenBank data bank under the accession numbers AB247566 (Bmbtl) and AB247567 (Sfbtl).
RESULTS
Cloning of Bmbtl and Sfbtl.
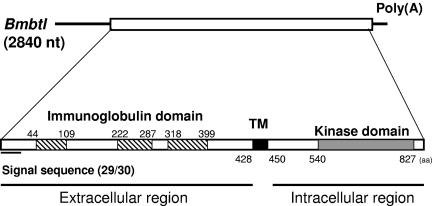
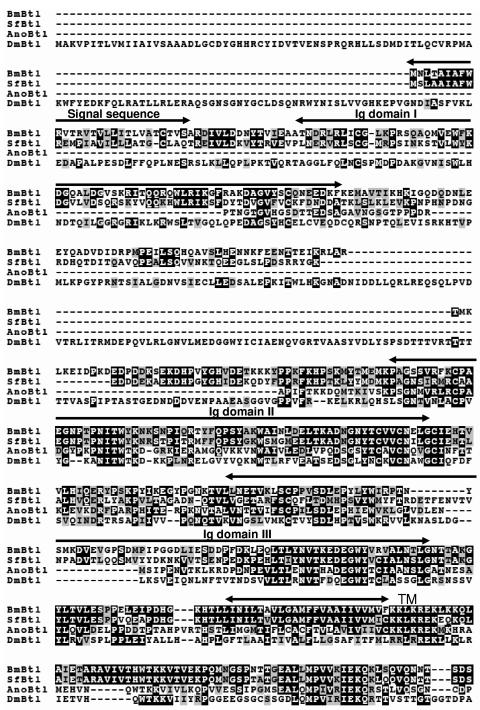
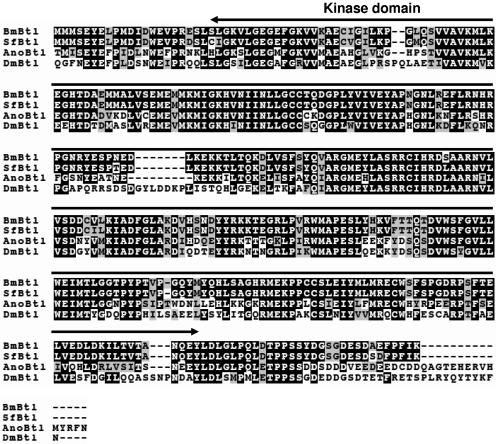
To identify a receptor for BmNPV vFGF, we attempted to clone a breathless (btl) homolog of B. mori (Bmbtl) from B. mori cDNA libraries, since vFGF has a high homology to Drosophila Branchless/FGF, a ligand for Btl (22). In a homology search with Drosophila Btl (DmBtl) as the query and B. mori cDNA sequences as the database, we identified the clone FWDP26_H17 from the B. mori wing disk cDNA library. Sequence analysis revealed that this clone contained a 2,840-bp cDNA, which possessed a 2,568-bp ORF. Deduced amino acid sequence showed that the putative protein was composed of 856 amino acid residues and potentially encoded a protein with a molecular mass of 97.3 kDa. As seen in Fig. 1, the protein has a typical signal peptide sequence at the N terminus, three immunoglobulin (Ig) domains in the extracellular region, a transmembrane domain, and a kinase domain in the intracellular region. This is a typical structure of the receptor-type tyrosine kinase. Thus, we designated this gene as Bmbtl. Using homology cloning, we also isolated a btl ortholog from the Sf-9 cell line (Sfbtl). SfBtl was composed of 847 amino acid residues and showed a high sequence homology to BmBtl (Fig. 2). The amino acid sequences of these lepidopteran Btls were aligned with Anopheles gambiae Btl (AnoBtl, accession number XM_562866) and DmBtl (accession number Q09147). As seen in Fig. 2, the alignment revealed that the kinase domain in the intracellular region was highly conserved between these four Btls. BmBtl has 71.6, 53.5, and 43.3% amino acid sequence identity to SfBtl, AnoBtl, and DmBtl, respectively.
FIG. 1.
Structure of BmBtl. Bmbtl is a 2,840-b-long mRNA containing an ORF encoding 856 amino acid residues. BmBtl has a typical signal peptide sequence at the N terminus, three immunoglobulin (Ig) domains in the extracellular region, a transmembrane domain (TM), and a kinase domain in the intracellular region.
FIG.2.
Alignment of BmBtl, SfBtl, Anopheles gambiae Btl (AnoBtl), and Drosophila Btl (DmBtl). In the sequences shown, black shading denotes identical residues, and gray shading indicates similarities among proteins. The signal sequence, Ig domains (I to III), transmembrane domain (TM), and kinase domain are also shown.
FIG. 2—
Continued.
Expression analysis of Bmbtl.
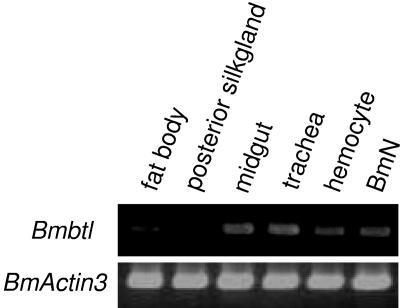
We performed expression analysis of Bmbtl in several tissues of the fifth-instar B. mori larvae. RT-PCR experiments showed that Bmbtl was abundantly expressed in the trachea and midgut, with moderate expression observed in the hemocytes and the B. mori cultured cell line BmN (Fig. 3). Also, we observed the expression of Sfbtl in Sf-9 cells (see below).
FIG. 3.
Bmbtl expression in B. mori. RT-PCR analysis of Bmbtl was performed with RNA prepared from several B. mori tissues or BmN cultured cells. B. mori Actin3 (BmActin3) (14) was used as the control.
Activation of BmBtl by vFGF.
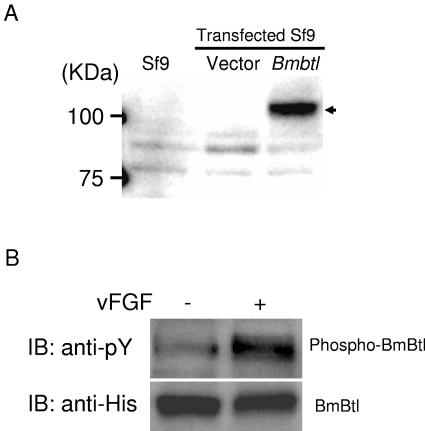
To examine whether BmBtl is a receptor for vFGF, we first generated Sf-9 cells that stably expressed His-tagged BmBtl. Western blot analysis with anti-His antibody showed that His-tagged BmBtl was expressed in Sf-9 cells with a molecular mass of approximately 110 kDa (Fig. 4A). Using this transfectant, we investigated whether or not BmBtl was phosphorylated by vFGF stimulation. As shown in Fig. 4B, Western blot analysis by anti-phospho-tyrosine antibody of the immunoprecipitated proteins clearly showed that BmBtl was markedly phosphorylated by vFGF stimulation.
FIG.4.
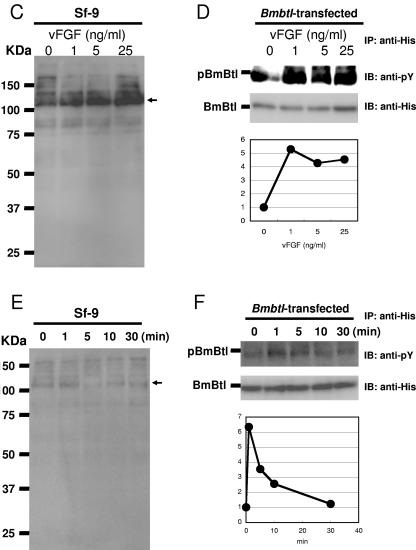
vFGF-induced phosphorylation of BmBtl. (A) Sf-9 cells were transfected with empty vector or BmBtl-His-pIZ. A Western blot analysis with anti-His antibody showed the expression of His-tagged BmBtl in Sf-9 cells transfected with BmBtl-His-pIZ. Protein size markers are shown on the left. (B) Sf-9 cells transfected with BmBtl-His-pIZ were serum starved for 48 h and stimulated with vFGF (5 ng/ml) for 1 min. Cells were then lysed and immunoprecipitated with anti-His antibody. Precipitates were immunoblotted with anti-phospho-tyrosine (anti-pY) or anti-His antibody. Similar results were obtained in three independent experiments. (C) Sf-9 cells were serum starved for 48 h and stimulated with various concentrations (0, 1, 5, or 25 ng/ml) of vFGF for 1 min. Cells were then lysed and immunoblotted with anti-phospho-tyrosine antibody (anti-pY). Arrowhead indicates a band of putative endogenous SfBtl. Similar results were obtained in two independent experiments. (D) Bmbtl-transfected Sf-9 cells were serum starved for 48 h and stimulated with various concentrations (0, 1, 5, or 25 ng/ml) of vFGF for 1 min. Cells were then lysed and immunoprecipitated with anti-His antibody. Precipitates were immunoblotted with anti-phospho-tyrosine (anti-pY) or anti-His antibody. Similar results were obtained in two independent experiments. (E) Sf-9 cells were serum starved for 48 h and stimulated with vFGF (5 ng/ml) for 0, 1, 5, 10 or 30 min. Cells were then lysed and immunoblotted with anti-phospho-tyrosine antibody (anti-pY). An arrow indicates a band of putative endogenous SfBtl. Similar results were obtained in two independent experiments. (F) Bmbtl-transfected Sf-9 cells were serum starved for 48 h and stimulated with vFGF (5 ng/ml) for 0, 1, 5, 10, or 30 min. Cells were then lysed and immunoprecipitated with anti-His antibody. Precipitates were immunoblotted with anti-phospho-tyrosine (anti-pY) or anti-His antibody. Similar results were obtained in two independent experiments.
Western blot analysis with anti-phospho-tyrosine antibody also detected a protein markedly phosphorylated by BmNPV vFGF stimulation in the Sf-9 cells (Fig. 4C). Approximately fivefold induction was observed compared to the basal level when Sf-9 cells were stimulated with 25 ng of BmNPV vFGF/ml. This host protein seemed to be SfBtl, since the size of this protein was approximately 110 kDa, and this protein was phosphorylated by BmNPV vFGF in a dose-dependent manner (1 to 25 ng of vFGF/ml) (Fig. 4C). This suggests that BmNPV vFGF may be able to activate SfBtl. In His-tagged BmBtl-transfected Sf-9 cells, the activation of exogenous BmBtl reached a plateau when 1 ng of vFGF/ml was added (Fig. 4D). Furthermore, in Sf-9 or Bmbtl-transfected Sf-9 cells, we observed maximal induction of endogenous SfBtl or exogenous BmBtl activation at 1 min after vFGF stimulation (Fig. 4E and F).
vFGF-induced cell migration via Btl.
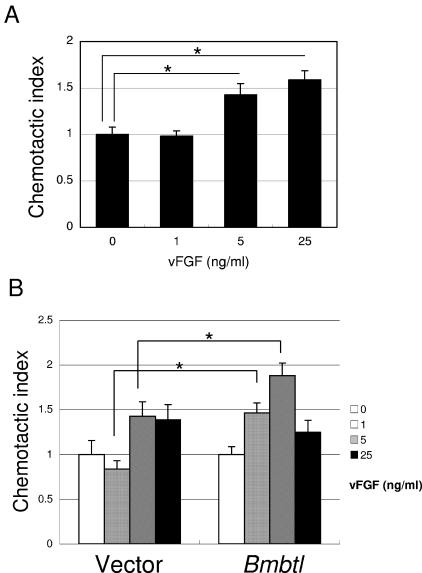
It was recently reported that AcMNPV vFGF had the ability to stimulate the migration of several different types of insect cells, Sf-21 cells, TN-368 cells, and T. ni hemocytes (4). Therefore, we examined whether vFGF-induced migration of host cells was mediated via Btl. Using a Boyden chamber assay, we found that BmNPV vFGF was able to induce migration of Sf-9 cells when 5 or 25 ng of vFGF/ml was added to the medium (Fig. 5A), suggesting that BmNPV vFGF had the ability to migrate Sf-9 cells presumably via SfBtl. Subsequently, we examined the effect of BmBtl overexpression on vFGF-induced cell migration. As shown in Fig. 5B, we observed that BmBtl overexpression in Sf-9 cells enhanced the migration activity at low concentrations of vFGF (1 or 5 ng/ml) compared to that observed in empty vector-transfected cells.
FIG. 5.
Overexpression of BmBtl enhanced the migration activity for vFGF. After incubation with various concentrations of vFGF (0, 1, 5, or 25 ng/ml) for 3 h, the migration activity of Sf-9 cells (A) or Sf-9 cells transfected with empty vector or BmBtl-His-pIZ (B) was examined by using a Boyden chamber assay. The data show means ± the standard errors of triplicates, and similar results were obtained in two independent experiments. ✽, P < 0.05.
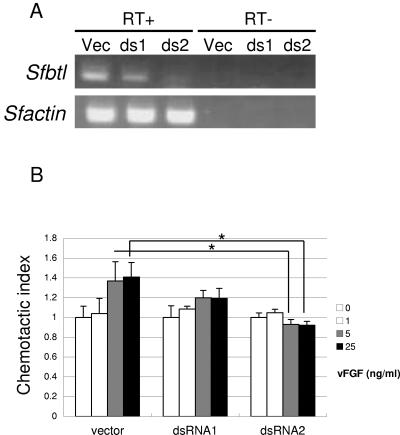
To verify the role of Btl in vFGF-induced cell migration in more detail, we attempted to generate Sf-9 cells in which endogenous Sfbtl was knocked down. Transfection of Sf-9 cells with the plasmids expressing dsRNA for Sfbtl resulted in the generation of dsRNA1 and dsRNA2 cells. We examined the Sfbtl expression level in these transfected cells by using RT-PCR and, as shown in Fig. 6A, Sfbtl was markedly knocked down in the dsRNA2 cells. In contrast, a moderate reduction was observed in the dsRNA1 cells. We performed migration assays with these cells and found that the activity was significantly reduced in dsRNA2 cells but not in dsRNA1 cells (Fig. 6B). This strongly suggests that SfBtl is required for vFGF-induced cell migration. When taken together, these results indicate that lepidopteran Btl is a receptor for vFGF.
FIG. 6.
Sfbtl knockdown reduced the migration activity for vFGF. (A) Transfection of Sf-9 cells with the plasmids expressing dsRNA for Sfbtl resulted in the generation of dsRNA1 (ds1) and dsRNA2 (ds2) cells. RT-PCR analysis of Sfbtl was performed with RNA prepared from the transfected cells. Spodoptera actin was used as a control. RT+, With RT; RT−, without RT. (B) Cell migration induced by vFGF was examined by using a Boyden chamber assay. The data show means ± the standard errors of triplicates, and similar results were obtained in two independent experiments. ✽, P < 0.05.
DISCUSSION
Previous studies have shown that the vFGFs of BmNPV and AcMNPV encode a terminal signal peptide and are secreted from cells (4, 13). This suggests that vFGF may act as an extracellular ligand in order to transduce its signals in the host cells. FGFs are known to function by binding heparin or heparan sulfate proteoglycans to form oligomers, with this complex then specifically interacting with cell surface FGFRs. Subsequently, several molecules are activated near the cell membrane, which leads to triggering of biological activities, such as cell growth and motility (9, 19). In the present study, we identified lepidopteran orthologs of Drosophila Btl as a receptor for vFGF. Also, through the use of overexpression and knockdown studies of the lepidopteran Btls, we were able to show that vFGF induces cell migration via its cell surface receptor Btl. As far as we know, this is the first report to identify a host receptor for a baculovirus-encoded protein.
Expression analysis showed that Bmbtl mRNA was abundantly expressed in the trachea, midgut, hemocytes, and BmN cells. Also, we observed the expression of Sfbtl in Sf-9 cells. These results are consistent with the previous report that the migration of Spodptera-derived Sf-21 cells and T. ni hemocytes was induced by AcMNPV vFGF (4). From our observation that Bmbtl was highly expressed in larval hemocytes, we speculated that vFGF might induce the migration of uninfected host cells (such as hemocytes) and attract these cells to the sites of infection (such as hemocytes and fat bodies) in the infected host larvae. A mutant BmNPV defective in the functional vfgf has been generated (7, 11), and in a preliminary examination we have characterized the properties of this mutant. We found that the 50% lethal time for vfgf-deleted mutant budded virus injection into the fifth-instar B. mori larvae of the mutant was about 1 day longer than that observed for the wild-type virus (S. Katsuma and T. Shimada, unpublished data). This supports our hypothesis that vFGF is involved in the spread efficiency of the virus in vivo. Taken together, we concluded that vFGF promotes cell migration of host insects via cell surface Btl, which presumably leads to enhancement of the virus infection.
Previous studies have reported that after occluded-derived virus infection of the midgut cells, tracheal epithelial cells become infected, and then budded viruses appear in the hemolymph, followed by the infection of other tissues (15, 25). Also, the insect tracheal system has been shown to serve as a conduit for systemic infection since the baculovirus can cross the basal lamina into the hemocoel of the host insect (5). The findings that Bmbtl is abundantly expressed in the trachea of B. mori larvae and that vfgf is expressed as an early gene during virus infection (4, 13) suggest that the vFGF-Btl signaling cascade may play an important role in the early stage of baculovirus infection. To clarify the role of Bmbtl in BmNPV infection in vivo, further studies, such as the generation of genetically modified B. mori in which Bmbtl expression is knocked down or enhanced only in the trachea, are required. Also, we are now constructing a polyhedron-positive mutant BmNPV expressing green fluorescent protein and lacking a functional vfgf. We will be able to know the role of the vFGF-BmBtl cascade in the process of oral infection by comparing pathogenesis in a time course study of a control virus and this green fluorescent protein-expressing vfgf-deficient BmNPV.
vfgf is conserved in all baculoviruses that have been sequenced to date that infect insects in the order Lepidoptera (6, 16). However, vfgf's are absent from baculoviruses that infect orders other than Lepidoptera: Culex nigripalpus NPV that infects insects in the order Diptera (1) and Neodiprion sertifer NPV and Neodiprion lecontei NPV that infect insects in the order Hymenoptera (6, 16). These baculoviruses are known to only replicate at the epithelial cells of the midgut, the primary site of infection, and do not spread systemically (18). Thus, it is theorized that ancestral lepidopteran baculoviruses might have acquired the fgf-related gene from the host genome, thereby allowing it to efficiently spread in the infected host while utilizing the lepidopteran insect tracheal system. To acquire further evidence on the possibility of horizontal transfer, we are in the process of isolating lepidopteran fgf's from baculovirus host insects, such as B. mori.
In conclusion, we identified BmBtl and SfBtl as the receptor for vFGF. Also, the present study indicates that the vFGF-Btl signaling cascade is required in order for vFGF-induced cell migration to occur. To explore the mechanism underlying the downstream pathway of the vFGF-Btl cascade, we are currently searching for molecules that could be activated by the vFGF-Btl signaling by using the B. mori microarray (14, 20).
Acknowledgments
We thank Michiyoshi Takahashi for constructing the Bombyx cDNA libraries and Naoko Omuro for the DNA sequencing.
This study was supported by grants from MEXT (no. 17018007) (to T.S.), JSPS for Scientific Research (no. 16658023), MAFF-NIAS (Insect Technology), and JST (Professional Program for Agricultural Bioinformatics) of Japan.
REFERENCES
- 1.Afonso, C. L., E. R. Tulman, Z. Lu, C. A. Balinsky, B. A. Moser, J. J. Becnel, D. L. Rock, and G. F. Kutish. 2001. Genome sequence of a baculovirus pathogenic for Culex nigripalpus. J. Virol. 75:11157-11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayres, M. D., S. C. Howard, J. Kuzio, M. Lopez-Ferber, and R. D. Possee. 1994. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 202:586-605. [DOI] [PubMed] [Google Scholar]
- 3.Daimon, T., S. Katsuma, M. Iwanaga, W. Kang, and T. Shimada. 2005. The BmChi-h gene, a bacterial-type chitinase gene of Bombyx mori, encodes a functional exochitinase that plays a role in the chitin degradation during the molting process. Insect Biochem. Mol. Biol. 35:1112-1123. [DOI] [PubMed] [Google Scholar]
- 4.Detvisitsakun, C., M. F. Berretta, C. Lehiy, and A. L. Passarelli. 2005. Stimulation of cell motility by a viral fibroblast growth factor homolog: proposal for a role in viral pathogenesis. Virology 336:308-317. [DOI] [PubMed] [Google Scholar]
- 5.Engelhard, E. K., L. N. Kam-Morgan, J. O. Washburn, and L. E. Volkman. 1994. The insect tracheal system: a conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc. Natl. Acad. Sci. USA 91:3224-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Maruniak, A., J. E. Maruniak, P. M. Zanotto, A. E. Doumbouya, J. C. Liu, T. M. Merritt, and J. S. Lanoie. 2004. Sequence analysis of the genome of the Neodiprion sertifer nucleopolyhedrovirus. J. Virol. 78:7036-7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomi, S., S. G. Kamita, and S. Maeda. 1999. Deletion analysis of all genes of Bombyx mori nucleopolyhedrovirus (BmNPV). RIKEN Rev. 22:39-41. [Google Scholar]
- 8.Gomi, S., K. Majima, and S. Maeda, S. 1999. Sequence analysis of the genome of Bombyx mori nucleopolyhedrovirus. J. Gen. Virol. 80:1323-1337. [DOI] [PubMed] [Google Scholar]
- 9.Itoh, N., and D. M. Ornitz. 2004. Evolution of the Fgf and Fgfr gene families. Trends Genet. 20:563-569. [DOI] [PubMed] [Google Scholar]
- 10.Iwanaga, M., K. Takaya, S. Katsuma, M. Ote, S. Tanaka, S. G. Kamita, W. Kang, T. Shimada, and M. Kobayashi. 2004. Expression profiling of baculovirus genes in permissive and nonpermissive cell lines. Biochem. Biophys. Res. Commun. 323:599-614. [DOI] [PubMed] [Google Scholar]
- 11.Kamita, S. G., K. Nagasaka, J. W. Chua, T. Shimada, K. Mita, M. Kobayashi, S. Maeda, and B. D. Hammock. 2005. A baculovirus-encoded protein tyrosine phosphatase gene induces enhanced locomotory activity in a lepidopteran host. Proc. Natl. Acad. Sci. USA 102:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsuma, S., Y. Noguchi, C. L. Zhou, M. Kobayashi, and S. Maeda. 1999. Characterization of the 25K FP gene of the baculovirus Bombyx mori nucleopolyhedrovirus: implications for post-mortem host degradation. J. Gen. Virol. 80:783-791. [DOI] [PubMed] [Google Scholar]
- 13.Katsuma, S., T. Shimada, and M. Kobayashi. 2004. Characterization of the baculovirus Bombyx mori nucleopolyhedrovirus gene homologous to the mammalian FGF gene family. Virus Genes 29:211-217. [DOI] [PubMed] [Google Scholar]
- 14.Katsuma, S., S. Tanaka, N. Omuro, L. Takabuchi, T. Daimon, S. Imanishi, S. Yamashita, M. Iwanaga, K. Mita, S. Maeda, M. Kobayashi, and T. Shimada. 2005. Novel macula-like virus identified in Bombyx mori cultured cells. J. Virol. 79:5577-5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keddie, B. A., G. W. Aponte, and L. E. Volkman. 1989. The pathway of infection of Autographa californica nuclear polyhedrosis virus in an insect host. Science 243:1728-1730. [DOI] [PubMed] [Google Scholar]
- 16.Lauzon, H. A., C. J. Lucarotti, P. J. Krell, Q. Feng, A. Retnakaran, and B. M. Arif. 2004. Sequence and organization of the Neodiprion lecontei nucleopolyhedrovirus genome. J. Virol. 78:7023-7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mita, K., M. Morimyo, K. Okano, Y. Koike, J. Nohata, H. Kawasaki, K. Kadono-Okuda, K. Yamamoto, M. G. Suzuki, T. Shimada, M. R. Goldsmith, and S. Maeda. 2003. The construction of an EST database for Bombyx mori and its application. Proc. Natl. Acad. Sci. USA 100:14121-14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moser, B., J. Becnel, S. White, C. Afonso, G. Kutish, S. Shanker, and E. Almira. 2001. Morphological and molecular evidence that Culex nigripalpus baculovirus is an unusual member of the family Baculoviridae. J. Gen. Virol. 82:283-297. [DOI] [PubMed] [Google Scholar]
- 19.Ornitz, D. M., and N. Itoh. 2001. Fibroblast growth factors. Genome Biol. 2:REVIEWS3005.1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ote, M., K. Mita, H. Kawasaki, M. Seki, J. Nohata, M. Kobayashi, and T. Shimada. 2004. Microarray analysis of gene expression profiles in wing discs of Bombyx mori during pupal ecdysis. Insect Biochem. Mol. Biol. 34:775-784. [DOI] [PubMed] [Google Scholar]
- 21.Powers, C. J., S. W. McLeskey, and A. Wellstein. 2000. Fibroblast growth factors, their receptors and signaling. Endocrinol. Relat. Cancer 7:165-197. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland, D., C. Samakovlis, and M. A. Krasnow. 1996. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 87:1091-1101. [DOI] [PubMed] [Google Scholar]
- 23.Takagaki, K., S. Katsuma, Y. Kaminishi, T. Horio, T. Tanaka, T. Ohgi, and J. Yano. 2005. Role of Chk1 and Chk2 in Ara-C-induced differentiation of human leukemia K562 cells. Genes Cells. 10:97-106. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka, H., M. Yamamoto, Y. Moriyama, M. Yamao, S. Furukawa, A. Sagisaka, H. Nakazawa, H. Mori, and M. Yamakawa. 2005. A novel Rel protein and shortened isoform that differentially regulate antibacterial peptide genes in the silkworm Bombyx mori. Biochim. Biophys. Acta 1730:10-21. [DOI] [PubMed] [Google Scholar]
- 25.Trudeau, D., J. O. Washburn, and L. E. Volkman. 2001. Central role of hemocytes in Autographa californica M nucleopolyhedrovirus pathogenesis in Heliothis virescens and Helicoverpa zea. J. Virol. 75:996-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada, M., S. Katsuma, T. Adachi, A. Hirasawa, S. Shiojima, T. Kadowaki, Y. Okuno, TA. Koshimizu, S. Fujii, Y. Sekiya, Y. Miyamoto, M. Tamura, W. Yumura, H. Nihei, M. Kobayashi, and G. Tsujimoto. 2005. Inhibition of protein kinase CK2 prevents the progression of glomerulonephritis. Proc. Natl. Acad. Sci. USA 102:7736-7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamauchi, J., Y. Miyamoto, H. Kokubu, H. Nishii, M. Okamoto, Y. Sugawara, A. Hirasawa, G. Tsujimoto, and H. Itoh. 2002. Endothelin suppresses cell migration via the JNK signaling pathway in a manner dependent upon Src kinase, Rac1, and Cdc42. FEBS Lett. 527:284-288. [DOI] [PubMed] [Google Scholar]